Abstract
Objective
Advanced maternal age (AMA) is an important factor in decreasing success of assisted reproductive technology by having a negative effect on the success rate of intra-cytoplasmic sperm injection (ICSI), particularly by increasing the rate of embryo aneuploidy. It has been suggested that the transfer of euploid embryos increases the implantation and pregnancy rates, and decreases the abortion rate. Preimplantation genetic screening (PGS) is a method for selection of euploid embryos. Past studies, however, have reported different results on the success of pregnancy after PGS in AMA. Investigating the pregnancy rate of ICSI with and without PGS in female partners over 35 years of age referred to infertility centers in Tehran.
Materials and Methods
In this randomized controlled trial, 150 couples with the female partner over age of 35 were included. Fifty couples underwent PGS and the remaining were used as the control group. PGS was carried out using fluorescent in situ hybridization (FISH) for chromosomes 13, 18, 21, X and Y. Results of embryo transfer following PGS were evaluated and compared with those in the control group.
Results
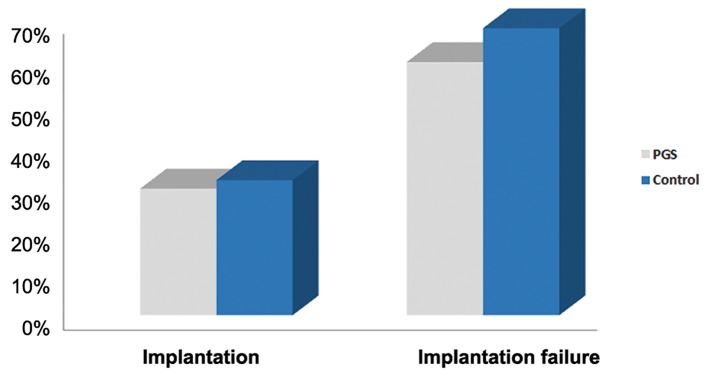
Implantation rates obtained in the PGS and control groups were 30 and 32% respectively and not significantly different (P>0.05).
Conclusion
PGS for chromosomes 13, 18, 21, X and Y does not increase implantation rate in women over 35 years of age and therefore the regular use of PGS in AMA is not recommended.
Keywords: Preimplantation Genetic Screening, Maternal Age, FISH Technique, Aneuploidies, ICSI
Introduction
As part of the lifestyle in the developed countries, women frequently decide to delay child bearing, which results in an increased incidence of agerelated fertility problems.
Without accounting for embryo morphology, embryos of women over 35 years of age have shown 40-80% higher rate of aneuploidy (1,3). Due to this increased risk, a low implantation rate and a high abortion rate have been observed after intra-cytoplasmic sperm injection (ICSI) treatment. This observation clearly demonstrates that the age of mother is one of the important factors in predicting a live birth after in vitro fertilization (IVF) (4,6). As a result, although assisted reproductive technology (ART) has been successful, women over 35 years of age still have a very low chance of pregnancy. Therefore, women over the age of 35 form a significant part of those who go under treatment with ICSI. It is thus suggested that screening a healthy embryo for chromosomal abnormalities may improve rate of pregnancy and decrease the possibility of aneuploidy among these individuals. Other factors including hormonal factors, physiological condition of uterus and oocyte quality are effective in the success rate of ART methods, however, chromosomal disorders are directly associated with the age of mother and low rate of ART success (7,9). Evidence shows older women who had failed ART with their own oocyte, became pregnant when they used donated oocytes of younger women (10,11).
In the IVF/ICSI method, embryo is usually transferred based on embryo development and morphology. However, it is possible that an embryo with a good morphology has a genetic disorder and does not grow. Although preimplantation genetic diagnosis (PGD) was used for couples with a hereditary genetic disorder to diagnose unaffected embryos (12,13), utilizing PGD has enormously evolved and couples who have undergone the IVF treatment opt for this method to select the best embryo. This decision is based on evidence which shows selecting an embryo with normal chromosomes increases the rate of pregnancy and decreases the rate of abortion, especially in older women (14,20).
Many observations in comparison with the control groups show after preimplantation genetic screening (PGS), the rate of implantation is increased the criterion of research is different based on number of researching chromosomes embryo transfer’s day and number of transferred embryo (21,23).
In Sweden, the law stipulates the legal transfer of one embryo unless in cases of aged mothers in which two embryos may be transferred.
A Swedish study, on the role of PGS in aged mothers, reported the low rate of pregnancy in the PGS group compared with the control group and concluded that PGS for aged mothers decreases the rate of successful IVF/ICSI (24). The result of another study in the United States also reported that PGS for aneuploidy not only increases the rate of successful IVF/ICSI, but it can also decrease the rate (25). In contrast to these two studies, the results of three random controlled studies show that PGS significantly improves the rate of pregnancy and implantation. In these studies approximately three embryos were transferred (26). Another study in Italy also reported positive outcome after PGD of aneuploidy in human embryos (19).
The aim of this study was to investigate the pregnancy rate of ICSI with and without PGS in female partners over 35 years old referred to Tehran infertility centers.
Materials and Methods
Subjects
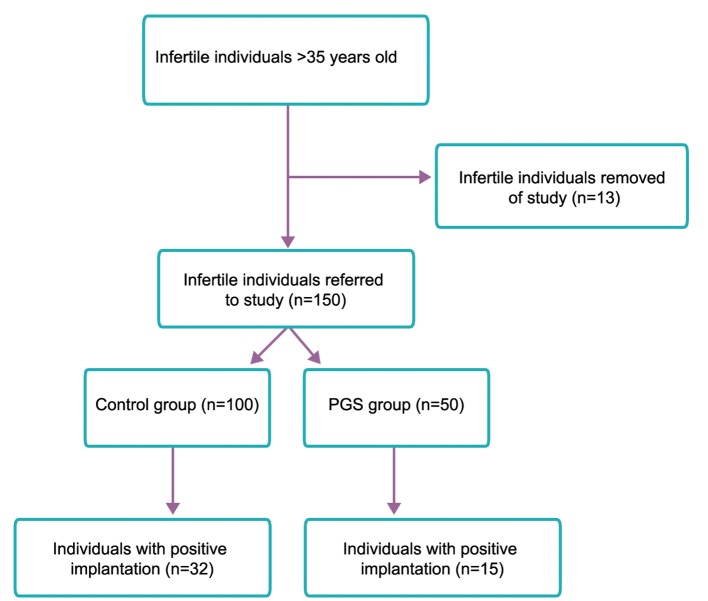
This randomized controlled trial study was carried out on 150 couples. 163 infertile females over 35 years of age were recruited at a specified time who reffered to Omid Infertility Clinic, Sara Infertility Clinic and the Embryology Laboratory of Aban Hospital in Tehran and this study has been approved by the Vice President for Research of Islamic Azad University, Tehran Medical Branch in accordance with Helsinki declaration and guideline of Iranian Ministry of Health and Medical Education. All the participants signed the consent form and were chosen randomly. Couples with infertility of female or male origin without history of recurrent abortion and with at least two embryos with high morphological quality for biopsy were included. Thirteen infertile individuals who had not undergone a suitable ICSI procedure and as a consequence did not have any embryo for examination, or who had insufficient embryos or their embryos were of low quality for biopsy were excluded from this study. 50 infertile individuals were tested by PGS and the other 100 were considered as the control group.
Ovarian stimulation
Primarily, all females were super-ovulated using a gonadotropin-releasing hormone agonist (GnRH) analogue suppression protocol (27) in combination with human menopausal gonadotropin (hMG). Ovulation was induced by a 10000 IU injection of human chorionic gonadotropin (hCG). Subsequently, follicular aspiration was performed 36-38 hours after the hCG was administered (28,29).
Embryo culture
The ICSI procedure was used to fertilize the oocytes and performed on mature metaphase II oocytes. Microinjected oocytes were incubated in 25 ml droplets of medium in a Petri dish at 37˚C and 5% CO2, 5% O2and 90% N2. Fertilization was recognized 16-18 hours after injection. Normal fertilization was confirmed by the presence of two distinct pronuclei and polar bodies (3).
Resulting embryos were cultured for three days. On the third day, tested embryos had a biopsy and underwent PGS examination by fluorescent in situ hybridization (FISH). On the fourth day, as a result of PGS examination, some embryos were reported healthy and among those a maximum of three embryos were transferred.
Embryo biopsy
Embryos of grade A, B and less than C with suitable quality were biopsied in third day after fertilization. Embryo biopsy was performed on all embryos that had 5 cells and, 20% fragmentation. Embryo biopsy was performed mechanically by a needle and a biopsy pipette (Fig.1). First, embryos were incubated in Ca²+ free biopsy medium for 15 minutes. Zona pellucida was mechanically drilled with needle and blastomeres were aspirated with a biopsy pipette. In the mechanical biopsy, part of the ruptured zona pellucida overlap after biopsy and heals the damage caused on the embryo. In most cases, one blastomere and in some cases, when there was no suitable nucleus for microscopic analysis, two blastomeres were biopsied. These blastomeres were then genetically tested at Omid Infertility Clinic.
Fig.1.

Mechanical biopsy at the cleavage stage of an embryo.
Fixation of interphase nucleus
The blastomeres were fixed on a poly-L-lysine cover slide using an acetic acid-methanol solution (1:3). After fixing the nucleus, the location of the nucleus was observed under a phase contrast microscope.
FISH test steps
Slides were put in 1% formalin (SigmaAldrich, USA) at 4˚C for better stabilization. Next, the slides were washed in 1x phosphate buffered saline (PBS, Sigma-Aldrich, USA) at room temperature for 5 minutes and were then placed in pepsin (Merck, USA) at 37˚C for 5 minutes for better cytoplasm digestion (pepsin solution contains 0.05 g pepsin in 100 ml HCL 0.01 N). The slides were rewashed for 5 minutes with 1x PBS buffer at room temperature. Subsequently, three ethanol solutions (SigmaAldrich, USA) (70, 85 and 100%) were used for 1 minute for better wash. Finally the slides were immersed in methanol for 5 minutes to be dehydrated.
Five color Vysis probe kit (Multi-Vysion™ PGT multi-color Probe Panel, Vysis Inc, USA) designated for chromosomes X, Y, 13, 18 and 21 marked with green, aqua, red, blue and gold colors was used to recognize 5 chromosomes at the same time.
Probes were poured on the cell nucleus and were covered by a cover slip and then fixed by paste. The cover slides were then placed at 76˚C heat for 5 minutes to denature the DNA and the probe elements. Heated slides at 37˚C were put into a moist chamber for 4 to 8 hours; lesser time made the signal weak and the more time increased the background color with both making the signal reorganization difficult. After, slides were washed in 0.4X saline-sodium citrate (SSC) buffer (Sigma-Aldrich, USA) at 73˚C for 5 minutes. The slides were then placed in 2X SSC solution for 1 minute and placed in distilled water for 1 minute.
Finally, slides were dried in room temperature and signals were observed under a fluorescent microscope by using Antifade II.
Observable scales in FISH examination
In size and light degree, they are considered two signals, when the signals were too weak or totally unrecognizable, it was reported as no signal, and when all five chromosomes analyzed were present in the correct numbers, the embryo was reported as normal (Fig.2) and when there were unequal numbers of chromosomes, abnormal embryo or aneuploidy was reported.
Fig.2.

Normal male embryo after PGS. Photo was taken by applied spectral imaging software. A blastomere nucleus hybridized with probes specific for chromosomes 13 (red), 18 (aqua), 21 (green) X (blue) and Y (gold). The signal pattern is consistent with normal male chromosome complement. PGS; Preimplantation genetic screening.
Embryo transfer
Generally, two normal embryos with the best morphologic qualities were selected for transfer on day four. Embryos were ranked according to their morphologic qualities with a focus on the regularity and number of blastomeres, and the percentage of fragmentation (29). In the control group, the selection of embryos for transfer was based only on morphologic qualities according to the scoring procedure described above. A maximum of three embryos were transferred four days after injection in both study groups although two embryos were usually transferred.
Outcome measures
Biochemical pregnancy and implantation was defined when serum β-hCG levels reach above 10 IU per liter after 3 weeks of embryo transfer. Clinical pregnancy was defined as the presence of a gestational sac confirmed by transvaginal ultrasound examination at a gestational age of 7 weeks. In this study, we evaluated implantation rate by measuring the level of β-hCG.
Statistical analysis
We used Fisher’s exact test for statistical analysis and generated 95% confidence intervals (CIs). P>0.05 was reported as not significant.
Results
Totally 50 infertile individuals were tested of which five, after PGS, did not obtain healthy embryos for transfer (non-transfer rate of 10%). Thirty individuals, following transfer of healthy embryo, did not become pregnant (implantation failure rate of 60%) and the other 15 individuals, after transfer, did not show a positive β-hCG test for biochemical pregnancy (implantation rate of 30%) (Fig.3).
Fig.3.
Patient flow diagram. PGS; Preimplantation genetic screening.
Of 50 infertile individuals who were tested, 324 embryos were biopsied and tested by FISH. A total of 279 embryos generated signals and no signal was observed in 45 embryos. From 279 tested embryos, 125 embryos (44.8%) were reported as abnormal and 154 embryos (55.2%) were reported healthy for the aneuploidy of chromosomes X, Y, 13, 18 and 21 (Table 1).
Table 1.
PGS cycle data
| FISHresults | n | % |
|---|---|---|
| Biopsied(day3,cleavagestage) | 324 | 100 |
| Nosignal/inconclusive | 45 | 13.88 |
| Normal | 154 | 47.53 |
| Abnormal | 125 | 38.58 |
| Aneuploidy chromosome 21 | 56 | 44.80 |
| Aneuploidy chromosome 18 | 45 | 36.00 |
| Aneuploidy chromosome 13 | 35 | 28.00 |
| Aneuploidy sex chromosome | 70 | 56.00 |
PGS; Preimplantation genetic screening and FISH; Fluorescent in situ hybridization.
In the control group from 100 mothers aged above 35, 32 mothers were pregnant (implantation rate of 32%) and 68 mothers were not biochemically pregnant (implantation failure rate of 68%) (Fig.4). The difference of implantation rate between cases and controls was 2%. This difference between the two groups was not significant, suggesting PGS had no effects on the implantation rate in mothers aged above 35.
Fig.4.
Comparison of implantation rates with and without the use of PGS in women aged above 35. PGS; Preimplantation genetic screening.
Discussion
In this study, we have shown that preimplantation genetic screening did not increase.
There are two randomized controlled trial which were published and did not represent any advantage for using PGS as an indicator of advanced maternal age (AMA) (3,26). In Staessen et al. (3), 400 women aged above 37 were randomly selected. The first result showed that there were no statistical difference in implantation rate, the rate of pregnancy per transfer and the rate of pregnancy per cycle between the PGS and the control groups. In a similar study by Stevens et al. (30), 40 women aged above 35 were randomly selected. There was no signification difference in clinical pregnancy or the rate of implantation of the PGS group compared with the controls.
It was hypothesized that selecting an embryo which has normal chromosome numbers will increase the rate of implantation and pregnancy and will decrease the rate of abortion, but both studies showed that PGS had no effect. Nevertheless, such a result cannot be exactly determined. There are many factors which affect this achievement of which one is embryo biopsy. Many studies have shown that the biopsy from embryo has no effect on the growth of embryo in laboratory conditions but the result of pregnancy rate indicated that it is still necessary to review this case (24). It is possible that blastomere biopsy on day three of embryonic stage prevents the potential of an embryo to successfully implant (31). However, the effect of biopsy alone on pregnancy rate has not been studied.
Moreover, the omission of one or two cells may have a detrimental effect, and this may prevent the growth of the embryo. On the other hand, the selection of an embryo with 5 normal chromosome sets (13, 18, 21, X and Y) can not guarantee the absolute growth and health of the embryo. FISH usually estimates between 5-8, rather than all 23, chromosome pairs (32). Recent studies have shown that embryonic aneuploidy occurs in clinically significant amounts for all 23 chromosome pairs (33). FISH is thus unable of diagnosing many of the chromosomal abnormalities generally found in the embryonic developing stage. Therefore, the limitation in the number of chromosomes that can be analyzed with FISH could lead to the transfer of normal embryos that are in fact aneuploid for one or several chromosomes not tested. This problem may be overcome in the future by the use of new techniques such as array comparative genomic hybridization (aCGH) in which the complete ploidy for a blastomere can be detected after biopsy (34).
PGS on the biopsied blastomeres from developing embryos presents its own challenges. Cell mosaicism is also a main factor in generating false results in PGS. A normal embryo may be incorrectly reported abnormal because of mosaicism, and may not be transferred, or vice versa. In contrast an abnormal embryo may be reported as normal and may be transferred but it will not grow and pregnancy will fail (24). In addition, many human embryos generated by ICSI may be mosaic (3,35,36) where chromosomal constitution of the one blastomere may not be representative of the entire embryo. Studies have repeatedly demonstrated that, at day three of development, embryos have high levels of mosaicism (37,38). Mosaicism is a situation in which a single embryo is made of more than one distinct genetic cell line. In other words, mosaic embryos may have both euploid (normal) and aneuploid (abnormal) cell lines inside them. Studies estimating this event have deduced that the majority of all embryos may be mosaic at day three of development (37,39). Therfore, a biopsy performed at day three of development may produce a result that is not representative of the overall embryo. Also, although mosaicism has been shown to exist at day five of embryo development (40), recent studies suggest that mosaicism may be much decreased by day five of development (28,41).
When we extract a normal cell from an abnormal embryo, this decreases the ratio of normal cells in the embryo and thus prevents the growth and progress of that embryo. On the contrary, when we extract an abnormal cell out of a normal embryo, the embryo grows better while this embryo was not transferred for the wrong diagnosis (24).
Another important factor is the day of biopsy. Blastocysts are more likely to be the appropriate source for biopsy, because more cells can then be produced and analyzed with accurate clinical results (42). However, usually for performing PGS, the most popular sources are either polar body biopsy (15,16) from the oocyte or cleavage stage biopsy on one or two blastomeres (12).
Transfer of embryos in the two study groups was made on distinct days. Transfer was performed on day four because we wanted to keep each step for the control group the same as our ordinary routine as much as possible.
Conclusion
We show that PGS, which is used to select embryos for transfer, does not improve the rate of implantation for advanced maternal age. Also the results showed that there was no significant difference in implantation rate for transfer between the PGS and control groups.
Our study was limited to women undergoing PGS as an indicator of advanced maternal age. It remains uncertain whether the results would be different for women with other indications for PGS.
The hypothesis that PGS in women of advanced age improves the implantation rate has not been supported by this study. Therefore, this study and previous randomized controlled trials alike show that the regular use of PGS in AMA is not recommended.
Acknowledgments
The authors would like to thank the Omid Infertility Clinic for financial support. There is no conflict of interest in this study.
References
- 1.Gianaroli L, Magli MC, Ferraretti AP, Munné S. Preimplantation diagnosis for aneupoloidies in patients undergoing in vitro fertilization with poor prognosis: identification of the categories to which it should be proposed. Fertil Steril. 1999;72(5):837–844. doi: 10.1016/s0015-0282(99)00377-5. [DOI] [PubMed] [Google Scholar]
- 2.Platteau P, Staessen C, Michiels A, Van Steirteghem A, Liebaers I, Devroey P. Preimplantation genetic diagnosis for aneuploidy screening in women older than 37 years. Fertil Steril. 2005;84(2):319–324. doi: 10.1016/j.fertnstert.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 3.Staessen C, Platteau P, Van Assche E, Michiels A, Tournaye H, Camus M, et al. Comparison of blastocyst transfer with or without Preimplantation genetic diagnosis for aneuploidy screening in couples with advanced maternal age: a prospective randomized controlled trial. Hum Reprod. 2004;19(12):2849–2858. doi: 10.1093/humrep/deh536. [DOI] [PubMed] [Google Scholar]
- 4.Piette C, de Mouzon J, Bachelot A, Spira A. In-vitro fertilization: influence of women’s age on pregnancy rates. Hum Reprod. 1990;5(1):56–59. doi: 10.1093/oxfordjournals.humrep.a137041. [DOI] [PubMed] [Google Scholar]
- 5.Schieve LA, Peterson HB, Meikle SF, Jeng G, Danel I, Burnett NM, et al. Live birth rates and multiple-birth risk using in vitro fertilization. JAMA. 1999;282(19):1832–1838. doi: 10.1001/jama.282.19.1832. [DOI] [PubMed] [Google Scholar]
- 6.Elizur SE, Lerner-Geva L, Levron J, Shulman A, Bider D, Dor J. Factors predicting IVF treatment outcome: a multivariate analysis of 5310 cycles. Reprod Biomed Online. 2005;10(5):645–649. doi: 10.1016/s1472-6483(10)61673-2. [DOI] [PubMed] [Google Scholar]
- 7.Meldrum DR. Female reproductive aging--ovarian and uterine factors. Fertil Steril. 1993;59(1):1–5. doi: 10.1016/s0015-0282(16)55608-8. [DOI] [PubMed] [Google Scholar]
- 8.Pellicer A, Simon C, Remohi J. Effects of aging on the female reproductive system. Hum Reprod. 1995;10(Suppl 2):77–83. doi: 10.1093/humrep/10.suppl_2.77. [DOI] [PubMed] [Google Scholar]
- 9.Saldeen P, Kallen K, Sundstrom P. The probability of successful IVF outcome after poor ovarian response. Acta Obstet Gynecol Scand. 2007;86(4):457–461. doi: 10.1080/00016340701194948. [DOI] [PubMed] [Google Scholar]
- 10.Navot D, Bergh PA, Williams MA, Garrisi GJ, Guzman I, Sandler B, et al. Poor oocyte quality rather than implantation failure as a cause of age-related decline in female fertility. Lancet. 1991;337(8754):1375–1377. doi: 10.1016/0140-6736(91)93060-m. [DOI] [PubMed] [Google Scholar]
- 11.Borini A, Bafaro G, Violini F, Bianchi L, Casadio V, Flamigni C. Pregnancies in postmenopausal women over 50 years old in an oocyte donation program. Fertil Steril. 1995;63(2):258–261. [PubMed] [Google Scholar]
- 12.Handyside AH, Pattinson JK, Penketh RJ, Delhanty JD, Winston RM, Tuddenham EG. Biopsy of human preimplantation embryos and sexing by DNA amplification. Lancet. 1989;1(8634):347–349. doi: 10.1016/s0140-6736(89)91723-6. [DOI] [PubMed] [Google Scholar]
- 13.Handyside AH, Lesko JG, Tarı´n JJ, Winston RM, Hughes MR. Birth of a normal girl after in vitro fertilization and preimplantation diagnostic testing for cystic-fibrosis. N Engl J Med. 1992;327(13):905–909. doi: 10.1056/NEJM199209243271301. [DOI] [PubMed] [Google Scholar]
- 14.Verlinsky Y, Cieslak J, Freidine M, Ivakhnenko V, Wolf G, Kovalinskaya L, et al. Pregnancies following pre-conception diagnosis of common aneuploidies by fluorescent in-situ hybridization. Hum Reprod. 1995;10(7):1923–1927. doi: 10.1093/oxfordjournals.humrep.a136207. [DOI] [PubMed] [Google Scholar]
- 15.Verlinsky Y, Cieslak J, Freidine M, Ivakhnenko V, Wolf G, Kovalinskaya L, et al. Polar body diagnosis of common aneuploidies by FISH. J Assis Reprod Genet. 1996;13(2):157–162. doi: 10.1007/BF02072538. [DOI] [PubMed] [Google Scholar]
- 16.Verlinsky Y, Cieslak J, Ivahnenko V, Lifchez A, Strom C, Kuliev A. Birth of healthy children after preimplantation diagnosis of common aneuploidies by polar body fluorescent in-situ hybridisation analysis.Preimplantation Genetics Group. Fertil Steril. 1996;66(1):126–129. doi: 10.1016/s0015-0282(16)58399-x. [DOI] [PubMed] [Google Scholar]
- 17.Gianaroli L, Magli MC, Ferraretti AP, Fiorentino A, Garrisi J, Munne´ S. Preimplantation genetic diagnosis increases the implantation rate in human in vitro fertilization by avoiding the transfer of chromosomally abnormal embryos. Fertil Steril. 1997;68(6):1128–1131. doi: 10.1016/s0015-0282(97)00412-3. [DOI] [PubMed] [Google Scholar]
- 18.Munne´ S, Magli C, Bache M, Fung J, Legator M, Morrison L, et al. Preimplantation diagnosis of the aneuploidies most commonly found in spontaneous abortions and live births: XY, 13, 14, 15, 16, 18, 21, 22. Prenat Diagn. 1998;18(13):1459–1466. doi: 10.1002/(sici)1097-0223(199812)18:13<1459::aid-pd514>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 19.Munne´ S, Magli C, Cohen J, Morton P, Sadowy S, Gianaroli L, et al. Positive outcome after preimplantation diagnosis of aneuploidy in human embryos. Hum Reprod. 1999;14(9):2191–2199. doi: 10.1093/humrep/14.9.2191. [DOI] [PubMed] [Google Scholar]
- 20.Kahraman S, Bahçe M, Samli H, Imirzalioğlu N, Yakisn K, Cengiz G, et al. Healthy births and ongoing pregnancies obtained by preimplantation genetic diagnosis in patients with advanced maternal age and recurrent implantation failure. Hum Reprod. 2000;15(9):2003–2007. doi: 10.1093/humrep/15.9.2003. [DOI] [PubMed] [Google Scholar]
- 21.Munné S, Sandalinas M, Escudero T, Velilla E, Walmsley R, Sadowy S, et al. Improved implantation after preimplantation genetic diagnosis of aneuploidy. Reprod Biomed Online. 2003;7(1):91–97. doi: 10.1016/s1472-6483(10)61735-x. [DOI] [PubMed] [Google Scholar]
- 22.Munné S, Garrisi J, Barnes F, Werlin L, Schoolcraft W, Kaplan B. Reduced spontaneous abortion and increased live birth rate after PGD for advanced maternal age. Fertil Steril. 2007;88(Suupl 1):S85–S86. [Google Scholar]
- 23.Munné S, Gianaroli L, Tur-Kaspa I, Magli C, Sandalinas M, Grifo J, et al. Substandard application of preimplantation genetic screening may interfere with its clinical success. Fertil Steril. 2007;88(4):781–784. doi: 10.1016/j.fertnstert.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Hardarson T, Hanson C, Lundin K, Hillensjö T, Nilsson L, Stevic J, et al. Preimplantation genetic screening in women of advanced maternal age caused a decrease in clinical pregnancy rate: a randomized controlled trial. Hum Reprod. 2008;23(12):2806–2812. doi: 10.1093/humrep/den217. [DOI] [PubMed] [Google Scholar]
- 25.Checa MA, Alonso-Coello P, Solà I, Robles A, Carreras R, Balasch J. IVF/ICSI with or without preimplantation genetic screening for aneuploidy in couples without genetic disorders: a systematic review and meta-analysis. J Assist Reprod Genet. 2009;26(5):273–283. doi: 10.1007/s10815-009-9328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donoso P, Staessen C, Fauser BC, Devroey P. Current value of preimplantation genetic aneuploidy screening in IVF. Hum Reprod Update. 2007;13(1):15–25. doi: 10.1093/humupd/dml043. [DOI] [PubMed] [Google Scholar]
- 27.Kolibianakis E, Osmanagaoglu K, Camus M, Tournaye H, Van Steirteghem A, Devroey P. Effect of repeated assisted reproductive technology cycles on ovarian response. Fertil Steril. 2002;77(5):967–970. doi: 10.1016/s0015-0282(02)02975-8. [DOI] [PubMed] [Google Scholar]
- 28.Brezina PR, Benner A, Rechitsky S, Kuliev A, Pomerantseva E, Pauling D, et al. Single-gene testing combined with single nucleotide polymorphism microarray preimplantation genetic diagnosis for aneuploidy: a novel approach in optimizing pregnancy outcome. Fertil Steril. 2011;95(5):1786.e5-8. doi: 10.1016/j.fertnstert.2010.11.025. e5-8. [DOI] [PubMed] [Google Scholar]
- 29.Brezina PR, Brezina DS, Kearns WG. Preimplantation genetic testing. BMJ. 2012;345:e5908–e5908. doi: 10.1136/bmj.e5908. [DOI] [PubMed] [Google Scholar]
- 30.Stevens J, Wale P, Surrey ES, Schoolcraft WB, Gardner DK. Is aneuploidy screening for patients aged 35 or over beneficial?. A prospective randomized trial. Fertil Steril. 2004;82:P–322. [Google Scholar]
- 31.De Vos A, Van Steirteghem A. Aspects of biopsy procedures prior to preimplantation genetic diagnosis. Prenat Diagn. 2001;21(9):767–780. doi: 10.1002/pd.172. [DOI] [PubMed] [Google Scholar]
- 32.Harper JC, Sengupta SB. Preimplantation genetic diagnosis: state of the art 2011. Hum Genet. 2012;131(2):175–186. doi: 10.1007/s00439-011-1056-z. [DOI] [PubMed] [Google Scholar]
- 33.Brezina P, Tobler K, Benner AT, Du L, Xu X, Kearns WG. All 23 chromosomes have significant levels of aneuploidy in recurrent pregnancy loss couples. Fertil Steril. 2012;97(3):S7–S7. [Google Scholar]
- 34.Mastenbroek S, Twisk M, van Echten-Arends J, Sikkema-Raddatz B, Korevaar JC, Verhoeve HR, et al. In vitro fertilization with preimplantation genetic screening. N Engl J Med. 2007;357(1):9–17. doi: 10.1056/NEJMoa067744. [DOI] [PubMed] [Google Scholar]
- 35.Coonen E, Derhaag JG, Dumoulin JC, van Wissen LC, Bras M, Janssen M, et al. Anaphase lagging mainly explains chromosomal mosaicism in human preimplantation embryos. Hum Reprod. 2004;19(2):316–324. doi: 10.1093/humrep/deh077. [DOI] [PubMed] [Google Scholar]
- 36.Munné S, Alikani M, Tomkin G, Grifo J, Cohen J. Embryo morphology, developmental rates, and maternal age are correlated with chromosome abnormalities. Fertil Steril. 1995;64(2):382–391. [PubMed] [Google Scholar]
- 37.Harton GL, Magli MC, Lundin K, Montag M, Lemmen J, Harper JC, et al. ESHRE PGD Consortium/Embryology Special Interest Group--best practice guidelines for polar body and embryo biopsy for preimplantation genetic diagnosis/ screening (PGD/PGS) Hum Reprod. 2011;26(1):41–46. doi: 10.1093/humrep/deq265. [DOI] [PubMed] [Google Scholar]
- 38.Munné S, Weier HU, Grifo J, Cohen J. Chromosome mosaicism in human embryos. Biol Reprod. 1994;51(3):373–379. doi: 10.1095/biolreprod51.3.373. [DOI] [PubMed] [Google Scholar]
- 39.van Echten-Arends J, Mastenbroek S, Sikkema-Raddatz B, Korevaar JC, Heineman MJ, van der Veen F, et al. Chromosomal mosaicism in human preimplantation embryos: a systematic review. Hum Reprod Update. 2011;17(5):620–627. doi: 10.1093/humupd/dmr014. [DOI] [PubMed] [Google Scholar]
- 40.Bielanska M, Tan SL, Ao A. High rate of mixoploidy among human blastocysts cultured in vitro. Fertil Steril. 2002;78(6):1248–1253. doi: 10.1016/s0015-0282(02)04393-5. [DOI] [PubMed] [Google Scholar]
- 41.Brezina PR, Sun Y, Anchan RM, Li G, Zhao Y, Kearns WG. Aneuploid embryos as determined by 23 single nucleotide polymorphism (SNP) microarray preimplantation genetic screening (PGS) possess the potential to genetically normalize during early development. Fertil Steril. 2012;98(3):S108–S108. [Google Scholar]
- 42.McArthur SJ, Leigh D, Marshall JT, de Boer KA, Jansen RP. Pregnancies and live births after trophectoderm biopsy and preimplantation genetic testing of human blastocysts. Fertil Steril. 2005;84(6):1628–1636. doi: 10.1016/j.fertnstert.2005.05.063. [DOI] [PubMed] [Google Scholar]