Abstract
Objective
The human OCT4 gene, the most important pluripotency marker, can generate at least three different transcripts (OCT4A, OCT4B, and OCT4B1) by alternative splicing. OCT4A is the main isoform responsible for the stemness property of embryonic stem (ES) cells. There also exist eight processed OCT4 pseudogenes in the human genome with high homology to the OCT4A, some of which are transcribed in various cancers. Recent conflicting reports on OCT4 expression in tumor cells and tissues emphasize the need to discriminate the expression of OCT4A from other variants as well as OCT4 pseudogenes.
Materials and Methods
In this experimental study, DNA sequencing confirmed the authenticity of transcripts of OCT4 pseudogenes and their expression patterns were investigated in a panel of different human cell lines by reverse transcription-polymerase chain reaction (RT-PCR).
Results
Differential expression of OCT4 pseudogenes in various human cancer and pluripotent cell lines was observed. Moreover, the expression pattern of OCT4-pseudogene 3 (OCT4-pg3) followed that of OCT4A during neural differentiation of the pluripotent cell line of NTERA-2 (NT2). Although OCT4-pg3 was highly expressed in undifferentiated NT2 cells, its expression was rapidly down-regulated upon induction of neural differentiation. Analysis of protein expression of OCT4A, OCT4-pg1, OCT4-pg3, and OCT4-pg4 by Western blotting indicated that OCT4 pseudogenes cannot produce stable proteins. Consistent with a newly proposed competitive role of pseudogene microRNA docking sites, we detected miR-145 binding sites on all transcripts of OCT4 and OCT4 pseudogenes.
Conclusion
Our study suggests a potential coding-independent function for OCT4 pseudogenes during differentiation or tumorigenesis.
Keywords: OCT4 Pseudogenes, Stem Cell, Cancers, miR-145
Introduction
OCT4, an important transcription factor in embryonic stem (ES) and embryonic carcinoma (EC) cells, has a crucial role in maintenance of pluripotency of stem cells and also in generating induced pluripotent stem (iPS) cells (1,3). There are three known variants of OCT4 (OCT4A, OCT4B and OCT4-B1) which are generated by different promoters or alternative splicing (4,5). OCT4A is localized in the nucleus of ES, EC, cancer stem and germinal cells, and germ cell tumors where it functions as the main transcription factor to sustain pluripotency and self-renewal of the cells (6,10). On the other hand, OCT4B is primarily expressed in the cytoplasm of tumor cells, and is unable to maintain pluripotency of stem cells (6,11). Recent studies have demonstrated the existence of an internal ribosome entry site (IRES) for OCT4B, which can produce three isoforms (OCT4B-265, OCT4B-190 and OCT4B-164) by alternative translation initiation (12,13). OCT4A and OCT4B-265 isoforms have identical POUDNA binding domain and C-terminal transactivation domain (CTD), but their N-terminal transactivation domain is different. OCT4B-190 and OCT4B-164 isoforms have the same CTD, but they have lost the N-terminal domain and a part of the POU-DNA binding domain (6,14). The newly discovered variant, OCT4B1, is localized in the nucleus and cytoplasm of pluripotent and undifferentiated cells (5,15). This isoform is generated by retaining intron2 of the OCT4B transcript as a cryptic exon. It has an in-frame stop codon (TGA) within its cryptic exon and thus produces a truncated protein with an N-terminal domain similar to OCT4B-265 as well as a part of POU specific (POUs) domain (5). In addition to pluripotent cells, further studies have demonstrated OCT4B1 expression in bladder, gastric and colorectal tumors where it acts as an anti-apoptotic factor (16,18). Pseudogenes have been traditionally described as non-functional genes which originate from protein coding genes. Currently, up to 20,000 pseudogenes have been detected in the human genome (19). Contrary to previous perceptions, current studies have indicated that some of these pseudogenes are transcribed. Aside from the possibility of having a biological function, they may aslo cause falsepositive signals in gene expression experiments such as reverse transcription-polymerase chain reaction (RT-PCR). Based on recent studies, some pseudogenes (i.e. PTEN-pg and OCT4-pg4) act as microRNA decoys and thereby regulate the effects of microRNAs on the corresponding protein-coding genes (20). Moreover, some pseudogenes have roles in gene silencing and thus regulate the expression of their parental genes. On the other hand, some transcribed pseudogenes are translated to generate truncated proteins or antigenic peptides. All of these findings indicate that pseudogenes are not junk DNA and can have important roles within normal and abnormal cells (20,22).
So far, seven pseudogenes have been discovered for the human OCT4 gene by bioinformatics and experimental analyses. All of them have been shown to be processed and transcribed in various cancer cell lines and tissues (11). OCT4-pseudogenes of OCT4-pg1, OCT4-pg3 and OCT4-pg4 have very similar exon structures to OCT4A, and hence could wrongly be detected as OCT4A.
In this study, we investigated the expression pattern of OCT4-pg1, OCT4-pg2, OCT4-pg3, OCT4-pg4, OCT4-pg5, OCT4-pg6 and OCT4-pg7 in different human pluripotent and cancer cell types. Moreover, we cloned the whole sequence of OCT4-pg1, OCT4-pg3 and OCT4-pg4 in eukaryotic expression vectors to perform functional analyses. Finally, the protein production of OCT4pg1, OCT4-pg3 and OCT4-pg4 were examined by Western blotting.
Materials and Methods
Cell culture
In this experimental study, 17 human cell lines were mainly provided by Pasteur Institute of Iran and Research Institute of Avicenna, included two human embryonic carcinoma cell lines, one normal fibroblast cell line (HS-5) and HEK293 (embryonic kidney) and 13 human tumor cell lines, namely U87MG and A172 (glioblastoma), Daoy (medulloblastoma), 1321N1 (brain astrocytoma), Jurkat (T-Cell lymphoma), Y79 (retinoblastoma), PC3 (prostate adenocarcinoma), Raji (Burkit’s lymphoma), Ovcar3 (ovary adenocarcinoma), HepG2 (hepatocellular carcinoma), MCF7 (breast adenocarcinoma), 5637 (urinary bladder carcinoma), HeLa (cervix adenocarcinoma), and NT2 and NCCIT (pluripotent embryonic carcinoma).
Jurkat, Raji, ovcar3, U87, 5637 and NCCIT were cultured in RPMI-1640 medium supplemented with 10% fetal bovin serum (FBS, Gibco, UK), penicillin (100 U/ml) and streptomycin (100 µg/ ml, Gibco, UK). The Y-79 cell line was cultured in RPMI-1640 medium supplemented with 20% FBS. HS5, HepG2, MCF7, NT2, HeLa, A172, Daoy and HEK293 cells were cultured in High Glucose Dulbecco’s Modified Eagle Medium (DMEM, Gibco, UK, 4500 mg/l) supplemented with 10% FBS, sodium pyruvate and penicillin/streptomycin as described above. All cell lines were incubated at 37˚C (humidified) and 5% CO2.
The human NT2 cells (kindly provided by Dr. Peter Andrews at Sheffield University) was propagated in DMEM/F-12 (Invitrogen, Gaithersburg, MD) supplemented with 10% FBS and 1% penicillin/streptomycin, and incubated at 37˚C in 5% CO2. NT2 cells were treated with all-trans retinoic acid (RA, Sigma-Aldrich, Germany) to induce their differentiation into neural-like phenotype as described previously (16). Briefly, 2 days before RA induction, cells were seeded in six-well plates at a density of 3-4×104in 2 ml growth medium per well. RA was added to the growth medium at a final concentration of 10 mM, and the differentiation medium was renewed twice a week. Cultured cells from three replicates at 0, 3, 7, 14 and 21 days after RA treatment were harvested for RNA extraction and subsequent RT-PCR experiments.
RNA extraction and cDNA synthesis
Total RNA was extracted using TRIzol (Invitrogen, UK) according to the manufacturer’s instruca tion. The quality and quantity of extracted RNA were examined by agarose gel electrophoresis and spectrophotometery respectively. All extracted RNA samples were treated with DNaseI (Fermentas, Lithuania) and incubated at 37˚C for 30 minn utes. The enzyme was then inactivated by the addition of Ethylenediaminetetraacetic acid (EDTA, 50 mM) and incubation at 65˚C for 10 minutes. Subsequently, 2 µg of each DNase-treated RNA was used to synthesize cDNA by using reverse transcriptase (Fermentase, Lithuania) and oligodT primers according to the manufacturer’s instruction. The efficiency of DNase treatment and lack of DNA contamination was tested by having a NoRT control in all of RT-PCR experiments.
Reverse transcription-polymerase chain reaction
RT-PCR analysis of OCT4 pseudogenes was carried out using specific primers (Table 1) that can exclusively amplify each OCT4 pseudogene transcript. Details of PCR conditions for each OCT4 pseudogene is summarized in Table 1. The PCR program included an intial step of 95˚C for 4 minc utes, followed by 35 cycles of denaturation at 95˚C for 30 seconds, annealing for 30 seconds and extension at 72˚C for 45 seconds, and a final extenn sion step of 72˚C for 7 minutes.
Table 1.
Details of primers and PCR conditions for amplification of OCT4A, OCT4 pseudogenes, and the internal control, GAPDH
| Primer name | Primer sequence | Annealing temp | Amplicon size (bp) |
|---|---|---|---|
| OCT4-Pg1 | F: 5´-CAT GCA RGC CCG AAA GAG AAA GCR AR-3´ | 60 | 107 |
| R: 5´-TGT GGC TGA TCT GCA GTG TGG G-3´ | |||
| OCT4-Pg2 | F: 5´-GTG TAC ATG TTT ATA AAG TTT GTG GTA GTG TTC-3´ | 58 | 380 |
| R: 5´-GGG TCG CTA GGT AAT TTT GTC ACT GG-3´ | |||
| OCT4-Pg3 | F: 5´-CTT CTC ACC CCC TCC AGG C-3´ | 62 | 841 |
| R: 5´-CCA CTG CTT GAT CGC TTG C-3´ | |||
| OCT4-Pg4 | F: 5´-GGG ACA CCT GGC TTC GGA TG-3´ | 56 | 172 |
| R: 5´-CCC CAC ACC TCA GAG CCT GA-3´ | |||
| OCT4-Pg5 | F: 5´-CAG TGA TTA TGC ACC ATG AGA GGA-3´ | 56 | 179 |
| R: 5´ GGG AAA GGC ACT AAG GAA CAC AG-3´ | |||
| OCT4-Pg6 | F: 5´-CCT AGC AAA ACC TCA ACG AGT CCC AG-3´ | 60 | 219 |
| R: 5´-CAA GAG GTT GTG GTG AGC GAA GG-3´ | |||
| OCT4-Pg7 | F: 5´-GCC AAA TTG CTG AAG CAG AAG GAT ATC-3´ | 58 | 347 |
| R: 5´-CTG GTC TTG TGA AAG GAT ACT CAG TGG-3´ | |||
| OCT4A | F: 5-CTT CTC GCC CCC TCC AGG T-3´ | 65 | 495 |
| R: 5´-AAA TAG AAC CCC CAG GGT GAG C-3´ | |||
| GAPDH | F: 5-GCCACATCGCTCAGACAC-3´ | 58 | 140 |
| R: 5´-GGCAACAATATCCACTTTACCAG-3´ | |||
PCR; Polymerase chain reaction.
Cloning and sequencing of transcribed OCT4 pseudogenes
The PCR products were separated on a 1.5% agarose gel and the bands were excised and extracted from the gel using DNA extraction kit (GeneAll Biotechnology, South Korea) and then cloned into the PTZ57R/T vector (Fermentase, Lithuania). The specificity and authenticity of the amplicons was further confirmed by DNA sequencing (Applied Biosystems, South Korea).
Constructing expression cassettes for OCT4-pg 1, 3 and 4
Using Pfu enzyme (GeneAll Biotechnology, South Korea) and specific primers for flanking regions of OCT4-pg1, OCT4-pg3 and OCT4-pg4 on genomic DNA, we amplified the corresponding genomic DNA by PCR. Nested-PCR was then performed by specific primers for coding sequences of each pseudogene. PCR products were then extracted from agarose gel, cloned in a TA cloning vector (PTZ57R/T) and their authenticity confirmed by DNA sequencing. Next, the amplified segments of the pseudogenes were digested by the NotI restriction enzyme and then cloned in the PCMV6-Neo expression vector. The specificity of the sequences and correct direction of cloned fragments inside the vector were further confirmed by DNA sequencing.
Western blotting
Untransfected HeLa, 5637, 1321, A172, NCCIT and NT2 cell cultures were lysed with 1 ml of lysis buffer [1% Triton X-100, 5 mM EDTA, 50 mM TrisHC1, pH=7.4, 150 mM NaCl, 0.1% sodium dodecyl sulfate (SDS)], 1% protease inhibitor cocktail and phosphatase inhibitor (Sigma, USA). Protein concentrations were measured by the Bradford Protein Assay reagent. Briefly, 20 µg of cell lysates were isolated on 12.5% SDS-PAGE and transferred onto a Polyvinylidene difluoride (PVDF) membrane (Millipore, USA). Blocking was carried out with 5% skim milk in tris-buffered saline (Sigma-Aldrich, Germany), containing 0.05% Tween20 (TBS-T). The membrane was then incubated for 1 hour with primary mouse anti-Oct-3/4 sc-5279 antibody (Santa Cruz, USA) diluted in 3% blocking buffer, and washed with PBS for 30 minutes at room temperature. A secondary HRPconjugated sheep-anti-mouse antibody (Avicenna Research Institute, Iran) was added next and incubated at RT for 1 hour. After washing, signals were detected using the Amersham ECL Prime Western Blotting Detection kit (GE Healthcare Life Sciences) and quantified by Gel Logic 2200 (Kodak, Japan).
Cell cycle analysis
HeLa cells were transfected with OCT4-pg1, OCT4-pg3, and OCT4-pg4 vectors separately, and collected 48 hours after transfection. Harvested cells were washed with phosphate buffered saline (PBS, Sigma-Aldrich, Germany) and fixed in 1 ml ice-cold 70% ethanol for 30 minutes. Cells were stained with 50 mg/ml of propidium iodide (PI) solution (SigmaAldrich) containing 0.1% Triton X-100 and 10 mg/ ml RNaseA (Takara, Japan), mixed well and incubated for 5 to 10 minutes at room temperature. Prepared cells were then analyzed by a flow cytometer instrument (Becton Dickinson Bioscience, San Jose, CA).
Results
Differential expression pattern of OCT4-pseudogenes in different human cell types
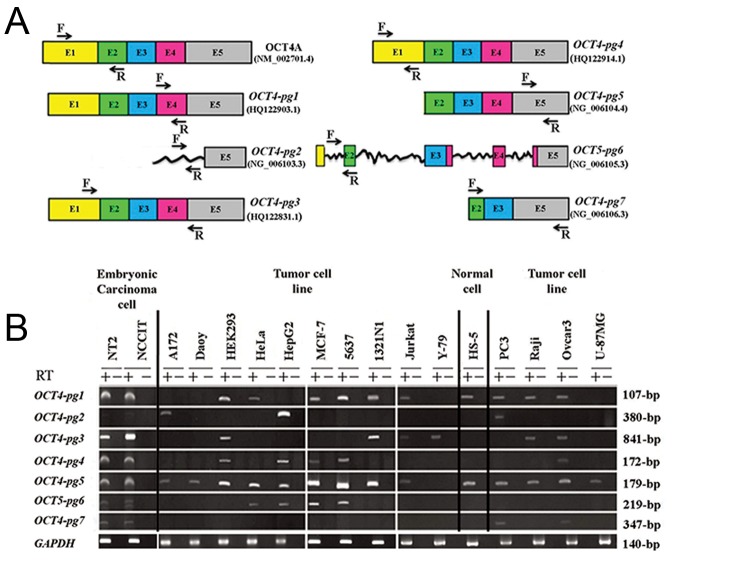
Based on RT-PCR data, OCT4-Pg1 was detected in NT2 and NCCIT, HS-5, and nine cancer cell lines (A172, HEK293, MCF-7, 5637, 1231N1, Jurkat, PC3, Raji, and Ovcar3) (Fig.1A). In contrast, OCT4-Pg2 expression was restricted only to A172, HepG2 and PC3 cells. OCT4-Pg3 was expressed in HEK293, 1321N1, Jurkat, Y79, Raji and Ovcar3 but showed a significant expression in EC cells. OCT4-Pg4 showed detectable signals in EC cells as well as in HEK293, HepG2, MCF-7, 5637 and Ovcar3 tumor cell lines. OCT4-Pg5 was expressed in all cell lines except Y-79. Expression of OCT4-Pg6 was detected in EC cells as well as in HeLa, HepG2, MCF-7 and 5637 cells. OCT4-Pg7 was also detected in EC cells, and PC3 and Ovcar3 cell lines (Fig.1B). DNA sequencing confirmed authenticity of all amplified PCR products.
Fig.1.
A. Schematic representation of OCT4 pseudogenes. OCT4-pg1, OCT4-pg3 and OCT4-pg4 have highly similar nucleotide sequences to that of the OCT4A transcript. OCT4-pg5 transcript lacks exon1, and OCT4-pg7 lacks exon1, exon4, and part of exon2. OCT4-pg2 has a part of exon5, and OCT4-pg6 has all five exons, incompletely. Rough lines in OCT4-pg2 and OCT4-pg6 are remained sequences which are derived from OCT4 introns and B. RT-PCR analysis of OCT4-pseudogenes in different human pluripotent and cancer cell lines by specific primer sets. GAPDH was used as an internal control. RT-PCR; Reverse transcriptase-polymerase chain reaction.
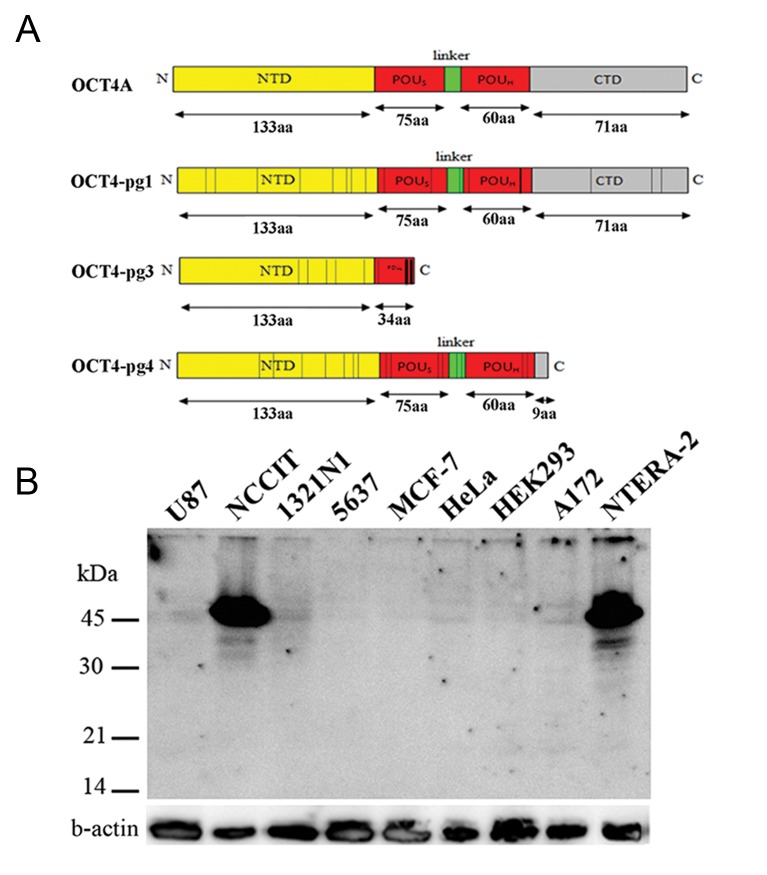
OCT4-pg1, OCT4-pg3 and OCT4-pg4 produce unstable proteins
OCT4-pg1, OCT4-pg3 and OCT4-pg4 might potentially produce proteins. For instance, OCT4-pg1 transcript can produce a protein similar to OCT4A, containing NTD, CTD and POU domain. Due to point mutations, OCT4-pg3 encodes a truncated protein with a complete NTD and a partial POUs domain. Hypothetical OCT4-pg4 protein misses a large part of CTD, but has intact NTD and POU domain. Therefore, we decided to experimentally examine the protein expression of these pseudogenes by Western blotting based on mouse anti-OCT3/4 sc-5279 monoclonal antibody (raised against amino acids 1-134 of OCT-3/4 of human origin), an antibody against NTD. Therefore, antibodies which recognize NTD of OCT4A can also detect OCT4-pg1, OCT4-pg3 and OCT4-pg4 but can be discriminated from OCT4A by size differences (Fig.2A). We used NT2 and NCCIT cells as positive controls, U-87MG as a negative control and six somatic cancer cell lines (A172, 5637, 1321N1, HeLa, HEK293, and MCF- 7) that express the OCT4 pseudogenes. We detected a high level of OCT4A expression in NT2 and NCCIT cells, but no detectable signal was observed for Fig.1: A. Schematic representation of OCT4 pseudogenes. OCT4-pg1, OCT4-pg3 and OCT4-pg4 have highly similar nucleotide sequences to that of the OCT4A transcript. OCT4-pg5 transcript lacks exon1, and OCT4-pg7 lacks exon1, exon4, and part of exon2. OCT4-pg2 has a part of exon5, and OCT4-pg6 has all five exons, incompletely. Rough lines in OCT4-pg2 and OCT4-pg6 are remained sequences which are derived from OCT4 introns and B. RT-PCR analysis of OCT4-pseudogenes in different human pluripotent and cancer cell lines by specific primer sets. GAPDH was used as an internal control. RT-PCR; Reverse transcriptase-polymerase chain reaction. OCT4 pseudogene potential proteins (Fig.2B).
Fig.2.
A. A schematic view of OCT4A protein structure, along with predicted protein structures of OCT4-Pg1, OCT4-Pg3, and OCT4-Pg4. The putative OCT4-Pg1 protein is similar to OCT4A, containing intact NTD, POU domain and CTD. OCT4-Pg3 can potentially produce a truncated protein with NTD and a part of the POUs domain. The predicted OCT4-pg4 protein contains NTD and POU domain, but lacks a large part of the C-terminal domain. Vertical lines within the depicted structures of OCT4 pseudogenes indicate the position of point mutations which have changed the amino acid sequences of the predicted proteins and B. Western blotting in different human cell types transfected with OCT4-Pg1, OCT4-Pg3 and OCT4-Pg4 expression vectors. The NCCIT and NT2 cell lines were used as positive controls for OCT4A, while U-87MG was used as a negative control for OCT4A and OCT4 pseudogenes. Using the sc-5279 antibody, we detected OCT4A protein exclusively in NCCIT and NTERA-2 cell lines but did not detect OCT4 pseudogenes in the cells which expressed them at the transcript level. Note that the internal control b-actin protein is detectable at similar intensities in all examined cell lines.
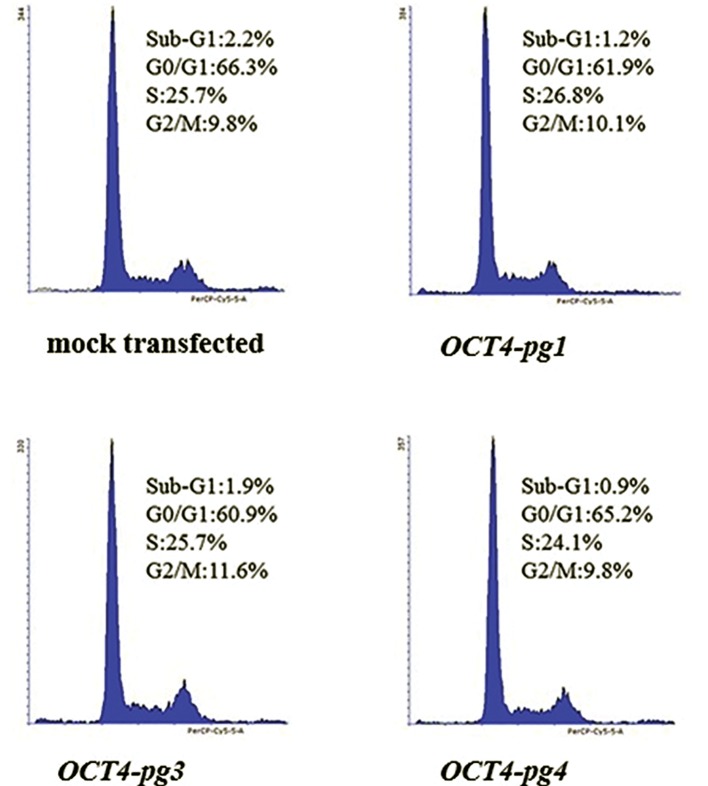
Cell cycle alteration following overexpression of OCT4-pg1, OCT4-pg3 and OCT4-pg4
To investigate the potential function of OCT4-pg1, OCT4-pg3 and OCT4-pg4, we overexpressed them in HeLa cells. Cell cycle analysis was then undertaken after staining the DNA content of the cells with PI. Compared with the control cells transfected with mock PCMV6-Neo vector, the transfected cells demonstrated subtle decline in distribution of cells in the G1 and sub-G1 phases, and a slight elevation of distributed cells in the S phase of cell cycle (Fig.3).
Fig.3.
Cell cycle analysis of HeLa cells transfected with either the mock p-EGFPC-1 expression vector or the same vector containing the sequence corresponding to OCT4-pg1, OCT4-pg 3 or OCT4-pg4.
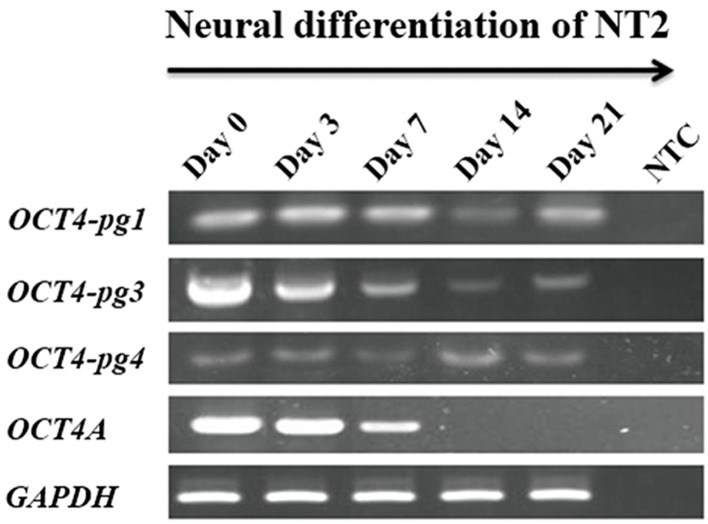
Downregulation of OCT4-pg3 during the course of neural differentiation of NT2 cells
As demonstrated in Figure 4, OCT4-pg3 was highly expressed in undifferentiated NT2 cells, and its expression is gradually diminished upon the induction of differentiation. The gene expression alteration of OCT4-pg3 correlated with that of OCT4A, suggesting a similar regulatory control for both genes. A similar decline in the expression pattern was not observed for OCT4-pg1 and OCT4-pg4 (Fig.4).
Fig.4.
RT-PCR analysis of OCT4-Pg1, OCT4-Pg3 and OCT4-Pg4 expression during neural differentiation of NT2 cells. Note that OCT4-Pg3 is expressed at high levels in undifferentiated cells but is down-regulated upon the initiation of differentiation. NTC; Non-template control and RT-PCR; Reverse transcriptase-polymerase chain reaction.
Conservation of miR-145 binding sites in OCT4A and OCT4-pseudogene sequences
Considering a newly proposed competing endogenous RNA (ceRNA) role for pseudogenes, we hypothesized a non-coding functional role for OCT4 pesudogene transcripts of sponging a well-known inhibitor of OCT4, miR-145. We scanned the sequences of the OCT4 pesudogenes to find potential conserved binding sites for miR-145. As shown in Table 2, the seed sequence of miR-145 exists on almost all OCT4 pesudogenes.
Table 2.
A sequence complementary pattern of OCT4 and OCT4-pgs with miR-145. The conserved nucleotides are marked with vertical lines between the two sequences. The seed sequence of miR-145 is highlighted by a green box. A conservation of the putative miR-145 binding site sequence is also provided for each pseudogene
| Gene | Targetsiteofhsa-miR-145-5P | Location |
|---|---|---|
| OCT4A | 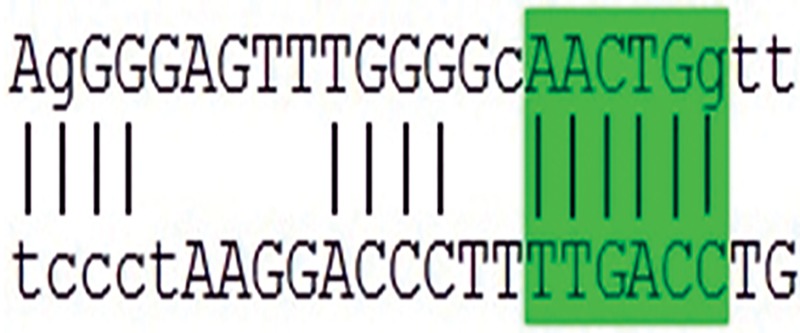 |
3´-UTR |
| OCT4-pg1 | AAACTGAG | 3´-UTR |
| OCT4-pg2 | GAAACTGG | Exon5 |
| OCT4-pg3 | AGACTGGA | Exon1 |
| OCT4-pg4 | GAAACTGGA | Exon1 |
| OCT4-pg5 | AACTGG | 3´-UTR |
| OCT4-pg6 | AACTGG | Exon5 |
| OCT4-pg7 | GGGAAACTGG | 3´-UTR |
Discussion
OCT4 is a crucial transcription factor with a key role in maintaining the stemness state of pluripotent cells (8, 13, 23, 24). It was initially believed that OCT4 is exclusively expressed in embryonic stem cells, however, its recent detection in some cancer cells and tissues ignited a dispute on the accuracy of the data (19, 25). A strong possible source for the conflicting reports on OCT4 may be due to non-specific primers which were unable to discriminate OCT4A from its pseudogenes (26). In other words, RT-PCR artifacts and misdetection of the OCT4A isoform could be partly derived by the amplification of highly homologous OCT4 pseudogenes at the transcript level (27). Therefore, using specific primers that can discriminate OCT4 pseudogenes from the OCT4A variant is crucial for RT-PCR analysis of OCT4 expression.
Considering the conflicting reports on expression analysis of OCT4A and OCT4 pseudogenes in embryonic stem cells, and cancer cell lines and tissues, we evaluated here the expression pattern of all known OCT4 pseudogenes in various human pluripotent and tumor cell types. Our data support the idea that misdetection of OCT4A in somatic cancer cell types may be caused by non-specific primers that could in addition amplify one or more OCT4 pseudogenes. Moreover, our data revealed that different OCT4 pseudogenes are differentially expressed in various tumor cell lines, suggesting a unique expression regulation for each of them. For instance, while OCT4-pg2 is barely detectable in most examined cell lines, it showed a very high level of expression in HepG2 cells.
Since OCT4 pseudogenes might have some functional activity at the transcript and/or protein levels (11), and are widely expressed in tumor cells and tissues, they might have a putative role in tumor cell proliferation or tumor progression (27). Our data demonstrated a lack of protein expression for pseudogenes of OCT4. However, the fact that OCT4-pg3 expression is regulated during the course of neural differentiation of NT2 cells, suggest a functional association, albeit at the transcript level. Interestingly, a coding-independent competing role has already been proposed for some pseudogenes. Accordingly, a sponge role for OCT4-pg4 in binding, and hence in releasing the inhibitory function of miR-145 is reported by Wang et al. (28). Given that we identified a conserved miR-145 binding site for almost all OCT4 pseudogenes and considering the role of miR-145 as a tumor-suppressor gene (29), it would be plausible that the wide expression of OCT4 pseudogenes in various cancer types may be associated with tumorigenesis. However, this hypothesis needs to be experimentally validated in different tumor cell lines.
Conclusion
We show that OCT4 pseudogenes are differentially expressed in various human pluripotent and tumor cell types. However, Western blotting revealed no protein expression for the OCT4 pseudogenes. This suggests that these pseudogenes may have a potential non-coding function, possibly by having a sponging effect on miR-145.
Acknowledgments
We are grateful to Dr. Mohamad Mehdi Akhundi, the head, and Dr. Fatemeh Ghaemimanesh the member of Avicenna Research Institute (Tehran) for their valuable help in providing some of the cancer and pluripotent cell lines. We also thank Dr. Javan for his kind help in Western Blotting. This work was financially supported by a research grant from the Iranian Council of Stem Cell Technology. The authors declare no conflicts of interest.
References
- 1.Schöler HR, Ruppert S, Suzuki N, Chowdhury K, Gruss P. New type of POU domain in germ line-specific protein Oct-4. Nature. 1990;344(6265):435–439. doi: 10.1038/344435a0. [DOI] [PubMed] [Google Scholar]
- 2.Rosner MH, De Santo RJ, Arnheiter H, Staudt LM. Oct-3 is a maternal factor required for the first mouse embryonic division. Cell. 1991;64(6):1103–1110. doi: 10.1016/0092-8674(91)90265-z. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Takeda J, Seino S, Bell GI. Human Oct3 gene family: cDNA sequences, alternative splicing, gene organization, chromosomal location, and expression at low levels in adult tissues. Nucleic Acids Res. 1992;20(17):4613–4620. doi: 10.1093/nar/20.17.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atlasi Y, Mowla SJ, Ziaee SA, Gokhale PJ, Andrews PW. OCT4 spliced variants are differentially expressed in human pluripotent and nonpluripotent cells. Stem Cells. 2008;26(12):3068–3074. doi: 10.1634/stemcells.2008-0530. [DOI] [PubMed] [Google Scholar]
- 6.Lee J, Kim HK, Rho JY, Han YM, Kim J. The human OCT-4 isoforms differ in their ability to confer self-renewal. J Biol Chem. 2006;281(44):33554–33565. doi: 10.1074/jbc.M603937200. [DOI] [PubMed] [Google Scholar]
- 7.Hansis C, Grifo JA, Krey LC. Oct-4 expression in inner cell mass and trophectoderm of human blastocysts. Mol Hum Reprod. 2000;6(11):999–1004. doi: 10.1093/molehr/6.11.999. [DOI] [PubMed] [Google Scholar]
- 8.Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 2005;26(2):495–502. doi: 10.1093/carcin/bgh321. [DOI] [PubMed] [Google Scholar]
- 9.Webster JD, Yuzbasiyan-Gurkan V, Trosko JE, Chang CC, Kiupel M. Expression of the embryonic transcription factor Oct4 in canine neoplasms: a potential marker for stem cell subpopulations in neoplasia. Vet Pathol. 2007;44(6):893–900. doi: 10.1354/vp.44-6-893. [DOI] [PubMed] [Google Scholar]
- 10.Lengner CJ, Camargo FD, Hochedlinger K, Welstead GG, Zaidi S, Gokhale S, et al. Oct4 expression is not required for mouse somatic stem cell self-renewal. Cell Stem Cell. 2007;1(4):403–415. doi: 10.1016/j.stem.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suo G, Han J, Wang X, Zhang J, Zhao Y, Zhao Y, et al. Oct4 pseudogenes are transcribed in cancers. Biochem Biophys Res Commun. 2005;337(4):1047–1051. doi: 10.1016/j.bbrc.2005.09.157. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Zhao Y, Xiao Z, Chen B, Wei Z, Wang B, et al. Alternative translation of OCT4 by an internal ribosome entry site and its novel function in stress response. Stem Cells. 2009;27(6):1265–1275. doi: 10.1002/stem.58. [DOI] [PubMed] [Google Scholar]
- 13.Cauffman G, Liebaers I, Van Steirteghem A, Van de Velde H. POU5F1 isoforms show different expression patterns in human embryonic stem cells and preimplantation embryos. Stem Cells. 2006;24(12):2685–2691. doi: 10.1634/stemcells.2005-0611. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Dai J. Concise review: isoforms of OCT4 contribute to the confusing diversity in stem cell biology. Stem Cells. 2010;28(5):885–893. doi: 10.1002/stem.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papamichos SI, Kotoula V, Tarlatzis BC, Agorastos T, Papazisis K, Lambropoulos AF. OCT4B1 isoform: the novel OCT4 alternative spliced variant as a putative marker of stemness. Mol Hum Reprod. 2009;15(5):269–270. doi: 10.1093/molehr/gap018. [DOI] [PubMed] [Google Scholar]
- 16.Asadi MH, Mowla SJ, Fathi F, Aleyasin A, Asadzadeh J, Atlasi Y. OCT4B1, a novel spliced variant of OCT4, is highly expressed in gastric cancer and acts as an antiapoptotic factor. Int J Cancer. 2011;128(11):2645–2652. doi: 10.1002/ijc.25643. [DOI] [PubMed] [Google Scholar]
- 17.Gazouli M, Roubelakis MG, Theodoropoulos GE, Papailiou J, Vaiopoulou A, Pappa KI, et al. OCT4 spliced variant OCT4B1 is expressed in human colorectal cancer. Mol Carcinog. 2012;51(2):165–173. doi: 10.1002/mc.20773. [DOI] [PubMed] [Google Scholar]
- 18.Atlasi Y, Mowla SJ, Ziaee SA, Bahrami AR. OCT-4, an embryonic stem cell marker, is highly expressed in bladder cancer. Int J Cancer. 2007;120(7):1598–1602. doi: 10.1002/ijc.22508. [DOI] [PubMed] [Google Scholar]
- 19.Liedtke S, Enczmann J, Waclawczyk S, Wernet P, Kögler G. Oct4 and its pseudogenes confuse stem cell research. Cell Stem Cell. 2007;1(4):364–366. doi: 10.1016/j.stem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Swami M. Small RNAs: Pseudogenes act as microRNA decoys. Nat Rev Genet. 2010;11(8):530–531. doi: 10.1038/nrg2835. [DOI] [PubMed] [Google Scholar]
- 21.Rutnam ZJ, Du WW, Yang W, Yang X, Yang BB. The pseudogene TUSC2P promotes TUSC2 function by binding multiple microRNAs. Nat Commun. 2014;5:2914–2914. doi: 10.1038/ncomms3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pink RC, Wicks K, Caley DP, Punch EK, Jacobs L, Carter DR. Pseudogenes: pseudo-functional or key regulators in health and disease? RNA. 2011;17(5):792–798. doi: 10.1261/rna.2658311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95(3):379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 24.Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122(6):947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liedtke S, Stephan M, Kögler G. Oct4 expression revisited: potential pitfalls for data misinterpretation in stem cell research. Biol Chem. 2008;389(7):845–850. doi: 10.1515/BC.2008.098. [DOI] [PubMed] [Google Scholar]
- 26.Kotoula V, Papamichos SI, Lambropoulos AF. Revisiting OCT4 expression in peripheral blood mononuclear cells. Stem Cells. 2008;26(1):290–291. doi: 10.1634/stemcells.2007-0726. [DOI] [PubMed] [Google Scholar]
- 27.Zhao S, Yuan Q, Hao H, Guo Y, Liu S, Zhang Y, et al. Expression of OCT4 pseudogenes in human tumours: lessons from glioma and breast carcinoma. J Pathol. 2011;223(5):672–682. doi: 10.1002/path.2827. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Guo ZY, Zhang R, Xin B, Chen R, Zhao J, et al. Pseudogene OCT4-pg4 functions as a natural micro RNA sponge to regulate OCT4 expression by competing for miR-145 in hepatocellular carcinoma. Carcinogenesis. 2013;34(8):1773–1781. doi: 10.1093/carcin/bgt139. [DOI] [PubMed] [Google Scholar]
- 29.Sachdeva M, Zhu S, Wu F, Wu H, Walia V, Kumar S, et al. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc Natl Acad Sci USA. 2009;106(9):3207–3212. doi: 10.1073/pnas.0808042106. [DOI] [PMC free article] [PubMed] [Google Scholar]