Abstract
Mitochondria—classically viewed as the powerhouses of the cell—have taken center stage in disease pathogenesis and resolution. Mitochondrial dysfunction, which originates from primary defects within the organelle or is induced by environmental stresses, plays a critical role in human disease. Despite their central role in human health and disease, there are no approved drugs that directly target mitochondria. We present possible new druggable targets in mitochondrial biology, including protein modification, calcium ion (Ca2+) transport, and dynamics, as we move into a new era of mitochondrial medicine.
As the powerhouse of the cell, the mitochondrion is essential for life. A large body of research in recent years has established that mitochondria are not simply static, passively producing adenosine 5′-triphosphate (ATP) for fuel, but that they sense and respond to changing cellular environments and stresses. The mitochondria thereby control critical decision points for cells, such as whether to live or die or whether to transform to malignant cancer cells. Acquired mitochondrial dysfunction has been implicated in numerous common diseases and conditions, such as cardiovascular disease; neurodegenerative diseases, including Alzheimer’s and Parkinson’s; metabolic disorders, such as diabetes and obesity; cancer; and even in normal aging. Despite the central role of mitochondria in human health and disease, there has been no successful therapy for defective mitochondrial function, which is devastating and often fatal. Looking at the pipeline of new drugs, as of the end of 2015, there were more than 200 trials on ClinicalTrials.gov that used “mitochondria” as a key word. However, drugs tested in most of these trials did not target mitochondria (e.g., Table 1); instead, they monitored mitochondrial function as an indicator of indirect effects of the treatments. This begs the question: Why is there a void of mitochondria-targeted therapies when its role in human disease is widely recognized?
Table 1. Selected clinical trials on drugs affecting mitochondrial function.
Trial statuses were confirmed as of 26 January 2016. AMP, adenosine-5′-monophosphate.
| Class | Compound name (manufacturer) | Targeting site | Disease | Phase | Outcome | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|---|
| Antioxidant | MitoQ | ROS scavenger | Chronic kidney disease (CKD) | 4* | Ongoing | NCT02364648 |
| Resveratrol | SIRT1 activator | CKD | 3 | Completed | NCT02433925 | |
| EPI-743 (Edison Pharmaceuticals) | Coenzyme Q10 (CoQ10)–based | Rett syndrome | 2 | Completed | NCT01822249 | |
| Idebenone (Santhera Pharmaceuticals) | CoQ10-based | Duchenne muscular dystrophy | 3 | Positive (74) | NCT01027884 | |
| Edaravone | Nonspecific ROS scavenger | Acute myocardial infarction (AMI) | 4 | Positive (75) | NCT00265239 | |
| Edaravone | Nonspecific ROS scavenger | Amyotrophic lateral sclerosis | 3 | Negative (76) | NCT00330681 | |
| CoQ10 | ROS scavenger | Parkinson’s disease | 3 | Negative (77) | NCT00740714 | |
| SS31 (bendavia) (Stealth BioTherapeutics Inc.) | Unknown | AMI | 2 | Negative† | NCT01572909 | |
|
| ||||||
| Metabolism modulator | Acipimox | Niacin derivative | Obesity | 2 | Negative (78) | NCT01488409 |
| Metformin | Type 1 diabetes | 4 | Ongoing | NCT01813929 | ||
| Metformin | Metabolism/unknown | Nonalcoholic fatty liver disease | 4 | Terminated | NCT00736385 | |
| Creatine | Metabolism | Huntington’s disease | 3 | Terminated‡ | NCT00712426 | |
| Acadesine (Merck Sharp & Dohme Corp.) | AMP-activated protein kinase, nonspecific | Myocardial infarction | 3 | Negative (79) | NCT00872001 | |
|
| ||||||
| Mitochondrial permeability transition pore (mPTP) inhibitor | Cyclosporine A | Cyclophilin D | Acute kidney injury | 2 | Ongoing | NCT02397213 |
| Cyclosporine A | Cyclophilin D | AMI | 3 | Negative (46) | NCT01502774 | |
| Dimebon (Pfizer) | Unknown | Alzheimer’s disease | 3 | Negative (80) | NCT00838110 | |
| TRO40303 (Trophos) | Mitochondrial translocator protein | AMI | 2 | Negative (81) | NCT01374321 | |
The compound entered a phase 4 trial on the basis that it is used as a nutritional supplement.
Terminated as unlikely to be effective.
Results reported at the American College of Cardiology 2015 Scientific Sessions.
To fuel the cell, mitochondria synthesize ATP by oxidative phosphorylation, generating reactive oxygen species (ROS) as a by-product. A long-standing hypothesis is that defective energy supply and/or excessive ROS generation accounts for the pathology caused by mitochondrial dysfunction. Several antioxidants or compounds aimed at boosting energy metabolism, through improving ATP production, have been tested clinically to improve mitochondrial function but have yielded disappointing or inconclusive results (Table 1) (1–4). Failures of the earlier trials were attributed to the loss of physiological ROS function or the inefficiency of delivering antioxidants to targets inside the mitochondria owing to the organelle’s double-membrane structure, which is virtually impermeable to the passive diffusion of most small molecules and hence a barrier for drug delivery.
Newer-generation compounds that bear positive charges [for example, MitoQ (5)] and/or have high affinity to the mitochondrial membrane lipids (6) are now in clinical trials (Table 1). One promising redox therapeutic from Edison Pharmaceuticals is EPI-743 (7, 8), a designed CoQ10 analog with much more potent antioxidant activity, and is being tested currently in pediatric patients with various mitochondrial diseases, including Rett syndrome, Friedreich’s ataxia, and Leigh syndrome (Table 1). Thus, the efficacy of these compounds in human diseases remains to be determined.
There are opportunities for innovation in mitochondrial medicine beyond the conventional focus on bioenergetics and oxidative stress [or, in the oncology field, selective destruction of mitochondria (9–12)]. Here, we highlight emerging studies in mitochondrial genetics, signaling, and physiology that lead to novel avenues of improving mitochondrial function and/or protect against mitochondria-mediated cell injury in human diseases.
MITOCHONDRIAL REPLACEMENT THERAPY
For mitochondrial disease caused by inborn mutations, mitochondrial genetics research has yielded remarkable progress, particularly advances in mitochondrial replacement therapy (MRT) (13–15). MRT prevents the passing of mutated mitochondrial DNA (mtDNA) from the mother to the offspring via a three-way in vitro fertilization in which the nuclear genome from a patient’s egg is transferred into an enucleated healthy egg with normal mtDNA before fertilization. In this way, mitochondrial diseases caused by mutant mtDNA can be prevented.
However, concerns remain regarding the long-term outcome of this technology. Several major issues, such as the interaction of mitochondrial and nuclear genome and the fate of carryover mutant mtDNA, remain to be clarified. There are several other limitations as well. MRT does not benefit mitochondrial diseases caused by mutations in the nuclear genome that encodes most of the proteins, only inherited diseases resulting from mutations of mtDNA, which encodes transfer RNAs, ribosomal RNAs, and mRNAs that make only 13 of ~1000 proteins in mitochondria. Thus, the target patient population is rather small. Furthermore, MRT only prevents the development of mitochondrial disease in the offspring of mutation carriers but does not treat existing mitochondrial disease or the more prevalent secondary mitochondrial dysfunction caused by maladaptive responses to environmental stresses. Therefore, the technology can potentially complement but not replace the unmet need: a mitochondria-targeted therapeutic.
MITOCHONDRIAL PROTEIN MODIFICATION
Mitochondrial metabolism integrates the energy demand of the cell with the nutrient availability, the redox state, and ion fluxes (Fig. 1) through a tight coupling of the TCA cycle flux with the rate of oxidative phosphorylation. Interruption of the coupling by stresses, such as hypoxia, changes in nutritional status, or energy demand, triggers mitochondrial responses to restore the homeostasis or to initiate cell death if the damage incurred is beyond repair. Mechanisms mediating such responses are poorly understood but are increasingly shown to involve protein modification by mitochondrial metabolites, including acetylation of lysine residue (LysAc), malonylation, and succinylation. LysAc results from the transfer of an acetyl group from acetyl-CoA to the ε-amino group of lysine, which neutralizes the positive charge. A putative mitochondrial acetyltransferase (GCN5L1) has been proposed for LysAc (16); however, nonenzymatic LysAc owing to the abundance of acetyl-CoA in mitochondria is likely the primary mechanism in protein acetylation (17) (Fig. 1). Enzymatic de-acetylation by sirtuins is another major determinant of LysAc level in mitochondria. There are three isoforms of sirtuins in the mitochondria, SIRT3/4/5, among which SIRT3 is the predominant deacetylase. SIRT4 is an ADP (adenosine 5′-diphosphate)–ribose transferase, whereas SIRT5 has been shown to function as desuccinylase, demalonylase, and deglutarylase (18). Thus, the level of lysine modifications reflects the availability of the thioester-CoAs and the sirtuin activities in the mitochondria (Fig. 1).
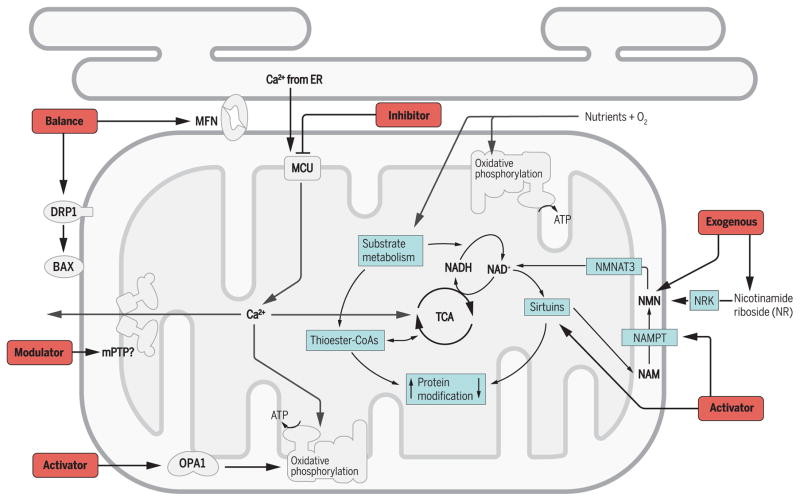
Fig. 1. Key players—and therapeutic targets—in mitochondrial protein modification, Ca2+ transport, and dynamics.
Mitochondrial protein can be modified by the thioester–coenzyme A (CoA) produced by substrate metabolism, for example, acetyl-CoA, malonyl-CoA, succinyl-CoA. The most commonly studied is the acetylation of lysine residue (LysAc). The LysAc level is determined by the availability of acetyl-CoA and the activity of deacetylases, sirtuins, which catalyze deacetylation at the expenses of nicotinamide adenine dinucleotide (NAD+). Mitochondrial NAD+ level is regulated by the activities of tricarboxylic acid (TCA) cycle and oxidative phosphorylation. Nicotinamide (NAM) generated from deacetylation reaction is converted to nicotinamide mononucleotide (NMN) by nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting enzyme. Alternatively, NMN is synthesized from nicotinamide riboside (NR) by nicotinamide riboside kinase (NRK). NMN is converted to NAD+ by nicotin-amide mononucleotide adenylyltransferase 3 (NMNAT3) in the mitochondria. Ca2+ is a key player in orchestrating metabolism and signaling function of the mitochondria. It is mainly stored in endoplasmic reticulum (ER) and transported into mitochondrial matrix by mitochondrial Ca2+ uniporter (MCU) to stimulate enzymes in TCA cycle and oxidative phosphorylation. Mitochondrial Ca2+ also triggers the opening of mPTP, which likely plays a physiological role in matrix Ca2+ release and a detrimental role in cell death. The mitochondrial dynamic regulatory proteins may bear new roles beyond fusion and fission. The outer membrane fusion protein mitofusin (MFN) tethers the mitochondria and ER membranes and through which facilitate mitochondrial Ca2+ uptake. The inner membrane fusion protein optic atrophy 1 (OPA1) controls the cristae structure and through which modulates mitochondrial respiratory chain activity. The fission protein dynamin-related protein 1 (DRP1) also regulates BAX (BCL2-associated X protein) and mPTP. The black arrows indicate potential targets for drug development.
Studies of the SIRT3-deficient mouse have provided the first evidence that mitochondrial LysAc is key to stress response. Sirt3−/− mice are normal under unstressed conditions but show higher susceptibility to obesity and cardiovascular disease (19–21). Sirtuins are NAD+-dependent enzymes; their activities are sensitive to the NAD+ level as well as the NAD+/NADH (reduced form of NAD+) ratio (22). NAD+ is a coenzyme for a variety of biochemical reactions, and in mitochondria, it also serves as the major electron carrier for oxidative phosphorylation. NAD(H) exists in either oxidized or reduced form; the partition of NAD+ and NADH in the mitochondria is determined by the balance of substrate metabolism that generates and the oxidative phosphorylation that consumes NADH. Under conditions of increased NADH production, such as in overnutrition/obesity/diabetes, or decreased NADH consumption, such as in mitochondrial disease with impaired oxidative phosphorylation, the balance of NAD+/NADH could be tilted toward a lower ratio and a concomitant increase in mitochondrial LysAc (Fig. 1 and Table 2) (22–25). There is increased sensitivity to stress in these and other mouse models of decreased NAD+/NADH ratio and increased mitochondrial LysAc (22, 25, 26). Conversely, the NAD+/NADH ratio increases, and LysAc decreases in animals subjected to caloric restriction, which has been attributed to the health benefits observed in these models (27, 28).
Table 2. Potential new therapeutic targets in mitochondria.
These new targets are shown in Fig. 1 and have been tested in preclinical studies.
| Compound | Compound type | Target site or mechanism | Disease | Reference |
|---|---|---|---|---|
| NMN | Small molecule | NAD+, protein acetylation | Heart failure, metabolic diseases, neurodegeneration, aging | (22, 30, 32) |
| NR | Small molecule | NAD+, protein acetylation | Metabolic diseases, aging | (24) |
| P7C3 chemicals | Small molecule | Activate NAMPT | Neurodegeneration | (31) |
| Honokiol | Small molecule | Activate SIRT3 | Cardiac hypertrophy | (82) |
| Ru360 | Small molecule | MCU | Myocardial infarction | (38, 40) |
| Cyclophilin D–specific inhibitors | Small molecule | mPTP | Ischemic disease | (83–86) |
| Mdivi-1 | Small molecule | DRP1 | Ischemic disease, neurodegeneration | (56, 87) |
| S3 | Small molecule | MFN | Neurodegeneration | (57) |
| P110 | Small peptide | DRP1 | Neurodegeneration | (55, 88) |
How does increased LysAc increase propensity of disease? This is a challenging question, because the observed phenotype is unlikely attributable to the hyperacetylation of either one single protein or all acetylated proteins. Nevertheless, the concept that protein modification mediates the response of mitochondrial metabolism to stress is an important one. It also proposes a role of NAD+ redox imbalance and sirtuin-regulated LysAc in the development and progression of diseases. Indeed, several strategies have been shown to improve stress response by targeting the NAD+-LysAc mechanism. Decreasing protein acetylation via the activation of sirtuins by pharmacological, genetic, or dietary interventions has demonstrated benefits in mouse models (29). Stimulating the NAD+ biosynthetic pathway also effectively increases the NAD+ levels and promotes protein deacetylation by the sirtuins (Fig. 1) (30, 31). Treating the mouse heart with complex I deficiency with the NAD+ precursor NMN normalizes the NAD+/NADH ratio and restores the sensitivity to stress (22). NMN treatment in mice also attenuates the pathophysiological changes in diabetes (30) and aging (32) (Table 2). Elevating NAD+ levels with another NAD+ precursor, NR, protected mice from high-fat diet–induced obesity (24). Recently, a small-molecule activator of NAMPT, the rate-limiting enzyme of the NAD+ biosynthetic pathway, was neuroprotective in mice (Table 2) (31).
Rebalancing the NAD+/NADH ratio and/or restoring LysAc may therefore be viable strategies in people for protection against downstream consequences of mitochondrial dysfunction (Fig. 1). Supplementation of the NAD+ precursors or stimulation of the key enzymes in the NAD+ salvage pathway is a logical approach (Table 2). One NAD+ precursor, NR, is already available for human consumption as a nutritional supplement. Clinical studies are underway to test its efficacy in raising intracellular NAD+ levels as well as its safety and tolerability in patients.
MITOCHONDRIAL CA2+ TRANSPORT
Calcium is a crucial regulator of mitochondrial function, stimulating dehydrogenases in the TCA cycle and the respiratory chain and triggering mitochondria-mediated cell death (Fig. 1). Insufficient mitochondrial calcium ion (Ca2+) uptake or Ca2+ overload can cause various human diseases, such as ischemia/reperfusion injury and neurodegeneration (33, 34). Although Ca2+ transport across the inner mitochondrial membrane has been known for decades, the exact protein identities of the channels or transporters involved were unknown until recently. The major Ca2+ uptake channel MCU was identified in 2011 (35, 36), and subsequently, the MCU complex that contains several regulatory proteins was described (Fig. 1) (37). These landmark discoveries allow further understanding of the regulation of mitochondrial Ca2+ and open the door for therapeutic intervention (Table 2).
A major motivation to intervene in mitochondrial Ca2+ handling is to protect against ischemia/reperfusion injury, where restoring blood supply to ischemic tissues in diseases such as stroke, myocardial infarction, and peripheral vascular disease causes additional damage. The mitochondrial cell death pathway—which occurs through Ca2+-triggered opening of mPTP, leading to the collapse of mitochondrial membrane potential and release of cytochrome C (Fig. 1)—is proposed to be an important mechanism of reperfusion injury. Preclinical studies have repeatedly shown the pathological roles of mitochondrial Ca2+ overload and the effectiveness of MCU blockade by small molecules, such as ruthenium red and Ru360, on ischemia/reperfusion injury (Table 2) (38–40). It is therefore unexpected that germline knockout of MCU in mice did not lead to protection from cardiac ischemia/reperfusion injury (41). However, inducible and cardiac-specific deletion of the MCU in adult mice did protect against acute ischemic injury (42, 43). Because both models reveal that mitochondria lacking MCU cannot take up Ca2+ in response to acute metabolic or signaling stress, the difference in these animals in protecting the heart from ischemia/reperfusion injury suggests that germline deletion of MCU might have triggered mechanisms other than mitochondrial Ca2+ transport for cell death. Perhaps one of the lessons we can learn from these studies is that genetic inhibition of MCU would not be a clinically preferred approach. Rather, the genetic models can aid the design and testing of small molecules that target MCU and mitochondrial Ca2+ uptake. Pharmacological modulators of MCU and its regulatory proteins may have broad utility as mitochondria-targeted therapies.
An end effector of mitochondrial Ca2+ overload is prolonged opening of the mPTP and subsequent cell death. The mitochondrial permeability transition phenomenon was first discovered more than 40 years ago, yet the molecular identity of the mPTP has not been fully revealed, making it difficult to develop a therapy. Preclinical studies have provided strong evidence for the causal role of mPTP in cell death, particularly in ischemic diseases (39). Pharmacological and genetic inhibitions of cyclophilin D, the confirmed regulator of mPTP, have shown beneficial effects against myocardial infarction in mice (Table 2) (44). Multiple clinical trials have tested mPTP inhibitors in various diseases, most notably cyclosporine A, a cyclophilin D inhibitor, for AMI (Table 1) (1, 2, 6). Cyclosporine A decreased infarct size in a small, pilot phase 2 clinical trial (45). In a recent trial with a larger patient population [CIRCUS (Cyclosporine and Prognosis in Acute Myocardial Infarction Patients) trial], cyclosporine A failed to improve clinical outcomes or prevent adverse left ventricular remodeling at 1 year after myocardial infarction (46). Other trials with different mPTP inhibitors or in different diseases also failed recently, raising the question whether the mPTP is a meaningful target (Table 1). Alternatively, there could be a fundamental flaw in the current strategy of drug development even though the mPTP is a promising target.
Strategies targeting mPTP components other than cyclophilin D, eliminating the nonspecific effects of cyclosporine A, and balancing the physiological and nonphysiological roles of mPTP are all in consideration for the development of new inhibitors of mPTP. It is increasingly evident that the mPTP has a physiological role, such as transient release of mitochondrial matrix Ca2+ (Fig. 1). Mitochondrial respiration and ROS production under resting conditions are coupled with physiological mPTP openings (47). Chronic suppression of physiological mPTP openings in cyclophilin D–deficient mice leads to increased mitochondrial matrix Ca2+, metabolic remodeling, and heart failure (26). Thus, the mixed results for cyclosporine in the clinical trials may stem from its nonselective inhibition of both physiological and pathological mPTP openings. Therefore, a therapy that specifically inhibits pathological mPTP function (prolonged and massive openings) while preserving physiological mPTP function (transient and controlled openings) would be highly desirable. Alternatively, targeting the sensitivity of mPTP during stress rather than blocking its opening entirely would be more favorable for maintaining the ion homeostasis. To achieve such a goal, again, the physical identity and molecular structure of mPTP are needed. Recent reports on the formation of mPTP by the Fo/F1 ATP synthase have raised new hope (48, 49). Before any drug is designed to target the ATP synthase, however, it is critical to fully understand how the most abundant and important protein in mitochondria can play dual and seemingly contradictory roles in the life and death of the cell (48, 49).
MITOCHONDRIAL DYNAMICS AND ITS REGULATORS
Research in the past decade has established that mitochondria are not static organelles; their size, shape, and intracellular location undergo constant changes, termed mitochondrial dynamics (50). These events are executed by a family of dynamin-related proteins that hydrolyze GTP (guanosine 5′-triphosphate), including the fusion proteins mitofusin (MFN1/2) and OPA1 and the fission protein DRP1 (51) (Fig. 1). Mitochondrial dynamics are essential for normal mitochondrial function, transporting and exchanging mitochondrial content and sequestering and removing the damaged mitochondrial subpopulation through mitophagy (52). Mutations of fission and fusion proteins have been associated with the inherited human diseases Charcot-Marie-Tooth neuropathy type 2A and dominant optic atrophy (52).
Initial studies of mitochondrial morphology during stress showed that fragmented mitochondria are associated with detrimental outcomes such as oxidative stress and cell death, but fusion is protective against stress-induced cell death (53). Thus, there is interest in developing fission inhibitors. Inhibition of DRP1 by a small molecule named Mdivi-1 (for mitochondrial division inhibitor) (54) or interfering the binding of DRP1 with its outer membrane receptor FIS1 by a small peptide has been shown to prevent excessive fission-induced cell dysfunction and ameliorate ischemia/reperfusion injury in the mouse heart and neurotoxicity in rodent neurons (Table 2) (55, 56). Recently, a compound called S3 that enhances MFN1/2 activity by inhibiting its deubiquitination has been shown to restore the morphology and function of mitochondria in fusion-deficient human and mouse cells in vitro (Table 2) (57). Continued success in pre-clinical studies using these compounds or similar may eventually lead to clinical trials.
More recent work has challenged the dogma that “fusion is always beneficial, whereas fission is detrimental.” Deletion of the fission protein DRP1 resulted in elongated but dysfunctional mitochondria, whereas deletion of MFN2 unexpectedly also yielded enlarged (rather than fragmented) mitochondria in the heart (58–61). In both cases, mitochondrial dysfunction was caused by inadequate mitophagy and poor quality control, suggesting that a healthy balance of fusion and fission is critical. Whether the modulators of DRP1 or MFN1/2 function described above can restore the balance of fusion and fission remains to be determined and is likely critical for their utility in therapeutic application. Furthermore, in some cell types, such as skeletal muscle or cardiac myocytes, the fission and fusion proteins are abundant despite infrequent morphological changes and/or movement of mitochondria. This observation raises the question as to whether the fission and fusion proteins play other roles in the regulation of mitochondrial function (Fig. 1).
Recently, the inner membrane fusion protein OPA1 has been shown to critically maintain the cristae structure of the inner membrane and to facilitate the expression and assembly of the protein complexes in the respiratory chain (Fig. 1) (62). Transgenic overexpression of Opa1 in mice partially rescued mitochondrial disease phenotypes caused by defects in respiration chain complexes (63, 64). Small molecules that enhance OPA1 function are not yet available but are attractive for mitochondrial diseases that exhibit defective respiration and abnormal cristae structures. Other dynamic proteins are also involved in modulating mitochondrial function outside of fission/fusion. For instance, DRP1 regulates BAX oligomerization and mPTP opening (56, 65), and MFN1/2 regulates mitochondrial Ca2+ uptake by tethering mitochondria with ER (Fig. 1) (66). Identification of these novel functions of the dynamic proteins opens a new window for innovative drug development.
The tethering of mitochondria and ER by MFN1/2 provides a mechanism to manipulate the distance between the two organelles and, as such, alter the local concentration of Ca2+ released from the ER. Because the ER is the major intracellular Ca2+ storage, a close association between mitochondria and ER would facilitate prompt and appropriate amount of mitochondrial Ca2+ uptake for metabolic stimulation (Fig. 1). Indeed, MFN2 has been shown to critically modulate mitochondrial bioenergetics response in Drosophila heart tubes during cardiac excitation-contraction coupling through the tethering mechanism (67). A recent report showed that such tethering facilitated OPA1 cleavage and cristae remodeling in mice response to nutritional stress (68). Excessive nutrition, such as in obesity, enhances mitochondria-ER tethering, which is responsible for mitochondrial Ca2+ overload and dysfunction in the liver (69). Thus, regulating the distance between mitochondria and ER impacts mitochondrial function and morphology (70, 71). Altering the distance between these two organelles through drug-inducible inter-organelle linkers could be a promising novel approach to manipulate the distance between the two organelles and to modify the space available for local Ca2+ transfer machinery (72). One potential clinical application of this approach is the treatment of heart failure, a chronic condition with drastically deranged T-tubule and sarco(endo)plasmic reticulum systems that lead to compromised intracellular Ca2+ handling (73). Small-molecule linkers that can reestablish the Ca2+ microdomains between sarco(endo)plasmic reticulum and mitochondria would provide double benefits at early stage of heart failure by enhancing the capacity of mitochondrial Ca2+ buffering and boosting mitochondrial metabolism to maintain the energy supply.
CONCLUSION AND PERSPECTIVES
In summary, the mitochondrial biology field has experienced fast-paced progress in recent years, yielding numerous opportunities to translate the discoveries to clinical medicine. Small-molecule compounds included in Table 2 represent a starting point for the new generation of therapies, which are expected to grow significantly in the near future. At the same time, we realize that the ultimate success of mitochondrial medicine is dependent on a thorough understanding of mitochondrial biology and function. ATP production by mitochondria and its role in Ca2+, ROS, and redox regulation are intertwined; mitochondrial morphology and function are likely coupled; and the regulators in mitochondrial function are multitasking and interacting with each other. Although modern science has revealed exciting new targets, many challenges are expected on the journey of translation, such as how to target a particular pathway without affecting the other functions of a protein and how to determine the effectiveness of a potential candidate. Thus, this is an exciting time in mitochondrial research and also the beginning of a long expedition.
Acknowledgments
Funding: This work is in part supported by grants from the NIH (HL114760 to W.W.; HL088634, HL110349, HL118989, HL126209, and HL129510 to R.T.) and from the American Heart Association (10SDG3450009to W.W.).
Footnotes
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Walters AM, Porter GA, Jr, Brookes PS. Mitochondria as a drug target in ischemic heart disease and cardiomyopathy. Circ Res. 2012;111:1222–1236. doi: 10.1161/CIRCRESAHA.112.265660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishimoto Y, Inagi R. Mitochondria: A therapeutic target in acute kidney injury. Nephrol Dial Transplant. 2015:gfv317. doi: 10.1093/ndt/gfv317. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Wang L-N. Mitochondrial enhancement for neurodegenerative movement disorders: A systematic review of trials involving creatine, coenzyme Q10, idebenone and mitoquinone. CNS Drugs. 2014;28:63–68. doi: 10.1007/s40263-013-0124-4. [DOI] [PubMed] [Google Scholar]
- 4.Ienco EC, LoGerfo A, Carlesi C, Orsucci D, Ricci G, Mancuso M, Siciliano G. Oxidative stress treatment for clinical trials in neurodegenerative diseases. J Alzheimers Dis. 2011;24(Suppl 2):111–126. doi: 10.3233/JAD-2011-110164. [DOI] [PubMed] [Google Scholar]
- 5.Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, Smith RAJ, Murphy MP. Selective targeting of a redox-active ubiquinone to mitochondria within cells: Antioxidant and antiapoptotic properties. J Biol Chem. 2001;276:4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 6.Frantz MC, Wipf P. Mitochondria as a target in treatment. Environ Mol Mutagen. 2010;51:462–475. doi: 10.1002/em.20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enns GM, Kinsman SL, Perlman SL, Spicer KM, Abdenur JE, Cohen BH, Amagata A, Barnes A, Kheifets V, Shrader WD, Thoolen M, Blankenberg F, Miller G. Initial experience in the treatment of inherited mitochondrial disease with EPI-743. Mol Genet Metab. 2012;105:91–102. doi: 10.1016/j.ymgme.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Shrader WD, Amagata A, Barnes A, Enns GM, Hinman A, Jankowski O, Kheifets V, Komatsuzaki R, Lee E, Mollard P, Murase K, Sadun AA, Thoolen M, Wesson K, Miller G. α-Tocotrienol quinone modulates oxidative stress response and the biochemistry of aging. Bioorg Med Chem Lett. 2011;21:3693–3698. doi: 10.1016/j.bmcl.2011.04.085. [DOI] [PubMed] [Google Scholar]
- 9.Weinberg SE, Chandel NS. Targeting mitochondria metabolism for cancer therapy. Nat Chem Biol. 2015;11:9–15. doi: 10.1038/nchembio.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ubah OC, Wallace HM. Cancer therapy: Targeting mitochondria and other sub-cellular organelles. Curr Pharm Des. 2014;20:201–222. doi: 10.2174/13816128113199990031. [DOI] [PubMed] [Google Scholar]
- 11.Patergnani S, Missiroli S, Marchi S, Giorgi C. Mitochondria-associated endoplasmic reticulum membranes microenvironment: Targeting autophagic and apoptotic pathways in cancer therapy. Front Oncol. 2015;5:173. doi: 10.3389/fonc.2015.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9:447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- 13.Craven L, Tuppen HA, Greggains GD, Harbottle SJ, Murphy JL, Cree LM, Murdoch AP, Chinnery PF, Taylor RW, Lightowlers RN, Herbert M, Turnbull DM. Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease. Nature. 2010;465:82–85. doi: 10.1038/nature08958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paull D, Emmanuele V, Weiss KA, Treff N, Stewart L, Hua H, Zimmer M, Kahler DJ, Goland RS, Noggle SA, Prosser R, Hirano M, Sauer MV, Egli D. Nuclear genome transfer in human oocytes eliminates mitochondrial DNA variants. Nature. 2013;493:632–637. doi: 10.1038/nature11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tachibana M, Amato P, Sparman M, Woodward J, Sanchis DM, Ma H, Gutierrez NM, Tippner-Hedges R, Kang E, Lee HS, Ramsey C, Masterson K, Battaglia D, Lee D, Wu D, Jensen J, Patton P, Gokhale S, Stouffer R, Mitalipov S. Towards germline gene therapy of inherited mitochondrial diseases. Nature. 2013;493:627–631. doi: 10.1038/nature11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott I, Webster BR, Li JH, Sack MN. Identification of a molecular component of the mitochondrial acetyltransferase programme: A novel role for GCN5L1. Biochem J. 2012;443:655–661. doi: 10.1042/BJ20120118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baeza J, Smallegan MJ, Denu JM. Site-specific reactivity of nonenzymatic lysine acetylation. ACS Chem Biol. 2015;10:122–128. doi: 10.1021/cb500848p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirschey MD, Zhao Y. Metabolic regulation by lysine malonylation, succinylation and glutarylation. Mol Cell Proteomics. 2015;14:2308–2315. doi: 10.1074/mcp.R114.046664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B, Stančáková A, Goetzman E, Lam MM, Schwer B, Stevens RD, Muehlbauer MJ, Kakar S, Bass NM, Kuusisto J, Laakso M, Alt FW, Newgard CB, Farese RV, Jr, Kahn CR, Verdin E. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell. 2011;44:177–190. doi: 10.1016/j.molcel.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, Sinclair DA. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging. 2010;2:914–923. doi: 10.18632/aging.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karamanlidis G, Lee CF, Garcia-Menendez L, Kolwicz SC, Jr, Suthammarak W, Gong G, Sedensky MM, Morgan PG, Wang W, Tian R. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab. 2013;18:239–250. doi: 10.1016/j.cmet.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alrob OA, Sankaralingam S, Ma C, Wagg CS, Fillmore N, Jaswal JS, Sack MN, Lehner R, Gupta MP, Michelakis ED, Padwal RS, Johnstone DE, Sharma AM, Lopaschuk GD. Obesity-induced lysine acetylation increases cardiac fatty acid oxidation and impairs insulin signalling. Cardiovasc Res. 2014;103:485–497. doi: 10.1093/cvr/cvu156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cantó C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, Gademann K, Rinsch C, Schoonjans K, Sauve AA, Auwerx J. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner GR, Pride PM, Babbey CM, Payne RM. Friedreich’s ataxia reveals a mechanism for coordinate regulation of oxidative metabolism via feedback inhibition of the SIRT3 deacetylase. Hum Mol Genet. 2012;21:2688–2697. doi: 10.1093/hmg/dds095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elrod JW, Wong R, Mishra S, Vagnozzi RJ, Sakthievel B, Goonasekera SA, Karch J, Gabel S, Farber J, Force T, Brown JH, Murphy E, Molkentin JD. Cyclophilin D controls mitochondrial pore–dependent Ca2+ exchange, metabolic flexibility, and propensity for heart failure in mice. J Clin Invest. 2010;120:3680–3687. doi: 10.1172/JCI43171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Guarente L. Calorie restriction and sirtuins revisited. Genes Dev. 2013;27:2072–2085. doi: 10.1101/gad.227439.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshino J, Mills KF, Yoon MJ, Imai S-I. Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14:528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang G, Han T, Nijhawan D, Theodoropoulos P, Naidoo J, Yadavalli S, Mirzaei H, Pieper AA, Ready JM, McKnight SL. P7C3 neuroprotective chemicals function by activating the rate-limiting enzyme in NAD salvage. Cell. 2014;158:1324–1334. doi: 10.1016/j.cell.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomes AP, Price NL, Ling AJY, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL, Sinclair DA. Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calì T, Ottolini D, Brini M. Mitochondrial Ca2+ and neurodegeneration. Cell Calcium. 2012;52:73–85. doi: 10.1016/j.ceca.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams GSB, Boyman L, Lederer WJ. Mitochondrial calcium and the regulation of metabolism in the heart. J Mol Cell Cardiol. 2015;78:35–45. doi: 10.1016/j.yjmcc.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Stefani D, Patron M, Rizzuto R. Structure and function of the mitochondrial calcium uniporter complex. Biochim Biophys Acta. 2015;1853:2006–2011. doi: 10.1016/j.bbamcr.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de García-Rivas GJ, Carvajal K, Correa F, Zazueta C. Ru360, a specific mitochondrial calcium uptake inhibitor, improves cardiac post-ischaemic functional recovery in rats in vivo. Br J Pharmacol. 2006;149:829–837. doi: 10.1038/sj.bjp.0706932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shintani-Ishida K, Inui M, Yoshida K-i. Ischemia–reperfusion induces myocardial infarction through mitochondrial Ca2+ overload. J Mol Cell Cardiol. 2012;53:233–239. doi: 10.1016/j.yjmcc.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 41.Pan X, Liu J, Nguyen T, Liu C, Sun J, Teng Y, Fergusson MM, Rovira II, Allen M, Springer DA, Aponte AM, Gucek M, Balaban RS, Murphy E, Finkel T. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol. 2013;15:1464–1472. doi: 10.1038/ncb2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luongo TS, Lambert JP, Yuan A, Zhang X, Gross P, Song J, Shanmughapriya S, Gao E, Jain M, Houser SR, Koch WJ, Cheung JY, Madesh M, Elrod JW. The mitochondrial calcium uniporter matches energetic supply with cardiac workload during stress and modulates permeability transition. Cell Rep. 2015;12:23–34. doi: 10.1016/j.celrep.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwong JQ, Lu X, Correll RN, Schwanekamp JA, Vagnozzi RJ, Sargent MA, York AJ, Zhang J, Bers DM, Molkentin JD. The mitochondrial calcium uniporter selectively matches metabolic output to acute contractile stress in the heart. Cell Rep. 2015;12:15–22. doi: 10.1016/j.celrep.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, II, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 45.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, André-Fouët X, Revel D, Kirkorian G, Monassier JP, Derumeaux G, Ovize M. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 46.Cung TT, Morel O, Cayla G, Rioufol G, Garcia-Dorado D, Angoulvant D, Bonnefoy-Cudraz E, Guérin P, Elbaz M, Delarche N, Coste P, Vanzetto G, Metge M, Aupetit JF, Jouve B, Motreff P, Tron C, Labeque JN, Steg PG, Cottin Y, Range G, Clerc J, Claeys MJ, Coussement P, Prunier F, Moulin F, Roth O, Belle L, Dubois P, Barragan P, Gilard M, Piot C, Colin P, De Poli F, Morice MC, Ider O, Dubois-Randé JL, Unterseeh T, Le Breton H, Béard T, Blanchard D, Grollier G, Malquarti V, Staat P, Sudre A, Elmer E, Hansson MJ, Bergerot C, Boussaha I, Jossan C, Derumeaux G, Mewton N, Ovize M. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med. 2015;373:1021–1031. doi: 10.1056/NEJMoa1505489. [DOI] [PubMed] [Google Scholar]
- 47.Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M, Yin J, Wang W, Mattson MP, Kao JP, Lakatta EG, Sheu SS, Ouyang K, Chen J, Dirksen RT, Cheng H. Superoxide flashes in single mitochondria. Cell. 2008;134:279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park H-A, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, Porter GA, Jr, Jonas EA. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci USA. 2014;111:10580–10585. doi: 10.1073/pnas.1401591111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabó I, Lippe G, Bernardi P. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci USA. 2013;110:5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedman JR, Nunnari J. Mitochondrial form and function. Nature. 2014;505:335–343. doi: 10.1038/nature12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hall AR, Burke N, Dongworth RK, Hausenloy DJ. Mitochondrial fusion and fission proteins: Novel therapeutic targets for combating cardiovascular disease. Br J Pharmacol. 2014;171:1890–1906. doi: 10.1111/bph.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan DC. Fusion and fission: Interlinked processes critical for mitochondrial health. Annu Rev Genet. 2012;46:265–287. doi: 10.1146/annurev-genet-110410-132529. [DOI] [PubMed] [Google Scholar]
- 53.Dorn GW., II Mitochondrial dynamism and heart disease: Changing shape and shaping change. EMBO Mol Med. 2015;7:865–877. doi: 10.15252/emmm.201404575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, Nunnari J. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qi X, Qvit N, Su YC, Mochly-Rosen D. A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J Cell Sci. 2013;126:789–802. doi: 10.1242/jcs.114439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 57.Yue W, Chen Z, Liu H, Yan C, Chen M, Feng D, Wu H, Du L, Wang Y, Liu J, Huang X, Xia L, Liu L, Wang X, Jin H, Wang J, Song Z, Hao X, Chen Q. A small natural molecule promotes mitochondrial fusion through inhibition of the deubiquitinase USP30. Cell Res. 2014;24:482–496. doi: 10.1038/cr.2014.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, Nomura M, Mihara K, Egashira K, Ohishi M, Abdellatif M, Sadoshima J. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ Res. 2015;116:264–278. doi: 10.1161/CIRCRESAHA.116.303356. [DOI] [PubMed] [Google Scholar]
- 59.Kageyama Y, Hoshijima M, Seo K, Bedja D, Sysa-Shah P, Andrabi SA, Chen W, Höke A, Dawson VL, Dawson TM, Gabrielson K, Kass DA, Iijima M, Sesaki H. Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. EMBO J. 2014;33:2798–2813. doi: 10.15252/embj.201488658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song M, Mihara K, Chen Y, Scorrano L, Dorn GW., II Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab. 2015;21:273–285. doi: 10.1016/j.cmet.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dorn GW, II, Song M, Walsh K. Functional implications of mitofusin 2-mediated mitochondrial-SR tethering. J Mol Cell Cardiol. 2015;78:123–128. doi: 10.1016/j.yjmcc.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cogliati S, Frezza C, Soriano ME, Varanita T, Quintana-Cabrera R, Corrado M, Cipolat S, Costa V, Casarin A, Gomes LC, Perales-Clemente E, Salviati L, Fernandez-Silva P, Enriquez JA, Scorrano L. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell. 2013;155:160–171. doi: 10.1016/j.cell.2013.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Varanita T, Soriano ME, Romanello V, Zaglia T, Quintana-Cabrera R, Semenzato M, Menabò R, Costa V, Civiletto G, Pesce P, Viscomi C, Zeviani M, Di Lisa F, Mongillo M, Sandri M, Scorrano L. The OPA1-dependent mitochondrial cristae remodeling pathway controls atrophic, apoptotic, and ischemic tissue damage. Cell Metab. 2015;21:834–844. doi: 10.1016/j.cmet.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Civiletto G, Varanita T, Cerutti R, Gorletta T, Barbaro S, Marchet S, Lamperti C, Viscomi C, Scorrano L, Zeviani M. Opa1 overexpression ameliorates the phenotype of two mitochondrial disease mouse models. Cell Metab. 2015;21:845–854. doi: 10.1016/j.cmet.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Montessuit S, Somasekharan SP, Terrones O, Lucken-Ardjomande S, Herzig S, Schwarzenbacher R, Manstein DJ, Bossy-Wetzel E, Basañez G, Meda P, Martinou JC. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell. 2010;142:889–901. doi: 10.1016/j.cell.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 67.Chen Y, Csordás G, Jowdy C, Schneider TG, Csordás N, Wang W, Liu Y, Kohlhaas M, Meiser M, Bergem S, Nerbonne JM, Dorn GW, II, Maack C. Mitofusin 2-containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via interorganelle Ca2+ crosstalk. Circ Res. 2012;111:863–875. doi: 10.1161/CIRCRESAHA.112.266585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sood A, Jeyaraju DV, Prudent J, Caron A, Lemieux P, McBride HM, Laplante M, Tóth K, Pellegrini L. A Mitofusin-2–dependent inactivating cleavage of Opa1 links changes in mitochondria cristae and ER contacts in the postprandial liver. Proc Natl Acad Sci USA. 2014;111:16017–16022. doi: 10.1073/pnas.1408061111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arruda AP, Pers BM, Parlakgül G, Güney E, Inouye K, Hotamisligil GS. Chronic enrichment of hepatic endoplasmic reticulum–mitochondria contact leads to mitochondrial dysfunction in obesity. Nat Med. 2014;20:1427–1435. doi: 10.1038/nm.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Korobova F, Ramabhadran V, Higgs HN. An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science. 2013;339:464–467. doi: 10.1126/science.1228360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eisner V, Csordás G, Hajnóczky G. Interactions between sarco-endoplasmic reticulum and mitochondria in cardiac and skeletal muscle–pivotal roles in Ca2+ and reactive oxygen species signaling. J Cell Sci. 2013;126:2965–2978. doi: 10.1242/jcs.093609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Csordás G, Várnai P, Golenár T, Roy S, Purkins G, Schneider TG, Balla T, Hajnóczky G. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol Cell. 2010;39:121–132. doi: 10.1016/j.molcel.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Song LS, Sobie EA, McCulle S, Lederer WJ, Balke CW, Cheng H. Orphaned ryanodine receptors in the failing heart. Proc Natl Acad Sci USA. 2006;103:4305–4310. doi: 10.1073/pnas.0509324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buyse GM, Voit T, Schara U, Straathof CSM, D’Angelo MG, Bernert G, Cuisset J-M, Finkel RS, Goemans N, McDonald CM, Rummey C, Meier T DELOS Study Group. Efficacy of idebenone on respiratory function in patients with Duchenne muscular dystrophy not using glucocorticoids (DELOS): A double-blind randomised placebo-controlled phase 3 trial. Lancet. 2015;385:1748–1757. doi: 10.1016/S0140-6736(15)60025-3. [DOI] [PubMed] [Google Scholar]
- 75.Tsujita K, Shimomura H, Kaikita K, Kawano H, Hokamaki J, Nagayoshi Y, Yamashita T, Fukuda M, Nakamura Y, Sakamoto T, Yoshimura M, Ogawa H. Long-term efficacy of edaravone in patients with acute myocardial infarction. Circ J. 2006;70:832–837. doi: 10.1253/circj.70.832. [DOI] [PubMed] [Google Scholar]
- 76.Abe K, Itoyama Y, Sobue G, Tsuji S, Aoki M, Doyu M, Hamada C, Kondo K, Yoneoka T, Akimoto M, Yoshino H Edaravone ALS Study Group. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:610–617. doi: 10.3109/21678421.2014.959024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parkinson Study Group QE3 Investigators. Beal MF, Oakes D, Shoulson I, Henchcliffe C, Galpern WR, Haas R, Juncos JL, Nutt JG, Voss TS, Ravina B, Shults CM, Helles K, Snively V, Lew MF, Griebner B, Watts A, Gao S, Pourcher E, Bond L, Kompoliti K, Agarwal P, Sia C, Jog M, Cole L, Sultana M, Kurlan R, Richard I, Deeley C, Waters CH, Figueroa A, Arkun A, Brodsky M, Ondo WG, Hunter CB, Jimenez-Shahed J, Palao A, Miyasaki JM, So J, Tetrud J, Reys L, Smith K, Singer C, Blenke A, Russell DS, Cotto C, Friedman JH, Lannon M, Zhang L, Drasby E, Kumar R, Subramanian T, Ford DS, Grimes DA, Cote D, Conway J, Siderowf AD, Evatt ML, Sommerfeld B, Lieberman AN, Okun MS, Rodriguez RL, Merritt S, Swartz CL, Martin WR, King P, Stover N, Guthrie S, Watts RL, Ahmed A, Fernandez HH, Winters A, Mari Z, Dawson TM, Dunlop B, Feigin AS, Shannon B, Nirenberg MJ, Ogg M, Ellias SA, Thomas CA, Frei K, Bodis-Wollner I, Glazman S, Mayer T, Hauser RA, Pahwa R, Langhammer A, Ranawaya R, Derwent L, Sethi KD, Farrow B, Prakash R, Litvan I, Robinson A, Sahay A, Gartner M, Hinson VK, Markind S, Pelikan M, Perlmutter JS, Hartlein J, Molho E, Evans S, Adler CH, Duffy A, Lind M, Elmer L, Davis K, Spears J, Wilson S, Leehey MA, Hermanowicz N, Niswonger S, Shill HA, Obradov S, Rajput A, Cowper M, Lessig S, Song D, Fontaine D, Zadikoff C, Williams K, Blindauer KA, Bergholte J, Propsom CS, Stacy MA, Field J, Mihaila D, Chilton M, Uc EY, Sieren J, Simon DK, Kraics L, Silver A, Boyd JT, Hamill RW, Ingvoldstad C, Young J, Thomas K, Kostyk SK, Wojcieszek J, Pfeiffer RF, Panisset M, Beland M, Reich SG, Cines M, Zappala N, Rivest J, Zweig R, Lumina LP, Hilliard CL, Grill S, Kellermann M, Tuite P, Rolandelli S, Kang UJ, Rao J, Cook MM, Severt L, Boyar K. A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: No evidence of benefit. JAMA Neurol. 2014;71:543–552. doi: 10.1001/jamaneurol.2014.131. [DOI] [PubMed] [Google Scholar]
- 78.Makimura H, Stanley TL, Suresh C, Luisa De Sousa-Coelho A, Frontera WR, Syu S, Braun LR, Looby SE, Feldpausch MN, Torriani M, Lee H, Patti M-E, Grinspoon SK. Metabolic effects of long-term reduction in free fatty acids with acipimox in obesity: A randomized trial. J Clin Endocrinol Metab. 2015 doi: 10.1210/jc.2015-3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Newman MF, Ferguson TB, White JA, Ambrosio G, Koglin J, Nussmeier NA, Pearl RG, Pitt B, Wechsler AS, Weisel RD, Reece TL, Lira A, RA Harrington; RED-CABG Steering Committee and Investigators. Effect of adenosine-regulating agent acadesine on morbidity and mortality associated with coronary artery bypass grafting: The RED-CABG randomized controlled trial. JAMA. 2012;308:157–164. doi: 10.1001/jama.2012.7633. [DOI] [PubMed] [Google Scholar]
- 80.Bezprozvanny I. The rise and fall of Dimebon. Drug News Perspect. 2010;23:518–523. doi: 10.1358/dnp.2010.23.8.1500435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Atar D, Arheden H, Berdeaux A, Bonnet JL, Carlsson M, Clemmensen P, Cuvier V, Danchin N, Dubois-Randé JL, Engblom H, Erlinge D, Firat H, Halvorsen S, Hansen HS, Hauke W, Heiberg E, Koul S, Larsen AI, Le Corvoisier P, Nordrehaug JE, Paganelli F, Pruss RM, Rousseau H, Schaller S, Sonou G, Tuseth V, Veys J, Vicaut E, Jensen SE. Effect of intravenous TRO40303 as an adjunct to primary percutaneous coronary intervention for acute ST-elevation myocardial infarction: MITOCARE study results. Eur Heart J. 2015;36:112–119. doi: 10.1093/eurheartj/ehu331. [DOI] [PubMed] [Google Scholar]
- 82.Pillai VB, Samant S, Sundaresan NR, Raghuraman H, Kim G, Bonner MY, Arbiser JL, Walker DI, Jones DP, Gius D, Gupta MP. Honokiol blocks and reverses cardiac hypertrophy in mice by activating mitochondrial Sirt3. Nat Commun. 2015;6:6656. doi: 10.1038/ncomms7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Argaud L, Gateau-Roesch O, Muntean D, Chalabreysse L, Loufouat J, Robert D, Ovize M. Specific inhibition of the mitochondrial permeability transition prevents lethal reperfusion injury. J Mol Cell Cardiol. 2005;38:367–374. doi: 10.1016/j.yjmcc.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 84.Hausenloy DJ, Duchen MR, Yellon DM. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia–reperfusion injury. Cardiovasc Res. 2003;60:617–625. doi: 10.1016/j.cardiores.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 85.Gomez L, Thibault H, Gharib A, Dumont JM, Vuagniaux G, Scalfaro P, Derumeaux G, Ovize M. Inhibition of mitochondrial permeability transition improves functional recovery and reduces mortality following acute myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2007;293:H1654–H1661. doi: 10.1152/ajpheart.01378.2006. [DOI] [PubMed] [Google Scholar]
- 86.Linkermann A, Bräsen JH, Darding M, Jin MK, Sanz AB, Heller JO, De Zen F, Weinlich R, Ortiz A, Walczak H, Weinberg JM, Green DR, Kunzendorf U, Krautwald S. Two independent pathways of regulated necrosis mediate ischemia–reperfusion injury. Proc Natl Acad Sci USA. 2013;110:12024–12029. doi: 10.1073/pnas.1305538110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rappold PM, Cui M, Grima JC, Fan RZ, de Mesy-Bentley KL, Chen L, Zhuang X, Bowers WJ, Tieu K. Drp1 inhibition attenuates neurotoxicity and dopamine release deficits in vivo. Nat Commun. 2014;5:5244. doi: 10.1038/ncomms6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Su YC, Qi X. Inhibition of excessive mitochondrial fission reduced aberrant autophagy and neuronal damage caused by LRRK2 G2019S mutation. Hum Mol Genet. 2013;22:4545–4561. doi: 10.1093/hmg/ddt301. [DOI] [PubMed] [Google Scholar]