Abstract
Purpose of review
With global demographic changes and an overall improved healthcare, more older end-stage-renal-disease (ESRD) patients receive kidney transplants. At the same time, organs from older donors are utilized more frequently. Those developments have and will continue to impact allocation, immunosuppression and efforts improving organ quality.
Recent findings
Findings mainly outside the field of transplantation have provided insights into mechanisms that drive immunosenescence and immunogenicity, thus providing a rationale for an age-adapted immunosuppression and relevant clinical trials in the elderly. With fewer rejections in the elderly, alloimmune responses appear to be characterized by a decline in effectiveness and an augmented unspecific immune response.
Summary
Immunosenescence displays broad and ambivalent effects in elderly transplant recipients. Those changes appear to compensate a decline in allospecific effectiveness by a shift towards an augmented unspecific immune response. Immunosuppression needs to target those age-specific changes to optimize outcomes in elderly transplant recipients,
Keywords: Immunosenescence, aging, kidney transplantation, rejection, tolerance
Introduction
Aging is of broad relevance and affects a wide range of physiological conditions. Consequences include a deteriorating and less effective immune response towards exogenous antigens. This phenomenon has been coined immunosenescence by the geriatrician Roy Walford [1]. To date, the concept of immunosenescence remains loosely defined, but is receiving increasing attention with the demographic shift and an overall improved healthcare.
In organ transplantation, data provided from the Organ Procurement and Transplantation Network (OPTN) emphasize on the relevance of the current demographics. Strikingly, patients on waiting lists for renal transplants > than 65 years have tripled during the last decade [2]. Similar tendencies have been observed for organ donors. [2,3].
Of clinical relevance, donor organ age has been identified as an independent risk factor for graft survival [4]. Demographic changes, in addition to the growing shortage of donor organs in general, have impacted organ allocation in the US and in Europe. In the US, the introduction of the Expanded Criteria Donors (ECD) classification attempted to increase the recruitment of marginal donor organs. Notably, age determined the principal criteria for the ECD classification [5]. The recently introduced Kidney Donor Profile Index (KDPI) determines the relative organ quality by calculating a numerical score from ten characteristics which include the donor age [6]. Along the same lines, Eurotransplant launched the European Senior Program (ESP) in 1999 that matched kidney recipients and deceased donor organs ≥65 years without emphasizing on HLA-matching while keeping cold ischemic times brief [7]. The introduction of the ESP led to a doubling of transplanted kidneys from donors older than 60 years from 1998 to 2002 [8].
The concept of immunosenescence in kidney transplantation
Immunosenescence affects both recipients’ immune response and organ aging with consequences on injury, repair and immunogenicity. For any detailed analysis, effects of increased prevalences of co-morbidities in the elderly need to be distinguished from changes related to immunosenescence. Although, mortality rates after kidney transplantation are elevated in older recipients, well-selected older patients clearly benefit from renal transplantation. Indeed, death-censored graft survival is best in older transplant recipients [9,10]. Moreover, rates of acute rejections are lowest in older recipients [11], effects clearly attributed to immunosenescence.
Alloimmunity in the elderly
Telomere length is playing a critical role in the aging of cellular compartments [12]. Telomere length is determined by telomerase activity, which is compromised by aging [13,14]. In experimental kidney models, for example, compromised regenerative capacities subsequent to Ischemia/Reperfusion Injury (IRI) have been linked to telomere attrition [15].
Lymphocyte subsets and proliferation capacity
The effect of aging on absolute lymphocyte counts remains controversial [16–18]. In general, it is presumed that changes of absolute numbers of T cells and NK cells play only a minor role in immunosenescence. Of relevance, numerous studies have characterized a modified distribution of subsets within these populations, in addition to a diminished proliferative response of T and NK cells to mitotic stimuli [18–22]. These compromised proliferative T cell responses limit the effectiveness of alloimmunity, thus characterizing functional consequences rather than numerical T cell responses in immunosenescence. Distributional changes within T cell populations are characterized by a shift from the naïve CCR7+ CD45RA+ subset to an effector memory CCR7− CD45RA− population [18]. This phenomenon can be explained, at least in part, by thymic involution and a subsequent decline in naïve T cell production [17]. The shift from naïve to effector memory T cell subsets emphasizes on the predominant role of ‘experienced’ T cells in aging while reflecting a compromised ‘de novo’ T cell response to new antigens in addition to an impaired chemotactic migration capacity towards secondary lymphoid organs. Thus, the elderly T cell response is mainly built on less effective memory responses that lack the migratory- and naïve de novo-production capacity of younger T cells [23].
Phenotypic changes in aging
The decline of CD28 expression on CD4+ and CD8+ T cells has been characterized as a hallmark of T cell senescence [24]. CD28 is a key co-stimulatory surface receptor and the blockade of this pathway has been shown to prolong graft survival in mice [25,26]. In elderly kidney recipients, an increased proportion of peripheral CD28− CD4+ has been associated with an absence of acute rejections [27]. More recently, the blockade of co-stimulation gained further clinical relevance with the approval of belatacept, a T lymphocyte antigen-4 immunoglobulin fusion protein (CTLA-4-Ig) that blocks the CD28 co-stimulatory pathway. Additional in vitro studies have shown that a loss of CD28 is accompanied by an increased gene expression of its antagonist, the CTLA-4 receptor, which potentially augments the already inhibitory effect [28,29].
The loss of CD28 may be compensated by a de novo expression of cytotoxic NK cell receptors on senescent T cells. Recently, de novo expression and transcriptional upregulation of the stimulatory NKG2D receptor on elderly CD4+ CD28− T cells have been reported clinically [30]. Of note, old CD8+ T cells displayed a transcriptional upregulation of activating killer cell lectin-like receptors (KLR) and killer-cell immunoglobulin-like receptors (KIRs). Together, it seems that CD8+ T cell resemble an innate NK cell receptor repertoire with aging [31]. These findings correlate with a general increase of CD3+ T cells that co-express NK cell receptors in elderly individuals [32]. Noteworthy, pre-existing or de novo synthesized antibodies against the MHC class I polypeptide-related sequence A (MICA) that bind to the NKG2D receptor have been linked to either an early graft loss or late graft dysfunction in kidney transplantation [33,34].
These alterations indicate that the increased expression of NK cell receptors will impact alloimmune responses in the elderly, potentially reflecting relevance of an augmented innate immune response. While the overall significance of NK cell receptors in kidney transplantation remains sparsely investigated, recent work has shown phenotypic changes of NK cell repertoires driven by immunosuppressive treatment [35].
NK cell senescence, in turn, is attributed to a distributional shift from the CD56bright subset to the cytotoxic CD56dim subset [19,36]. Moreover, CD56dim NK cells of elderly individuals have been shown to increasingly express the senescence-associated surface molecule CD57 [37,38]. The expression of CD57 was additionally identified on CD8+ CD28− T cells [39]. The CD57 subset is associated with an advanced cytotoxic and proinflammatory cytokine capacity and several studies have reported on a potential link between circulating CD57+ CD28− CD8+ T cells, HLA mismatch and late kidney graft dysfunction, although the impact of de novo expressed NK cell receptors has not been investigated [40*,41**–44].
Furthermore, the tendency for a high CD28− CD57+ CD4+ T cell frequency in kidney recipients treated with polyclonal anti-thymocyte globulin (ATG) has recently been associated with acute rejection, whereas ATG was thought to accelerate cellular senescence [45*]. Another recent study indicated that the expression of CD57 on CD8+ T cells might have utility as a predictive marker for the development of cutaneous squamous cell carcinoma in renal transplant recipients [46*]. Thus, despite an impaired per cell NK cell activity, the overall de novo synthesis of NK cell receptors on T cells, CD57 expression and the general CD56dim shift may enhance an overall, however less specific cytotoxic capacity during immunosenescence, [47–49]. Moreover, the ability of CD56dim NK cells to bind anti-HLA antibodies (donor-specific antibodies, DSA) has been linked to complement-independent pathways of antibody-mediated rejections (AMR) in kidney transplantation, leading to the assumption that NK cells contribute to a chronic active antibody-mediated rejection [50,51].
Cytokine capacity
Maintenance immunosuppression critically relies on calcineurin inhibitors that specifically target the production of IL-2 in T cells. Strikingly, it has been shown that both IL-2 cytokine capacity and sensitivity of CD4+ T cells decreases with aging, at least in murine models [52–54]. This effect may be most likely accounted for by the distributional shift to memory T cells. Indeed, naïve CD4+ T cells responded with an unimpaired IL-2 production to neoantigenic stimulation in the elderly [55]. Moreover, age-dependent downregulation of CD28 on CD4+ and CD8+ T cells correlated with an impaired IL-2 production as the co-stimulatory cell surface receptor CD28 is critical for the activation of T cells and their subsequent production of IL-2.
Taken together, loss of CD28 and diminished IL-2 release may represent critical drivers of a compromised alloimmune response in the elderly affecting both, immunosuppression and tolerance protocols.
B cells and humoral responses
It is noteworthy to recognize that B cell development and humoral responses depend on interactions between provided by T- and dendritic cells (DC). The downmodulation of CD154 (CD40L) and CD28 on CD4+ T cells, in addition to a diminished IL-2 production may lead to an impaired B cell proliferation and antibody production [56–58]. Moreover, compromised T cell help will impair B cell diversity linked to a hampered humoral response and affinity of antibodies [59]. Similarly, as with age-mediated shifts in T cell compartments, distributional shifts of B cell subsets are observed. Moreover, the production of naïve B cells appears affected by shifts towards myelopoiesis at the expense of the lymphopoiesis [60–62]. Furthermore, aging-associated CD11b+ CD11c+ B cells (ABC) with innate immune responsive characteristics have been identified as unique B cell subset in the periphery of elderly mice and were linked to autoimmunity [63,64].
In summary, immunosenescence appears to alter B cell development contributing to a compromised de novo donor specific HLA antibody (DSA) production that has been linked to allograft function [65]. Although DSAs have not been shown to be impacted by age, the de novo DSA genesis has not been studied in an age-dependent approach [66].
Innate immunity in the elderly
Initial studies on immunosenescence focused on alterations within the adaptive immune system and have only marginally investigated effects on the innate immunity. It has now become evident that aging will not only affect innate immunity, but also equips adaptive immune components with tools of innate immune responses, potentially enabling players of adaptive immunity to act in an antigen-independent manner. DCs with their cardinal feature as antigen presenting cells are potent instigators of T cell stimulation and it is presumed that the change of the absolute numbers of DCs plays an inferior role in aging [67]. Depending on their maturity, DCs in rodents have been shown to critically impact rejection or tolerance in kidney transplantation [67,68]. Several studies have addressed TLR expression on old DCs and neutrophilic granulocytes, and at least for TLR-4, a downmodulation over age has not been identified [69,70]. However, migration, cytokine and phagocytotic responses of DCs encountering new antigens had been compromised, linked to an impaired functional status [69]. Similarly, function of neutrophilic granulocytes appears to diminish with age in parallel with a compromised capacity for phagocytosis, Fcγ receptor type III (CD16) expression and subsequent superoxide production [71,72]. Of interest, an elevated basal cytokine production of DCs and macrophages has been reported in elderly individuals [73]. This phenomenon touches upon the “paradox” of aging and immune functions with a general decline of immune functions and effectiveness appears balanced with an overall augmented reactivity towards self antigens, a process called inflamm-aging [69].
Organ age and immunogenicity
While old macrophages and DCs appear to have a compromised capacity for phagocytosis, this aspect may contribute to a diminished clearance of apoptotic cells [74]. Indeed, a prolonged presence of apoptotic cells may lead to proteolytic degradation and a subsequent release of intracellular damage associated molecular pattern molecules (DAMPs), potentially enhancing immunogenicity [75]. Mounting evidence acknowledges the link between renal IRI in the elderly linked to an augmented release of DAMPs [76,77]. DAMPs in aging are more likely of mitochondrial origin related to a direct exposure to the reactive oxygen species (ROS) [78]. The severity of IRI thereby depends on the level of ROS, again shown to be elevated in elderly individuals [79]. The subsequent recruitment and inflammatory degranulation of neutrophilic granulocytes is facilitated through the binding of DAMPs to Pattern Recognition Receptors, most notably TLRs. More recently, we have been able to show that old donor DCs accelerated rejection, demonstrating the relevance of an augmented immunogenicity when utilizing older organs for transplantation (unpublished data). Of additional relevance, young donor organs may induce significantly more tolerogenic ILT4+ DCs compared to older donor organs [80]. Others have suggested that cellular senesescence might contribute to an impaired physiological potential of old nephrons to cope with stress stimuli [81].
Conclusion
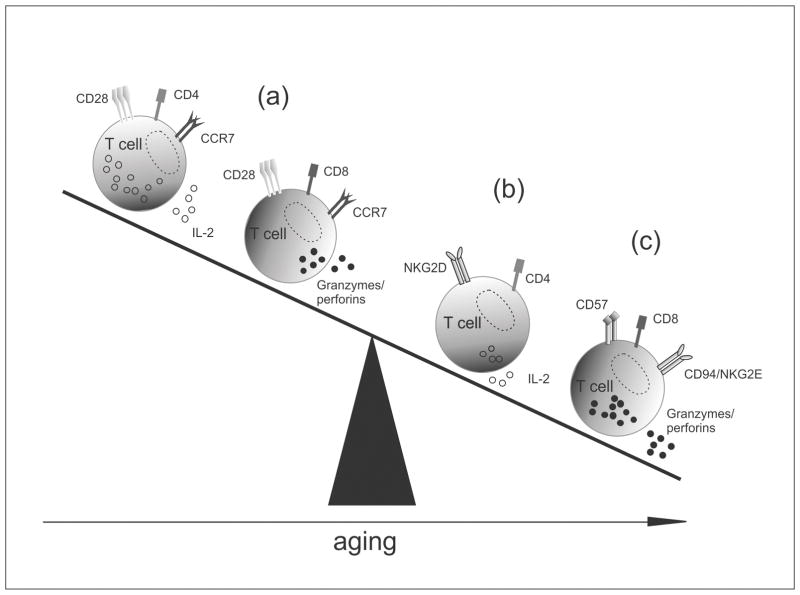
The immunology of elderly recipients presents an ambivalent picture of changes. On the one hand, an impaired naïve T and B cell alloresponse through the diminished production of high affinity antibodies as well as less effective CD28− memory T cell subsets may be the driving force behind less potent immune responses in elderly recipients. On the other hand, the aging immune response appears to promote a compensation of lost allospecific functions through the expression of innate immune receptors in memory subsets, providing a more unspecific immune response (Fig. 1).
Figure 1.
Concept of a less effective allospecific but augmented unspecific immune response in the elderly. (a) T cell subsets in the healthy individuals. (b) Old CD4+ T cell with diminished alloresponse potential, including an impaired IL-2 capacity and CD28 downregulation coincide with an increasing NK cell receptor equipment [30,52–54]. (c) Enhanced cytotoxic capacity of old CD28− CD8+ T cells that upregulate CD57 while co-expressing NK cell receptors [43,44].
Understanding this ambivalent concept of immunosenescence bears the potential of developing age-adjusted immunosuppressive therapies for older transplant recipients, as their less effective adaptive response may not be the primary target for treatment.
With regards to donor organ age, recent studies indicate that advanced age may augment injuries subsequent to ischemia and reperfusion, potentially eliciting the release of DAMPs, which in turn can lead to an increased inflammatory response and eventually enhance a potential rejection.
Key points.
Immunosenescence may shift the balance between innate and adaptive immunity towards more dominant unspecific immune responses.
Key players of adaptive immunity acquire receptors characteristic of innate immunity
Enhanced expression of CD57 on CD8+ T cells has been linked to rejection, yet current studies remain rather descriptive and mechanisms require a more detailed investigation.
Changes of co-stimulatory pathways in addition to compromised IL-2 responses provide additional opportunities for age-specific treatments.
Acknowledgments
None.
Financial support and sponsorship
This work has been supported by grants from the National Institutes of Health (R01AG039449 to SGT). M.S. was supported by the German National Academic Foundation and International Academy of Life Sciences/Biomedical Sciences Exchange Program.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
References
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Effros RB. Telomerase induction in T cells: a cure for aging and disease? Exp Gerontol. 2007;42:416–20. doi: 10.1016/j.exger.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tso PL. Access to renal transplantation for the elderly in the face of new allocation policy: a review of contemporary perspectives on “older” issues. Transplant Rev. 2014;28:6–14. doi: 10.1016/j.trre.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Nathan HM, Conrad SL, Held PJ, et al. Organ donation in the United States. Am J Transplant. 2003;3:29–40. doi: 10.1034/j.1600-6143.3.s4.4.x. [DOI] [PubMed] [Google Scholar]
- 4.Schold JD, Kaplan B, Baliga RS, et al. The broad spectrum of quality in deceased donor kidneys. Am J Transplant. 2005;5:757–65. doi: 10.1111/j.1600-6143.2005.00770.x. [DOI] [PubMed] [Google Scholar]
- 5.Nyberg SL, Baskin-Bey ES, Kremers W, et al. Improving the prediction of donor kidney quality: deceased donor score and resistive indices. Transplantation. 2005;80:925–9. doi: 10.1097/01.tp.0000173798.04043.af. [DOI] [PubMed] [Google Scholar]
- 6.Tanriover B, Mohan S, Cohen DJ, et al. Kidneys at higher risk of discard: expanding the role of dual kidney transplantation. Am J Transplant. 2014;14:404–15. doi: 10.1111/ajt.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frei U, Noeldeke J, Machold-Fabrizii V, et al. Prospective age-matching in elderly kidney transplant recipients--a 5-year analysis of the Eurotransplant Senior Program. Am J Transplant. 2008;8:50–7. doi: 10.1111/j.1600-6143.2007.02014.x. [DOI] [PubMed] [Google Scholar]
- 8.Fritsche L, Hörstrup J, Budde K, et al. Old-for-old kidney allocation allows successful expansion of the donor and recipient pool. Am J Transplant. 2003;3:1434–9. doi: 10.1046/j.1600-6135.2003.00251.x. [DOI] [PubMed] [Google Scholar]
- 9.Kauffman HM, McBride MA, Cors CS, et al. Early mortality rates in older kidney recipients with comorbid risk factors. Transplantation. 2007;83:404–10. doi: 10.1097/01.tp.0000251780.01031.81. [DOI] [PubMed] [Google Scholar]
- 10.Ponticelli C. Should renal transplantation be offered to older patients? Nephrol Dial Transplant. 2000;15:315–7. doi: 10.1093/ndt/15.3.315. [DOI] [PubMed] [Google Scholar]
- 11.Tullius SG, Tran H, Guleria I, et al. The combination of donor and recipient age is critical in determining host immunoresponsiveness and renal transplant outcome. Ann Surg. 2010;252:662–74. doi: 10.1097/SLA.0b013e3181f65c7d. [DOI] [PubMed] [Google Scholar]
- 12.Shammas MA. Telomeres, lifestyle, cancer, and aging. Curr Opin Clin Nutr Metab Care. 2011;14:28–34. doi: 10.1097/MCO.0b013e32834121b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Y, Damjanovic A, Metter EJ, et al. Age-associated telomere attrition of lymphocytes in vivo is co-ordinated with changes in telomerase activity, composition of lymphocyte subsets and health conditions. Clin Sci. 2015;128:367–77. doi: 10.1042/CS20140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaszubowska L. Telomere shortening and ageing of the immune system. J Physiol Pharmacol. 2008;59 (Suppl 9):169–86. [PubMed] [Google Scholar]
- 15.Westhoff JH, Schildhorn C, Jacobi C, et al. Telomere shortening reduces regenerative capacity after acute kidney injury. J Am Soc Nephrol. 2010;21:327–36. doi: 10.1681/ASN.2009010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan J, Greer JM, Hull R, et al. The effect of ageing on human lymphocyte subsets: comparison of males and females. Immun Ageing. 2010;7:4. doi: 10.1186/1742-4933-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–9. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 18.Koch S, Larbi A, Derhovanessian E, et al. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun Ageing. 2008;5:6. doi: 10.1186/1742-4933-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Garff-Tavernier M, Béziat V, Decocq J, et al. Human NK cells display major phenotypic and functional changes over the life span. Aging Cell. 2010;9:527–35. doi: 10.1111/j.1474-9726.2010.00584.x. [DOI] [PubMed] [Google Scholar]
- 20.Douziech N, Seres I, Larbi A, et al. Modulation of human lymphocyte proliferative response with aging. Exp Gerontol. 37:369–87. doi: 10.1016/s0531-5565(01)00204-2. [DOI] [PubMed] [Google Scholar]
- 21.Jiang J, Gross D, Elbaum P, et al. Aging affects initiation and continuation of T cell proliferation. Mech Ageing Dev. 2007;128:332–9. doi: 10.1016/j.mad.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Morbach H, Eichhorn EM, Liese JG, et al. Reference values for B cell subpopulations from infancy to adulthood. Clin Exp Immunol. 2010;162:271–9. doi: 10.1111/j.1365-2249.2010.04206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park J, Miyakawa T, Shiokawa A, et al. Attenuation of migration properties of CD4+ T cells from aged mice correlates with decrease in chemokine receptor expression, response to retinoic acid, and RALDH expression compared to young mice. Biosci Biotechnol Biochem. 2014;78:976–80. doi: 10.1080/09168451.2014.910099. [DOI] [PubMed] [Google Scholar]
- 24.Moro-García MA, Alonso-Arias R, López-Larrea C. When Aging Reaches CD4+ T-Cells: Phenotypic and Functional Changes. Front Immunol. 2013;4:107. doi: 10.3389/fimmu.2013.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turka LA, Linsley PS, Lin H, et al. T-cell activation by the CD28 ligand B7 is required for cardiac allograft rejection in vivo. Proc Natl Acad Sci U S A. 1992;89:11102–5. doi: 10.1073/pnas.89.22.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang T, Fresnay S, Welty E, et al. Selective CD28 blockade attenuates acute and chronic rejection of murine cardiac allografts in a CTLA-4-dependent manner. Am J Transplant. 2011;11:1599–609. doi: 10.1111/j.1600-6143.2011.03624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trzonkowski P, Debska-Slizie A, Jankowska M, et al. Immunosenescence increases the rate of acceptance of kidney allotransplants in elderly recipients through exhaustion of CD4+ T-cells. Mech Ageing Dev. 2010;131:96–104. doi: 10.1016/j.mad.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Parish ST, Wu JE, Effros RB. Sustained CD28 expression delays multiple features of replicative senescence in human CD8 T lymphocytes. J Clin Immunol. 2010;30:798–805. doi: 10.1007/s10875-010-9449-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leng Q, Bentwich Z, Borkow G. CTLA-4 upregulation during aging. Mech Ageing Dev. 2002;123:1419–21. doi: 10.1016/s0047-6374(02)00077-5. [DOI] [PubMed] [Google Scholar]
- 30.Alonso-Arias R, Moro-García MA, López-Vázquez A, et al. NKG2D expression in CD4+ T lymphocytes as a marker of senescence in the aged immune system. Age (Dordr) 2011;33:591–605. doi: 10.1007/s11357-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen G, Lustig A, Weng N-P. T cell aging: a review of the transcriptional changes determined from genome-wide analysis. Front Immunol. 2013;4:121. doi: 10.3389/fimmu.2013.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarazona R, DelaRosa O, Alonso C, et al. Increased expression of NK cell markers on T lymphocytes in aging and chronic activation of the immune system reflects the accumulation of effector/senescent T cells. Mech Ageing Dev. 2001;121:77–88. doi: 10.1016/s0047-6374(00)00199-8. [DOI] [PubMed] [Google Scholar]
- 33.Zou Y, Stastny P, Süsal C, et al. Antibodies against MICA antigens and kidney-transplant rejection. N Engl J Med. 2007;357:1293–300. doi: 10.1056/NEJMoa067160. [DOI] [PubMed] [Google Scholar]
- 34.Cox ST, Stephens HAF, Fernando R, et al. Major histocompatibility complex class I-related chain A allele mismatching, antibodies, and rejection in renal transplantation. Hum Immunol. 2011;72:827–34. doi: 10.1016/j.humimm.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Neudoerfl C, Mueller BJ, Blume C, et al. The Peripheral NK Cell Repertoire after Kidney Transplantation is Modulated by Different Immunosuppressive Drugs. Front Immunol. 2013;4:46. doi: 10.3389/fimmu.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chidrawar SM, Khan N, Chan YLT, et al. Ageing is associated with a decline in peripheral blood CD56bright NK cells. Immun Ageing. 2006;3:10. doi: 10.1186/1742-4933-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brenchley JM, Karandikar NJ, Betts MR, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–20. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 38.Borrego F, Alonso MC, Galiani MD, et al. NK phenotypic markers and IL2 response in NK cells from elderly people. Exp Gerontol. 1999;34:253–265. doi: 10.1016/s0531-5565(98)00076-x. [DOI] [PubMed] [Google Scholar]
- 39.Bandrés E, Merino J, Vázquez B, et al. The increase of IFN-gamma production through aging correlates with the expanded CD8(+high)CD28(−)CD57(+) subpopulation. Clin Immunol. 2000;96:230–5. doi: 10.1006/clim.2000.4894. [DOI] [PubMed] [Google Scholar]
- 40*.Ducloux D, Courivaud C, Bamoulid J, et al. Alloimmune responses and atherosclerotic disease after kidney transplantation. Transplantation. 2015;99:220–5. doi: 10.1097/TP.0000000000000346. This study gives indications that the increased circulation of CD57+ CD8+ T cells may be associated with HLA mismatches in kidney transplantation. [DOI] [PubMed] [Google Scholar]
- 41**.Yap M, Boeffard F, Clave E, et al. Expansion of highly differentiated cytotoxic terminally differentiated effector memory CD8+ T cells in a subset of clinically stable kidney transplant recipients: a potential marker for late graft dysfunction. J Am Soc Nephrol. 2014;25:1856–68. doi: 10.1681/ASN.2013080848. First clinical study that links the CD57+ CD8+ T cells and its associated high cytotoxic potential to late graft dysfunction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez-Vergès S, Milush JM, Pandey S, et al. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116:3865–74. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le Priol Y, Puthier D, Lécureuil C, et al. High cytotoxic and specific migratory potencies of senescent CD8+ CD57+ cells in HIV-infected and uninfected individuals. J Immunol. 2006;177:5145–54. doi: 10.4049/jimmunol.177.8.5145. [DOI] [PubMed] [Google Scholar]
- 44.Chattopadhyay PK, Betts MR, Price DA, et al. The cytolytic enzymes granyzme A, granzyme B, and perforin: expression patterns, cell distribution, and their relationship to cell maturity and bright CD57 expression. J Leukoc Biol. 2009;85:88–97. doi: 10.1189/jlb.0208107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.Crepin T, Carron C, Roubiou C, et al. ATG-Induced Accelerated Immune Senescence: Clinical Implications in Renal Transplant Recipients. Am J Transplant. 2015;15:1028–38. doi: 10.1111/ajt.13092. This study suggests that the CD57 expression on CD4+ T cells is associated with the occurence of rejection in ATG-treated patients and links it to the cellular senescence triggered by ATG. [DOI] [PubMed] [Google Scholar]
- 46*.Bottomley M, Harden P, Wood K. CD57 expression in CD8 T cells and development of cutaneous squamous cell carcinoma in renal transplant recipients: a prospective cohort study. Lancet. 2015;385:S23. doi: 10.1016/S0140-6736(15)60338-5. This study is the first one to indicate that the CD57 expression on CD8+ T cells may be used as a marker for the prediction of the cutaneous squamous cell carcinoma in kidney recipients. [DOI] [PubMed] [Google Scholar]
- 47.Borrego F, Alonso MC, Galiani MD, et al. NK phenotypic markers and IL2 response in NK cells from elderly people. Exp Gerontol. 1999;34:253–65. doi: 10.1016/s0531-5565(98)00076-x. [DOI] [PubMed] [Google Scholar]
- 48.Onyema OO, Njemini R, Bautmans I, et al. Cellular aging and senescence characteristics of human T-lymphocytes. Biogerontology. 2012;13:169–81. doi: 10.1007/s10522-011-9366-z. [DOI] [PubMed] [Google Scholar]
- 49.Focosi D, Bestagno M, Burrone O, et al. CD57+ T lymphocytes and functional immune deficiency. J Leukoc Biol. 2010;87:107–16. doi: 10.1189/jlb.0809566. [DOI] [PubMed] [Google Scholar]
- 50.Hirohashi T, Chase CM, Della Pelle P, et al. A novel pathway of chronic allograft rejection mediated by NK cells and alloantibody. Am J Transplant. 2012;12:313–21. doi: 10.1111/j.1600-6143.2011.03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akiyoshi T, Hirohashi T, Alessandrini A, et al. Role of complement and NK cells in antibody mediated rejection. Hum Immunol. 2012;73:1226–32. doi: 10.1016/j.humimm.2012.07.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elrefaei M, Blank KJ, Murasko DM. Decreased IL-2, IFN-gamma, and IL-10 production by aged mice during the acute phase of E55+ retrovirus infection. Virology. 2002;299:8–19. doi: 10.1006/viro.2002.1367. [DOI] [PubMed] [Google Scholar]
- 53.Haynes L, Linton PJ, Eaton SM, et al. Interleukin 2, but not other common gamma chain-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J Exp Med. 1999;190:1013–24. doi: 10.1084/jem.190.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gong Z, Liu T, Wan Y, et al. Decreased c-rel activation contributes to aberrant interleukin-2 expression in CD4(+)T cells of aged rats. Mol Immunol. 2014;61:1–6. doi: 10.1016/j.molimm.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 55.Gomez I, Marx F, Gould EA, et al. T cells from elderly persons respond to neoantigenic stimulation with an unimpaired IL-2 production and an enhanced differentiation into effector cells. Exp Gerontol. 2004;39:597–605. doi: 10.1016/j.exger.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 56.Eaton SM, Burns EM, Kusser K, et al. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med. 2004;200:1613–22. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frasca D, Riley RL, Blomberg BB. Humoral immune response and B-cell functions including immunoglobulin class switch are downregulated in aged mice and humans. Semin Immunol. 2005;17:378–384. doi: 10.1016/j.smim.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 58.Blaeser A, McGlauchlen K, Vogel LA. Aged B lymphocytes retain their ability to express surface markers but are dysfunctional in their proliferative capability during early activation events. Immun Ageing. 2008;5:15. doi: 10.1186/1742-4933-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goenka R, Scholz JL, Naradikian MS, et al. Memory B cells form in aged mice despite impaired affinity maturation and germinal center kinetics. Exp Gerontol. 2014;54:109–15. doi: 10.1016/j.exger.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rossi DJ, Bryder D, Zahn JM, et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 2005;102:9194–9. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dykstra B, Olthof S, Schreuder J, et al. Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. J Exp Med. 2011;208:2691–703. doi: 10.1084/jem.20111490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Veneri D, Ortolani R, Franchini M, et al. Expression of CD27 and CD23 on peripheral blood B lymphocytes in humans of different ages. Blood Transfus. 2009;7:29–34. doi: 10.2450/2008.0007-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hao Y, O’Neill P, Naradikian MS, et al. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118:1294–304. doi: 10.1182/blood-2011-01-330530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rubtsov AV, Rubtsova K, Fischer A, et al. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c+ B-cell population is important for the development of autoimmunity. Blood. 2011;118:1305–15. doi: 10.1182/blood-2011-01-331462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hidalgo LG, Campbell PM, Sis B, et al. De novo donor-specific antibody at the time of kidney transplant biopsy associates with microvascular pathology and late graft failure. Am J Transplant. 2009;9:2532–41. doi: 10.1111/j.1600-6143.2009.02800.x. [DOI] [PubMed] [Google Scholar]
- 66.Rodríguez Ferrero ML, Arroyo D, Panizo N, et al. Monitoring of circulating antibodies in a renal transplantation population: preliminary results. Transplant Proc. 2012;44:2548–50. doi: 10.1016/j.transproceed.2012.09.072. [DOI] [PubMed] [Google Scholar]
- 67.Xia MJ, Shan J, Li YP, et al. Adoptive transfusion of tolerant dendritic cells prolong the survival of renal allografts: a systematic review. J Evid Based Med. 2013;6:250–64. doi: 10.1111/jebm.12070. [DOI] [PubMed] [Google Scholar]
- 68.Lechler RI, Batchelor JR. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J Exp Med. 1982;155:31–41. doi: 10.1084/jem.155.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Agrawal A, Gupta S. Impact of aging on dendritic cell functions in humans. Ageing Res Rev. 2011;10:336–45. doi: 10.1016/j.arr.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hajishengallis G. Too old to fight? Aging and its toll on innate immunity. Immun ageing. 2010;25:25–37. doi: 10.1111/j.2041-1014.2009.00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Butcher SK, Chahal H, Nayak L, et al. Senescence in innate immune responses: reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J Leukoc Biol. 2001;70:881–6. [PubMed] [Google Scholar]
- 72.Fülöp T, Fóris G, Wórum I, et al. Age-dependent alterations of Fc gamma receptor-mediated effector functions of human polymorphonuclear leucocytes. Clin Exp Immunol. 1985;61:425–32. [PMC free article] [PubMed] [Google Scholar]
- 73.Panda A, Qian F, Mohanty S, et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol. 2010;184:2518–27. doi: 10.4049/jimmunol.0901022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hearps AC, Martin GE, Angelovich TA, et al. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell. 2012;11:867–75. doi: 10.1111/j.1474-9726.2012.00851.x. [DOI] [PubMed] [Google Scholar]
- 75.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–89. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rabadi MM, Ghaly T, Goligorksy MS, et al. HMGB1 in renal ischemic injury. Am J Physiol Renal Physiol. 2012;303:F873–85. doi: 10.1152/ajprenal.00092.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosin DL, Okusa MD. Dangers within: DAMP responses to damage and cell death in kidney disease. J Am Soc Nephrol. 2011;22:416–25. doi: 10.1681/ASN.2010040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–95. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 79.Fan Q, Chen M, Fang X, et al. Aging might augment reactive oxygen species (ROS) formation and affect reactive nitrogen species (RNS) level after myocardial ischemia/reperfusion in both humans and rats. Age (Dordr) 2013;35:1017–26. doi: 10.1007/s11357-012-9421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Benito MJ, Lopez-Hoyos M, Fernandez-Fresnedo G, et al. Changes in the expression of the immunoglobulin-like transcript 3 (ILT3) and ILT4 receptors in renal allograft recipients: effect of donor and recipient aging. Transplant Proc. 2008;40:2894–6. doi: 10.1016/j.transproceed.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 81.Melk A, Halloran PF. Cell senescence and its implications for nephrology. J Am Soc Nephrol. 2001;12:385–93. doi: 10.1681/ASN.V122385. [DOI] [PubMed] [Google Scholar]