Abstract
Introduction
A major goal of neonatal medicine is to identify neonates at highest risk for morbidity and mortality. Previously, we developed PhysiScore (Saria et al., 2010), a novel tool for preterm morbidity risk prediction. We now further define links between overall individual morbidity risk, specific neonatal morbidities, and placental pathologies.
Methods
102 placentas, including 38 from multiple gestations, were available from the previously defined PhysiScore cohort (gestational age ≤ 34 weeks and birth weight ≤ 2000 grams). Placentas were analyzed for gross and histologic variables including maternal malperfusion, amniotic fluid infection sequence, chronic inflammation, and fetal vascular obstruction. Risk as determined by PhysiScore and recorded neonatal morbidities were tested for statistical association with placental findings.
Results
In pair-wise correlations, respiratory distress syndrome, bronchopulmonary dysplasia, acute hemodynamic instability, post-hemorrhagic hydrocephalus, culture positive sepsis, and necrotizing enterocolitis each significantly correlated with at least one placenta histology variable. Amniotic fluid infection sequence (p = 0.039), specifically the fetal inflammatory response (p = 0.017), correlated with higher PhysiScores (greater morbidity) but was not independent of gestational age and birth weight. In multivariate analyses correlating variables with all nine morbidities, gestational age (p <0.001), placental size <10th percentile, (p = 0.031) full thickness perivillous fibrin deposition (p = 0.001), and amniotic fluid infection sequence (umbilical arteritis, p = 0.031; ≥2 chorionic plate vessels with vasculitis, p = 0.0125), each were significant associations.
Discussion
Amniotic fluid infection sequence plays a significant role in neonatal morbidity. Less neonatal morbidity was observed in older and heavier infants and those with small placental size and full thickness perivillous fibrin deposition. The combined assessment of placental gross and histologic findings together with physiologic risk evaluation may allow more precise prediction of neonatal morbidity risk soon after delivery.
Keywords: preterm infant, morbidity, placenta, fetal inflammatory response, histology
INTRODUCTION
The clinical outcome of prematurity varies widely in terms of infant morbidity, and predicting an individual neonate’s morbidity risk will allow tailored interventions. Many different prediction methods have been developed to try to determine the risk of severe morbidity or mortality in neonates. Recently, PhysiScore was published as a robust tool for integrating physiologic data within the first 3 hours of life in order to predict morbidity in the premature infant (Saria et al. [1]). Specifically, PhysiScore uses the mean, base, and residual variability of the heart rate and respiratory rate, mean oxygen saturation and cumulative hypoxia time, gestational age, and birth weight to calculate a probability score between 0 and 1, with higher scores indicating a higher risk of severe morbidity due to cardiopulmonary and infectious complications. Increased gestational age and birth weight have long been known to decrease the risk of death and illnesses including respiratory distress syndrome (RDS), high grade intraventricular hemorrhage (IVH), necrotizing enterocolitis (NEC), and sepsis [2; 3]. However, PhysiScore is the first method to also take into account physiologic data derived from routine monitoring.
Placental evidence of antenatal conditions also likely correlates with morbidity, but studies are few, and many fail to incorporate newer entities in placental pathology. Of the published studies, there is conflicting evidence of placental histology correlating with neonatal morbidities. For instance, one study correlates chorioamnionitis with many morbidities including bronchopulmonary dysplasia (BPD) [4], whereas other studies refute a significant relationship between chorioamnionitis and BPD [5; 6]. Placental histologic features of maternal malperfusion, including distal villous hypoplasia, and chronic inflammation, including chronic chorionitis, are not often cited or examined as risk factors for neonatal disease.
In this study, we report a detailed retrospective review of placental histology from the PhysiScore cohort of preterm NICU neonates. Using 18 placental gross and histologic variables to represent the more severe or novel placental findings, we performed a comprehensive analysis to identify links between neonatal physiology, risk of morbidities associated with preterm birth, and specific pathologies, testing the hypothesis that placental pathological features correlate with postnatal physiology predictive of risk in preterm infants.
METHODS
Preterm Cohort
The preterm cohort employed the same infants as those in the PhysiScore study [1]. In brief, infants admitted to the NICU at Lucile Packard Children’s Hospital between March 2008 and March 2009 were eligible for enrollment. With approval of Stanford’s Panel on Human Subjects, a total of 138 neonates were enrolled, meeting the criteria of gestational age ≤34 weeks, birth weight ≤2000 grams, available cardiorespiratory monitor data within the first three hours of birth, and without major malformations. Electronic medical records, imaging studies, and laboratory values were noted. Gender, 5 minute Apgar score, and multiple gestations were recorded. Documented morbidities included RDS, pneumothorax, BPD, acute hemodynamic instability (characterized by hypotension requiring 3 or more days of pressor support or adrenal insufficiency requiring hydrocortisone), retinopathy of prematurity (ROP), IVH, post-hemorrhagic hydrocephalus (PHH), culture positive sepsis, and NEC. Criteria for identifying BPD, ROP, IVH, and NEC are as previously described [7–10]. The highest unilateral stage or grade for ROP and IVH respectively, were noted. For analyses, only moderate and severe BPD, high stage (≥2) ROP, and high grade (≥3) IVH were included. Of these nine morbidities, 7 were considered severe: bronchopulmonary dysplasia, acute hemodynamic instability, retinopathy of maturity, intraventricular hemorrhage, post-hemorrhagic hydrocephalus, culture positive sepsis, and necrotizing enterocolitis. PhysiScores were recorded as previously calculated [1].
Placentas
Of the 138 neonates in the preterm cohort, 102 infants had placentas submitted for macroscopic and microscopic examination that were available for re-review. Histologic sections included sections of umbilical cord, membrane rolls, and at least 2 full-thickness placenta sections. Blinded to clinical data, the placenta reports and slides were reviewed by a perinatal pathologist for a comprehensive set of 73 gross and microscopic features. Of these features, 18 major findings were chosen for further analysis, as we hypothesized that more severe placental pathology would correlate with significant neonatal morbidity. In addition, we included some more recently described entities such as chronic chorionitis [11] to determine if there were any associations with the neonatal clinical findings. The gross variables included a marginal (within 1 cm of the margin) or velamentous umbilical cord insertion, and trimmed placental weight, from which weights ≤10th percentile and ≥90th percentile were determined using the reference values for singleton and twin placental weights [12]. Placenta weight:birth weight ratios were calculated. For twins with fused placentas (no triplets had three fused placentas), the placenta weights were divided by two. Two fetal and two maternal variables were classified as features of amniotic fluid infection sequence: fetal inflammatory responses (umbilical arteritis and ≥2 chorionic plate vessel with vasculitis) and maternal inflammatory responses (acute subchorionitis with abscess formation, and subacute or necrotizing acute chorioamnionitis) [13]. Of note, all of the cases found to have ≥2 chorionic plate vessel with vasculitis also demonstrated umbilical arteritis. Maternal malperfusion was delineated by four features: placental size ≤10th percentile, distal villous hypoplasia, severe maternal decidual vasculopathy (fibrinoid necrosis and/or acute atheromatous changes) and ≥2 infarcts [14]. Full thickness perivillous fibrin was noted when at least one slide demonstrated an exaggeration of Langhan’s stria encasing both stem villi and regional distal villi from the chorionic plate to basal fibrin [15]. Two features of chronic inflammation (chronic chorionitis and basal chronic villitis, and parenchymal chronic villitis), were chosen as features of an aberrant maternal immune response. Chronic chorionitis, a more recently described entity, was diagnosed based upon the presence of band-like infiltrates of mononuclear cells associated with membranous cytotrophoblast cell dropout, and/or lymphohistiocytic inflammation of the basal chorionic plate obvious on low power. Basal chronic villitis is chronic villitis limited to the area adjacent to or at the decidual basalis, and is commonly associated with lymphoplasmacytic deciduitis [11; 16]. The cases of parenchymal chronic villitis were all villitis of unknown etiology, representing more than 10 villi per focus on more than one slide (high-grade villitis) [16]; some of these cases were also associated with fetal vasculopathy. No viral cytopathic effects or plasma cells were present in these cases of chronic villitis to suggest an infectious etiology. The two variables of large vessel thrombi and villous damage from fetal ischemia (avascular villi or villous stromal-vascular karyorrhexis) were grouped under the heading of fetal vascular obstruction [17]. In addition to a placental size ≥90th percentile, the morphologic variable of villous edema was subgrouped under a large, edematous placenta. Diffuse chorionic hemosiderosis was an additional variable, as defined by Redline and Wilson-Costello [18]. Lastly, nucleated red blood cells enumerated a >10/10 high power fields was recorded as an indicator of fetal hypoxia [19].
Statistics
The baseline and disease characteristics of the study cohort were summarized with mean and standard deviation for continuous variables and actual counts for binary variables. The two-sided Wilcox rank test was used to test the difference in gestational age between infants with and without each morbidity of interest. The Fisher’s exact test was used to test the association between binary histology measures and comorbidities. The odds ratio and the corresponding 95% confidence intervals were obtained. The proportional odds regression model was used to test associations between histology characteristics and PhysiScore, with or without the adjustment of gestational age and birth weight, where PhysiScore was treated as an ordinal response. The association of interest was summarized by odds ratio of having a high PhysiScore versus low PhysiScore and the associated 95% confidence interval. Similarly, associations between histology characteristics and the number of morbidities were examined. The statistical analysis was performed using R 3.1.3 (The R Foundation for Statistical Computing).
RESULTS
Correlation of Neonatal Outcomes with Placental Histology
Table 1 details the characteristics and morbidities of the 102 preterm infants in this study. Thirty-eight infants (37.2%) were the products of multiple gestations. As listed, the most common morbidity was RDS (77%). In total, 9.8% of infants had moderate or severe BPD, 8.8% had high stage (stage II or III) ROP in at least one eye, and 20.6% high grade (grade 3 or 4) IVH detected on radiography. Sepsis was suspected in many infants, but only proven by culture in 5.9%. No severe morbidities were identified in 18.6% infants.
Table 1.
Baseline and disease characteristics of the study cohort.
| Infant Characteristics | Number |
|---|---|
| Subjects | 102 |
| Birth weight (average, g) | 1405 ± 403 |
| Gestational age (average, weeks) | 30.2 ± 2.7 |
| Gender, M:F | 58:44 |
| Apgar score at 5 min (average) | 7.56 ± 1.82 |
| Multiple gestation | |
| Total | 38 |
| Twins | 32 |
| Triplets | 6 |
| PhysiScore (average) | 0.236 ± 0.315 |
| Placenta weight (average) | 309.7 ± 83.3 |
| Placenta weight: birth weight ratio (average) | 0.23 ± 0.08 |
|
| |
| Maternal Characteristics | Number |
|
| |
| Maternal age (years) | 32.6 ± 6.3 |
| Gravida (average) | 2.8 ± 1.8 |
| Para (average) | 0.9 ± 1.2 |
| Pre-eclampsia, HELLP | 24 |
| Preterm premature rupture of membranes | 36 |
| Spontaneous preterm labor | 56 |
| Induction of labor | 10 |
| Clinical placental abruption | 10 |
| Cesarean section mode of delivery | 76 |
|
| |
| Infant Morbidities | Number |
|
| |
| Respiratory distress syndrome | 79 |
| Pneumothorax | 8 |
| Bronchopulmonary dysplasia, total | 20 |
| NOSa | 2 |
| Mild | 8 |
| Moderate | 4 |
| Severe | 6 |
| Acute hemodynamic instabilityb | 16 |
| Retinopathy of prematurity (ROP)b, total | 15 |
| Stage I | 6 |
| Stage II | 6 |
| Stage III | 3 |
| Intraventricular hemorrhage (IVH)c, total | 21 |
| Grade 1 | 11 |
| Grade 2 | 5 |
| Grade 3 | 2 |
| Grade 4 | 3 |
| Post-hemorrhagic hydrocephalus | 5 |
| Culture-positive Sepsis | 6 |
| Necrotizing enterocolitis, total | 6 |
| Stage 1 | 2 |
| Stage 2 | 2 |
| Stage 3 | 2 |
| No disease | 19 |
| Died | 1 |
Infants with oxygen requirement at 28 days for whom oxygen requirement was not known at 36 weeks after menstrual age; NOS, not otherwise specified
Acute hemodynamic instability is characterized by hypotension requiring ≥3 days of pressor support or adrenal insufficiency requiring hydrocortisone.
ROP is counted by the most severe stage in either eye during the hospitalization
IVH is counted by the most severe grade in either cerebral hemisphere by Papile classification
Placentas from the 102 infants were subjected to gross and histological review. Figure 1 displays some of the identified histologic features. Table 2 lists the percent of each infant morbidity showing each histologic feature, using pair-wise associations with two-tailed T-tests; this table also lists the mean gestational age, PhysiScore, and placenta weight:birth weight ratio for each morbidity.
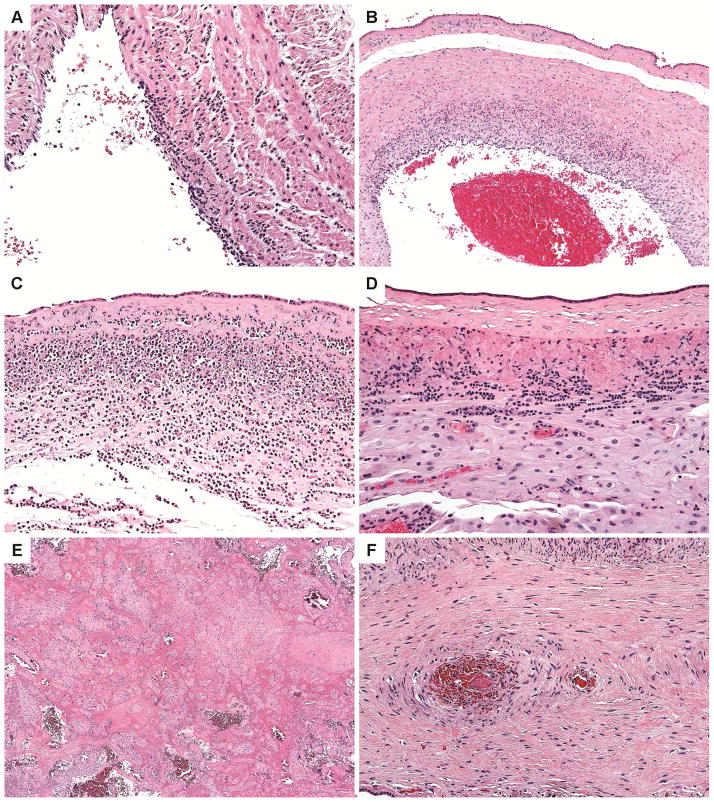
Figure 1. Placental Histology.
Fetal inflammatory response features include umbilical arteritis (A) and chorionic plate vessels with vasculitis (B). A maternal inflammatory response includes necrotizing acute chorioamnionitis (C). Lymphocytic inflammation can be characterized by chronic chorionitis (D). Full thickness perivillous fibrin deposition was present in some placentas (E). A stem villous thrombus is identified (F).
Table 2.
Premature Infant Morbidities Pair-Wise Correlated with Placental Histology
| Placental Histology | Total | RDSa | Pneumothorax | BPDb | AHIc | ROPd | IVHe | PHHf | Culture+Sepsis | NECg |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Total Number (percent of total) | 102 | 79 (77.5%) | 8 (7.8%) | 10 (9.8%) | 16 (15.7%) | 9 (8.8%) | 5 (4.9%) | 5 (4.9%) | 6 (5.9%) | 6 (5.9%) |
|
| ||||||||||
| Gestational age in weeks (mean) | 30.2 | 29.5* | 27.0* | 26.4* | 26.4* | 25.6* | 29.2 | 26.7* | 25.3* | 26.0* |
|
| ||||||||||
| PhysiScore (mean) | 0.236 | 0.291* | 0.718* | 0.770* | 0.726* | 0.853* | 0.364 | 0.597* | 0.909* | 0.724* |
|
| ||||||||||
| Placenta weight:birth weight ratio (mean) | 0.23 | 0.24 | 0.31* | 0.33* | 0.32* | 0.33* | 0.24 | 0.31* | 0.29 | 0.37* |
|
| ||||||||||
| Amniotic Fluid Infection Sequence | 22/102 (21.6%) | 26.6%* | 37.5% | 40.0% | 37.5% | 44.4% t | 40.0% | 40.0% | 66.7%* | 50.0% |
| Fetal inflammatory response | 21/102 (20.6%) | 25.3%* | 37.5% | 30.0% | 37.5% t | 44.4% t | 40.0% | 40.0% | 50.0%* | 50.0% |
| Umbilical Arteritis | 20/101 (19.8%) | 24.1% t | 37.5% | 30.0% | 37.5% t | 44.4% t | 40.0% | 40.0% | 50.0% t | 50.0% t |
| ≥2 Chorionic plate vessels with vasculitis | 16/99 (16.2%) | 20.5%* | 25.0% | 20.0% | 31.3% | 33.3% | 40.0% | 40.0% | 50.0% t | 33.3% |
| Maternal inflammatory response | 18/102 (17.6%) | 21.5% t | 25.0% | 30.0% | 25.0% | 22.2% | 20.0% | 20.0% | 50.0% t | 33.3% |
| Acute subchorionitis with abscess formation | 9/102 (8.8%) | 10.1% | 0.0% | 0.0% | 6.3% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Subacute or necrotizing acute chorioamnionitis | 15/102 (14.7%) | 17.7% | 25.0% | 30.0% | 18.8% | 22.2% | 20.0% | 20.0% | 50.0* | 33.3% |
|
| ||||||||||
| Maternal malperfusion | 46/102 (45.1%) | 41.8% | 50.0% | 40.0% | 50.0% | 11.1%* | 20.0% | 20.0% | 16.7% | 66.7% |
| Size ≤10th percentile | 13/101 (12.9%) | 7.7% * | 0.0% | 10.0% | 6.3% | 0.0% | 20.0% | 0.0% | 16.7% | 0.0% |
| Distal villous hypoplasia | 34/102 (33.3%) | 31.6 | 37.5% | 30.0% | 37.5% | 11.1% | 0.0% | 0.0% | 0.0% | 50.0% |
| ≥2 Infarcts | 10/102 (9.8%) | 7.6% | 0.0% | 10.0% | 0.0% | 11.1% | 0.0% | 0.0% | 0.0% | 0.0% |
| Maternal decidual vasculopathy, severe | 11/83 (13.3%) | 9.4% | 20.0% | 0.0% | 8.3% | 0.0% | 0.0% | 25.0% | 0.0% | 25.0% |
|
| ||||||||||
| Full thickness perivillous fibrin | 10/102 (9.8%) | 3.8% * | 0.0% | 0.0% | 0.0% | 33.3% | 0.0% | 0.0% | 0.0% | 0.0% |
|
| ||||||||||
| Chronic inflammation | 34/102 (33.3%) | 34.2% | 12.5% | 30.0% | 25.0% | 33.3% | 60.0% | 80.0%* | 83.3%* | 16.7% |
| Chronic chorionitis and basal chronic villitis | 32/101 (31.7%) | 33.3% | 12.5% | 30.0% | 25.0% | 33.3% | 60.0% | 80.0%* | 83.3%* | 16.7% |
| Parenchymal chronic villitis | 9/102 (8.8%) | 7.6% | 12.5% | 10.0% | 12.5% | 11.1% | 20.0% | 40.0% t | 16.7% | 16.7% |
|
| ||||||||||
| Fetal vascular obstruction | 26/102 (25.5%) | 25.3% | 37.5% | 50.0% | 31.3% | 44.4% | 0.0% | 20.0% | 0.0% | 50.0%* |
| Large vessel thrombi | 11/101 (10.9%) | 11.4% | 25.0% | 30.0% t | 18.8% | 33.3% t | 0.0% | 20.0% | 0.0% | 50.0%* |
| Villous damage from fetal ischemia | 17/102 (16.7%) | 16.5% | 25.0% | 30.0% | 18.8% | 22.2% | 0.0% | 0.0% | 0.0% | 33.3% |
|
| ||||||||||
| Marginal or velamentous cord insertion | 21/100 (21.0%) | 20.8% | 0.0% | 55.6%* | 35.7% | 50.0% | 2.0% | 20.0% | 16.7% | 25.0% |
|
| ||||||||||
| Large, edematous placenta | 38/102 (37.3%) | 44.3%* | 37.5% | 10.0% t | 43.8% | 33.3% | 60.0% | 40.0% | 16.7% | 50.0% |
| Size ≥90th percentile | 24/101 (23.8%) | 29.5%* | 37.5% | 10.0% | 25.0% | 33.3% | 40.0% | 40.0% | 16.7% | 33.3% |
| Villous edema | 23/102 (22.5%) | 26.6% t | 25.0% | 10.0% | 43.8%* | 33.3% | 40.0% | 40.0% | 16.7% | 50.0% |
|
| ||||||||||
| Diffuse chorionic hemosiderosis | 3/101 (3.0%) | 3.8% | 12.5% | 20.0%* | 6.3% | 11.1% | 0.0% | 0.0% | 16.7% | 16.7% |
|
| ||||||||||
| Nucleated red blood cells >10/10 high power fields | 29/101 (28.7%) | 28.2% | 28.6% | 50.0% | 18.8% | 33.3% | 40.0% | 20.0% | 0.0% | 50.0% |
considered statistically significant
trending p-value (between 0.050 and 0.100)
RDS: respiratory distress syndrome
BPD: moderate and severe bronchopulmonary dysplasia
AHI: acute hemodynamic instability characterized by hypotension requiring ≥3 days of pressor support or adrenal insufficiency requiring hydrocortisone
ROP: high stage (≥2) retinopathy of maturity
IVH: high grade (≥3) intraventricular hemorrhage
PHH: post-hemorrhagic hydrocephalus
NEC: necrotizing enterocolitis
Increased gestational age was significantly protective (p ≤ 0.01) of all morbidities except for IVH; similarly PhysiScores were significantly increased (p ≤ 0.05) in all morbidities except for IVH. Placenta weight:birth weight ratios were significantly increased in all morbidities compared to unaffected infants, except for those with RDS, IVH, and culture positive sepsis. Placental size less than 10th percentile and increased full-thickness perivillous fibrin were significantly protective against RDS. Of note, out of 46 cases with placental features of maternal malperfusion, only 20 (43%) had clinical maternal pre-eclampsia (data not shown).
The risk of RDS was significantly increased in the presence of amniotic fluid infection sequence, specifically fetal inflammatory response, especially multifocal chorionic plate vasculitis. Placental weight greater than 90th percentile also correlated with an increased risk of RDS. The risk of high grade BPD was significantly elevated in placentas with a marginal or velamentous cord insertion and diffuse chorionic hemosiderosis; BPD also trended with large vessel thrombi. Acute hemodynamic instability was significantly correlated with villous edema. High grade ROP trended with large vessel thrombi and marginal or velamentous cord insertion, and was significantly associated with the absence of features of maternal malperfusion. Chronic inflammation in general, and chronic chorionitis and basal chronic villitis specifically, significantly increased the risk of PHH. An increased risk of culture-positive sepsis was evident in infants whose placentas demonstrated amniotic fluid infection sequence, specifically a fetal inflammatory response and a maternal inflammatory response of subacute or necrotizing acute chorioamnionitis. In addition, culture-positive sepsis correlated with chronic inflammation, specifically chronic chorionitis and basal chronic villitis. Lastly, NEC was increased in those infants with placentas demonstrating fetal vascular obstruction, specifically large vessel thrombi.
Comparing the multiple gestation infants (n=38) to the singleton infants (n=74) showed few statistically significant differences. The multiple gestations infants were born at increased gestational age (31.1 weeks versus 29.7 weeks, p = 0.01), and had mothers with increased maternal age (35.1 years versus 31.2 years, p = 0.002). The only morbidity with any statistically significant difference between these infants was retinopathy of prematurity, which was decreased in the multiple gestations (p = 0.025), likely due to increased gestational age. The multiple gestation infants also had fewer features of amniotic fluid infection: umbilical arteritis (7.9% vs 27.0%, p = 0.022), ≥2 chorionic plate vessel with vasculitis (2.7% vs 24.2%, p = 0.004), and subacute or necrotizing acute chorioamnionitis (5.3% vs 20.3%, p = 0.045). Multiple infarcts were very uncommon in twins (0.0% vs 15.6%, p = 0.012). Interestingly, there was no statistically significant difference in rate of marginal or velamentous cord insertion (29.7% vs 15.8%, p = 0.129), a finding often identified in multiple gestation pregnancies [20].
Correlation with PhysiScore with placental histology
PhysiScores had been previously calculated [1] and ranged from 0.0170 to 1.000, with a median of 0.0576, and average of 0.236; the higher the PhysiScore, the higher the risk of severe morbidity. To determine if the calculated PhysiScores correlated with the placental gross or histologic features, a proportional odds regression model was used to test for associations, with and without adjustment for gestational age and birth weight (Table 3). Amniotic fluid infection sequence was associated with higher unadjusted PhysiScores, indicating loss of physiological variability and increased risk of morbidity. Full thickness perivillous fibrin deposition was associated with lower unadjusted PhysiScores. Maternal malperfusion, chronic inflammation, fetal vascular obstruction, and large and/or edematous placenta, were not statistically associated with PhysiScore. When amniotic fluid infection sequence components were assessed, only the fetal inflammatory response was associated with unadjusted PhysiScore. However, when PhysiScore was adjusted for both gestational age and birth weight, these fetal inflammatory responses and full thickness perivillous fibrin deposition associations were lost.
Table 3.
Placental Histology Characteristics associated with PhysiScore
| Placental Histology | Average GA (weeks) | Average BW (g) | Average PhysiScore | Association with PhysiScore unadjusted | Association with PhysiScore adjusted for GA and BW | ||
|---|---|---|---|---|---|---|---|
| ORc | p-value | OR | p-value | ||||
|
| |||||||
| Amniotic Fluid Infection Sequence | 28.4* | 1271 | 0.42* | 2.996 | 0.017 ** | 1.330 | 0.554 |
| Fetal inflammatory response | 28.5* | 1296 | 0.40* | 2.598 | 0.039 ** | 1.347 | 0.544 |
| Maternal inflammatory response | 28.4* | 1259 | 0.40* | 2.422 | 0.071 | 0.737 | 0.534 |
|
| |||||||
| Maternal malperfusion | 30.2 | 1322* | 0.24 | 1.618 | 0.167 | 0.970 | 0.936 |
|
| |||||||
| Full thickness perivillous fibrin | 32.6* | 1555 | 0.04* | 0.330 | 0.049 ** | 0.724 | 0.598 |
|
| |||||||
| Chronic inflammation | 29.6 | 1371 | 0.25 | 1.310 | 0.459 | 0.576 | 0.128 |
|
| |||||||
| Fetal vascular obstruction | 29.9 | 1292 | 0.29 | 1.619 | 0.220 | 1.138 | 0.751 |
| Large vessel thrombi | 28.7 | 1286 | 0.38 | 2.453 | 0.117 | 1.813 | 0.358 |
| Villous damage from fetal ischemia | 30.1 | 1281 | 0.27 | 1.370 | 0.489 | 1.026 | 0.955 |
|
| |||||||
| Large and/or edematous placenta | 29.6 | 1399 | 0.29 | 1.673 | 0.155 | 1.822 | 0.132 |
|
| |||||||
| Diffuse chorionic hemosiderosis | 27.2 | 1077 | 0.64* | 6.639 | 0.085 | 1.271 | 0.686 |
GA: gestational age; BW: birth weight
OR: odds ratio
considered statistically significant in comparison to those without placental histology
considered statistically significant
Correlation of Neonatal Outcomes with PhysiScore
In performing proportional odds regression model analyses of PhysiScore, gestational age, birth weight, and placental variables against the group of all nine morbidities and against the group of the 7 severe morbidities, older gestational age and higher birth weight decreased the risk of morbidities (Table 4). Small placental size and full thickness perivillous fibrin deposition also each decreased the risk of morbidities (when all nine morbidities were grouped together). The fetal inflammatory responses of umbilical arteritis and multifocal chorionic plate vasculitis each increased the risk of morbidities (when analyzed against all 9 morbidities and the 7 severe morbidities). Diffuse chorionic hemosiderosis trended with increased morbidities when compared to both groups of morbidities.
Table 4.
Associations with Total Number of Morbidities
| Variable | All 9 morbiditiesa | 7 severe morbiditiesb | ||
|---|---|---|---|---|
| ORc (CId) | p-value | ORc (CId) | p-value | |
|
| ||||
| PhysiScore | 2.028 (1.656–2.484) | <0.001* | 1.912 (1.563–2.339) | <0.001* |
|
| ||||
| Gestational age | 0.437 (0.351–0.545) | <0.001* | 0.441 (0.337–0.577) | <0.001* |
|
| ||||
| Birth weight | 0.996 (0.995–0.998) | <0.001* | 0.995 (0.994–0.996) | <0.001* |
|
| ||||
| Amniotic Fluid Infection Sequence | ||||
| Fetal inflammatory response | ||||
| Umbilical Arteritis | 3.075 (1.209–7.822) | 0.018* | 2.942 (1.047–8.268) | 0.041* |
| ≥2 Chorionic plate vessels with vasculitis | 3.440 (1.272–9.307) | 0.015* | 3.092 (1.036–9.225) | 0.043* |
| Maternal inflammatory response | ||||
| Acute subchorionitis with abscess formation | 0.813 (0.251–2.630) | 0.730 | 0.327 (0.039–2.726) | 0.302 |
| Subacute or necrotizing acute chorioamnionitis | 2.620 (0.948–7.237) | 0.063 | 2.572 (0.836–7.912) | 0.100 |
|
| ||||
| Maternal malperfusion | ||||
| Size ≤10th percentile | 0.270 (0.082–0.884) | 0.031* | 0.770 (0.200–2.970) | 0.705 |
| Distal villous hypoplasia | 0.776 (0.360–1.674) | 0.518 | 0.811 (0.314–2.095) | 0.665 |
| ≥2 Infarcts | 0.445 (0.146–1.359) | 0.155 | 0.369 (0.049–2.783) | 0.334 |
| Maternal decidual vasculopathy, severe | 0.299 (0.085–1.051) | 0.060 | 0.355 (0.042–2.988) | 0.341 |
|
| ||||
| Full thickness perivillous fibrin | 0.084 (0.020–0.351) | 0.001* | 0.000 (0.000-inf) | 0.992 |
|
| ||||
| Chronic inflammation | ||||
| Chronic chorionitis and basal chronic villitis | 1.175 (0.537–2.573) | 0.686 | 1.162 (0.444–3.043) | 0.760 |
| Parenchymal chronic villitis | 1.042 (0.251–4.327) | 0.954 | 1.843 (0.431–7.886) | 0.410 |
|
| ||||
| Fetal vascular obstruction | ||||
| Large vessel thrombi | 1.984 (0.561–7.022) | 0.288 | 2.543 (0.670–9.642) | 0.170 |
| Villous damage from fetal ischemia | 0.935 (0.348–2.512) | 0.894 | 0.985 (0.292–3.321) | 0.981 |
|
| ||||
| Marginal or velamentous cord insertion | 1.338 (0.530–3.382) | 0.538 | 2.077 (0.729–5.914) | 0.171 |
|
| ||||
| Large, edematous placenta | ||||
| Size ≥90th percentile | 1.713 (0.748–3.923) | 0.203 | 0.835 (0.276–2.524) | 0.749 |
| Villous edema | 2.032 (0.863–4.784) | 0.105 | 1.897 (0.704–5.110) | 0.206 |
|
| ||||
| Diffuse chorionic hemosiderosis | 8.783 (0.968–79.70) | 0.053 | 7.681 (0.941–62.731) | 0.057 |
|
| ||||
| Nucleated red blood cells >10/10 high power fields | 0.986 (0.438–2.209) | 0.967 | 0.995 (0.368–2.687) | 0.992 |
All 9 morbidities: respiratory distress syndrome, pneumothorax, moderate and severe bronchopulmonary dysplasia, acute hemodynamic instability, high stage (≥2) retinopathy of maturity, high grade (≥3) intraventricular hemorrhage, post-hemorrhagic hydrocephalus, culture positive sepsis, necrotizing enterocolitis
7 severe morbidities: Moderate and severe bronchopulmonary dysplasia, acute hemodynamic instability, high stage (≥2) retinopathy of maturity, high grade (≥3) intraventricular hemorrhage, post-hemorrhagic hydrocephalus, culture positive sepsis, and necrotizing enterocolitis
OR: odds ratio
CI: confidence interval
considered statistically significant
DISCUSSION
In this study, we correlated placental gross and histologic features in a preterm cohort with our previously calculated risk predictor, PhysiScore. Higher PhysiScores, adjusted for birth characteristics, primarily reflect short-term decreased variability in heart rate and respiratory rate [1]. PhysiScore helps to predict with high accuracy short and longer-term morbidities, including infection and cardiopulmonary problems. We find that this decreased physiological variability (autonomic dysfunction) presented by PhysiScore is strongly associated with placental evidence of amniotic fluid infection sequence (p = 0.017), and more specifically, the fetal inflammatory response (p = 0.039). PhysiScore reflects acute perinatal autonomic instability and therefore, correlation with acute placental pathology such as amniotic fluid infection sequence suggests that the autonomic instability is associated with that pathology. Other than full thickness perivillous fibrin deposition, PhysiScore did not have any statistically significant associations with other placental histology, suggesting that chronic pathologic conditions such as FTV and maternal malperfusion might not be as readily detected by PhysiScore. Fetal inflammatory response, as exemplified by plasma interleukin-6 (IL-6) levels, has been linked to higher rates of severe neonatal morbidity in prior literature reports. Gomez et al. [21] identified that fetuses with plasma IL-6 greater than 11 pg/mL had significantly higher rates of overall severe neonatal morbidity, as well as RDS and proven or suspected sepsis. Further studies have reported the significant relationship between umbilical arteritis/severe fetal inflammatory response and severe neonatal morbidity [22; 23]. Our findings are in agreement with the prior reports that the fetal inflammatory response is highly correlated with neonatal morbidity.
Interestingly, when using multivariate analyses to correct for both gestational age and birth weight, the significant association between PhysiScore and fetal inflammatory response was lost. This finding suggests that increased fetal inflammatory response correlates with younger gestational age and lower birth weight, and that the overall increased morbidity associated with fetal inflammatory response is actually due to earlier premature birth. Previously, chorionic vasculitis was found to correlate with infants of younger gestational age at birth (22–27 weeks) versus older but still premature infants (28–33 weeks at birth) [24].
In the pair-wise associations part of this study, we identified increased gestational age to be significantly protective of almost all morbidities. This finding is consistent with multiple prior reports [2; 3; 21], including Robertson et al. [2] who reported that the incidence of RDS, NEC, and sepsis decreased with increasing gestational age and birth weight.
The placenta weight:birth weight ratios were statistically significantly elevated in pneumothorax, BPD, acute hemodynamic instability, ROP, PHH, and NEC compared to the unaffected infants. This elevation is not surprising since, recently, Shehata et al. [25] linked high placenta weight:birth weight (PW/BW) ratios to an increased risk of short-term adverse perinatal outcomes, including neonatal intensive care unit admissions. Interestingly, the current study did not find an association between IVH, gestational age, or placenta weight:birth weight ratio. Since there is a well-documented association between increasing gestational age and a decreasing risk of IVH [2; 26], the lack of gestational age and IVH association is likely due to the small number of IVH cases (n=5) in the current study.
Our study did identify a significant correlation between chronic chorionitis, a more recently described entity, and basal chronic villitis with PHH. Interestingly, when we performed individual correlations between chronic chorionitis alone and each of the neonatal morbidities, we not only identified chronic chorionitis to be statistically significantly correlated with PHH, but to also trend with an increased risk of IVH (p = 0.072, data not shown). As PHH follows IVH, this finding suggests a true correlation between chronic chorionitis and these two entities. Histologic features of chronic inflammation in the placenta are thought to represent maternal anti-fetal cellular rejection [27]. Studies have shown elevated concentrations of the chemokines CXCL9, CXCL10, CXCL11, and CXCL13 in the plasma of fetuses born preterm with placentas demonstrating chronic inflammation [28; 29]. While preterm IVH has been previously linked to acute inflammation (acute chorioamnionitis) in the placenta with increased inflammatory factors including IL-6, IL-1β, and TNF-α in the neonate, it has not been associated so far with chronic inflammation in the placenta [30]. While based on a very limited sample set, this new association is worthy of further investigation.
RDS had multiple associations and was the only disease to be correlated with placental weight. In RDS, a placenta less than tenth percentile in weight was protective, and a placenta greater than 90th percentile in weight increased the risk. Prior reports have correlated acute antenatal hypoxia and RDS with placentomegaly [31]. In addition, villous edema has been associated with umbilical cord arterial blood pH values, low Apgar scores, resuscitation at birth, assisted ventilation, increased hyaline membrane disease, and neonatal mortality [32]. Villous edema can be nearly impossible to distinguish from delayed maturation in the premature placenta. Both conditions may be associated with a heavy placenta.
We confirmed a significant role for prolonged amniotic fluid infection in RDS and neonatal sepsis. In this study, the fetal inflammatory response, specifically multiple chorionic plate vessels with vasculitis, was correlated with an increased risk of RDS, and trended with an increased risk of culture positive sepsis. In addition to overall severe neonatal morbidity, Gomez et al. [21] also looked at specific morbidities, including RDS and proven or suspected sepsis, both of which were also correlated with increased fetal plasma IL-6. The current study also identified a trend between maternal inflammatory response increasing RDS and culture positive sepsis, as well as a significant correlation between culture-positive sepsis and subacute or necrotizing acute chorioamnionitis. The relationship between neonatal sepsis and chorioamnionitis has previously been established [4; 33].
Interestingly, no other significant relationships existed between each other neonatal morbidity and features of amniotic fluid infection sequence; however, fetal inflammatory response, in general, and specifically umbilical arteritis, trended with acute hemodynamic instability, ROP, and NEC. Prior reports have linked fetal inflammatory response to sepsis [21; 22; 34], BPD [22; 35], IVH [22; 24; 36–38], and ROP [38]. In addition, the maternal inflammatory response, specifically chorioamnionitis, has been correlated with BPD, IVH, and ROP [4; 33; 39; 40]. The lack of any of these associations in this study could be due to the smaller number of cases (n=102). This study also only performed correlations between findings considered higher grade in the placenta as well as some novel entities.
BPD was significantly correlated with diffuse chorionic hemosiderosis. Diffuse chorionic hemosiderosis, defined as hemosiderin-laden macrophages in the membranes and chorionic plate, is a chronic lesion associated with marginal placental abruption, recurrent maternal bleeding in earlier trimesters, and oligohydramnios [18]. This placental finding has been reported to be increased in infants with persistent pulmonary hypertension of the newborn, pulmonary hypoplasia, and chronic lung disease [41; 42]. Lung injury in these cases may be due to the effects of oligohydramnios on the developing lung as well as possible toxic effects of hemoglobin breakdown in the aspirated amniotic fluid.
Fetal vascular obstruction has been associated with poor outcomes including neonatal death, major thrombotic events, and fetal cardiac abnormalities [43–47]. In addition, some studies have identified a relationship between NEC and fetal thrombotic vasculopathy or fetal vascular obstructive lesions [4; 48]. In our study, fetal large vessel thrombi were significantly associated with an increased risk of NEC and trended with an increased risk of ROP and BPD. In addition, marginal or velamentous cord insertion significantly correlated with BPD and trended with ROP. This abnormal cord insertion has been associated with disrupted cord blood flow, stasis, thrombosis within the fetal vasculature, and avascular villi [49; 50]. The data presented here support the hypothesis that large vessel thrombi within the placenta may be associated with systemic vasculopathy. Thrombi or microthrombi in the placenta are continuous with the circulation of the neonate, resulting in a disruption of oxygen distribution and hypoxia. Interestingly, these three morbidities associated with fetal vascular obstruction – ROP, NEC, and BPD – were classified as “oxygen radical disease of neonatology” by Saugstad [51; 52] and their pathogenesis appears to involve oxidative stress.
Proportional odds regression model analyses of gestational age and placental variables against all nine morbidities identified gestational age, small placenta size, and full thickness perivillous fibrin as independently associated with a decreased risk of morbidities. These three findings may all have their basis in accelerated placental maturation. Full thickness perivillous fibrin is uncommon in the premature placenta, and is an exaggeration of Langhan’s stria (accumulation of fibrin surrounding large stem villi), a normal finding of placental maturation. It is important to distinguish this finding from massive perivillous fibrin deposition, which we did not diagnose in any of the cases from this cohort. In this study, full thickness perivillous fibrin correlated strongly with increased gestational age. We suggest that in these cases, the association of small for gestational age placentas and full thickness perivillous fibrin with fewer morbidities may reflect accelerated maturation of the placenta, likely as a result of maternal stressors such as uteroplacental malperfusion. Conversely, the fetal inflammatory response features of umbilical arteritis and multifocal chorionic plate vasculitis were associated with an increased risk of the morbidities. Similar decreased risks (gestational age, birth weight) and increased risks (placental histology of fetal inflammatory response) were noted when comparing gestational age and placental variables against the 7 severe morbidities. This data complements the finding that features of amniotic fluid infection correlates with PhysiScore.
Our study did have limitations. Most importantly, we had a small number of cases (n=102), and many of the morbidities only affected a small group of this cohort, greatly decreasing statistical power. Given that we studied nine neonatal morbidities and 18 placental variables, there was a risk for false associations; however, as discussed above, many of our findings are substantiated by other studies. Our identified associations should be confirmed in independent studies with higher numbers of cases. Another limitation of this study was that we did not do any corrections for placentas demonstrating overlapping pathologic findings (for example, placentas demonstrating both amniotic fluid infection and fetal vascular obstruction). However, review of our results does not suggest that any histology group carried another histology group to any statistically significant associations. Another disadvantage of this study was potential selection bias of placentas available for review. Of the 138 infants originally used in the PhysiScore paper [1], only 102 (74%) had placentas submitted to pathology for gross and histologic examination. An additional disadvantage of this study was the inclusion of such a high fraction of twins and triplets; in total 38 of 102 infants were the results of multiple gestations. However, there were few statistically significant differences between these multiple gestations infants and singleton infants.
In this study, both small placental size and full thickness perivillous fibrin correlated with decreased number of morbidities, using multivariate analyses and were protective of the single morbidity RDS, using pair-wise analyses. Both of these features are associated with accelerated maturation of the placenta, suggesting that the infant who has adapted to in utero stressors resulting in accelerated placental maturation at the expense of further placental growth, may be more “fit” for prematurity than one born because of amniotic fluid infection. This hypothesis would need further study to substantiate. However, features of amniotic fluid infection sequence did correlate with increased number of morbidities using multivariate analysis and pair-wise analysis.
Use of a combination of information available shortly after birth – rapid placental exam and early physiological risk assessment - may allow more precise prediction of individual risk well beyond general predictions based on gestational age and birth weight. Placental exam adds information reflecting both the acute and chronic in utero environment while PhysiScore reflects the neonate’s immediate physiological state in the first hours of life, physiology presumably shaped by this in utero environment. Moreover, some morbidities, such as PHH, may be better predicted by pathological assessment rather than physiology. Such risk prediction of morbidity allows individually targeted treatment and improved parental counseling. As the tools for personalized risk prediction grow, preventions and therapies can be better targeted to specific risks that individual preterm neonates face.
Highlights.
Placentas from premature infants show a spectrum of pathologies.
Increased gestational age is protective of neonatal morbidities.
Amniotic fluid infection sequence is associated with higher morbidity by PhysiScore.
Small placental size is associated with a low number of morbidities.
Full thickness perivillous fibrin is associated with a low number of morbidities.
Acknowledgments
This work was supported in part by a NIH Director’s New Innovator Award (1DP2OD006457; AAP). We also thank the March of Dimes Prematurity Research Center at Stanford University for their support.
Abbreviations
- BPD
bronchopulmonary dysplasia
- IVH
intraventricular hemorrhage
- NEC
necrotizing enterocolitis
- PHH
post-hemorrhagic hydrocephalus
- RDS
respiratory distress syndrome
- ROP
retinopathy of prematurity
Footnotes
Financial disclosure: All authors have indicated they have no financial relationships relevant to this article to disclose.
Conflict of interest: All authors have indicated they have no conflicts of interest relevant to this article to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saria S, Rajani AK, Gould J, Koller D, Penn AA. Integration of early physiological responses predicts later illness severity in preterm infants. Sci Transl Med. 2010;2:48–65. doi: 10.1126/scitranslmed.3001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robertson PA, Sniderman SH, Laros RK, Jr, Cowan R, Heilbron D, Goldenberg RL, et al. Neonatal morbidity according to gestational age and birth weight from five tertiary care centers in the United States, 1983 through 1986. Am J Obstet Gynecol. 1992;166:1629–41. doi: 10.1016/0002-9378(92)91551-k. discussion 41–5. [DOI] [PubMed] [Google Scholar]
- 3.Tyson JE, Parikh NA, Langer J, Green C, Higgins RD National Institute of Child Health and Human Development Neonatal Research Network. Intensive care for extreme prematurity--moving beyond gestational age. N Engl J Med. 2008;358:1672–81. doi: 10.1056/NEJMoa073059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogunyemi D, Murillo M, Jackson U, Hunter N, Alperson B. The relationship between placental histopathology findings and perinatal outcome in preterm infants. J Matern Fetal Neonatal Med. 2003;13:102–9. doi: 10.1080/jmf.13.2.102.109. [DOI] [PubMed] [Google Scholar]
- 5.Kent A, Dahlstrom JE. Chorioamnionitis/funisitis and the development of bronchopulmonary dysplasia. J Paediatr Child Health. 2004;40:356–9. doi: 10.1111/j.1440-1754.2004.00366.x. [DOI] [PubMed] [Google Scholar]
- 6.Prendergast M, May C, Broughton S, Pollina E, Milner AD, Rafferty GF, et al. Chorioamnionitis, lung function and bronchopulmonary dysplasia in prematurely born infants. Arch Dis Child Fetal Neonatal Ed. 2011;96:F270–4. doi: 10.1136/adc.2010.189480. [DOI] [PubMed] [Google Scholar]
- 7.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–60. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 8.International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123:991–9. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 9.Kliegman RM, Walsh MC. Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr. 1987;17:213–88. doi: 10.1016/0045-9380(87)90031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–34. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 11.Kim CJ, Romero R, Kusanovic JP, Yoo W, Dong Z, Topping V, et al. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol. 2010;23:1000–11. doi: 10.1038/modpathol.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinar H, Sung CJ, Oyer CE, Singer DB. Reference values for singleton and twin placental weights. Pediatr Pathol Lab Med. 1996;16:901–7. doi: 10.1080/15513819609168713. [DOI] [PubMed] [Google Scholar]
- 13.Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C, et al. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6:435–48. doi: 10.1007/s10024-003-7070-y. [DOI] [PubMed] [Google Scholar]
- 14.Redline RW, Boyd T, Campbell V, Hyde S, Kaplan C, Khong TY, et al. Maternal vascular underperfusion: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2004;7:237–49. doi: 10.1007/s10024-003-8083-2. [DOI] [PubMed] [Google Scholar]
- 15.Katzman PJ, Genest DR. Maternal floor infarction and massive perivillous fibrin deposition: histological definitions, association with intrauterine fetal growth restriction, and risk of recurrence. Pediatr Dev Pathol. 2002;5:159–64. doi: 10.1007/s10024001-0195-y. [DOI] [PubMed] [Google Scholar]
- 16.Redline RW. Villitis of unknown etiology: noninfectious chronic villitis in the placenta. Hum Pathol. 2007;38:1439–46. doi: 10.1016/j.humpath.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 17.Redline RW, Ariel I, Baergen RN, Desa DJ, Kraus FT, Roberts DJ, et al. Fetal vascular obstructive lesions: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2004;7:443–52. doi: 10.1007/s10024-004-2020-x. [DOI] [PubMed] [Google Scholar]
- 18.Redline RW, Wilson-Costello D. Chronic peripheral separation of placenta. The significance of diffuse chorioamnionic hemosiderosis. Am J Clin Pathol. 1999;111:804–10. doi: 10.1093/ajcp/111.6.804. [DOI] [PubMed] [Google Scholar]
- 19.Redline RW. Elevated circulating fetal nucleated red blood cells and placental pathology in term infants who develop cerebral palsy. Hum Pathol. 2008;39:1378–84. doi: 10.1016/j.humpath.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Ebbing C, Kiserud T, Johnsen SL, Albrechtsen S, Rasmussen S. Prevalence, risk factors and outcomes of velamentous and marginal cord insertions: a population-based study of 634,741 pregnancies. PLoS One. 2013;8:e70380. doi: 10.1371/journal.pone.0070380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 22.Kim CJ, Yoon BH, Romero R, Moon JB, Kim M, Park SS, et al. Umbilical arteritis and phlebitis mark different stages of the fetal inflammatory response. Am J Obstet Gynecol. 2001;185:496–500. doi: 10.1067/mob.2001.116689. [DOI] [PubMed] [Google Scholar]
- 23.Salas AA, Faye-Petersen OM, Sims B, Peralta-Carcelen M, Reilly SD, McGwin G, Jr, et al. Histological characteristics of the fetal inflammatory response associated with neurodevelopmental impairment and death in extremely preterm infants. J Pediatr. 2013;163:652–7. e1–2. doi: 10.1016/j.jpeds.2013.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta R, Nanjundaswamy S, Shen-Schwarz S, Petrova A. Neonatal morbidity and placental pathology. Indian J Pediatr. 2006;73:25–8. doi: 10.1007/BF02758255. [DOI] [PubMed] [Google Scholar]
- 25.Shehata F, Levin I, Shrim A, Ata B, Weisz B, Gamzu R, et al. Placenta/birthweight ratio and perinatal outcome: a retrospective cohort analysis. BJOG. 2011;118:741–7. doi: 10.1111/j.1471-0528.2011.02892.x. [DOI] [PubMed] [Google Scholar]
- 26.Salafia CM, Minior VK, Rosenkrantz TS, Pezzullo JC, Popek EJ, Cusick W, et al. Maternal, placental, and neonatal associations with early germinal matrix/intraventricular hemorrhage in infants born before 32 weeks’ gestation. Am J Perinatol. 1995;12:429–36. doi: 10.1055/s-2007-994514. [DOI] [PubMed] [Google Scholar]
- 27.Kim CJ, Romero R, Chaemsaithong P, Kim JS. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol. 2015;213:S53–69. doi: 10.1016/j.ajog.2015.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim MJ, Romero R, Kim CJ, Tarca AL, Chhauy S, LaJeunesse C, et al. Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. J Immunol. 2009;182:3919–27. doi: 10.4049/jimmunol.0803834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J, Romero R, Chaiworapongsa T, Dong Z, Tarca AL, Xu Y, et al. Characterization of the fetal blood transcriptome and proteome in maternal anti-fetal rejection: evidence of a distinct and novel type of human fetal systemic inflammatory response. Am J Reprod Immunol. 2013;70:265–84. doi: 10.1111/aji.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ment LR, Aden U, Bauer CR, Bada HS, Carlo WA, Kaiser JR, et al. Genes and environment in neonatal intraventricular hemorrhage. Semin Perinatol. 2015;39:592–603. doi: 10.1053/j.semperi.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naeye RL. Do placental weights have clinical significance? Hum Pathol. 1987;18:387–91. doi: 10.1016/s0046-8177(87)80170-3. [DOI] [PubMed] [Google Scholar]
- 32.Naeye RL, Maisels MJ, Lorenz RP, Botti JJ. The clinical significance of placental villous edema. Pediatrics. 1983;71:588–94. [PubMed] [Google Scholar]
- 33.Arayici S, Kadioglu Simsek G, Oncel MY, Eras Z, Canpolat FE, Oguz SS, et al. The effect of histological chorioamnionitis on the short-term outcome of preterm infants </=32 weeks: a single-center study. J Matern Fetal Neonatal Med. 2014;27:1129–33. doi: 10.3109/14767058.2013.850668. [DOI] [PubMed] [Google Scholar]
- 34.Keenan WJ, Steichen JJ, Mahmood K, Altshuler G. Placental pathology compared with clinical outcome: a retrospective blind review. Am J Dis Child. 1977;131:1224–7. doi: 10.1001/archpedi.1977.02120240042009. [DOI] [PubMed] [Google Scholar]
- 35.Yoon BH, Romero R, Kim KS, Park JS, Ki SH, Kim BI, et al. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1999;181:773–9. doi: 10.1016/s0002-9378(99)70299-1. [DOI] [PubMed] [Google Scholar]
- 36.DiSalvo D. The correlation between placental pathology and intraventricular hemorrhage in the preterm infant. The Developmental Epidemiology Network Investigators. Pediatr Res. 1998;43:15–9. doi: 10.1203/00006450-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Martinez E, Figueroa R, Garry D, Visintainer P, Patel K, Verma U, et al. Elevated Amniotic Fluid Interleukin-6 as a Predictor of Neonatal Periventricular Leukomalacia and Intraventricular Hemorrhage. J Matern Fetal Investig. 1998;8:101–7. [PubMed] [Google Scholar]
- 38.Moscuzza F, Belcari F, Nardini V, Bartoli A, Domenici C, Cuttano A, et al. Correlation between placental histopathology and fetal/neonatal outcome: chorioamnionitis and funisitis are associated to intraventricular haemorrage and retinopathy of prematurity in preterm newborns. Gynecol Endocrinol. 2011;27:319–23. doi: 10.3109/09513590.2010.487619. [DOI] [PubMed] [Google Scholar]
- 39.Dessardo NS, Mustac E, Dessardo S, Banac S, Peter B, Finderle A, et al. Chorioamnionitis and chronic lung disease of prematurity: a path analysis of causality. Am J Perinatol. 2012;29:133–40. doi: 10.1055/s-0031-1295654. [DOI] [PubMed] [Google Scholar]
- 40.Zanardo V, Vedovato S, Suppiej A, Trevisanuto D, Migliore M, Di Venosa B, et al. Histological inflammatory responses in the placenta and early neonatal brain injury. Pediatr Dev Pathol. 2008;11:350–4. doi: 10.2350/07-08-0324.1. [DOI] [PubMed] [Google Scholar]
- 41.Ohyama M, Itani Y, Yamanaka M, Goto A, Kato K, Ijiri R, et al. Maternal, neonatal, and placental features associated with diffuse chorioamniotic hemosiderosis, with special reference to neonatal morbidity and mortality. Pediatrics. 2004;113:800–5. doi: 10.1542/peds.113.4.800. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida S, Kikuchi A, Sunagawa S, Takagi K, Ogiso Y, Yoda T, et al. Pregnancy complicated by diffuse chorioamniotic hemosiderosis: obstetric features and influence on respiratory diseases of the infant. J Obstet Gynaecol Res. 2007;33:788–92. doi: 10.1111/j.1447-0756.2007.00657.x. [DOI] [PubMed] [Google Scholar]
- 43.Chisholm KM, Heerema-McKenney A. Fetal thrombotic vasculopathy: significance in liveborn children using proposed society for pediatric pathology diagnostic criteria. Am J Surg Pathol. 2015;39:274–80. doi: 10.1097/PAS.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 44.Kaplan CG. Fetal and maternal vascular lesions. Semin Diagn Pathol. 2007;24:14–22. doi: 10.1053/j.semdp.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 45.Redline RW, Pappin A. Fetal thrombotic vasculopathy: the clinical significance of extensive avascular villi. Hum Pathol. 1995;26:80–5. doi: 10.1016/0046-8177(95)90118-3. [DOI] [PubMed] [Google Scholar]
- 46.Saleemuddin A, Tantbirojn P, Sirois K, Crum CP, Boyd TK, Tworoger S, et al. Obstetric and perinatal complications in placentas with fetal thrombotic vasculopathy. Pediatr Dev Pathol. 2010;13:459–64. doi: 10.2350/10-01-0774-OA.1. [DOI] [PubMed] [Google Scholar]
- 47.Tantbirojn P, Saleemuddin A, Sirois K, Crum CP, Boyd TK, Tworoger S, et al. Gross abnormalities of the umbilical cord: related placental histology and clinical significance. Placenta. 2009;30:1083–8. doi: 10.1016/j.placenta.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Dix L, Roth-Kleiner M, Osterheld MC. Placental vascular obstructive lesions: risk factor for developing necrotizing enterocolitis. Patholog Res Int. 2010;2010:838917. doi: 10.4061/2010/838917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benirschke K. Obstetrically important lesions of the umbilical cord. J Reprod Med. 1994;39:262–72. [PubMed] [Google Scholar]
- 50.Chan JS, Baergen RN. Gross umbilical cord complications are associated with placental lesions of circulatory stasis and fetal hypoxia. Pediatr Dev Pathol. 2012;15:487–94. doi: 10.2350/12-06-1211-OA.1. [DOI] [PubMed] [Google Scholar]
- 51.Saugstad OD. Hypoxanthine as an indicator of hypoxia: its role in health and disease through free radical production. Pediatr Res. 1988;23:143–50. doi: 10.1203/00006450-198802000-00001. [DOI] [PubMed] [Google Scholar]
- 52.Saugstad OD. Oxidative stress in the newborn--a 30-year perspective. Biol Neonate. 2005;88:228–36. doi: 10.1159/000087586. [DOI] [PubMed] [Google Scholar]