Abstract
Vascular endothelial growth factor A (VEGFA) plays an important role in tumour angiogenesis and its angiogenic action is mainly mediated through its VEGF receptor 2 (VEGFR-2). Therefore drugs targeting VEGFA/VEGFR-2 are being presently used in the clinics for treatment of several types of solid malignant tumours. We here in report that low dose of chebulagic acid (CA), a hydrolysable tannin found in myrobalan fruits can inhibit VEGFA induced vascular permeability, endothelial cell proliferation, migration, tube formation and thereby, angiogenesis by suppressing VEGFR-2 phosphorylation. CA may thus be an effective and useful natural inhibitor of VEGFA mediated angiogenesis.
VEGFA induced new blood vessel formation is essential for promotion, progression and metastasis of solid tumours1,2. VEGFA by acting mainly via its VEGFR-2 present in the endothelial cells promotes angiogenesis by inducing microvascular permeability, endothelial cell proliferation, migration and tube formation1,2. Accordingly, several anti-angiogenic agents are being presently used in the clinics targeting this cytokine and VEGFR-2 for the treatment of several types of solid tumours3,4. However, these anti-angiogenic drugs are very expensive and it is therefore prudent to identify newer, inexpensive and effective anti-angiogenic molecules5.
We have very recently demonstrated that Triphala (THL), a mixture of three myrobalan fruits can significantly inhibit VEGFA induced angiogenesis6,7. Previous reports have indicated that in addition to gallic acid and ellagic acid, chebulagic acid (CA), benzopyran tannin is also a major constituent of THL8,9. Since plasma levels and bioavailability of gallic acid and ellagic acid following oral ingestion are poor10,11, therefore it is possible that other bioactive constituents of THL may be responsible for the anti-VEGFA actions of this mixture7. This study thus investigated the effects of CA at a concentration present in THL on VEGFA mediated regulation of proangiogenic endothelial cells functions.
Results and Discussion
Plasma concentration of CA in mice after oral feeding of THL
Since our previous report indicated significant inhibition of VEGFA mediated angiogenesis by THL and because CA present in the THL may have anti-VEGFA effects, we therefore at first investigated the plasma level of CA after gavaging mice with THL7,8,9. Our LC-MS/MS data indicated that the concentrations of CA in plasma were 4341.67 ng/ml (~5 μM) at 10 min, 2098.33 ng/ml (~2 μM) at 30 min and 1795.00 ng/ml (~1 μM) at 2 hr after oral feeding of these animals with a single non-toxic dose of 100 mg/kg of THL containing 22.2 mg/kg of CA (Figure 1).
Figure 1. The plasma concentration of chebulagic acid (CA).
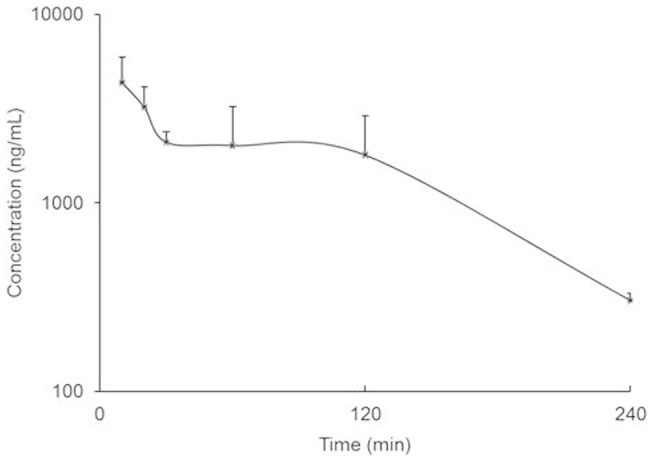
The plasma concentration-time profile of chebulagic acid in mice after oral ingestion of a single dose (100 mg/kg) of Triphala (n = 3). The error bars represent SEM.
Cytotoxic effects of CA on endothelial cells
Thereafter, the effects of 1, 2 and 5 μM of CA on the viability of human umbilical vein endothelial cells (HUVEC) were determined because these plasma concentrations of CA reached when mice were gavaged with a single non toxic VEGFA inhibitory dose (100 mg/kg) of THL (Figure 1). Our results indicated that 1, 2 and 5 μM of CA had no effects on cell viability (Figure 2). Therefore 1, 2 and 5 μM of CA were selected for our in vitro experiments.
Figure 2. The effects of chebulagic acid (CA) on the viability of endothelial cells.
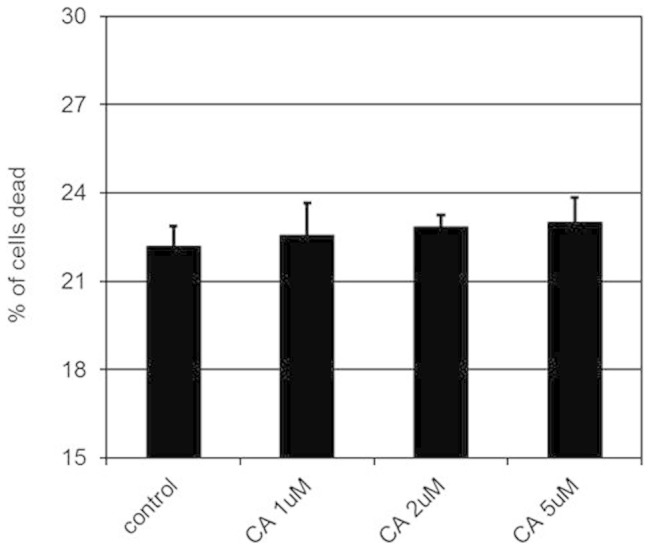
The cytotoxic effects of 1, 2 and 5 μM of chebulagic acid (CA) on human umbilical vein cells (HUVEC) (*, p < 0.05). The error bars represent SEM. The data represent six separate experiments.
Effects of CA on endothelial cell functions
Since VEGFA induces angiogenesis by stimulating endothelial cell permeability, proliferation, migration and tube formation1,2, we thus investigated the effects of plasma levels (1, 2 and 5 μM) of CA detected in mice following THL intake on these VEGFA mediated endothelial cell functions. We observed that 2 and 5 μM of CA significantly inhibited VEGFA (20 ng/ml) induced endothelial cell proliferation (Figure 3), migration (Figure 4a and 4b) and tube formation (Figure 5a, 5b, 5c, 5d, 5e and 5f). In addition, 2 and 5 μM of CA also significantly inhibited VEGFA mediated endothelial cell permeability in vitro (Figure 6). On the contrary, no such effects were observed when these cells were treated only with CA (data not shown). In addition, we observed that 2 and 5 μM of CA could significantly inhibit VEGFA induced VEGFR-2 phosphorylation in these cells (Figure 7a and 7b), thereby suggesting the molecular mechanism of VEGFA inhibitory actions of this natural compound. However, 1 μM of CA had no such significant effects on VEGFA mediated activation of VEGFR-2 (Figure 7c and 7d).
Figure 3. The effects of various concentrations of chebulagic acid (CA) on vascular endothelial growth factor A (VEGFA) mediated proliferation of endothelial cells.
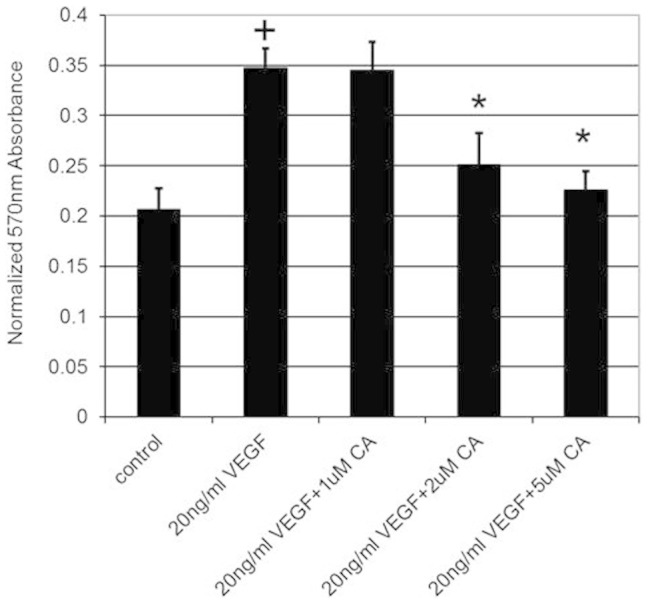
VEGFA stimulated proliferation of human umbilical vein cells (HUVEC) when compared to untreated control (+, p < 0.05). However, this stimulatory effect was inhibited when these cells were also treated with 2 and 5 μM of CA (*, p < 0.05). The error bars represent SEM. The data represent six separate experiments.
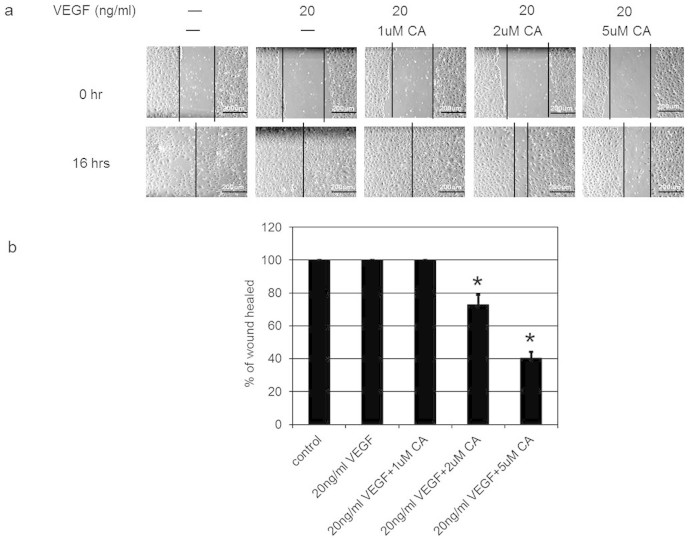
Figure 4. The effects of chebulagic acid (CA) on vascular endothelial growth factor A (VEGFA) induced migration of human umbilical vein cells (HUVEC).
Phase-contrast photomicrographs demonstrated VEGFA induced complete wound closure or healing at 16 hr. On the contrary, this effect of VEGFA was abrogated when these cells were treated with 2 and 5 μM of CA. The wound healing was calculated as the distance covered by the cells in relation to the initial wound distance at 0 hr and is expressed as the percentage of initial distance at 0 hr. (*, P < 0.05). The error bars represent SEM. Scale bars in a, 200 um. The data represent six separate experiments.
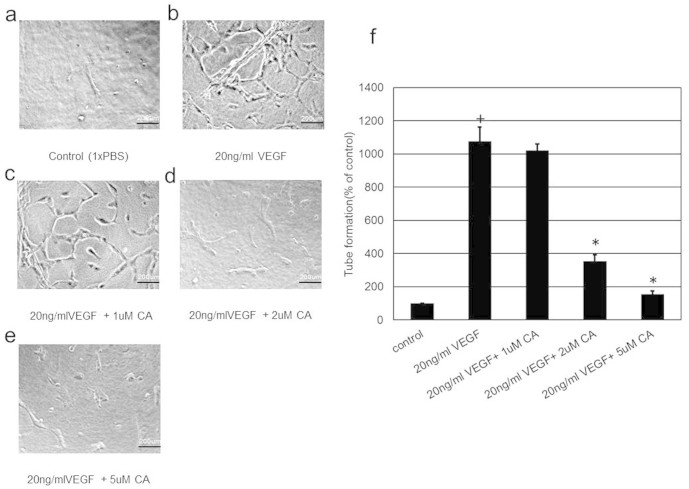
Figure 5. The effects of chebulagic acid (CA) on vascular endothelial growth factor A (VEGFA) mediated tube formation in endothelial cells.
VEGFA stimulated tube formation in human umbilical vein cells (HUVEC) when compared to untreated control (+, p < 0.05). On the contrary, treatment with 2 and 5 μM of CA inhibited VEGFA induced endothelial cell tube formation (*, p < 0.05). Scale bars in (a), (b), (c), (d) and (e), 200 um. The data represent six separate experiments.
Figure 6. The effects of chebulagic acid (CA) on vascular endothelial growth factor A (VEGFA) induced permeability in endothelial cells.
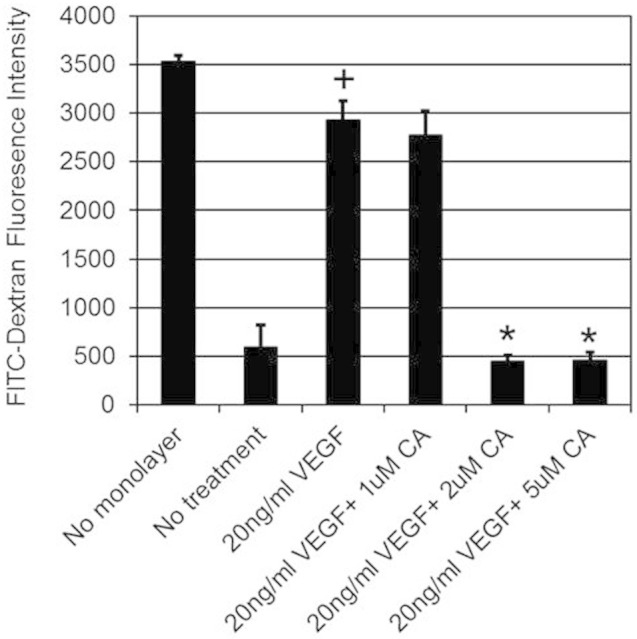
In comparison to untreated control, VEGFA induced significant permeability in human umbilical vein cells (HUVEC) (+, p < 0.05). On the contrary, treatment with 2 or 5 μM of CA significantly inhibited VEGFA stimulated permeability in HUVEC (*, p < 0.05). The data represent six separate experiments.
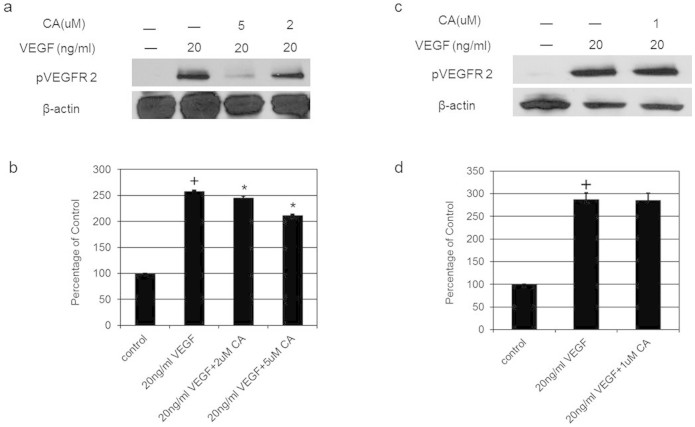
Figure 7. The effects of chebulagic acid (CA) on vascular endothelial growth factor A (VEGFA) induced phosphorylation of VEGF receptor 2 (VEGFR-2).
Western blot analysis shows that in comparison to untreated control, VEGFA induced significant VEGFR-2 phosphorylation (+, p < 0.05). However, there was also a significant inhibition of VEGFA induced phosphorylation of VEGFR2 after treatment with 2 or 5 μM of CA (*, p < 0.05). The blot was re-probed with an antibody to β actin for comparison of equal protein load. Cropped gel images have been used in this figure and the gels were run under the same experimental conditions. The data represent six separate experiments. Full-length blots are presented in Supplementary Figure S1.
Effects of CA on angiogenesis in chick chorioallantoic membrane (CAM) assay
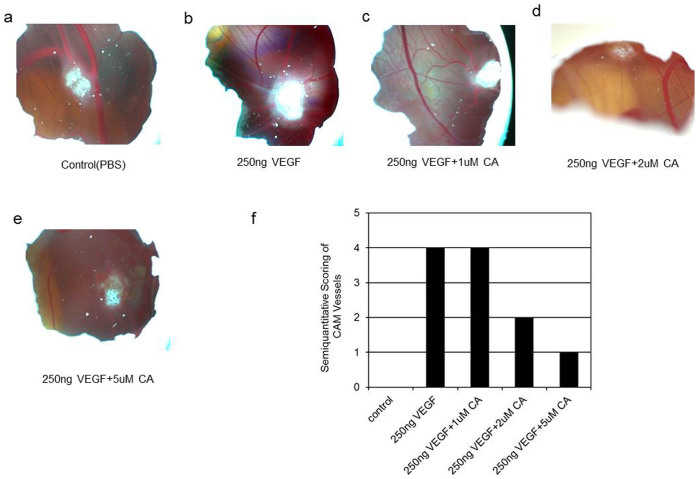
Furthermore as our in vitro data indicated that CA could significantly inhibit the pro-angiogenic actions of VEGFA (Figure 3, 4, 5, 6, 7), we next examined the effects of CA (1, 2 and 5 μM) on VEGFA induced angiogenesis using CAM assay7,12,13. New blood vessel formation or angiogenesis was not detected on treatment with the vehicle (phosphate buffered saline solution) (Figure 8a and 8f). In contrast, prominent angiogenesis was seen on treatment with 250 ng of VEGFA (Figure 8b and 8f). Furthermore, although 1 μM of CA had no prominent effects, there was significant inhibition of VEGFA mediated angiogenesis after treatment with 2 and 5 μM of CA (Figure 8c, 8d, 8e and 8f). Importantly, CA alone had no effects on angiogenesis (data not shown). These observations were made on Day 4 after treatment with CA.
Figure 8. The effects of chebulagic acid (CA) on vascular endothelial growth factorA (VEGFA) induced new blood formation or angiogenesis in the CAM assay.
(a, f) Phosphate buffered saline (PBS) used as control did not induce blood vessel formation. (b, f) On the contrary, VEGFA stimulated new blood vessel formation. (c, f) 1 μM of CA did not inhibit VEGFA induced angiogenesis. (d, e, f) 2 or 5 μM of CA inhibited VEGFA induced new blood vessel formation. The photograph represents six separate experiments.
In conclusion, this study demonstrates that CA by suppressing VEGFA mediated activation of VEGFR-2 inhibits the pro-angiogenic actions of this cytokine on endothelial cells. Because angiogenesis plays an important role in many pathological conditions such as malignant tumours, rheumatoid arthritis and diabetic retinopathy, therefore this natural molecule may be used as an effective anti-angiogenic agent for the treatment of these diseases in future14.
Methods
Reagents
Triphala and more than 90% pure CA were purchased from Dabur, New Delhi, India and Natural Remedies, Bangalore, India respectively. The recombinant human VEGFA was procured from R&D systems, MN, USA. The CA and other solutions used for the experiments were free from endotoxin as determined by gel-clot limulus amebocyte lysate assay (Charles River, MA, USA)15. All other chemicals were from Sigma, MO, USA.
Detection of plasma CA in mice
All animal experiments were undertaken following the approved protocol of Institutional Animal Care and Use Committee of the Ohio State University. 6–8 wks old, C57BL/6 male mice were at first gavaged with a single non-toxic dose (100 mg/kg) of THL7. Blood was then collected by cardiac puncture at different time intervals followed by separation of plasma. The level of CA in plasma was finally determined by LC-MS/MS7.
Endothelial cell culture
HUVEC were cultured in endothelial basal medium (EBM) supplemented with growth factors and 2% FBS (Lonza, CA, USA). All HUVEC experiments unless specified were undertaken in 24 hrs serum and growth factor starved cells7,16,17.
In vitro toxicity assay
In order to determine the cytotoxicity of CA, trypan blue dye exclusion test was done to examine the viability of the cells. 2 × 104 endothelial cells were at first treated with 1, 2 and 5 μM of CA. 200 μl of 0.4% w/v trypan blue (Sigma-Aldrich, MO, USA) was then added to these cells followed by counting of the stained cells12.
Proliferation assay
5 × 103 HUVEC were either untreated or treated with VEGFA (20 ng/ml) or VEGFA (20 ng/ml) + CA (1, 2 and 5 μM) for 24 hr. Prestoblue™ Cell Viability reagent (Invitrogen, NY, USA) was used as per instructions of the manufacturer to detect proliferation of these cells7,18.
Wound healing assay
After wounding the HUVEC monolayers with a 200 μl pipette, the effects of CA on VEGFA mediated migration were determined in both untreated cells or cells treated with VEGFA (20 ng/ml) or VEGFA (20 ng/ml) + CA (1, 2 and 5 μM). The closure of wound was determined at 16 hr by the distance covered by these cells in relation to the initial distance. The results were expressed as percentage of wound healed7,17.
Endothelial cell tube formation assay
In vitro angiogenesis assay kit (Millipore, CA, USA) was used as per the manufacturers instructions to determine the effect of CA on HUVEC tube formation. In brief, HUVEC treated either with phosphate buffered saline or VEGFA (20 ng/ml) or VEGFA (20 ng/ml) + CA (1, 2 and 5 μM) were seeded on extra cellular matrix to form capillary tubes. The tubular structures were manually quantified by counting the numbers of connected cells in randomly selected fields and the total tube numbers in the control group was designated as 100%7,19.
In vitro endothelial cell permeability assay
This assay was undertaken as per the instructions of the manufacturer using in vitro vascular permeability assay kit (Millipore, CA, USA). 2 × 105 HUVEC were seeded onto collagen coated inserts in 24 well plates. The respective inserts containing HUVEC were then pre-treated with 1, 2 and 5 μM of CA for 24 hr. Thereafter, FITC-Dextran containing phenol free medium containing 20 ng/ml of VEGFA was added and the fluorescence was measured at 485 nm/535 nm7,17.
Western Blot
Rabbit monoclonal antibody against phospho VEGFR-2 purchased from Cell Signaling, MA, USA was used to detect VEGFR-2 phosphorylation. The protein loads were normalized using antibody for β actin (Sigma, MO, USA) and the antibody reactive bands were quantified by densitometry7,20,21.
Chick chorioallantoic membrane (CAM) assay
Semi-quantitative CAM assay was utilized to determine the effects of CA on VEGFA stimulated new blood vessel formation or angiogenesis. At first, windows were created on the shells of Leghorn chicken eggs (Ohio State University Farms, Columbus, USA) at day 3 of fertilization to expose the CAM. 1-mm3 sterilized gelatin sponge (Pfizer, MI, USA) soaked either phosphate buffered saline (PBS) (control), or VEGFA (250 ng) or VEGFA (250 ng) + CA (1, 2 and 5 μM) was then aseptically inserted onto the CAM on day 8. Formation of new blood vessel formation was scored on day 12 as negative (0); change in vessel architecture (0.5); partial spoke of a wheel (1/3 of circumference exhibited directional angiogenesis) (1); spoke of a wheel (2); strong and fully spoke of a wheel (≥3)7,12,13. Nikon D70 camera with AF Micro Nikkor 105 mm lens was used to take the photographs7.
Statistical analysis
ANOVA and unpaired Student's t test or Dunn's multiple comparison tests were used to analyze the differences among the groups. P < 0.05 was considered to be significant16,20.
Supplementary Material
Full Length Blot of Figure 7
Acknowledgments
This study is supported by the National Institutes of Health, USA grants to SB (R01CA124763 and R01CA169158. We acknowledge the Pharmacoanalytical Shared Resource Facility (Biomedical Mass Spectrometry laboratory at the College of Pharmacy) of the Ohio State University for detection of chebulagic acid in the plasma of mice.
Footnotes
Author Contributions S.B. conceived the idea. K.L. and S.B. participated in the design of the study. K.L. performed the experiments, K.L. and S.B. prepared the manuscript. Finally, both K.L. and S.B. read and approved the manuscript.
References
- Dvorak H. F. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J. Clin. Oncol. 20, 4368–4380 (2002). [DOI] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor. Arterioscler. Thromb. Vasc. Biol. 29, 789–791 (2009). [DOI] [PubMed] [Google Scholar]
- Sloan B. & Scheinfeld N. S. Pazopanib, a VEGF receptor tyrosine kinase inhibitor for cancer therapy. Curr. Opin. Investig. Drugs. 9, 1324–1335 (2008). [PubMed] [Google Scholar]
- Sullivan L. A. & Brekken R. A. The VEGF family in cancer and antibody-based strategies for their inhibition. MAbs. 2, 165–175 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niraula S., Amir E., Vera-Badillo F., Seruga B., Ocana A. & Tannock I. F. Risk of incremental toxicities and associated costs of new anticancer drugs: a meta-analysis. J. Clin. Oncol. 32, 3634–3642 (2014). [DOI] [PubMed] [Google Scholar]
- Baliga M. S. Triphala, Ayurvedic formulation for treating and preventing cancer: a review. J. Altern. Complement. Med. 16, 1301–1308 (2010). [DOI] [PubMed] [Google Scholar]
- Lu K. et al. Triphala and its active constituent chebulinic acid are natural inhibitors of vascular endothelial growth factor-A mediated angiogenesis. PLoS One 7, e43934 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D. P., Govindarajan R. & Rawat A. K. High-performance liquid chromatography as a tool for the chemical standardisation of Triphala- an Ayurvedic formulation. Phytochem. Anal. 19, 164–168 (2008). [DOI] [PubMed] [Google Scholar]
- Pawar V., Lahorkar P. & Anantha Narayana D. B. Development of a RP-HPLC method for analysis of Triphala Curna and its applicability to test variations in Triphala Curna preparations. Indian J. Pharm. Sci. 71, 382–386 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferruzzi M. G. et al. Bioavailability of gallic acid and catechins from grape seed polyphenol extract is improved by repeated dosing in rats: implications for treatment in Alzheimer's disease. J. Alzheimers. Dis. 18, 113–124 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeram N. P., Lee R. & Heber D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin. Chim. Acta. 348, 63–68 (2004). [DOI] [PubMed] [Google Scholar]
- Hussain S. et al. Anti-angiogenic activity of sesterterpenes; natural product inhibitors of FGF-2-induced angiogenesis. Angiogenesis 11, 245–256 (2008). [DOI] [PubMed] [Google Scholar]
- Ribatti D., Nico B., Vacca A. & Presta M. The gelatin sponge-chorioallantoic membrane assay. Nat. Protoc. 1, 85–91 (2006). [DOI] [PubMed] [Google Scholar]
- Hoeben A. et al. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 56, 549–580 (2004). [DOI] [PubMed] [Google Scholar]
- Li L., Wilbur C. L. & Mintz K. L. Kinetics of hydrothermal inactivation of endotoxins. Appl. Environ. Microbiol. 77, 2640–2647 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. et al. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat. Med. 7, 569–574 (2001). [DOI] [PubMed] [Google Scholar]
- Chakroborty D. et al. Dopamine stabilizes tumor blood vessels by up-regulating angiopoietin 1 expression in pericytes and Kruppel-like factor-2 expression in tumor endothelial cells. Proc. Natl. Acad. Sci. U S A 108, 20730–20735 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istivan T. S. et al.Biological effects of a de novo designed myxoma virus peptide analogue: evaluation of cytotoxicity on tumor cells. PLoS One 6, e24809 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B. et al. VEGF-C differentially regulates VEGFA expression in ocular and cancer cells; promotes angiogenesis via RhoA mediated pathway. Angiogenesis 14, 371–380 (2011). [DOI] [PubMed] [Google Scholar]
- Sarkar C., Chakroborty D., Chowdhury U. R., Dasgupta P. S. & Basu S. Dopamine increases the efficacy of anticancer drugs in breast and colon cancer preclinical models. Clin. Cancer Res. 14, 2502–2510 (2008). [DOI] [PubMed] [Google Scholar]
- Antonescu C. R. et al. (2009) KDR activating mutations in human angiosarcomas are sensitive to specific kinase inhibitors. Cancer Res. 69, 7175–7179 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full Length Blot of Figure 7