Abstract
Aim:
Gastrointestinal (GI) nematodes are considered as a major constraint for successful sheep production. Control of these parasites heavily relies on the use of chemical anthelmintics. Over the past decades, the development of anthelmintic resistance to various groups of anthelmintics and problem of drug residues in animal products has awakened interest in medicinal plants as an alternative source of anthelmintics. Hence, this study was undertaken to evaluate the anthelmintic efficacy of Indigofera tinctoria by scientifically validated in vitro and in vivo tests approved by the World Association for the Advancement of Veterinary Parasitology.
Materials and Methods:
In vitro assays such as egg hatch assay for ovicidal and larval migration inhibition and larval development assay for larvicidal properties were used to investigate in vitro effect of extracts on strongyle egg and larvae, respectively. Fecal egg count reduction test was conducted in vivo to evaluate the therapeutic efficacy of the extracts administered orally at dose rates of 125, 250, 500 mg/kg to sheep naturally infected with mixed GI nematodes.
Results:
Ethanolic extract of I. tinctoria demonstrated significant (p<0.01) inhibition on egg hatching at concentrations of 40 mg/ml and 80 mg/ml. In in vivo assay, the ethanolic extract of I. tinctoria reduced the fecal egg count ranging between 30.82% and 47.78% at various doses (125, 250 and 500 mg/kg). Although there was a slight variation, all the hematological parameters were within the normal range reported for sheep. Except for alanine transaminase, the overall mean of all the serum biochemical profile was within the normal range for sheep.
Conclusion:
Based on the results obtained by in vitro and in vivo assay, the ethanolic extract of I. tinctoria possesses anthelmintic activity and could replace the chemical anthelmintics used presently.
Keywords: anthelmintic, evaluation, gastrointestinalnematodes, Indigofera tinctoria, sheep
Introduction
Sheep production plays a vital role in augmenting socio-economic status of the rural masses, particularly the small landholders and landless farmers, who rely on these animals for their animal protein source and income for their livelihood [1]. However, mismanagement, poor hygiene, and precarious housing conditions all contributed to the incidence of disease and high mortality. Parasitic diseases, especially gastrointestinal (GI) nematodes, are among the factors that limit sheep production worldwide, accounting for thelargest economic losses due to retarded growth, weight loss, reduced food consumption, lower milk production, impaired fertility, and in cases of massive infections, highmortality rates [2].
Currently, nematode control programs in sheep depend mainly through the use of anthelmintics. Development of anthelmintic resistance [3], increased public awareness over the drug residues in animal products and toxicity problems [4] has necessitated an intensified effort to find alternative endoparasite control measures that are both feasible and economical for the farmers [5]. Among the alternative strategies, there has been considerable and expanding interest in traditional herbal dewormers in both developed and developing countries. Several studies have showed that plant species can effectively reduce the degree of parasite infestation in livestock and are promising alternatives to conventional anthelmintics [6,7] that are both sustainable and environmentally acceptable.
Hence, the present study was envisaged to evaluate the anthelmintic properties of Indigofera tinctoria plant extracts by scientifically validated in vitro and in vivo techniques.
Materials and Methods
Ethical approval
The study was conducted after the approval of the Institutional Animal Ethics Committee.
Collection of plant materials and extraction
I. tinctoria is a medicinal plant belonging to the family Papilionaceae and extensively used for its blue dye indigo. It is a small erect shrub cultivated in all parts of India, especially in the Southern India, Tamil Nadu. The plant is a purgative, antiseptic and astringent. In Ayurveda and Siddha systems, the entire plantsareused for the treatment of helminthic infections. The leaves of I. tinctoria were collected from the local field and certified by a botanist. The collected plant materials were shade dried, powdered and stored in airtight container for further extraction. The aqueous extract was prepared as prescribed by Onyeyili et al. [8]. The dried extract wascollected in stoppered vials and stored at 4°C until use. The ethanolic extract was prepared as described by Wang and Waller [9].
Gas chromatography–mass spectrometry (GC-MS)analysis
GC-MS analysis was carried out on a GC clarus 500 Perkin Elmer system comprising an AOC-20i autosampler and GC-MS instrument employing the following conditions: Column Elite-1 fused silica capillarycolumn (30 mm ID × 0.25 mm ID × 1 µM df), composed of 100% dimethyl poly diloxide, operating in electron impact mode at 70eV; helium (99.99%) was used as carrier gas at a constant flow of 1 ml/min and an injection volume of 0.5 µl was employed (split ratio of 10:1) injector temperature 250°C; ionsource temperature 280°C. The oven temperature was programed from 110°C (isothermal for 2 min), with an increase of 10°C/min, to 200°C, then 5°C/min to 280°C, ending with a 9 min isothermal at 280°C. Mass spectra were taken at 70 eV; a scan interval of 0.5 s and fragments from 40 to 450 Da. Total GC running time is 36 mininterpretation on mass spectrum GC-MS was conducted using the database of the National Institute Standard and Technology (NIST) having more than 62,000 patterns. The spectrum of the unknown component was compared with the spectrum of the known components stored in the NIST library. The name, molecular weight and structure of the components of the test materials were ascertained.
Preparation plant/drug stock solution
Pure thiabendazole and levamisole (0.1 g, Sigma, USA) were transferred into a 100 ml volumetric flask through a small glass funnel and rinsed twice, each with 10 ml of dimethyl sulfoxide (DMSO). A further 20 ml of DMSO was added, and the total volume was made up to 100 ml with distilled water. Using the stock solution, a suitable working solution of thiabendazole with a final concentration of 200 µg/ml was prepared and used as positive control. Stock solutions of crudeaqueousandethanolicextracts of I. tinctoria initially were prepared by dissolving the crude extracts in DMSO so as to improve their solubility in water. Aliquots of stock solution (100 mg/ml) were further diluted to obtain final concentrations of 10 (1%), 20 (2%), 40 (4%), and 80.0 (8%) mg/ml for each extract.
In vitro tests
Plant extracts at concentrations of 10, 20, 40 and 80 mg/ml were used for all in vitro assays.
Egg hatch assay - Pooled fecal samples were obtained by mixing several samples collected per rectum from a number of sheep naturally infected with mixed GI nematodes. About 40 ml of water was added to the fecal sample and kneaded thoroughly. The macerated fecal material was then suspended in 1 L of tap water and the fecal suspension was washed over a series of sieves of decreasing sizes (500, 75 and 35 µm). The retentate in the 35 µm sieve, which contained the nematode eggs was washed and collected in a polyallomer tube and centrifuged at 1000 rpm for 1-2 min. After removing the supernatant, the sediment was resuspended in 10-12 ml of saturated sodium chloride solution. After thorough and gentle mixing, the suspension was centrifuged again at 1000 rpm for 1-2 min. Using artery forceps, the polyallomer tube was clamped just below the meniscus and the contents above the clamp were transferred into a 15 ml polystyrene tube and washed twice with distilled water. Isolated eggs were pooled and made up to a volume of 10 ml. From this suspension, 100 µl was pipetted eggs counted and resuspended in such a manner that 100 µl of the suspension contained approximately 100 eggs. The assay was performed in 24 well plates as per the method described by Jackson et al. [10]. The percentage of hatch for each concentration was calculated, and the results were subjected to probit analysis to obtain effective dose 50% values.
In larval development assay, eggs were harvested from the pooled fecal samples and the concentration of eggs was estimated in 100 µl samples and adjusted to 100 eggs per 100 µl. The assay was conducted as per the methods designed by Hubert and Kerboeuf [11]. The mean larval development for each drug concentration and the lethal dose 50% value was determined by plotting the percentage larval development and drug concentration.
Larval migration assay was conducted as per the method described by Jackson et al. [10]. The number of larvae retained by the mesh (Nr) and those that had migrated (Nm) through the mesh was counted. The drug concentration against percentage migration was plotted over a graph and the lethal mutation 50% values were derived.
In vivo tests
Vembur sheep maintained under semi-intensive system of management at Instructional Livestock Farm Complex, Veterinary College and Research Institute, Ramayanpatti, Tirunelveli were utilized for this study. 30 vembur lambs of 6-12 months age which showed eggs per gram of feces (EPG) from 1000 to 2700 before treatment were selected and randomly distributed into five treatment groups each comprising of six animals. Three groups were treated with doses of plant extracts at 125, 250 and 500 mg/kg, respectively, while the fourth and the fifth group served as positive and negative controls. Fecal samples were collected from each animal on day 0 and at day 12 post-treatment and fecal egg count reduction (FECR) was assessed as recommended by the World Association for the Advancement of Veterinary Parasitology [12].
Estimation of hematological and serum parameters
Blood samples were collected on day 0, 3, and 12 post-treatment from each animal and hematological parameters were determined as described by Jain [13]. Serum biochemical profiles were determined using standard diagnostic kits obtained from Span Diagnostics Ltd. Pooled fecal samples were cultured and identified as described by MAFF [14].
Statistical analysis
For in vitro assays, probit transformation was performed to transform a typical sigmoid dose-response curve to linear function [11]. Fecal egg count, hematological and serum biochemical parameters were analyzed by the statistical methods as described by Snedecor and Cochran [15].
Results
The results of in vitro assays were shown in Table-1. Of the four doses (10, 20, 40 and 80 mg/ml) tested, ethanolic extract of I. tinctoria induced a significant egg hatch inhibition at 40 and 80 mg/ml and aqueous extract induced only marginal inhibition at all the concentrations tested. On the other hand, both aqueous and ethanolic extracts of I. tinctoria did not induce significant inhibition of larval development andmigration. Only, ethanolic extract of I. tinctoria which was proved to be effective in vitro egg hatch assay was further evaluated by in vivo assay (FECR test).
Table-1.
Mean percent efficacy of aqueous and ethanolic extracts of I. tinctoriaon nematode egg hatch, larval migration and larval development inhibition.
| Name of the plant extracts | Concentration of the plant extracts | Positive control | Negative control | |||
|---|---|---|---|---|---|---|
| 1% | 2% | 4% | 8% | |||
| Egg hatch assay | ||||||
| Aqueous extract | 7.50c±0.35 | 12.50bc±0.35 | 18.50ab±0.35 | 24.50a±4.50 | 97.03a±0.82 | 8.79d±1.05 |
| Ethanolic extract | 50.00c±8.00 | 61.00b±1.00 | 68.50ab±0.50 | 73.50a±0.50 | 97.03a±0.82 | 8.79b±1.05 |
| Larval migration inhibtion assay | ||||||
| Aqueous extract | 25.60b±4.17 | 34.09b±3.16 | 30.20±2.20 | 34.52±5.11 | 96.22a±0.35 | 8.27c±0.98 |
| Ethanolic extract | 5.78c±1.19 | 8.15bc±0.65 | 15.10a±1.78 | 14.40a±1.47 | 96.22a±0.35 | 8.27c±0.98 |
| Larval development assay | ||||||
| Aqueous extract | 12.50±0.90 | 13.50±3.10 | 19.80±2.00 | 17.70±1.10 | 93.46a±1.82 | 5.30b±1.12 |
| Ethanolic extract | 20.20±3.40 | 18.90±1.70 | 21.60±3.60 | 22.30±2.10 | 93.46a±1.82 | 5.30d±1.12 |
Values sharing any one common superscript in a row (overall) do not differ significantly (p<0.01). NS=Not significant, I. tinctoria=Indigofera tinctoria
The percentage reduction in fecal egg counts of sheep treated with different doses of ethanolic extract of I. tinctoria and albendazole are presented (Table-2). The ethanolic extract of I.tinctoria produced a dose-dependent reduction in EPG on 12 days post-treatment with higher reduction of 47.78% at 500 mg/kg. Sheep drenched with albendazole (albomar - positive control) at 7.0 mg/kg showed 93.25% reduction in EPG.
Table-2.
Effect of I. tinctoria ethanolic extracton percent FECR (mean±SE) in sheep naturally infected with GI nematodes.
| Dose (mg/kg) | Ethanolic extract of I. tinctoria | Albendazole (7 mg/kg) |
|---|---|---|
| 125 | 30.82cB±1.90 | 93.25a±1.15 |
| 250 | 41.41bA±2.06 | 93.25a±1.15 |
| 500 | 47.78bA±2.04 | 93.25a±1.15 |
(P<0.01) Values sharing any one common superscript in a row (small letters) and column (capital letters) do not differ significantly. I. tinctoria=Indigofera tinctoria, FECR=Fecal egg count reduction, GI=Gastrointestinal, SE=Standard error
Coproculture
Oesophagostomum columbianum was the primary GI nematode infecting animals with 71%. Haemonchus contortus was the second averaging 27% followed by Bunostomum spp. (1%).
Effect of plant extracts on hematological values of sheep
The mean values of hemogram and serum profilein sheep treated with ethanolic extract of I. tinctoria and albendazole (albomar) (positive control) are presented (Tables-3 and 4). There was not much variation in hemogram and serum biochemical levels before and after treatment at all the doses tested.
Table-3.
Effect of I. tinctoria ethanolic extracton blood parameters (mean±SE) in sheep naturally infected with GI nematodes.
| Serum parameters | Period | Dose (mg/kg) | Positive control | Negative control | ||
|---|---|---|---|---|---|---|
| 125 | 250 | 500 | ||||
| PCV (%) | 0-day | 29.33±0.71 | 28.90±0.25 | 29.47±0.58 | 26.73±2.01 | 27.47±2.11 |
| 3rd-day | 25.00±4.36 | 30.10±4.97 | 36.00±4.51 | 27.77±2.10 | 28.07±1.65 | |
| 12th-day | 22.00±10.54 | 26.67±1.76 | 27.67±1.67 | 29.00±2.45 | 29.07±1.77 | |
| Overall | 25.44bcd±3.47 | 28.56ab±1.61 | 31.04a±1.89 | 27.83abc±1.14 | 28.20ab±0.95 | |
| Hb concentration (g/dL) | 0-day | 9.70±0.29 | 9.87±0.12 | 9.97±0.17 | 9.33±0.73 | 9.57±0.88 |
| 3rd-day | 9.50±0.75 | 10.73±1.45 | 10.37±0.24 | 9.77±0.74 | 9.97±0.86 | |
| 12th-day | 11.17±1.57 | 10.57±1.19 | 10.30±0.29 | 10.13±0.78 | 10.23±0.88 | |
| Overall | 10.12b±0.57 | 10.39a±0.56 | 10.21ab±0.13 | 9.74abcd±0.39 | 9.92abc±0.45 | |
| TEC (106/μl) | 0-day | 8.88±0.34 | 8.98±0.13 | 9.02±0.20 | 8.63±0.76 | 8.47±0.75 |
| 3rd-day | 9.10±0.35 | 9.08±0.16 | 9.17±0.33 | 8.76±0.76 | 8.69±0.80 | |
| 12th-day | 9.20±0.25 | 8.86±0.13 | 9.20±0.21 | 8.96±0.76 | 9.07±0.72 | |
| Overall | 9.06ab±0.17 | 8.97abc±0.08 | 9.13a±0.13 | 8.78abcde±0.38 | 8.74abcde±0.39 | |
| TLC (103/μl) | 0-day | 8.80±0.17 | 8.77±0.24 | 8.17±0.81 | 6.77±0.52 | 8.67±0.79 |
| 3rd-day | 9.10±0.21 | 9.10±0.21 | 8.43±0.84 | 7.60±0.25 | 9.33±0.66 | |
| 12th-day | 9.00±0.12 | 8.97±0.19 | 8.40±0.64 | 8.00±0.21 | 9.23±0.33 | |
| Overall | 8.97ab±0.10 | 8.94bc±0.12 | 8.33d±0.39 | 7.46e±0.25 | 9.08a±0.33 | |
Values sharing any one common superscript in a row (overall) do not differ significantly (p<0.01). NS=Not significant (p<0.05), PCV=Packed cell volume, TEC=Total erythrocyte count, TLC=Total leukocyte count, I. tinctoria=Indigofera tinctoria, GI=Gastrointestinal, SE=Standard error
Table-4.
Effect of I. tinctoira ethanolic extract on serum parameters (mean±SE) in sheep naturally infected with GI nematodes.
| Serum parameters | Period | Dose (mg/kg) | Positive control | Negative control | ||
|---|---|---|---|---|---|---|
| 125 | 250 | 500 | ||||
| BUN | 0-day | 49.07±3.50 | 48.77±0.26 | 50.77±1.82 | 43.37±1.78 | 47.23±6.89 |
| 3rd-day | 38.90±1.85 | 38.93±1.10 | 50.70±3.00 | 52.90±2.25 | 42.47±11.11 | |
| 12th-day | 48.17±5.74 | 48.70±1.46 | 49.33±2.03 | 47.10±1.46 | 49.43±1.15 | |
| Overall | 45.38abcd±2.59 | 45.47abcd±1.72 | 50.27ab±1.19 | 47.79abcd±1.67 | 46.38abcd±3.93 | |
| Serum creatinine | 0-day | 1.47±0.12 | 1.37±0.03 | 1.53±0.03 | 1.40±0.06 | 1.40±0.12 |
| 3rd-day | 1.30±0.00 | 1.33±0.03 | 1.47±0.07 | 1.53±0.09 | 1.57±0.09 | |
| 12th-day | 1.50±0.12 | 1.47±0.03 | 1.53±0.03 | 1.43±0.03 | 1.47±0.03 | |
| Overall | 1.42bc±0.06 | 1.39bc±0.03 | 1.51abc±0.03 | 1.46bc±0.04 | 1.48bc±0.05 | |
| SGOT | 0-day | 115.83±2.89 | 103.23±2.57 | 135.13±10.87 | 124.67±2.11 | 109.20±12.01 |
| 3rd-day | 106.67±8.70 | 115.67±14.28 | 125.43±7.97 | 103.97±3.98 | 97.53±12.16 | |
| 12th-day | 121.63±7.86 | 96.77±5.62 | 116.70±9.50 | 113.47±17.02 | 90.27±5.49 | |
| Overall (NS) | 114.71±4.11 | 105.22±5.28 | 125.76±5.45 | 114.03±5.90 | 99.00±5.87 | |
| SGPT | 0-day | 29.00±2.30 | 20.90±4.08 | 13.40±3.38 | 27.83±5.98 | 20.70±1.88 |
| 3rd-day | 24.40±3.92 | 30.00±3.25 | 23.03±6.93 | 23.40±3.80 | 18.90±3.09 | |
| 12th-day | 18.13±2.10 | 21.70±2.15 | 20.97±0.45 | 21.10±1.00 | 17.00±0.61 | |
| Overall (NS) | 23.84±2.14 | 24.20±2.18 | 19.13±2.67 | 24.11±2.29 | 18.87±1.19 | |
Values sharing any one common superscript in a row (overall) do not differ significantly (p<0.01). NS=Not significant (p<0.05), SGPT=Serum glutamic pyruvic transaminase, SGOT=Serum glutamicoxaloacetic transaminase, BUN=Blood urea nitrogen, I. tinctoria=Indigofera tinctoria, GI=Gastrointestinal, SE=Standard error
Phytocomponents
About 10 phytocomponents were identified in the phytochemical screening of the ethanolic extract of I. tinctoira (Table-5 and Figure-1).
Table-5.
Phytocomponents identified in the ethanolic extract of I. tinctoira.
| RT | Name of the compound | Molecular formula | MW | Peak area % |
|---|---|---|---|---|
| 7.01 | 1,3,5-Triazine-2,4,6 (1H,3H,5H)trione | C3H3N3O3 | 129 | 2.31 |
| 7.95 | Hydrazine, 1,2-dimethyl- | C2H8N2 | 60 | 13.33 |
| 10.14 | Myo-Inositol, 2-Cmethyl- | C7H14O6 | 194 | 30.00 |
| 11.61 | 2-Tetradecyne | C14H26 | 194 | 1.54 |
| 12.10 | Z-2-Dodecenol | C12H24O | 184 | 0.51 |
| 13.07 | Ethaneperoxoic acid, 1-cyano-1-[2-(2-phenyl-1,3-dioxolan-2-yl) ethyl] pentyl ester | C19H25NO5 | 347 | 0.26 |
| 14.83 | 3-Butyn-2-ol | C4H6O | 70 | 0.51 |
| 14.96 | Orthoformic acid, tri-2-butenyl ester | C13H22O3 | 226 | 0.26 |
| 20.87 | 1,2-Benzenedicarboxylic acid, diisooctyl ester | C24H38O4 | 390 | 25.64 |
| 24.73 | 1,5-Heptadiene, 2,6-dimethyl- | C9H16 | 124 | 25.64 |
I. tinctoria=Indigofera tinctoria, MW=Molecular weight
Figure-1.
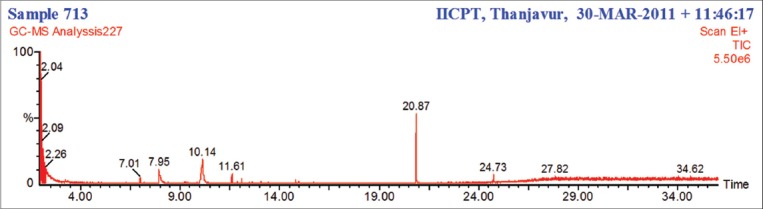
Gas chromatography-mass spectrometry chromatogram of the ethanolic extract of Indigofera tinctoria.
Discussion
Ethanolic extract of I. tinctoria demonstrated significant (p<0.05) inhibition of egg hatching at 40 and 80 mg/ml. Increasing concentration of the plant extracts resulted in increased inhibition of egg hatching indicating dose-dependent activity. Similar dose-dependent in vitro egg hatching inhibition was evaluated using aqueous extract of Caryocar brasiliense camb [16] and aqueous and methanolic extract of Ocimum sanctum [17] against H. contortus. The findings are in agreement with Balamurugan and Selvarajan [18] who reported that the methanolic extract of I. tinctoria revealed maximum activity against the Indian earthworm Pheretima posthuma owing to its anatomical and physiological resemblance with the intestinal roundworm of humans.
Both the extracts of I. tinctoria inhibited migration of H. contortus larvae only to the level of 5.78-34.52%. In contrast, Bendixsen et al. [19] reported larvicidal activity by LMIA with aqueous extracts obtained from Caliandra spp., Leucaena glauca and Acacia farnesiana at 0.8 mg/ml.
Larval development was not inhibited by both aqueous and ethanolic extracts of I. tinctoira which was comparable with the results recorded by Ademola et al. [20] when aqueous and ethanolic extracts of Nauclea latifolia were used.
The highest percent FECR (47.78%) recorded with ethanolic extract of I. tinctoria was in agreement with Soro et al. [21] who recorded a 81% FECR at a single oral dose of 80 mg/kg 3 weeks post-treatment using ethanolic extract of Anogeissus leiocarpus in sheep naturally infected with GI nematodes. The results were in par with the findings recorded by Mesquita Mde and Batista [22] with Eucalyptus staigeriana essential oil, banana crop residues [23] and Lespedeza cuneata [24].
The observed increase in the hemoglobin (Hb) levels in animals was in accordance with the findings of Hossain et al. [25] who reported increased Hb content in sheep when treated with neem leaves and which might be due to increased absorption of iron. Similar increase in Hb and total erythrocyte count were also recorded by Rahman [26] with neem, betel leaf and jute leaves in goats and Rob et al. [27] with aqueous extract of neem leaves in sheep. The increase in total leukocyte count in the present study was in accordance with the result obtained by Agaie and Onyeyili [28] with A. leiocarpus which might probably be due to the immune response to infection or sensitization.
Blood urea nitrogen and serum creatinine values were within normal in treated animals which implies that plant extracts used in the current study have no adverse effects on the kidney. Higher serum alanine transaminase values above normal in this study as recorded by Ajala et al. [29] with Millettia thonningii leaves in bucks, gradually decreased during the observation period which is comparable to values recorded in the control animal group. This implies that these extracts have no toxic effects on liver and heart.
Phytochemical analysis of the ethanolic extract of I. tinctoira revealed that the mechanism of action is not fully understood. However, the collective or individual presence of bioactive compounds in the extract may possibly constitute the basis for the profound anthelmintic activity exhibited by the plant extract as opined by Ruben et al. [30].
Conclusion
Ethanolic extract of I. tinctoira possess potential anthelmintic activity and offer an alternative source for the control of GI nematodes of sheep.
Authors’ Contributions
TJH designed the experiment, sample collection and experiment was performed by AM under the supervision of TJH. Manuscript preparation was supervised, reviewed and edited by TJH and TA. All authors read and approved the final manuscript.
Acknowledgments
The authors are grateful to the Dean, Veterinary College and Research Institute, Namakkal, Tamil Nadu for kind permission and providing necessary permission and facilities for undertaking this work and the authorities of Tamil Nadu Veterinary and Animal Sciences University, Chennai for providing funds to carry out the work.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Lateef M. Trichostrongyloide nematodes of sheep:Epidemiological aspects and evaluation of anthelmintic activity of indigenous plants. Faisalabad, Pakistan: Ph.D. Thesis. University of Agricultur; 2003. [Google Scholar]
- 2.Cavalcante C.R, Vieira L.S, Chagas A.C.S, Molento M.B. Parasitic Diseases of Goats and Sheep:Epidemiology & Control. 1st ed. Brasilia: Embrapa Information Technology; 2009. p. 603. [Google Scholar]
- 3.Taylor M.A, Learmount J, Lunn E, Morgan C, Craig B.H. Multiple resistance to anthelmintics in sheep nematodes and comparison of methods used for their detection. Small Rumin. Res. 2009;86:67–70. [Google Scholar]
- 4.Muhammad G, Abdul J, Khan M.Z, Saqib M. Use of neostigmine in massive ivermectin toxicity in cats. Vet. Hum. Toxicol. 2004;46:28–29. [PubMed] [Google Scholar]
- 5.Ademola I.O, Eloff J.N. In vitro anthelmintic activity of Combretum molle (RBr. ex G. Don) (Combretaceae) against Haemonchus contortus ova and larvae. Vet. Parasitol. 2010;169:198–203. doi: 10.1016/j.vetpar.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 6.Eguale T, Tadesse D, Giday M. In vitro anthelmintic activity of crude extracts of five medicinal plants against egg-hatching and larval development of Haemonchus contortus. J. Ethnopharmacol. 2011;137(1):108–113. doi: 10.1016/j.jep.2011.04.063. [DOI] [PubMed] [Google Scholar]
- 7.Jorge F.S.F, Peaden P, Keiser J. In vitro trematocidal effects of crude alcoholic extracts of Artemisia annua, A. absinthium, Asimina triloba and Fumaria officinalis Trematocidal plant alcoholic extracts. Parasitol. Res. 2011;109:1585–1592. doi: 10.1007/s00436-011-2418-0. [DOI] [PubMed] [Google Scholar]
- 8.Onyeyili P.A, Nwosu C.O, Amin J.D, Jibike J.I. Anthelmintic activity of crudea aqueous extract of Nauclea latifolia stems bark against ovine nematodes. Fitoterapi. 2001;72:12–21. doi: 10.1016/s0367-326x(00)00237-9. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Waller C.L. Recent advances in extraction of naturaceuticals from plants. Trends Food Sci. Technol. 2006;17:300–312. [Google Scholar]
- 10.Jackson F, Jackson E, Coop R.L. Larval migration inhibition assay for determination of susceptibility of nematodes to levamisole. In: Halto D.W, Behnke J.M, Marshall I, editors. Practical Exercise in Parasitology. Cambridge: Cambridge University Press; 2001. pp. 321–327. [Google Scholar]
- 11.Hubert J, Kerboeuf D. A microlarval development assay for the detection of anthelmintic resistance in sheep nematodes. Vet. Rec. 1992;130:442–446. doi: 10.1136/vr.130.20.442. [DOI] [PubMed] [Google Scholar]
- 12.Coles G.C, Bauer C, Borgsteede F.H.M, Geerts S, Taylor M.A, Waller P.J. World Association for the Advancement of Veterinary Parasitology methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 1992;44:35–44. doi: 10.1016/0304-4017(92)90141-u. [DOI] [PubMed] [Google Scholar]
- 13.Jain N.C, editor. Schalm’s Veterinary Haematology. 4th ed. Philadelphia: Lea and Febige; 1986. p. 1221. [Google Scholar]
- 14.MAFF. Manual of Veterinary Parasitological Laboratory Techniques. London: Ministry of Agricultur., Fisheries and Food, Technical Bulletin, HMSO; 1971. pp. 36–42. [Google Scholar]
- 15.Snedecor G.W, Cochran W.G. Statistical Methods. 6th ed. Calcutta: Oxford and IBH Publishing Co; 1976. [Google Scholar]
- 16.Nogueira F.A, Fonseca L.D, Silva R.B. In vitro and in vivo efficacy of aqueous extract of Caryocar brasiliense camb to control gastrointestinal nematodes in sheep. Parasitol. Res. 2012;111(1):325–330. doi: 10.1007/s00436-012-2843-8. [DOI] [PubMed] [Google Scholar]
- 17.Sujith S, Sreedevi R, Priya M.N, Deepa C.K, Darsana U, Sreeshitha S.G, Suja R.S, Juliet S. Anthelmintic activity of three Indian medicinal plants. Int. J. Pharmacogn. Phytochem. Res. 2015;7(2):361–364. [Google Scholar]
- 18.Balamurugan G, Selvarajan S. Preliminary phytochemical screening and anthelmintic activity of Indigofera tinctoria Linn. Int. J. Drug Dev. Res. 2009;1:157–160. [Google Scholar]
- 19.Bendixsen T, Hanh H.T, Van Dong V, van Binh D, Lien P.S. Proceedings of International Workshop on Small Ruminant Production and Development in South East Asia Held at Vietnam. 2005 Mar;2-4:97–99. [Google Scholar]
- 20.Ademola I.O, Fagbemi B.O, Idowu S.O. Anthelmintic efficacy of Nauclea latifolia extract against gastrointestinal nematodes of sheep. In vitro and in vivo studies. Afr. J. Trad. Compliment. Altern. Med. 2007;4:148–156. [PMC free article] [PubMed] [Google Scholar]
- 21.Soro M, Kone W.M, Bonfoh B. In vivo anthelmintic activity of Anogeissus leicarpus Guill & Perr (Combretaceae) against nematodes in naturally infected sheep. Parasitol. Res. 2013;112(7):2681–2688. doi: 10.1007/s00436-013-3435-y. [DOI] [PubMed] [Google Scholar]
- 22.Mesquita Mde A, Batista Je S.J. Anthelmintic activity of Eucalyptus staigeriana encapsulated oil on sheep gastrointestinal nematodes. Parasitol. Res. 2013;112(9):3161–3165. doi: 10.1007/s00436-013-3492-2. [DOI] [PubMed] [Google Scholar]
- 23.Nogueira F.A, Fonseca L.D. Anthelminthic efficacy of banana crop residues on gastrointestinal nematodes of sheep In vitro and in vivo tests. Parasitol. Res. 2012;111(1):317–323. doi: 10.1007/s00436-012-2842-9. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed M, Laing M.D, Nsahlai I.V. In vivo effect of selected medicinal plants against gastrointestinal nematodes of sheep. Trop. Anim. HealthProd. 2014;46(2):411–417. doi: 10.1007/s11250-013-0506-0. [DOI] [PubMed] [Google Scholar]
- 25.Hossain S.A, Mostofa M, Alam M.N, Awal M.A, Ahmed N.U. Comparative efficacy of modern anthelmintics and neem (leaves and seeds) in the treatment of bovine nematodiasis. Pharmacogn. Agric. 1996;7:29–33. [Google Scholar]
- 26.Rahman M. In vitro and in vivo anthelmintic effects of some plants against gastrointestinal nematodes of goats. Mymensingh: M.Sc. Thesis. Submitted to the Department of Parasitolog., Bangladesh Agricultural University; 2002. [Google Scholar]
- 27.Rob S, Mostafa M, Awal M.A, Shahiduzzaman M, Sardar S.A. Comparative efficacy of albendazole (Endokil) and neem (Azadirachta indica) leaves extract against haemonchosis in sheep. Pharmacogn. Agric. 2004;15:33–39. [Google Scholar]
- 28.Agaie B.M, Onyeyili P.A. Anthelmintic activity of the crude aqueous leaf extracts of Anogeissus leiocarpus in sheep. Afr. J. Biotech. 2007;6:1511–1515. [Google Scholar]
- 29.Ajala O.O, Oyeyemi M.O, Oke A.O, Alakpa C.O. Haematological and biochemical parameters in West African dwarf (WAD) bucks fed diets containing Millettia thonningii. Afr. J. Biomed. Res. 2000;3:121–124. [Google Scholar]
- 30.Ruben D.K, Baltini S, Andrew W, Abdulrahman F.I. Preliminary phytochemical screening and in vitro anthelmintic effects of aqueous extracts of Salvadora persica and Terminalia avicennoides against strongye nematodes of small ruminants in Nigeria. J. Anim. Vet. Sci. 2011;10:437–442. [Google Scholar]


