Abstract
Rationale
N-acetylcysteine can increase extrasynaptic glutamate and reduce nicotine self-administration in rats and smoking rates in humans.
Objectives
The aim of this study was to determine if N-acetylcysteine modulates the development of nicotine place conditioning and withdrawal in mice.
Methods
N-acetylcysteine was given to nicotine-treated male ICR mice. Experiment 1: reward-like behavior. N-acetylcysteine (0, 5, 15, 30, or 60 mg/kg, i.p.) was given 15 min before nicotine (0.5 mg/kg, s.c.) or saline (10 ml/kg, s.c.) in an unbiased conditioned place preference (CPP) paradigm. Conditioning for highly palatable food served as control. Experiment 2: spontaneous withdrawal. The effect of N-acetylcysteine (0, 15, 30, 120 mg/kg, i.p.) on anxiety-like behavior, somatic signs, and hyperalgesia were measured 18 - 24 hrs after continuous nicotine (24 mg/kg/day, 14 days). Experiment 3: Mecamylamine-precipitated, withdrawal-induced aversion. The effect of N-acetylcysteine (0, 15, 30, 120 mg/kg, i.p.) on mecamylamine (3.5 mg/kg, i.p.) precipitated withdrawal was determined after continuous nicotine (24 mg/kg, i.p., 28 days) using the conditioned place aversion (CPA) paradigm.
Results
Dose-related reductions in the development of nicotine CPP, somatic withdrawal signs, hyperalgesia, and CPA were observed after N-acetylcysteine pretreatment. No effect of N-acetylcysteine were found on palatable food CPP, anxiety-like behavior, or motoric capacity (crosses between plus maze arms). Finally, N-acetylcysteine did not affect any measure in saline-treated mice at doses effective in nicotine-treated mice.
Conclusions
These are the first data suggesting that N-acetylcysteine blocks specific mouse behaviors associated with nicotine reward and withdrawal, which adds to the growing appreciation that N-acetylcysteine may have high clinical utility in combating nicotine dependence.
Keywords: nicotine, reward, withdrawal, conditioned place preference, conditioned place aversion, mice, N-acetylcysteine
1. Introduction
Despite the efficacy of some current pharmacotherapies to abate tobacco dependence, relapse rates remain high, and tobacco smoking remains the leading cause of preventable death worldwide (Samet 2013; Shiffman et al. 2008). These statistics indicate that more effective medications and/or novel approaches are needed. Because a better understanding of the neural substrates underlying nicotine addiction should inform these approaches, we used behavioral pharmacology to study mechanisms underlying the development of nicotine-conditioned, reward-like behavior and withdrawal signs in the mouse.
Adaptations in the neurobiological machinery that encodes reinforcement and withdrawal are thought to contribute to the development of a nicotine addiction (Watkins et al. 2000). Amongst the many neurotransmitter systems engaged by nicotine, glutamate appears to be critically involved in reinforcement and withdrawal (Liechti and Markou 2008). For example, nicotine self-administration alters mesocorticolimbic glutamate receptor expression (Kenny et al. 2009; Wang et al. 2007). Furthermore, nicotine self-administration can be decreased by decreasing glutamatergic neurotransmission via blockade of the excitatory glutamate receptors mGlu5 (Liechti and Markou 2007; Paterson et al. 2003) or N-methyl-D-aspartate (NMDA, (Kenny et al. 2009) or via activation of inhibitory mGlu2/3 receptors (Liechti et al. 2007). Nicotine withdrawal is also mediated, at least in part, via glutamatergic signaling. For example, somatic nicotine withdrawal signs are worsened by pharmacological blockade of the mGlu5 receptor (Liechti and Markou 2007)), but see contradictory studies in genetically modified mice (Stoker et al. 2012). Conversely, increasing synaptic glutamate via blockade of inhibitory mGlu2/3 receptors alleviated withdrawal-associated reward deficits (Liechti and Markou 2008). Thus, synaptic glutamate regulates both nicotine reinforcement and withdrawal.
Many aspects of nicotine addiction are thought to stem from an imbalance between synaptic and extrasynaptic glutamate release and clearance (Liechti and Markou 2008; Kalivas 2009). Intriguingly, microdialysis experiments revealed that the level of extrasynaptic glutamate was largely unaffected by blocking synaptic transmission (Timmerman and Westerink 1997), suggesting that astrocytes may be the predominant source of extrasynaptic glutamate. Astrocytes are well known for their role in regulating extracellular glutamate (Parpura et al. 2012), and increasing attention is being paid to astrocyte-modulated neurotransmission (Santello et al. 2012). One pharmacological approach to increasing astrocytic glutamate release into the extrasynaptic space is N-acetylcystine. In brief, N-acetylcystine is hydrolyzed into cystine that is taken up into astrocytes by the cystine–glutamate antiporter (xCT or xc-) in exchange for one glutamate molecule (McBean 2002). Thus, N-acetylcystine increases extrasynaptic glutamate.
N-acetylcystine is a drug and nutritional supplement that is used as a mucolytic agent (Hurst et al. 1967) and in the management of acetaminophen overdose (Prescott et al. 1977; Dringen and Hirrlinger 2003). N-acetylcysteine is also showing promise for a number of psychiatric conditions including addiction (Dean et al. 2011), and xCT has been shown to play an important role in nicotine addiction. For example, xCT expression is reduced in the rat nucleus accumbens and ventral tegmental area by nicotine self-administration (Knackstedt et al. 2009). Moreover, N-acetylcystine administration dose-dependently reduced intravenous rat nicotine self-administration and the reinstatement of nicotine-seeking behavior (Ramirez-Nino et al. 2013) as well as cigarette smoking in humans (Knackstedt et al. 2009). Thus, extrasynaptic glutamate also appears to regulate nicotine reinforcement.
Taken together, these studies suggest that decreased synaptic glutamate neurotransmission decreases nicotine self-administration and worsens withdrawal. Whereas, increased extrasynaptic glutamate neurotransmission decreased both nicotine self-administration and withdrawal. Based on these studies, we sought to test the hypothesis that N-acetylcysteine will decrease the development of nicotine-conditioned, reward-like behavior and abate nicotine withdrawal-associated behaviors in mice. To test this hypothesis, we measured the effects of N-acetylcysteine administration on the conditioned rewarding effects of nicotine using the conditioned place preference (CPP) paradigm. Moreover, we examined the effect of N-acetylcysteine on both the physical (somatic signs, hyperalgesia) and affective (anxiety-related behaviors and aversion) signs of withdrawal in nicotine-dependent mice.
2. Materials and Methods
2.1. Animals
Drug-naive, ICR male mice (9 weeks upon arrival; Harlan Laboratories; Indianapolis, IN) served as subjects. Mice were housed five per cage with ad libitum access to food and water on a 12 h light cycle in a humidity and temperature controlled vivarium that was approved by the Association for Assessment and Accreditation of Laboratory Animal Care. Experiments were performed during the light cycle and were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University and followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
2.2. Drugs
(−)-Nicotine hydrogen tartrate [(−)-1-methyl-2-(3-pyridyl)pyrrolidine (+)-bitartrate], N-acetylcysteine, and mecamylamine HCl were purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). Drugs were dissolved in physiological saline. The nicotine solution pH was neutralized with sodium bicarbonate as needed. Freshly prepared solutions were given to mice at 10 ml/kg, s.c. Doses are expressed as the free base of the drug. Nicotine was given in a 0.5 mg/kg subcutaneous bolus because we found that this dose produced significant CPP in the ICR mouse (Kota et al. 2007). For spontaneous withdrawal studies, 24 mg/kg/day nicotine or saline was continuously perfused for 14 days using subcutaneous osmotic minipumps (model 2000; Alzet Corporation, Cupertino, CA) that were implanted under isoflurane anesthesia. Precipitated nicotine withdrawal studies used 28 day pumps (model 2004). We found that this chronic nicotine administration regimen produced significant spontaneous withdrawal syndrome in all three behavioral paradigms used here (Damaj et al. 2003; Jackson et al. 2009). In other mice, mecamylamine (3.5 mg/kg, s.c.) was used to precipitate withdrawal because this dose produced the most reproducible withdrawal (Jackson et al. 2009). N-acetylcysteine doses are within the range previously used to decrease nicotine self-administration (Ramirez-Nino et al. 2013).
2.3. Nicotine conditioned place preference (CPP) studies
An unbiased CPP paradigm was performed, as we previously described (Kota et al. 2007). Briefly, the CPP apparatus consisted of three chambers in a linear arrangement (Med Associates, St Albans, VT). The CPP apparatus (Med-Associates, St. Albans, VT, ENV3013) consisted of white and black chambers (20 × 20 × 20 cm each), which differed in overall color and floor texture (white mesh or black rod). These chambers were separated by a smaller grey chamber with a smooth PVC floor. Partitions could be removed to allow access from the grey chamber to the black and white chambers. On day 1, animals were confined to the middle chamber for a 5 min habituation and then allowed to freely move between all 3 chambers for 15 min. Time spent in each chamber was recorded, and these data were used to populate groups of approximately equal bias in baseline chamber preference. Twenty min conditioning sessions occurred twice a day (Days 2-4). During conditioning sessions, mice were confined to one of the larger chambers. The saline groups received saline in one large chamber in the morning and saline in the other large chamber in the afternoon. The nicotine group received nicotine in one large chamber and saline in the other large chamber. Treatments were counterbalanced equally in order to ensure that some mice received the unconditioned stimulus in the morning while others received it in the afternoon. The nicotine-paired chamber was randomized amongst all groups. Sessions were 7 hours apart and were conducted by the same investigator. To determine the effect of N-acetylcysteine on nicotine place conditioning, separate cohorts were generated by pretreating with either saline or N-acetylcysteine 15 min before nicotine. Day 5 was the drug free test day, and the procedure was the same as Day 1 where animals were allowed to freely explore the apparatus after the 5 min habituation period. Time spent in each chamber was measured and statistically compared as a preference score.
2.4 Highly palatable food CPP studies
To determine if N-acetylcysteine broadly affects conditioning, food place conditioning was measured for highly palatable food (Kraft Classic Philadelphia Cheesecake, Deerfield, IL) or standard laboratory chow (Harlan, Laboratories; Indianapolis, IN), as we previously described (Sanjakdar et al. 2014). Briefly, mice were conditioned as above, with the following exceptions. Immediately after establishing baseline side preference (Day 1), mice were allowed to consume the highly palatable food (or standard chow) for the next 4-6 hr in the home cage. This process minimizes occurrence of neophobia within the test environment, which can impair conditioned responding (Sharma et al. 2012). Next, the highly palatable food (or standard chow) was paired with one large chamber and standard chow was paired with the other large chamber during daily 40 min sessions that occurred for the next 6 days. Time spent in each chamber was measured and statistically compared as a preference score.
2.5. Spontaneous nicotine withdrawal
Spontaneous nicotine withdrawal occurred following removal of the osmotic minipump after 14 days of continuous nicotine administration. No analgesic was given after minipump removal, as this would interfere with hyperalgesia testing. One day after minipump removal (18 – 24 hrs), mice were treated with either vehicle or N-acetylcysteine (15, 30, and 120 mg/kg). Higher N-acetylcysteine doses were used in withdrawal assays in an attempt to reverse the decreased in open arm time during nicotine withdrawal. Fifteen min later, mice were observed for physical and affective nicotine withdrawal signs as we have described (Damaj et al. 2003). Mice were first evaluated in the plus maze test for anxiety-related behavior over 5 min. Time spent on the closed arms of the plus maze was interpreted as a measure of anxiety-related behavior. The number of crosses between the open and closed arms were counted as a measure of locomotor activity. The plus maze assessment was immediately followed by a 20-min observation of somatic signs that included paw and body tremors, head shakes, backing, jumps, curls, and ptosis (Damaj et al. 2003). The total number of somatic signs was tallied for each mouse and the average number of somatic signs during the observation period plotted for each group. Hyperalgesia was evaluated using the hot plate assay immediately following the somatic sign observation period. Mice were placed into a 10-cm wide glass cylinder on top of a hot plate (Thermojust Apparatus, Richmond, VA) that was maintained at 52°C. The latency to reaction time (jumping or paw licking) was recorded. The specific testing sequence was based on our prior studies showing that this order of testing reduced within-group variability and produced the most consistent results (Jackson et al. 2008). All studies were performed by an observer blinded to experimental treatment.
2.6. Precipitated nicotine withdrawal measured by the conditioned place avoidance (CPA) test
The CPA protocol was conducted using the CPP apparatus, as we previously described (Jackson et al. 2009). Mice that had received continuous nicotine treatment via osmotic minipump for 28 days served as subjects in which conditioning was conducted over the course of four days in a biased fashion. On Day 1, mice were confined to the middle chamber for a 5 min habituation. Next, mice were allowed to freely explore the apparatus in order to determine baseline preference in a 15 min session. The pre-preference score was used to pair mecamylamine with the preferred chamber of each mouse. On Days 2 and 3 of CPA conditioning, all mice received saline injections in the morning and mecamylamine in the afternoon. All mice were pretreated (15 min) with N-acetylcysteine (15, 30, and 120 mg/kg, s.c.) or vehicle (s.c.). On the afternoon of Day 4, mice were allowed to move freely between chambers, and time spent in each chamber was used to calculate the CPA score (time spent in the initially preferred chamber at baseline minus the amount of time spent in the initially preferred compartment on test day). A reduction in time spent in the initially preferred chamber was interpreted as a CPA.
2.7. Statistical analysis
For conditioned place studies, a preference score was calculated by subtracting time spent in the nicotine-, highly palatable food-, or mecamylamine-paired chamber post-conditioning minus time spent pre-conditioning. A positive value indicated a preference for the nicotine-paired compartment, whereas a negative value indicated an avoidance of the nicotine-paired compartment. A number at or near zero indicated no preference. Data are expressed as mean ± S.E.M. for each group. All studies were analyzed via a two-way analyses of variance (ANOVA; Prism 6; GraphPad Software, La Jolla, CA, USA) with nicotine (or saline) or n-acetylcysteine (or vehicle) as the between subject factor. p values < 0.05 were considered to be significant. Significant results were further described by the Student Neuman-Keuls post-hoc test.
3. Results
3.1. N-acetylcysteine attenuated nicotine conditioned place preference
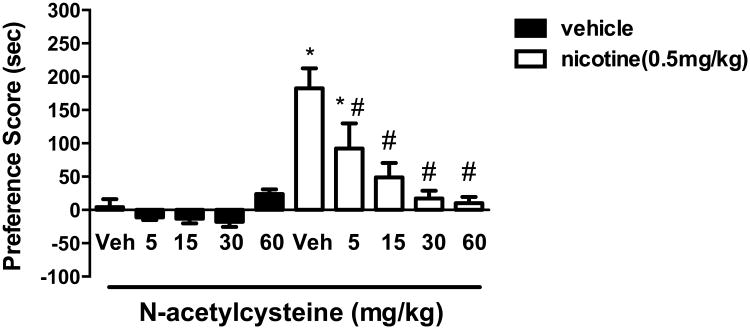
In order to determine if N-acetylcysteine affected the development of nicotine place conditioning, mice were pre-treated with N-acetylcysteine during conditioning days. A two-way ANOVA followed by a Neuman-Keuls multiple comparison test indicated that nicotine produced a significant CPP and that there was a dose-responsive reduction in nicotine place preference following N-acetylcysteine pretreatment [F(9, 63) = 11.73; p < 0.001] (Figure 1). Specifically, nicotine CPP was reduced at 5 mg/kg N-acetylcysteine, and completely blocked at 30 mg/kg, i.p. and above. Moreover, Figure 1 also illustrates that no effect of N-acetylcysteine was observed in vehicle CPP (p > 0.05). These data suggest that N-acetylcysteine, itself, does not alter conditioning, but that drug-pretreatment significantly reduced nicotine CPP.
Figure 1. N-acetylcysteine attenuates nicotine CPP in a dose-related manner.
Mice were conditioned with either saline or nicotine (0.5 mg/kg, s.c.) for 3 days. A robust CPP was observed in nicotine conditioned mice pre-treated with the N-acetylcysteine vehicle (saline, 10 ml/kg, i.p.). In contrast N-acetylcysteine blocked expression of nicotine CPP in a dose related manner. Time spent in the nicotine- vs. saline- paired chambers were measured, and the preference score expressed as time spent in the nicotine-paired chamber post-conditioning minus time spent in the nicotine-paired chamber pre-conditioning.* Denotes p < 0.05 from vehicle/vehicle; # Denotes p < 0.05 from nicotine/vehicle. Each point represents the mean ± SEM of n = 6-8 mice per group.
3.2. N-acetylcysteine did not alter highly palatable food conditioning
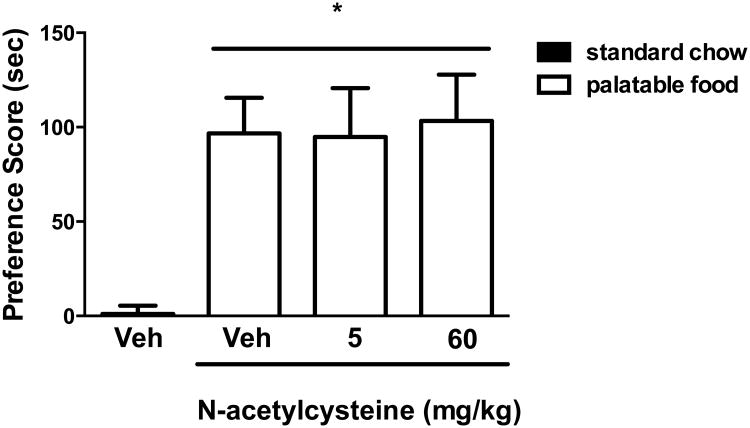
To begin testing the specificity of the N-acetylcysteine effect described above for the development of nicotine place conditioning, development of place conditioning for highly palatable food was examined. A two-way ANOVA indicated that a significant CPP was observed for highly palatable food compared to standard chow [F(3,21) = 7.452; p< 0.001] (Figure 2). However, in contrast to nicotine, no effect of N-acetylcysteine pretreatment was observed on palatable food conditioning (p > 0.05) at doses that decreased nicotine CPP. These data suggest that N-acetylcysteine does not generally alter reward-related behaviors.
Figure 2. N-acetylcysteine had no effect on highly palatable food CPP.
Mice were conditioned with either standard chow or highly palatable food for 4 days. A robust CPP was observed in palatable food conditioned mice pre-treated with the N-acetylcysteine vehicle (saline, 10 ml/kg, i.p.). No effect of N-acetylcysteine at 5 and 60 mg/kg, i.p. was found on the expression of CPP for highly palatable food. Time spent in the highly palatable food- vs. standard chow-paired chambers were measured, and the preference score expressed as time spent in the nicotine-paired chamber post-conditioning minus time spent in the nicotine-paired chamber pre-conditioning * Denotes p < 0.05 from vehicle/standard chow group. Each point represents the mean ± SEM of n = 6-8 mice per group.
3.3 N-acetylcysteine attenuated physical, but not anxiety-like spontaneous nicotine withdrawal signs
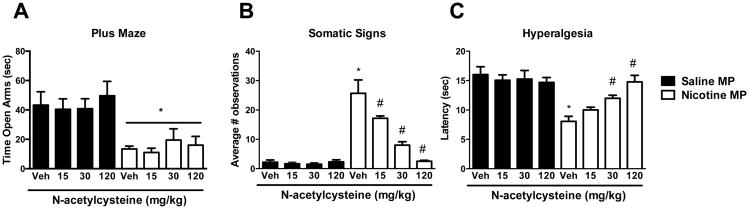
The capacity of N-acetylcysteine to attenuate nicotine-associated withdrawal signs was further evaluated with measures of spontaneous withdrawal that encompassed behaviors associated with both affective (anxiety-related behavior) and physical (somatic and hyperalgesia) withdrawal signs. Both affective and physical withdrawal signs were observed following continuous nicotine treatment (Figure 3), as measured by decreased time spent in the open arms of the elevated plus maze, increased somatic signs, and increased hyperalgesia (decreased response latency in the hotplate assay. However, no effect of N-acetylcysteine (15 – 120 mg/kg, i.p.) was observed on anxiety-like behavior, as measured by the elevated plus maze (p > 0.05, Figure 3A). Moreover, none of these N-acetylcysteine doses affected total crosses between arms (Table 1) despite evaluation of doses two times higher than what was effective in the conditioned place preference assay. The arm crossing data suggest that the lack of effect of N-acetylcysteine on anxiety-like behavior was not due to motoric affects. In contrast, a two-way ANOVA followed by the Neuman-Keuls test indicated that there was a dose-related decrease in both the number of somatic withdrawal signs (Figure 3B, [F (7, 35) = 26.36; p < 0.0001]) and hyperalgesia (Figure 3C, [F (7, 35) = 8.917; p< 0.0001]) following N-acetylcysteine treatment, with the dose of 120 mg/kg, i.p. N-acetylcysteine completely reversing these measures. Importantly, the 120 mg/kg dose of N-acetylcysteine did not alter any of these behaviors in mice that were continuously treated with saline instead of nicotine.
Figure 3. N-acetylcysteine dose-relatedly reduced physical nicotine withdrawal signs, but no effect was observed on anxiety-related behavior.
Nicotine withdrawn ICR mice treated with N-acetylcysteine (15, 30 or 120 mg/kg, i.p.) displayed A) anxiety-related behavior similar to that observed in vehicle-treated counterparts, but B) an attenuation of total somatic signs and C) hyperalgesia. Each point represents the mean ± S.E.M. of n = 6 mice per group. MP: minipump; * denotes p < 0.05 from vehicle/vehicle; # denotes p < 0.05 from nicotine/vehicle.
Table 1. N-acetylcysteine did not impact the number of arm crosses in the elevated plus maze.
Mice undergoing spontaneous nicotine withdrawal were treated with vehicle or N-acetylcysteine (15, 30, or 120 mg/kg, ip), and the number of crosses between arms of the plus maze was counted. Numbers are presented as the mean ± SEM for n = 6 mice per group. MP: mini pump.
| Treatment | arm crosses ± SEM |
|---|---|
| Saline MP, vehicle | 6.7 ± 0.6 |
| Saline MP, N-acetylcysteine (120 mg/kg) | 7.0 ± 0.7 |
| Nicotine MP, vehicle | 6.2 ± 1.3 |
| Nicotine MP, N-acetylcysteine (15 mg/kg) | 6.7 ± 0.8 |
| Nicotine MP, N-acetylcysteine (30 mg/kg) | 6.3 ± 0.6 |
| Nicotine MP, N-acetylcysteine (120 mg/kg) | 6.0 ± 0.5 |
3.4. N-acetylcysteine blocked conditioned place aversion expressed during precipitated nicotine withdrawal
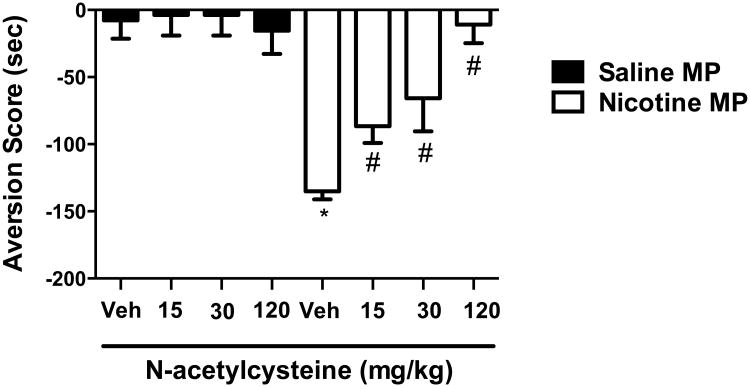
Next, the effect of N-acetylcysteine on mecamylamine-precipitated, nicotine withdrawal-induced aversion was examined. Nicotine withdrawal can be precipitated in mice that have been continuously treated with nicotine by administering the non-selective acetylcholine nicotinic receptor antagonist mecamylamine (Jackson et al. 2009). A two-way ANOVA followed by the Neuman-Keuls post hoc test indicated that mecamylamine had no effect on place conditioning in mice continuously treated with saline (Figure 4, p > 0.05). In contrast, Figure 4 also illustrates that N-acetylcysteine dose-relatedly reduced the CPA that was observed following mecamylamine conditioning [F(7,42) = 10.59; p < 0.0001]. Specifically, CPA was attenuated by N-acetylcysteine at 15 and 30 mg/kg, i.p. N-acetylcysteine and completely abolished at 120 mg/kg.
Figure 4. N-acetylcysteine dose-dependently reversed.
mecamylamine-precipitated nicotine withdrawal CPA. Mecamylamine (3.5 mg/kg, i.p.) precipitated a significant CPA in chronic nicotine-treated ICR mice. However, N-acetylcysteine pretreatment reversed mecamylamine-precipitated aversion in a dose-related manner. Each point represents the mean ± S.E.M. of n = 6 mice per group. Time spent in the mecamylamine-vs. saline-paired chamber was measured, and the preference score expressed as time spent in the nicotine-paired chamber post-conditioning minus time spent in the nicotine-paired chamber pre-conditioning. MP: minipump; * denotes p < 0.05 vs. saline group/vehicle; # denotes p < 0.05 from nicotine/vehicle.
4. Discussion
Here, we examined the effect of N-acetylcysteine on mouse behaviors that are associated with nicotine reward and withdrawal. We found that N-acetylcysteine dose-relatedly reduced the development of nicotine conditioned rewarding effects. In contrast, development of the conditioned rewarding effects of highly palatable food were unaffected by N-acetylcysteine. Effects of N-acetylcysteine on nicotine withdrawal were measured in both spontaneous and mecamylamine-precipitated withdrawal models. While no effect of N-acetylcysteine was observed on anxiety-like behaviors, as measured in the elevated plus maze, a dose-related reduction in both somatic signs and hyperalgesia were seen. Moreover, we observed a dose-related reduction in place aversion conditioned by precipitated nicotine withdrawal. Importantly, no effect of N-acetylcysteine was observed in saline-treated mice at doses that were effective in nicotine-treated mice. These data suggest that N-acetylcysteine can decrease both nicotine conditioned reward as well as many of the behaviors that are associated with nicotine withdrawal.
Our data with CPP extend earlier work by Markou's group showing that an acute dose of 30 mg/kg N-acetylcysteine and higher reduced intravenous rat nicotine self-administration on a fixed ratio schedule of reinforcement (Ramirez-Nino et al. 2013). While place conditioning and self-administration are not isomorphic and not likely to impact glutamatergic signaling equally (Wang et al. 2008; Lenoir and Kiyatkin 2013; Reid et al. 2000), CPP is thought to model the reward-like aspects of drug-conditioned cues (Bardo and Bevins 2000). Thus, our data extend the impact of N-acetylcysteine on other aspects of reward-like behaviors. Moreover, since we found no effect on conditioning for highly palatable food at doses up to 60 mg/kg, this suggests that N-acetylcysteine exhibits some behavioral specificity. Our data also builds upon Markou's finding that Noyes food pellet self-administration was unaffected by acute N-acetylcysteine (Ramirez-Nino et al. 2013).
An additional distinction between the two studies is we observed that N-acetylcysteine doses as low as 5 mg/kg disrupted mouse nicotine place conditioning. Markou's group did not test below 30 mg/kg. While precise cross-species and cross-paradigm comparisons are difficult, taking these data together is promising and potentially clinically relevant given that humans generally tolerate N-acetylcysteine well (Deepmala et al. 2015; McClure et al. 2014). N-acetylcysteine has also been shown to reduce cocaine- (Baker et al. 2003), heroin-(Zhou and Kalivas 2008), and nicotine-seeking (Ramirez-Nino et al. 2013) behavior in laboratory models and efficacy in some substance abuse clinical trials (Deepmala et al. 2015; McClure et al. 2014; Knackstedt et al. 2009) including a reduction in the number of cigarettes smoked by daily smokers (Knackstedt et al. 2009).
In addition to N-acetylcysteine reducing nicotine reward-related behaviors, we also observed reductions in several behaviors associated with nicotine withdrawal. Specifically, we observed reductions in the number of somatic signs, hyperalgesia, and conditioned place aversion. While lower doses were partially effective, these measures were completely blocked at 120 mg/kg. However, no effect of N-acetylcysteine was observed on anxiety-like behavior, as measured by time spent in open arms of the elevated plus maze, at doses that were effective in the other withdrawal models. Moreover, no effect on anxiety-like behavior was seen at doses up to twice as high (120 mg/kg) as doses that abolished nicotine place conditioning. While additional measures of anxiety-like behavior may be needed, these data are important for two reasons. First, withdrawal effects play a large role in drug recidivism (Watkins et al. 2000). Second, this is the first study of nicotine withdrawal paradigms using pharmacological reagents that can modulate, and perhaps normalize, extrasynaptic glutamate (Moussawi et al. 2009; Madayag et al. 2007; Baker et al. 2003). Thus, our data significantly extend earlier work showing that direct-acting glutamate receptor ligands modulate nicotine withdrawal, as discussed below (Liechti and Markou 2007; Liechti and Markou 2008), and add to the growing appreciation that N-acetylcysteine may at least encompass the molecular scaffold of a highly efficacious therapeutic intervention for addiction (Deepmala et al. 2015; McClure et al. 2014). That we failed to observe an effect of N-acetylcysteine on anxiety-like behavior likely reflects that these signs may be mediated by different neural substrates. For example, studies have shown that somatic nicotine withdrawal signs are mediated by both central and peripheral nicotinic receptors, while anhedonia and aversion are mediated solely through central nicotinic receptor populations such as the nucleus accumbens (NAc) and habenulo-interpeduncular system (Watkins et al. 2000; De Biasi and Dani 2011). Finally, corticotropin-releasing factor-1 (CRF1) receptors in the central nucleus of the amygdala (CeA) and the mesohabenular pathway through the interpeduncular nucleus seem to play an important role in the emergence of anxiety-like behaviors in nicotine-dependent animals (Cohen et al. 2015; Zhao-Shea et al. 2015). Alternatively, these data may reflect a limitation of the elevated plus maze to detect differences under the conditions examined. Indeed, an increase in anxiety-like behaviors with nicotine withdrawal was reported in multiple anxiety tests beside the elevated plus-maze, such as light-dark boxes, novelty-induced hypophagia, and marble burying test (Turner et al. 2014; Stoker et al. 2008).
While the precise neural substrates engaged here have not been identified, predictions can be made. N-acetylcysteine is widely used as a cystine prodrug (Baker et al. 2003; Zhou and Kalivas 2008; Ramirez-Nino et al. 2013; Knackstedt et al. 2009). Cystine is transported into astrocytes in exchange for glutamate that is released into the extracellular space in a 1:1 ratio via xCT (McBean 2002; Lo et al. 2008). Thus, the effects of N-acetylcysteine reported here may seem at odds with the literature given that inhibition of glutamatergic neurotransmission is known to reduce nicotine reinforcement and exacerbate withdrawal (Liechti and Markou 2008; Kalivas 2009). For example, systemic administration of either a NMDA (Kenny et al. 2009; Blokhina et al. 2005) or mGlu5 (Paterson et al. 2003) antagonist reduced nicotine self-administration in both mice and rats. Moreover, systemic administration of MPEP, an mGlu5 receptor antagonist, worsened somatic nicotine withdrawal signs (Liechti and Markou 2007). Given this apparent discrepancy, it is important to note that glutamate uptake transporters can largely protect synaptic glutamate receptors from activation by extrasynaptic glutamate in addition to preventing synaptic spillover from activating extrasynaptic glutamate receptors (Barbour 2001; Warr et al. 1999; Haydon 2001). Thus, N-acetylcysteine can be conceptualized as predominantly increasing extrasynaptic glutamate. In support of this mechanism of action, N-acetylcysteine has been shown to activate mGlu2/3 glutamate receptors that in turn reduce synaptic glutamate (Baker et al. 2003; Xi et al. 2002). Thus, our findings are in line with both the antagonist literature discussed above as well as studies indicating that systemic administration of mGlu2/3 glutamate receptor agonists reduced nicotine self-administration (Liechti et al. 2007). Moreover, given the effects on nicotine conditioning observed here, it is intriguing to note that nicotine self-administration reduced xCT expression in mesolimbic regions that mediate nicotine reinforcement (Knackstedt et al. 2009), and that N-acetylcysteine restored cocaine-induced reductions in xCT function in the nucleus accumbens (Moussawi et al. 2009; Madayag et al. 2007; Baker et al. 2003). Thus, our data may stem from N-acetylcysteine reversing some nicotine-induced deficits in xCT function. Directly testing this hypothesis will be an exciting avenue of future research.
Our data also indicate that several behavioral measures of nicotine withdrawal were alleviated by N-acetylcysteine. These data do not support the proposed mechanism of activating extrasynaptic mGlu2/3 glutamate receptors because nicotine withdrawal-associated reward deficits are worsened by mGlu2/3 glutamate receptor agonists, but improved by mGlu2/3 antagonists (Kenny et al. 2003). Thus, it is possible that the results observed here may at least partially stem from non-glutamatergic actions of N-acetylcysteine. These non-glutamatergic effects of N-acetylcysteine have proven clinical utility. For example, N-acetylcysteine is an effective mucolytic agent due to the ability of the reduced sulfhydryl moiety to disrupt mucous disulfide bonds (Hurst et al. 1967). The sulfhydryl donor is also effective in counteracting acetaminophen poisoning (Prescott et al. 1977). N-acetylcysteine is also thought to help combat acetaminophen poisoning by increasing intracellular antioxidant capacity via facilitating the rate-limiting step of glutathione synthesis (Prescott et al. 1977; Dringen and Hirrlinger 2003). Because our data cannot rule out these non-glutamatergic actions, future studies will have to elucidate the precise mechanism(s) whereby N-acetylcysteine reduced mouse behaviors associated with nicotine conditioning and withdrawal.
In conclusion, the results presented here establish that repeated N-acetylcysteine dose-relatedly reduced both nicotine reinforcement and many nicotine withdrawal-associated behaviors without altering food reward-related behavior in mice. These data support efforts to determine the utility of N-acetylcysteine in abating substance use disorders by indicating that N-acetylcysteine can reduce important aspects of nicotine dependence such as reinforcement and withdrawal.
Acknowledgments
MSB is supported by the Alcohol Beverage Medical Research Foundation, a Center for Translational Research Award (UL1 TR000058), the National Institutes for Alcohol Abuse and Alcoholism (P50 AA022537), and ABMRF/The Foundation for Alcohol Research. MID is supported by the National Institutes for Drug Abuse (R01 DA032246 and R21 DA032246).
Footnotes
Disclosures: The authors have no competing or conflicting interests to declare.
References
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Barbour B. An evaluation of synapse independence. J Neurosci. 2001;21:7969–7984. doi: 10.1523/JNEUROSCI.21-20-07969.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Blokhina EA, Kashkin VA, Zvartau EE, Danysz W, Bespalov AY. Effects of nicotinic and NMDA receptor channel blockers on intravenous cocaine and nicotine self-administration in mice. Eur Neuropsychopharmacol. 2005;15:219–225. doi: 10.1016/j.euroneuro.2004.07.005. 10.1016/j.euroneuro.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Cohen A, Treweek J, Edwards S, Leao RM, Schulteis G, Koob GF, George O. Extended access to nicotine leads to a CRF1 receptor dependent increase in anxiety-like behavior and hyperalgesia in rats. Addict Biol. 2015;20:56–68. doi: 10.1111/adb.12077. 10.1111/adb.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther. 2003;307:526–534. doi: 10.1124/jpet.103.054908. 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- De Biasi M, Dani JA. Reward, addiction, withdrawal to nicotine. Annu Rev Neurosci. 2011;34:105–130. doi: 10.1146/annurev-neuro-061010-113734. 10.1146/annurev-neuro-061010-113734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean O, Giorlando F, Berk M. N-acetylcysteine in psychiatry: current therapeutic evidence and potential mechanisms of action. J Psychiatry Neurosci. 2011;36:78–86. doi: 10.1503/jpn.100057. 10.1503/jpn.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepmala Slattery J, Kumar N, Delhey L, Berk M, Dean O, Spielholz C, Frye R. Clinical trials of N-acetylcysteine in psychiatry and neurology: A systematic review. Neurosci Biobehav Rev. 2015;55:294–321. doi: 10.1016/j.neubiorev.2015.04.015. 10.1016/j.neubiorev.2015.04.015. [DOI] [PubMed] [Google Scholar]
- Dringen R, Hirrlinger J. Glutathione pathways in the brain. Biol Chem. 2003;384:505–516. doi: 10.1515/BC.2003.059. 10.1515/BC.2003.059. [DOI] [PubMed] [Google Scholar]
- Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Hurst GA, Shaw PB, LeMaistre CA. Laboratory and clinical evaluation of the mucolytic properties of acetylcysteine. Am Rev Respir Dis. 1967;96:962–970. doi: 10.1164/arrd.1967.96.5.962. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Kota DH, Martin BR, Damaj MI. The role of various nicotinic receptor subunits and factors influencing nicotine conditioned place aversion. Neuropharmacology. 2009;56:970–974. doi: 10.1016/j.neuropharm.2009.01.023. 10.1016/j.neuropharm.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther. 2008;325:302–312. doi: 10.1124/jpet.107.132977. 10.1124/jpet.107.132977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chartoff E, Roberto M, Carlezon WAJ, Markou A. NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology. 2009;34:266–281. doi: 10.1038/npp.2008.58. 10.1038/npp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Gasparini F, Markou A. Group II metabotropic and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate glutamate receptors regulate the deficit in brain reward function associated with nicotine withdrawal in rats. J Pharmacol Exp Ther. 2003;306:1068–1076. doi: 10.1124/jpet.103.052027. 10.1124/jpet.103.052027. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65:841–845. doi: 10.1016/j.biopsych.2008.10.040. 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther. 2007;322:399–407. doi: 10.1124/jpet.107.121616. 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Kiyatkin EA. Intravenous nicotine injection induces rapid, experience-dependent sensitization of glutamate release in the ventral tegmental area and nucleus accumbens. J Neurochem. 2013;127:541–551. doi: 10.1111/jnc.12450. 10.1111/jnc.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Markou A. Interactive effects of the mGlu5 receptor antagonist MPEP and the mGlu2/3 receptor antagonist LY341495 on nicotine self-administration and reward deficits associated with nicotine withdrawal in rats. Eur J Pharmacol. 2007;554:164–174. doi: 10.1016/j.ejphar.2006.10.011. 10.1016/j.ejphar.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Markou A. Role of the glutamatergic system in nicotine dependence : implications for the discovery and development of new pharmacological smoking cessation therapies. CNS Drugs. 2008;22:705–724. doi: 10.2165/00023210-200822090-00001. [DOI] [PubMed] [Google Scholar]
- Lo M, Wang YZ, Gout PW. The x(c)- cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J Cell Physiol. 2008;215:593–602. doi: 10.1002/jcp.21366. 10.1002/jcp.21366. [DOI] [PubMed] [Google Scholar]
- Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, Grier MD, Baker DA. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci. 2007;27:13968–13976. doi: 10.1523/JNEUROSCI.2808-07.2007. 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBean GJ. Cerebral cystine uptake: a tale of two transporters. Trends Pharmacol Sci. 2002;23:299–302. doi: 10.1016/s0165-6147(02)02060-6. [DOI] [PubMed] [Google Scholar]
- McClure EA, Gipson CD, Malcolm RJ, Kalivas PW, Gray KM. Potential role of N-acetylcysteine in the management of substance use disorders. CNS Drugs. 2014;28:95–106. doi: 10.1007/s40263-014-0142-x. 10.1007/s40263-014-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG, Stout RFJ, Spray DC, Reichenbach A, Pannicke T, Pekny M, Pekna M, Zorec R, Verkhratsky A. Glial cells in (patho)physiology. J Neurochem. 2012;121:4–27. doi: 10.1111/j.1471-4159.2012.07664.x. 10.1111/j.1471-4159.2012.07664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Semenova S, Gasparini F, Markou A. The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology (Berl) 2003;167:257–264. doi: 10.1007/s00213-003-1432-z. 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- Prescott LF, Park J, Ballantyne A, Adriaenssens P, Proudfoot AT. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet. 1977;2:432–434. doi: 10.1016/s0140-6736(77)90612-2. [DOI] [PubMed] [Google Scholar]
- Ramirez-Nino AM, D'Souza MS, Markou A. N-acetylcysteine decreased nicotine self-administration and cue-induced reinstatement of nicotine seeking in rats: comparison with the effects of N-acetylcysteine on food responding and food seeking. Psychopharmacology (Berl) 2013;225:473–482. doi: 10.1007/s00213-012-2837-3. 10.1007/s00213-012-2837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Fox L, Ho LB, Berger SP. Nicotine stimulation of extracellular glutamate levels in the nucleus accumbens: neuropharmacological characterization. Synapse. 2000;35:129–136. doi: 10.1002/(SICI)1098-2396(200002)35:2<129::AID-SYN5>3.0.CO;2-D. 10.1002/(SICI)1098-2396(200002)35:2<129∷AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Samet JM. Tobacco smoking: the leading cause of preventable disease worldwide. Thorac Surg Clin. 2013;23:103–112. doi: 10.1016/j.thorsurg.2013.01.009. 10.1016/j.thorsurg.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Sanjakdar SS, Maldoon PP, Marks MJ, Brunzell DH, Maskos U, McIntosh JM, Bowers MS, Damaj MI. Differential Roles of alpha6beta2* and alpha4beta2* Neuronal Nicotinic Receptors in Nicotine- and Cocaine-Conditioned Reward in Mice. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.177. 10.1038/npp.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M, Cali C, Bezzi P. Gliotransmission and the tripartite synapse. Adv Exp Med Biol. 2012;970:307–331. doi: 10.1007/978-3-7091-0932-8_14. 10.1007/978-3-7091-0932-8_14. [DOI] [PubMed] [Google Scholar]
- Sharma S, Hryhorczuk C, Fulton S. Progressive-ratio responding for palatable high-fat and high-sugar food in mice. J Vis Exp. 2012:e3754. doi: 10.3791/3754. 10.3791/3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Use of smoking-cessation treatments in the United States. Am J Prev Med. 2008;34:102–111. doi: 10.1016/j.amepre.2007.09.033. 10.1016/j.amepre.2007.09.033. [DOI] [PubMed] [Google Scholar]
- Stoker AK, Olivier B, Markou A. Involvement of metabotropic glutamate receptor 5 in brain reward deficits associated with cocaine and nicotine withdrawal and somatic signs of nicotine withdrawal. Psychopharmacology (Berl) 2012;221:317–327. doi: 10.1007/s00213-011-2578-8. 10.1007/s00213-011-2578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker AK, Semenova S, Markou A. Affective and somatic aspects of spontaneous and precipitated nicotine withdrawal in C57BL/6J and BALB/cByJ mice. Neuropharmacology. 2008;54:1223–1232. doi: 10.1016/j.neuropharm.2008.03.013. 10.1016/j.neuropharm.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman W, Westerink BH. Brain microdialysis of GABA and glutamate: what does it signify? Synapse. 1997;27:242–261. doi: 10.1002/(SICI)1098-2396(199711)27:3<242::AID-SYN9>3.0.CO;2-D. 10.1002/(SICI)1098-2396(199711)27:3<242∷AID-SYN9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Turner JR, Ray R, Lee B, Everett L, Xiang J, Jepson C, Kaestner KH, Lerman C, Blendy JA. Evidence from mouse and man for a role of neuregulin 3 in nicotine dependence. Mol Psychiatry. 2014;19:801–810. doi: 10.1038/mp.2013.104. 10.1038/mp.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Chen H, Sharp BM. Neuroadaptive changes in the mesocortical glutamatergic system during chronic nicotine self-administration and after extinction in rats. J Neurochem. 2008;106:943–956. doi: 10.1111/j.1471-4159.2008.05456.x. 10.1111/j.1471-4159.2008.05456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Chen H, Steketee JD, Sharp BM. Upregulation of ionotropic glutamate receptor subunits within specific mesocorticolimbic regions during chronic nicotine self-administration. Neuropsychopharmacology. 2007;32:103–109. doi: 10.1038/sj.npp.1301033. 10.1038/sj.npp.1301033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr O, Takahashi M, Attwell D. Modulation of extracellular glutamate concentration in rat brain slices by cystine-glutamate exchange. J Physiol. 1999;514:783–793. doi: 10.1111/j.1469-7793.1999.783ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res. 2000;2:19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther. 2002;300:162–171. doi: 10.1124/jpet.300.1.162. [DOI] [PubMed] [Google Scholar]
- Zhao-Shea R, DeGroot SR, Liu L, Vallaster M, Pang X, Su Q, Gao G, Rando OJ, Martin GE, George O, Gardner PD, Tapper AR. Increased CRF signalling in a ventral tegmental area-interpeduncular nucleus-medial habenula circuit induces anxiety during nicotine withdrawal. Nat Commun. 2015;6:6770. doi: 10.1038/ncomms7770. 10.1038/ncomms7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Kalivas PW. N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol Psychiatry. 2008;63:338–340. doi: 10.1016/j.biopsych.2007.06.008. 10.1016/j.biopsych.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]