Abstract
Background
Tobacco use is common among ED patients, many of whom are low-income. Our objective was to study the efficacy of an intervention incorporating motivational interviewing, nicotine replacement, and quitline referral for adult smokers in an ED.
Methods
A two-arm randomized clinical trial conducted from October 2010–December 2012, at a 90,000 visit/year urban ED in the northeastern U.S. Eligible subjects were age 18 years or older who smoked and were self-pay or had Medicaid insurance. Intervention subjects received a motivational interview by a trained research assistant, 6 weeks of nicotine patches and gum initiated in the ED, a faxed referral to the state smokers’ quitline, a booster call, and a brochure. Control subjects received the brochure, which provided quitline information. The primary outcome was biochemically confirmed tobacco abstinence at three months. Secondary endpoints included quitline utilization.
Results
Of 778 enrolled subjects, 774 (99.5%) were alive at three months. The prevalence of biochemically confirmed abstinence was 12.2% (47/386) in the intervention arm vs. 4.9% (19/388) in the control arm, for a difference in quit rates of 7.3% (95% CI 3.2%, 11.5%). In multivariable logistic modeling controlling for age, sex, and race/ethnicity, study subjects remained more likely to be abstinent than controls (OR 2.72, 95% CI 1.55, 4.75).
Conclusions
An intensive intervention improved tobacco abstinence rates in low-income ED smokers. Because approximately 20 million smokers, many of whom are low-income, visit US EDs annually, these results suggest ED-initiated treatment may be an effective technique to treat this group of smokers.
Background
Cigarette smoking remains the leading cause of preventable death and illness in the United States. In 2012, 18.1% of all American adults smoked, and 480,000 died from smoking-related illnesses.1,2 Cessation is associated with significant individual and societal benefits.
Smokers are disproportionately from low-income households, and commonly receive care in hospital emergency departments (ED), either for medical consequences of smoking or for comorbid medical and psychiatric conditions. These patients often have limited access to primary care providers,3,4 who tend to undertreat tobacco use.5 Therefore, the ED visit may represent an ideal opportunity for screening, intervention and referral for treatment, particularly given the greater prevalence of smoking in ED patients than in the general population.6,7
In 2010, 129.8 million individuals visited US EDs.8 Recent reports from the Institute of Medicine,9 the federal government,10 and the 2008 Public Health Service tobacco treatment guideline,11 include EDs as effective loci for tobacco control. Screening followed by brief intervention and referral to treatment has had success in reducing high risk behaviors such as problem drinking.12
EDs have been the focus of tobacco control efforts for 15 years. A recent meta-analysis of 7 studies containing 1,986 subjects found enhanced abstinence at one month, with the odds for tobacco abstinence in the intervention arm of 1.47 (95% CI 1.06–2.06), compared to controls.13 At subsequent time points of 3, 6, and 12 months, however, the effect was nonsignificant. The interventions in these studies included combinations of printed materials, brief counseling, motivational interviewing, and post-discharge phone calls. Medications were not offered. An additional study found that smokers presenting to the ED with a tobacco-related ICD 9 code, or who thought they had a tobacco-related reason for the ED visit, were more likely to quit at three months than others.14
We hypothesized that a more potent intervention, including ED-initiated “facilitated” referral to a quitline and initiation of pharmacotherapy, might result in sustained abstinence.
The goal of this randomized controlled trial was to compare two models of brief intervention: 1) standard care, versus 2) screening, brief intervention, and facilitated referral (SBIRT) to the quitline with initiation of nicotine replacement therapy (NRT). Our primary hypotheses were that: (1) at three months, a higher proportion of subjects in the intervention arm would be abstinent than in the control arm; and (2) at three months, intervention arm subjects would be smoking fewer cigarettes/day than controls. Secondary hypotheses were: (1) Subjects who believed their ED visit was related to tobacco use, or who had a tobacco-related ICD9 code, would be more likely to be abstinent than others; (2) the intervention would reduce overall health care service utilization, and (3) the intervention would be cost-effective, relative to standard care. The latter two hypotheses will be the subjects of a separate report.
Methods
This was a single-hospital 2-arm randomized controlled trial of a multicomponent intervention for adult smokers presenting to the ED, with blinded outcome assessment. The intervention consisted of a brief motivational interview, provision of six weeks of NRT, initiation of NRT in the ED, active referral to a smokers’ quitline, a booster phone call 3 days after enrollment, and provision of a smoking cessation brochure. The control arm received the brochure alone. The study was conducted an urban, teaching ED with 90,000 visits per year, located in a medically underserved community. The Institutional Review Board approved the study. All subjects provided written informed consent. The trial is registered in www.clinicaltrials.gov (Identifier: NCT01328431).
Patients were eligible if they were at least 18 years of age, spoke English, had Medicaid or no insurance, were able to give written informed consent, smoked at least 100 cigarettes lifetime and were currently every- or some-day smokers, averaging at least 5 cigarettes/day. Both admitted and treated/released patients were eligible. Research staff continued to follow subjects admitted to inpatient units.
Patients were excluded if they lived outside Connecticut, were too ill to give consent, presented primarily with a psychiatric problem, were pregnant or nursing, in police custody, had a history of allergy to nicotine replacement products, were currently in treatment for tobacco dependence, or were leaving the ED against medical advice.
An on-line random plan generator (www.randomization.com) was used to generate an allocation schedule, with an allocation ratio of 1:1, and a block size of 6. A blinded staff member prepared a set of opaque, consecutively numbered envelopes with the treatment group indicated inside.
Subjects in the Usual Care arm received a brochure prepared by the state Department of Public Health that provides general information about smoking cessation, and the phone number of the toll-free state smokers’ quitline. They received no other study-specific treatment.
In addition to the brochure, subjects in the Intervention arm received:
A 10–15 minute brief motivational interview delivered by a research assistant (RA) trained in motivational techniques.15 Motivational interviewing interventions provide patients with feedback, enhance self-efficacy, offer brief advice and treatment options in a nonjudgmental, empathic fashion, allow for self-reflection, and allow the patient to assume responsibility for change.
Six weeks of nicotine replacement therapy (NRT) including 42 patches and 300 pieces of gum, all of which was given to the subject after randomization. The dose was tailored to the subject’s degree of nicotine addiction, as measured by the Heaviness of Smoking Index.16 Dosing for patches included 7mg, 14mg, and 21mg; gum dosing included 2mg and 4mg. Subjects were instructed to apply one patch daily, and to use up to 10 pieces of gum daily, per manufacturer’s directions. The first dose of patch or gum was administered by a nurse during the ED visit. Study medications were kept in a research-only medication storage system in the ED. Treatment initiation in the ED was designed to remove barriers to use of pharmacotherapy, demonstrate its rapid effects on alleviation of withdrawal, and to convert short-term abstinence into a quit attempt.
A referral to the state Smokers’ Quitline. This toll-free service offers all smokers, regardless of insurance, up to five free counseling sessions by individuals trained in motivational techniques, starter doses of NRT, and printed materials. The service is multi-lingual and available seven days a week. RAs faxed the referral from the ED to the Quitline; the Quitline called subjects within a few days of enrollment.
A phone call three days after the ED visit, from a study nurse, to answer any questions about treatment or study procedures. The nurse did not provide any additional motivational enhancement.
At enrollment, all subjects were given $10 gift cards for a local retailer, with payments of $25 at each telephone follow-up, at 1, 3, and 12 months. At 3 months, subjects self-reporting tobacco abstinence were given an additional $100 for providing biochemical confirmation of abstinence.
Research associates (RAs) screened patients to identify potential subjects, and were present in the ED 7 days/week, 12 hours/day. Patients were screened and enrolled in consecutive fashion. RAs had bachelor’s or master’s degrees, and were trained in motivational interviewing and tobacco dependence treatment by study investigators. All subject encounters were audiotaped; tapes were reviewed weekly with RAs by a psychologist to ensure fidelity to protocol.
Baseline variables recorded included age, sex, race/ethnicity, insurance status, marital/relationship status, level of education, and employment status. Clinical information included chief complaint, primary and secondary ICD-9 diagnoses, and the modified Patient Health Questionaire-2 (PHQ-2) depression screen.17 Tobacco use history included daily cigarette consumption, the Heaviness of Smoking Index16,18, daily cigarette consumption, beliefs addressing the relationship between smoking and illness, and reason for ED use. Data on alcohol and drug use were collected via the Rapid Alcohol Problems Screen19 and Rapid Drug Problems Screen.20 Biochemical verification of tobacco use was performed by measurement of exhaled carbon monoxide with the Bedfont Micro+ breath carbon monoxide monitor.
The ICD-9 codes used to designate smoking-related illnesses are those listed in the 1989 Surgeon General’s report, and have been used previously in ED-based studies.14,21,22 Any primary or secondary ICD-9 codes designated the subject as having a smoking-related reason for the ED visit. Self-reported post-enrollment data on use of healthcare services were collected via the Treatment Service Review,23 in order to analyze the cost effectiveness of the intervention. This will be the subject of a separate report.
The primary endpoint was the proportion of subjects who were confirmed abstinent at 3 months. Subjects who self-reported past 7-day abstinence were asked to return to the ED to measure exhaled breath carbon monoxide (CO). Those who recorded a CO level of less than 7 ppm, a standard cutoff recommended by the manufacturer, were considered abstinent. Carbon monoxide is an accurate, easy-to-measure biomarker that has been used for decades. It is detectable up to 24 hours after inhalation of burned tobacco.24 Outcome assessment was performed by study assistants blinded to group assignment.
Secondary endpoints included the change in daily cigarette consumption, the proportion of subjects with a tobacco-related ICD9 code who were abstinent, the proportion of subjects who thought their ED visit was tobacco-related who were abstinent, and the abstinence rates at one and 12 months.
Based on prior work25,26, we defined a clinically significant difference in the proportion of abstinent subjects between treatment arms of 8%. Setting a 2-sided alpha at 0.05 and 80% power yielded a sample size of 353 in each arm. Allowing for 10% loss to follow-up at 3 months yielded a final planned sample size of 778 patients. Power and sample size calculations were performed with NCSS/PASS.
Data were analyzed with SPSS 19.0 and EpiInfo 7.1. Univariate and bivariate data are reported with parametric and nonparametric statistics, as appropriate. Logistic models were fitted with variables selected for inclusion based on clinical criteria. An alpha value of 0.05 was chosen, and all tests were two-sided. Subjects with missing values or who were lost to follow-up were coded as smoking, in this intention-to-treat analysis.
Trial results are reported in accordance with the CONSORT statement.27
Results
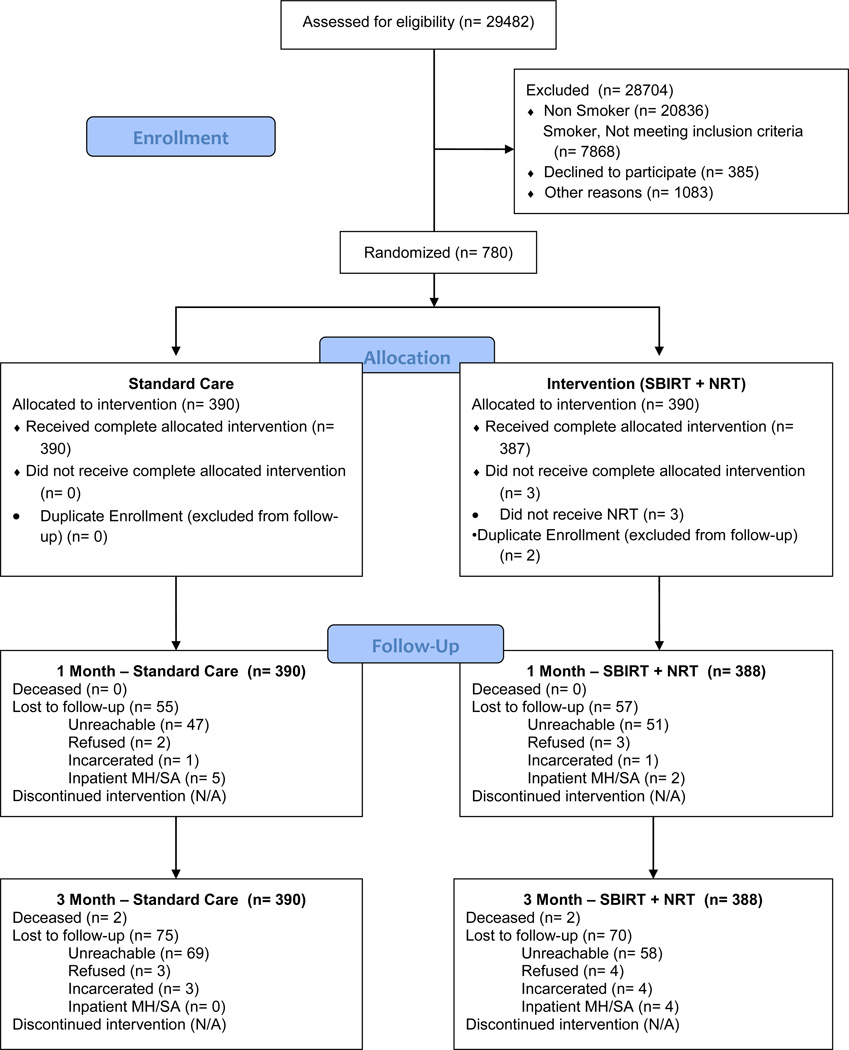
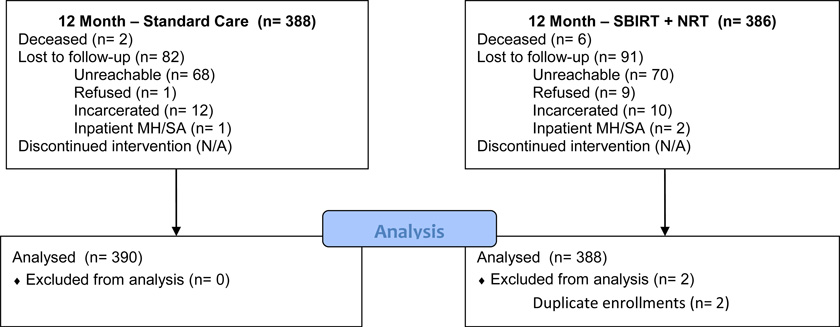
From October 2010 to December 2012, 778 subjects were enrolled, of whom 774 (99.5%) were alive at 3 months. The Figure and Table 1, respectively, provide a flow diagram and baseline characteristics of study subjects. Baseline characteristics were comparable between treatment groups, although minor imbalances exist in sex, insurance status and depression screen. More than half the study subjects were nonwhite. Median daily cigarette consumption was comparable to that found in other studies.14,28
Figure.
Flow of patients through the study.
Table 1.
Baseline patient characteristics.
| Variable | Usual Care (N = 390) |
Intervention (N = 388) |
|---|---|---|
| Age, mean, years | 40.2 | 40.8 |
| Sex, no. male (%) | 201 (51.5%) | 170 (43.8%) |
| Race/ethnicity, N (%) | ||
| White | 157 (40.3%) | 148 (38.1%) |
| African-American | 153 (39.2) | 155 (39.9) |
| Hispanic | 77 (19.8) | 81 (20.9) |
| Asian/Other | 3 (0.8) | 4 (1.1.) |
| Insurance, N (%) | ||
| Self-pay/none | 71 (18.2%) | 51 (13.1%) |
| Medicaid | 319 (81.8) | 337 (86.9) |
| Depression Screen (+), N (%) | 207 (53.1%) | 225 (58.0%) |
| Rapid Alcohol Screen (+), N (%) | 98 (25.2%) | 99 (25.6%) |
| Rapid Drug Screen (+), N (%) | 80 (20.5%) | 79 (20.4%) |
| Cigarettes/day, median, IQR | 11 (8, 20) | 11 (10, 20) |
| Carbon monoxide, median, IQR | 7 (5, 12) | 7 (4, 12) |
| Heavy Smoking Index ≥ 4, N (%) | 163 (41.8%) | 155 (39.9%) |
| Patients admitted, N (%) | 115 (29.5%) | 116 (29.9%) |
| Subject believes ED visit related to tobacco, N (%) | 101 (26.0%) | 89 (23.0%) |
| Subject believes medical illness related to tobacco, N (%) | 191 (49.2%) | 185 (47.8%) |
| Tobacco-related ICD9 | 125 (32.1%) | 117 (30.2%) |
The primary endpoint was biochemically confirmed tobacco abstinence at 3 months. As shown in Table 2, the proportion of abstinent subjects at 3 months in the Intervention and Usual Care arms was, respectively, 12.2% (47/386) and 4.9% (19/388), P<0.001, with a difference in quit rates of 7.3% (95% CI 3.2%, 11.5%). In addition, all other measures of cigarette consumption or abstinence showed greater improvement in the intervention arm compared to the control arm. Ninety-seven subjects self-reported tobacco abstinence at 3 months. Of these, 92 (94.8%) returned to the ED for biochemical testing.
Table 2.
Primary and secondary endpoints at three months.*
| Variable | Usual Care (N = 390) |
Intervention (N = 388) |
Difference between groups (95% CI) |
|---|---|---|---|
| 7-day abstinence, biochemically verified, N (%) | 19 (4.9%) | 47 (12.2%) | 7.3% (3.3, 11.3%) |
| 24 hour quit attempt since ED visit, N (%) | 217 (55.9%) | 264 (68.4%) | 12.5% (5.6, 19.1%) |
| 7-day abstinence, self-report, N (%) | 33 (8.5%) | 64 (16.6%) | 8.1% (3.4, 12.8%) |
| Change in daily cigarette consumption, mean (95% CI) | −5.9 (−4.9, −6.9) | −9.1 (−8.2, −10.0) | 3.2 (1.9, 4.5) |
| Used quitline, N (%) | 73 (18.7%) | 124 (32.0%) | 13.3% (7.2, 19.3%) |
continued smoking imputed to missing cases
Table 3 presents the quit rates in prespecified secondary analyses. These include the abstinence rates in subjects with a tobacco-related ICD-9 code, and those with a self-reported tobacco-related reason for the ED visit, and in those who believed they had a tobacco-related illness. In each, the quit rates between treatment arms were comparable.
Table 3.
Seven-day abstinence at three months, biochemically verified, for prespecified subgroups.
| Variable | Yes | No | P value |
|---|---|---|---|
| Tobacco-related ICD9 | 9.1% | 8.2% | 0.68 |
| ED visit related to tobacco (patient belief) | 8.9% | 8.5% | 0.77 |
| Medical illness related to tobacco (patient belief) | 8.7% | 8.3% | 0.89 |
| Depression screen (+) | 8.4% | 8.7% | 0.90 |
Quitline utilization was substantially greater in the intervention group. The proportion of subjects using quitline services in the intervention and control arms was, respectively, 32.0% (124/386) and 18.7% (73/388) (OR 2.04, 95% CI 1.46, 2.84).
Table 4 shows the results of a logistic model, with biochemically verified abstinence at 3 months as the dependent variable. To avoid overfitting,29 age, sex, race/ethnicity, and study arm were chosen for inclusion in the model. Of these, only assignment to Enhanced Care was associated with increased odds of abstinence at 3 months. The model showed adequate goodness-of-fit, with a Hosmer-Lemeshow statistic of 0.30.
Table 4.
Multivariate logistic model for odds of three-month abstinence
| Variable | OR | 95% CI | |
|---|---|---|---|
| Lower | Upper | ||
| Intervention (Usual Care is referent group) | 2.72 | 1.55 | 4.75 |
| Male Gender (Female is referent) | 1.41 | 0.84 | 2.37 |
| Age | 1.01 | 0.99 | 1.04 |
| Race/Ethnicity (Other is referent) | |||
| White/non-Hispanic | 0.70 | 0.09 | 5.74 |
| White/Hispanic | 1.36 | 0.16 | 11.30 |
| African-American/Non-Hispanic | 1.61 | 0.20 | 12.87 |
At one year, the self-reported abstinence rates in the Enhanced and Usual Care arms were, respectively, 62/380 (16.3%) vs. 45/386 (11.7%) (OR 1.47, 95% CI 0.97, 2.23). Of note, the study was neither designed nor powered to detect a difference in tobacco use at one year.
Subjects lost to follow-up had similar demographic and clinical characteristics to those retained. In addition, subjects lost to follow-up were similar between study arms.
Discussion
We report sustained 3-month tobacco abstinence from an ED-initiated intervention. Prior studies13 have found shorter-term improvements in tobacco abstinence, typically of one month’s duration. Our own prior work did not find a statistically significant difference at three months,14 although that study did find greater efficacy of a motivational interview and provision of NRT, without ED initiation) in subjects with self-reported alcohol or substance use disorders.30
The intervention in this report was likely successful for several reasons. First, all treatments offered to study subjects were evidence-based, and recommended by the Public Health Service.11 These included dual medications, in-person counseling using motivational techniques delivered by a trained interventionist, and an accessible telephone quitline. Second, although medication and counseling are each effective, combination treatment often offers greater efficacy.11
Third, we initiated medication management in the ED. Applying the first nicotine patch, or administering the first piece of gum, allowed us to demonstrate to the subject their ease of use, ability to alleviate nicotine withdrawal, and excellent tolerability. Of note, this practice contravenes traditional models of tobacco dependence treatment, in which patients are asked to set a quit date several weeks after the initial visit, and smoking is generally tapered prior to the quit, perhaps with concurrent initiation of NRT.31 Our approach tries to reinforce the “teachable moment” of the ED visit, in which the patient’s event-related fear may enhance the likelihood of a successful quit.32 Further, additional work has shown that most successful quits occur spontaneously, without extensive prior planning.33
Fourth, we provided two forms of NRT: long-acting, and short-acting. Patches provide a steady, basal replacement of nicotine, but usually not enough to prevent cravings and some symptoms of withdrawal. Nicotine from gum crosses mucosal and blood-brain barriers faster than nicotine from patches, and is indicated for cravings and withdrawal symptoms. Using both forms of NRT is increasingly recognized as a particularly effective treatment.34
Last, our baseline assessment was quite brief, with only 34 items that took about 15 minutes for the RAs to deliver. Prior work on brief behavioral interventions has suggested that extensive, lengthy baseline assessments may constitute a form of intervention, as study subjects, including those randomized to the control arm, are given the opportunity to discuss and reflect on their risky health behavior.35,36
A wealth of evidence highlights the efficacy of medication and behavioral counseling in tobacco dependence treatment, and the synergistic effects of both.11 Our goal was to offer both to adult smokers visiting an ED for an acute health event, which the Sentinel Events model of behavior change suggests may be an opportune time for an intervention for someone with an adverse consequence of a risky health behavior.32
The intervention’s efficacy nearly reached statistical significance at one year. Although this was an exploratory endpoint, it suggests that sustained quits from an ED-initiated intervention may be possible. Our data are consistent with the recommendations of a Cochrane review, which found that tobacco control interventions for hospitalized smokers result in sustained quits only if the interventions persist for at least 30 days after discharge.37 Providing the patient with six weeks of NRT and referring to the quitline extend the duration of treatment to 30 days or more. Thus, future studies should be powered to detect a difference at one year, perhaps with extended use of NRT.
Of note, in contrast to our earlier work, we did not find higher quit rates among smokers who self-identified a tobacco-related reason for the ED visit, or who had a tobacco-related diagnostic code for that visit. The reasons for this are unclear, but may reflect the intervention’s efficacy overwhelming any other between-group differences. The study was powered to allow inclusion of a limited number of covariates in the multivariable model. The findings imply that ED-initiated tobacco control need not be limited to these clinical subgroups, but should be offered to all adult smokers who visit the ED.
The study has limitations. It was performed at a single site. Although our low-income patients are diverse with respect to race, ethnicity, and gender, it is unclear whether our intervention would yield similar results in other populations.
The generalizability of the intervention may be limited. With dedicated non-clinical personnel, we were able to offer a comprehensive program that incorporated evidence-based treatment to promote tobacco abstinence: motivational interviewing, two forms of free NRT, proactive quitline referral, booster calls, and a brochure. A more real-world intervention that employed clinical staff might be limited to brief advice using motivational techniques, provision of literature, and a passive quitline referral, perhaps with a starter dose of NRT. Future work should focus on identification of the most effective components of the intervention, and developing strategies to implement them in real-world clinical ED environments. Use of mobile health technologies, such as texting, may offer an additional strategy to leverage the ED visit into a teachable moment for tobacco dependence treatment. In addition, electronic referral of smokers to quitlines is now possible.38
The lack of efficacy at one year is not surprising. The efficacy of tobacco dependence interventions often attenuates over time, and is greatest while the treatment is being delivered.39 Longer-term treatment may be necessary to induce sustained abstinence. It may not be reasonable to expect a relatively brief ED-initiated intervention to produce long-term cessation. In our economic analysis, we will examine the impact on abstinence rates of ongoing tobacco treatment in the outpatient setting.
A multicomponent intervention consisting of two forms of NRT, a brief motivational interview, active quitline referral, booster phone call, and quitline brochure literature yielded a higher quit rate at three months in low-income smokers than usual care. This intervention may offer a new approach for treating the difficult-to-reach population of low-income smokers. With continued implementation of the Affordable Care Act, which mandates Medicaid coverage of smoking cessation medications, and declining rates of uninsurance, initiation of tobacco dependence treatment in the ED and linkage to aftercare may be particularly timely. Given that over 20 million smokers are treated in US EDs annually, ED-based tobacco interventions represent an important opportunity to increase national rates of tobacco abstinence.
Acknowledgments
SLB conceived the study and obtained research funding. SLB, GD, SO, SB and RM designed the trial. SLB, JR, BT and MVP supervised the conduct of the trial and data collection. SLB, JR, and BT managed the data, including quality control. RM and SB provided statistical advice on study design and analyzed the data, along with SLB. SLB drafted the manuscript, and all authors contributed substantially to its revision. SLB takes responsibility for the paper as a whole.
This study was supported by grant R01CA141479 from the National Cancer Institute of the National Institutes of Health.
We wish to thank Michael C. Fiore, MD, MPH, MBA, and Edwin D. Boudreaux, PhD, for their consultative expertise, and the many research associates who contributed to this study.
Footnotes
This work was presented in partial form at the 2014 annual meeting of the Society for Research on Nicotine and Tobacco, in Seattle, WA, and the 2014 meeting of the Society for Academic Emergency Medicine, in Dallas, TX.
The authors report no conflicts of interest.
References
- 1.CDC. Current Cigarette Smoking Among Adults — United States, 2005–2012. MMWR. 2014;63:29–34. [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services. U.S. Department of Health and Human Services CfDCaP. Atlanta, GA: National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. The Health Consequences of Smoking--50 Years of Progress. A Report of the Surgeon General. [Google Scholar]
- 3.Glauser J. Rationing and the role of the emergency department as society's safety net. Acad Emerg Med. 2001;8:1101–1106. doi: 10.1111/j.1553-2712.2001.tb01123.x. [DOI] [PubMed] [Google Scholar]
- 4.Gordon JA. Emergency care as safety net. Health Affairs. 2000;19:277. doi: 10.1377/hlthaff.19.2.277-a. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein SL, Yu S, Post LA, Dziura J, Rigotti NA. Undertreatment of Tobacco Use Relative to Other Chronic Conditions. Am J Public Health. 2013;103:E59–E65. doi: 10.2105/AJPH.2012.301112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lowenstein S, Tomlinson D, Koziol-McLain J, Prochazka A. Smoking habits of emergency department patients: an opportunity for disease prevention. Acad Emerg Med. 1995;2:165–171. doi: 10.1111/j.1553-2712.1995.tb03189.x. [DOI] [PubMed] [Google Scholar]
- 7.Lowenstein SR, Koziol-McLain J, Thompson M, et al. Behavioral risk factors in emergency department patients: a multisite study. Acad Emerg Med. 1998;5:781–787. doi: 10.1111/j.1553-2712.1998.tb02504.x. [DOI] [PubMed] [Google Scholar]
- 8.National Hospital Ambulatory Medical Care Survey: 2010 Emergency Department Summary Tables. [Accessed 26 March 2013];2013 at http://www.cdc.gov/nchs/ahcd/web_tables.htm#2010. (Accessed at.
- 9.Bonnie RJ, Stratton K, Wallace R. Ending the Tobacco Problem: A Blueprint for the Nation. Washington, DC: National Academies Press; 2007. [Google Scholar]
- 10.US Department of Health and Human Services. Ending the Tobacco Epidemic. Washington, DC: 2010. [Google Scholar]
- 11.Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: US Department of Health and Human Services; 2008. [Google Scholar]
- 12.D’Onofrio G, Fiellin D, Pantalon M, et al. A Brief Intervention Reduces Hazardous and Harmful Drinking in Emergency Department Patients. Ann Emerg Med. 2012;60:181–192. doi: 10.1016/j.annemergmed.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rabe GL, Wellmann J, Bagos P, et al. Efficacy of Emergency Department–Initiated Tobacco Control—Systematic Review and Meta-analysis of Randomized Controlled Trials. Nicotine & Tobacco Research. 2013;15:643–655. doi: 10.1093/ntr/nts212. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein SL, Bijur P, Cooperman N, et al. A randomized trial of a multicomponent cessation strategy for emergency department smokers. Acad Emerg Med. 2011;18:575–583. doi: 10.1111/j.1553-2712.2011.01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2d. New York: Guilford Press; 2002. [Google Scholar]
- 16.Heatherton T, Kozlowski L, Frecker R, Rickert W, Robinson J. Measuring the heaviness of smoking using self-reported time to first cigarette of the day and number of cigarettes smoked per day. Brit J Addiction. 1989;86:1119–1127. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- 17.Whooley MA, Simon GE. Managing depression in medical outpatients. New Engl J Med. 2000;343:1942–1950. doi: 10.1056/NEJM200012283432607. [DOI] [PubMed] [Google Scholar]
- 18.Chabrol H, Niezboral M, Chastan E, Leon Jd. Comparison of the Heavy Smoking Index and of the Fagerstrom Test for Nicotine Dependence in a sample of 749 cigarette smokers. Addictive Behaviors. 2005;30:1474–1477. doi: 10.1016/j.addbeh.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Cherpitel CJ. A brief screening instrument for problem drinking in the emergency room: the RAPS4. Rapid Alcohol Problems Screen. J Stud Alcohol. 2000;61:447–449. doi: 10.15288/jsa.2000.61.447. [DOI] [PubMed] [Google Scholar]
- 20.Cherpitel CJ, Borges G. Screening for drug use disorders in the emergency department: performance of the rapid drug problems screen (RDPS) Drug & Alcohol Dependence. 2004;74:171–175. doi: 10.1016/j.drugalcdep.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Bernstein SL. The impact of smoking-related illness in the ED: an attributable risk model. American Journal of Emergency Medicine. 2002;20:161–164. doi: 10.1053/ajem.2002.32645. [DOI] [PubMed] [Google Scholar]
- 22.U S Department of Health and Human Services. US Dept. of Health and Human Services publication no. 89-8411. Rockville, MD: Public Health Service, Office on Smoking and Health; 1989. Reducing the Health Consequences of Smoking: 25 Years of Progress: A Report of the Surgeon General. [Google Scholar]
- 23.McLellan AT, Alterman AI, Cacciloa J, Metzger D, O'Brien CP. A new measure of substance abuse treatment: initial studies of the Treatment Service Review. J Nervous Mental Disease. 1992;180:101–110. doi: 10.1097/00005053-199202000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Middleton ET, Morice AH. Breath carbon monoxide as an indication of smoking habit*. Chest. 2000;117:758–763. doi: 10.1378/chest.117.3.758. [DOI] [PubMed] [Google Scholar]
- 25.Hollis JF, McAfee TA, Fellows JL, Zbikowski SM, Stark M, Riedlinger K. The effectiveness and cost effectiveness of telephone counselling and the nicotine patch in a state tobacco quitline. Tobacco Control. 2007;16:i53–i59. doi: 10.1136/tc.2006.019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katz DA, Muehlenbruch DR, Brown RL, Fiore MC, Baker TB. Effectiveness of implementing the Agency for Healthcare Research and Quality Smoking Cessation Clinical Practice Guideline: a randomized, controlled trial. J Natl Cancer Inst. 2004;96:594–603. doi: 10.1093/jnci/djh103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulz K, Altman D, Moher D, Group tC. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Medicine. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernstein SL, Boudreaux ED, Cabral L, et al. Nicotine dependence, motivation to quit, and diagnosis among adult emergency department patients who smoke: A national survey. Nicotine & Tobacco Res. 2008;10:1277–1282. doi: 10.1080/14622200802239272. [DOI] [PubMed] [Google Scholar]
- 29.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiology. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 30.Bernstein SL, Arnsten JH, Bijur PE, et al. Concurrent use of alcohol or illicit substances improves response to ED SBIRT for adult smokers. J Substance Abuse Treatment. 2013;44:139–142. doi: 10.1016/j.jsat.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abrams DB, Niaura R, Brown RA, Emmons KM, Goldstein MG, Monti PM. The Tobacco Dependence Treatment Handbook: A Guide to Best Practices. New York: Guilford Press; 2003. [Google Scholar]
- 32.Boudreaux ED, Baumann BM, Camargo CA, Jr, O'Hea E, Ziedonis DM. Changes in smoking associated with an acute health event: theoretical and practical implications. Annals of Behavioral Medicine. 2007;33:189–199. doi: 10.1007/BF02879900. [DOI] [PubMed] [Google Scholar]
- 33.West R, Sohal T. "Catastrophic" pathways to smoking cessation: findings from national survey. BMJ. 2006;332:458–460. doi: 10.1136/bmj.38723.573866.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. 2012 doi: 10.1002/14651858.CD000146.pub3. Cochrane Database of Systematic Reviews. [DOI] [PubMed] [Google Scholar]
- 35.Bernstein JA, Bernstein E, Heeren TC. Mechanisms of change in control group drinking in clinical trials of brief alcohol intervention: Implications for bias toward the null. Drug and Alcohol Review. 2010;29:498–507. doi: 10.1111/j.1465-3362.2010.00174.x. [DOI] [PubMed] [Google Scholar]
- 36.McCambridge J, Kypri K, Elbourne D. In randomization we trust? There are overlooked problems in experimenting with people in behavioral intervention trials. Journal of Clinical Epidemiology. 2014;67:247–253. doi: 10.1016/j.jclinepi.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rigotti NA, Munafo MR, Stead LF. Interventions for smoking cessation in hospitalised patients. [update of Cochrane Database Syst Rev. 2003;(1):CD001837; PMID: 12535418] 2007:CD001837. doi: 10.1002/14651858.CD001837. Cochrane Database of Systematic Reviews. [DOI] [PubMed] [Google Scholar]
- 38.Adsit R, Fox B, Tsiolis T, et al. Using the electronic health record to connect primary care patients to evidence-based telephonic tobacco quitline services: a closed-loop demonstration project. Behav Med Pract Policy Res. 2014:1–9. doi: 10.1007/s13142-014-0259-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker TB, Mermelstein R, Collins LM, et al. New Methods for Tobacco Dependence Treatment Research. ann behav med. 2011;41:192–207. doi: 10.1007/s12160-010-9252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]