Abstract
Mycobacteriosis is a bacterial disease that is common in captive, wild and research fish. There is no one causative agent of mycobacteriosis, as several strains and species of Mycobacterium have been identified in zebrafish. With increased usage and investment in wild-type and mutant zebrafish strains, considerable value is placed on preserving zebrafish health. One control measure used to prevent mycobacterial spread within and between zebrafish facilities is egg disinfection. Here we investigate the effectiveness of three disinfectants [chlorine bleach, hydrogen peroxide, and povidone iodine (PVPI)] commonly included in egg disinfection protocols for laboratory fish as well as aquaculture fish and compare the knockdown effect of these treatments on Mycobacterium spp. in vitro. Despite current usage, comparison of these disinfection regimes’ abilities to prevent mycobacterial growth has not been tested. We found that the germicidal effect of different disinfectants vary by Mycobacterium spp.. Hydrogen peroxide was the least effective disinfectant, followed by unbuffered chlorine bleach, which is commonly used to disinfect embryos in zebrafish facilities. Disinfection with 25 ppm PVPI for 5 min was very effective, and may be an improved alternative to chlorine bleach for embryo disinfection. Results from this study can be utilized by laboratory fish facilities in order to prevent the spread of mycobacteriosis in research fish.
Keywords: zebrafish, embryo disinfection, husbandry, iodine, chlorine bleach, hydrogen peroxide, mycobacteriosis, biosecurity
1.0 Introduction
Mycobacteriosis is a chronic bacterial disease caused by Mycobacterium species and is common in laboratory zebrafish colonies (Astrofsky et al., 2000; Kent et al., 2004; Kent, 2012; Whipps et al., 2012). Mycobacteria are facultative pathogens that can persist both within the host and in the environment and are readily isolated from surface biofilms (Falkinham, 2009; Falkinham et al., 2001). There is no single etiological agent for zebrafish mycobacteriosis and several species; both rapid-growing and slow-growing, of Mycobacterium have been implicated in zebrafish infections (Astrofsky et al., 2000; Kent et al., 2004; Whipps et al., 2012; Whipps et al., 2008). The manifestation of mycobacterial infections is species-specific and variable ranging from acute, severe epizootic outbreaks with significant colony mortality to chronic, low-level infections presenting no clinical signs of disease (Watral and Kent, 2007; Whipps et al., 2007a; Whipps et al., 2007b; Whipps et al., 2012). Thus, mycobacterial infections are detrimental to research when severe outbreaks cause population loss, but they are also concerning as a source of uncontrolled experimental variance in the case of chronic, low-level, sub-clinical infections (Kent et al., 2004; Whipps et al., 2012).
Control recommendations for mycobacteriosis in zebrafish colonies emphasize the importance of disease prevention through quarantine, disinfection, UV sterilization, and sentinel programs for monitoring disease (Kent et al., 2009; Whipps, 2012). Included in these recommendations is the surface disinfection of eggs through bleaching (Westerfield, 2000; Lawrence, 2007; Kent et al., 2009). Also, investigations involving the generation of gnotobiotic zebrafish include surface disinfection of embryos using immersion in bleach followed by an iodine solution (Milligan-Myhre et al., 2011). However, the efficacy of these disinfection treatments against Mycobacterium spp. from zebrafish is unknown (Whipps et al., 2012).
Disinfectant is a term that usually describes a chemical agent that prevents infection through the destruction of harmful microorganisms, but that may not eliminate bacterial spores (Block, 2001). The Centers for Disease Control and Prevention prescribes three main levels for disinfection: high-level disinfectants killing all microorganisms (including spores); intermediate-level disinfectants that kill vegetative cells, most viruses and some spores; and low-level disinfectants that kill vegetative cells (not including spores) (Garner and Favero, 1986). Many disinfectants currently used in aquaculture provide an intermediate-level of disinfection (Noga, 2010). Compared to sterilization, disinfection is a less lethal process as not all forms of life are destroyed (Block, 2001; Noga, 2010). The effectiveness of a disinfectant is specific to the infectious agent in question as their susceptibilities vary (Block, 2001). Therefore considerations should be given to the required application prior to the selection of a particular disinfectant (Block, 2001).
Mycobacteria are considered to be resistant to disinfection and they fall between vegetative bacteria and endospores in terms of their resistance to chemical disinfection and are generally susceptible to intermediate-to-high-level disinfectants (Block, 2001; Widmer and Frei, 2003). This degree of resistance can be attributed to the extremely resilient waxy mycobacterial cell wall that is highly hydrophobic (Russell, 1996). This hydrophobicity prevents hydrophilic antimicrobials and chemical disinfectants from penetrating the cell wall, protecting the mycobacteria from elimination (Russell, 1996; Block, 2001). In addition to this, biofilm formation has been shown to increase mycobacterial resistance to disinfection (Bardouniotis et al., 2003; Steed and Falkinham, 2006). Also, the susceptibility of mycobacteria in biofilms to disinfection is species specific (Russell, 1996; Block, 2001; Bardouniotis et al., 2003; Steed and Falkinham, 2006).
Chlorine bleaching of zebrafish embryos is already an established and accepted practice (Westerfield, 2000). Most zebrafish laboratories use concentrations of 25-100 ppm chlorine and dose embryos for up to 10 minutes (Westerfield, 2007; Harper and Lawrence, 2011; Kent et al., 2014). More recently, an increased chlorine concentration from 50 ppm to 100 ppm has been recommended to increase the killing of Pseudoloma neurophilia spores, another common zebrafish pathogen (Ferguson et al., 2007; Kent et al., 2014). Buffered bleach solutions have also been shown to be more effective at killing P. neurophilia (Ferguson et al., 2007); however, buffered bleach is more toxic to zebrafish embryos (Kent et al., 2014). It is not known if, like P. neurophilia, buffering bleach results in decreased mycobacterial survival compared to the currently utilized unbuffered solutions. In general, zebrafish embryo bleach disinfection involves the immersion of embryos in a 25-100 parts per million (ppm) chlorine bleach solution for up to 10 minutes followed by either rinsing in either system water (Westerfield, 2007), neutralization in sodium thiosulfate and rinsing in embryo medium (Detrich et al., 2011), or rinsing in sterile embryo media for the derivation of gnotobiotic fish (Milligan-Mhyre et al., 2011). Hydrogen peroxide is another disinfectant that is often used for embryos of other fish species, particularly in catfish, at a concentration of 250-500 ppm in both bath and flow-through treatments (Small, 2003). For Mycobacterium spp., however, a higher concentration of hydrogen peroxide seems to be necessary as recommendations for using hydrogen peroxide to disinfect Mycobacterium tuberculosis include the usage of concentrations greater than 30,000 ppm (Noga, 2010). Iodine disinfection of embryos is a widely used and accepted practice in salmonid fisheries with a recommended immersion in 100 ppm povidone-iodine (PVPI) for 10 minutes (Wood, 1979; Alaska Department of Fish and Game, 1983, 1988; United States Fish and Wildlife Service, 2004; Wagner et al., 2008). Immersion in PVPI is also used for generating gnotobiotic zebrafish at 100 pm for 2 minutes (Milligan-Mhyre et al. 2011). Despite the usage of these disinfectant regimes on fish embryos, much remains to be understood about the effectiveness of these treatments on preventing the spread of fish mycobacteriosis.
Mycobacteria are documented to be susceptible to the following chemical disinfectants: alcohols, aldehydes, some alkalis, halogens (including chlorine and iodine compounds), some peroxygen compounds and some phenols (Block, 2001; Widmer and Frei, 2003; Noga, 2010). Because most investigations into the effectiveness of chemical disinfectants on mycobacteria are clinically oriented, information specific to the Mycobacterium spp. found in zebrafish facilities is limited to studies investigating Mycobacterium marinum and Mycobacterium fortuitum (Bardouniotis et al., 2003). Therefore, more information is needed regarding the susceptibility of zebrafish mycobacteria to disinfection and the efficacy of currently utilized disinfection practices at preventing mycobacterial spread.
The aim of this study was to investigate the susceptibility of several Mycobacterium spp. isolated from zebrafish research facilities in the United States to chemical disinfection in vitro. Chemical disinfection regimes were chosen with a focus on methods already utilized within the fish community for egg disinfection. We hypothesize that Mycobacterium spp. will show differential susceptibility to different disinfectants and there will be species-specific susceptibilities similar to what has been previously shown in literature for non-zebrafish mycobacteria.
2.0 Methods
2.1 Bacterial culture and growth media
Isolates of Mycobacterium abscessus, Mycobacterium chelonae, Mycobacterium gordonae, and Mycobacterium peregrinum maintained in our culture collection were used. All were isolated from zebrafish facilities in the United States and were previously identified based on hsp65 gene sequencing as previously described (Kent et al. 2004; Poort et al. 2006; Whipps et al. 2007a; Whipps et al. 2007b). All isolates were grown at 28-30°C for seven days on solid-phase Middlebrook 7H10 (MB 7H10) agar (BD Biosciences 262710) supplemented with oleic albumin dextrose catalase (OADC, BD Biosciences 211886), prior to preparation for disinfection treatments.
2.2 Disinfection treatment and analysis
Chlorine Bleach (unbuffered)
We treated Mycobacterium spp. isolates with chlorine bleach at concentrations of 100 and 150 ppm chlorine bleach (Clorox®) for 10 minutes. Isolated M. abscessus, M. chelonae, M. gordonae, and M. peregrinum colonies were individually inoculated with a sterile loop into sterile culture tubes containing 3 ml of 100% Middlebrook 7H9 broth and incubated for 7 days at 30°C and 300 rpm. Following incubation, broth culture was diluted in sterile water to reach a concentration of 105 colony forming units (CFU) per milliliter measured using a nephelometer (Sensititre). Chlorine bleach treatment solutions (100 ppm, 150 ppm, control) were prepared by diluting Clorox® bleach in autoclaved Milli-Q® filtered water to a 1000 ppm concentration. Chlorine concentration was verified using a chlorine meter (Extech CL200). For each treatment, 1.0 mL of diluted broth culture was added to a sterile 2 ml Eppendorf tube and 1000 ppm chlorine bleach in Milli-Q® was added to bring treatment solutions to a final concentration of 100 ppm, 150 ppm chlorine bleach. For the control, sterile Milli-Q water was added to the broth culture. The pH of the treatment solution was measured before and after treatment. Tubes were incubated for 10 minutes at room temperature with gentle mixing. Following exposure time, an equal volume of 1% sodium thiosulfate (Na2HPO4, Fisher Scientific S-446) made in autoclaved Milli-Q® water was added to neutralize the chlorine with gentle mixing. Neutralizing activity of the sodium thiosulfate was confirmed using a chlorine meter. Following neutralization, serial dilutions of these solutions were prepared at 100, 10−1, 10−2 for treatment tubes and 10−3, 10−4, and 10−5 for control tubes. Finally, 100 μl of each dilution was plated onto MB 7H10 agar plates in triplicate using a sterile spreader. Plates were then incubated at 28°C for 7 days and colonies were counted. This experiment was repeated two more times.
Chlorine Bleach (buffered)
In addition to the chlorine experiment described above, we conducted another trial of chlorine disinfection in order to compare disinfection with buffered and unbuffered bleach. Cultures were prepared to 105 CFU/ml as above. For each treatment, 1 ml of this diluted culture was added to a sterile 2.0 ml tube and centrifuged to form a pellet. Following centrifugation the supernatant was removed and was replaced with 1 mL of treatment solutions [100 ppm, 150 ppm, or 0 ppm chlorine bleach prepared in autoclaved chlorine demand free buffer (CDFB), prepared by mixing 42% 0.05M KH2PO4, and 58% 0.05M Na2HPO4, or in autoclaved Milli-Q® water as a control for comparison]. The same inoculating cultures were used for both treatments. M. chelonae and M. peregrinum cultures were used for this second trial for 5 and 10 minute exposure times at 100 ppm or 150 ppm concentrations for both buffers. Following exposures, neutralization, serial dilution and plating were carried out as in the previously described trial. This experiment was then repeated two more times.
Hydrogen peroxide
We treated Mycobacterium spp. isolates to 15,000 ppm and 30,000 ppm hydrogen peroxide (H2O2, drugstore brand) for 5 minutes. Cultures were prepared to 105 CFU/ml as above. For each treatment, 1 ml of this diluted culture was added to a sterile 1.5 ml tube and centrifuged to form a pellet. Following centrifugation the supernatant was removed and was replaced with 1 ml of freshly made hydrogen peroxide (H2O2, drugstore brand) treatment solution (15,000 ppm or 30,000 ppm) or an equal volume of sterile water for the control treatment. Hydrogen peroxide treatment solutions were undiluted (30,000 ppm) and diluted with sterile water (15,000 ppm). Treatment tubes were vortexed to break pellet apart and tubes with incubated for 5 minutes at room temperature in the dark. Following incubation tubes were centrifuged again to re-pellet cells and the treatment/control solutions were replaced with 1 ml of sterile water. Tubes were vortexed to resuspend cells and serial dilutions of 10−7, 10−6, 10−5, 10−4, and 10−3 were prepared and 100 μl of each dilution was plated onto MB 7H10 in triplicate. These plates were incubated at 28°C for 7 days and colonies were counted. This experiment was repeated two more times.
Iodine
We treated Mycobacterium spp. isolates to 12.5-100 ppm PVPI for 5 minutes. Cultures were prepared to 105 CFU/ml. For each treatment, 1 ml of this diluted culture was pelleted in a sterile 1.5 ml tube. Following centrifugation the supernatant was removed and was replaced with 1 ml of freshly made iodine treatment solution made in sterile Milli-Q® water. Initially, M. chelonae was tested at four concentrations (12.5 ppm, 25 ppm, 50 ppm, or 100 ppm) of PVPI (10%, drugstore brand). Following this trial, 25 ppm PVPI was chosen as the optimal treatment to be tested on M. abscessus, M. gordonae, and M. peregrinum. Control treatments consisted of an equal volume of sterile water. All treatment solution concentrations were verified using iodine test paper (LaMotte). Treatment tubes were vortexed to break pellet apart and tubes with incubated for 5 minutes at room temperature in the dark. Following incubation tubes were centrifuged again to re-pellet cells and the treatment/control solutions were replaced with 1 ml of sterile water. Tubes were vortexed to resuspend cells and serial dilutions of 10−7, 10−6, 10−5, 10−4, and 10−3 were prepared, and 100 μl of each dilution was plated onto MB 7H10 agar plates in triplicate. Plates were incubated at 28°C for 7 days and colony counts conducted. This experiment was repeated two more times.
2.3 Statistics
The following analysis was carried out to compare differences between species for the same disinfectant treatment and also to compare different disinfectant treatments for each species separately. The same statistical method was utilized for all analyses using R 3.1.0 (R Core Team, 2013) and R Studio (R Studio, 2012). Colony count data were entered into a spread sheet where percent survival for each treatment was determined by comparing treatment counts (CFU/mL) to control counts (CFU/mL); Percent Survival = [(Treatment Count/Control Average Count )*100]. Data were then sorted by treatment or species and saved as individual text files for analysis. For each data file descriptive statistics were obtained using the “psych” package (Revelle, 2014). Data were also checked for normality equal variances using the “stats” package (R Core Team, 2013) and “car” package (Fox and Weisberg, 2011) respectively. Since all data sets were found to have non-normal distributions (p<0.05) and unequal variances (p<0.05) the non-parametric a Kruskal-Wallis rank sum test was used to compare percent survival values using the “stats” package (R Core Team, 2013). In the case of a significant result, indicating differences between disinfection treatments, post-hoc tests for pairwise multiple comparisons of the ranked data was performed using the “PMCMR” package (Pohlert, 2015). Data were then visualized as a clustered bar graph using the “sciplot” package (Morales et al., 2012).
3.0 Results
3.1 Chlorine bleach disinfection
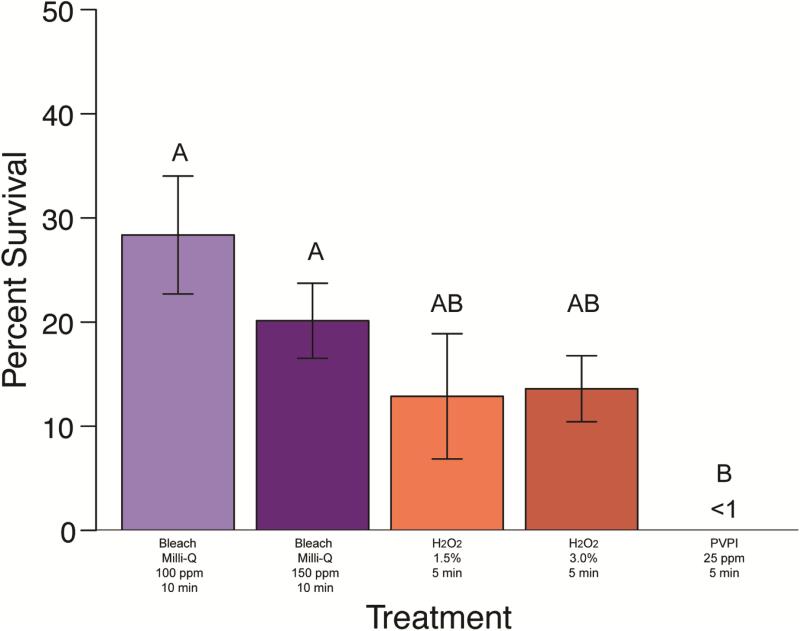
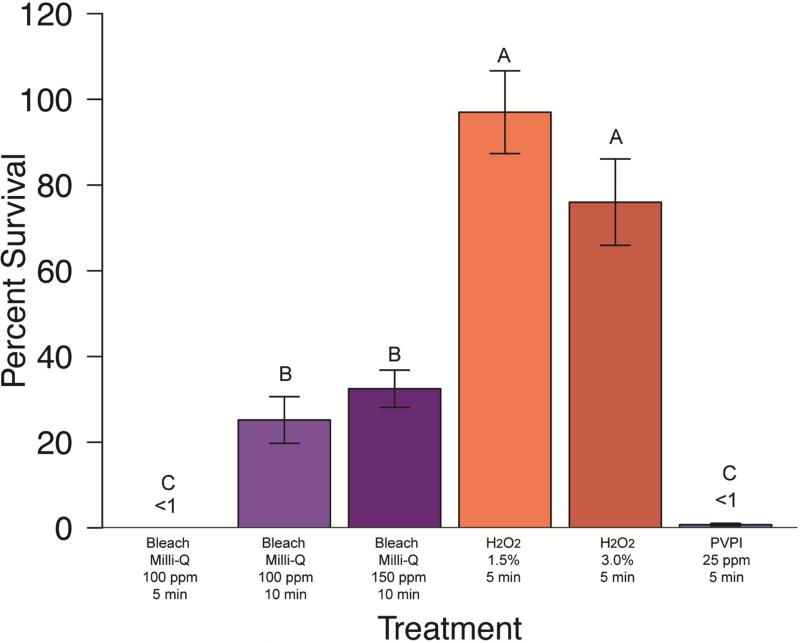
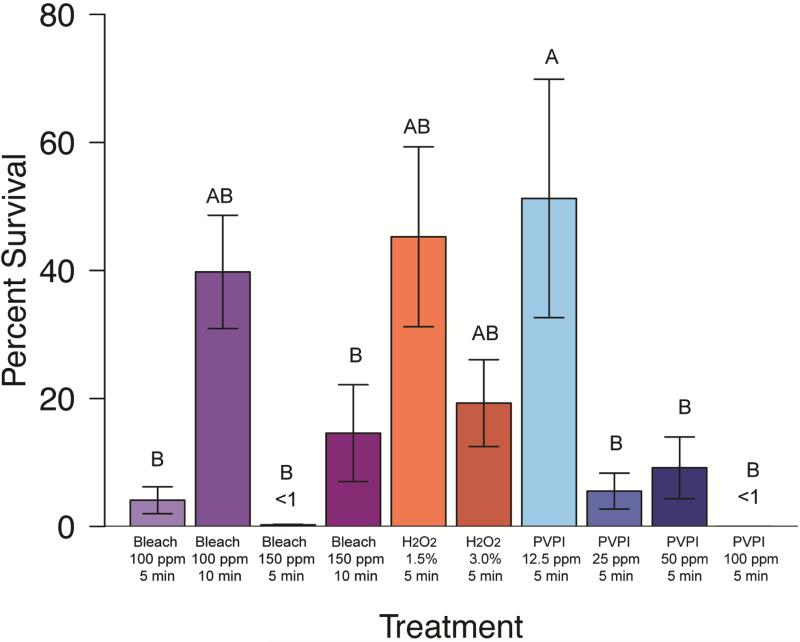
Treatment of M. abscessus, M. chelonae, M. gordonae, and M. pergrinum with 100ppm Clorox® chlorine bleach diluted in Milli-Q® water resulted in a minimum average survival of 2.94% for M. gordonae, followed by18.89% for M. peregrinum. Average survival of M. abscessus and M. chelonae was 27-40% (Fig 1-4). There was no significant difference between species for this treatment (p>0.05). Treatment with 150 ppm Clorox® chlorine bleach diluted in Milli-Q® water resulted in a similar trend in survival with a minimum average survival of less than 1% for M. gordonae. Mycobacterium chelonae had an average survival of 14.59%, M. abscessus had a 20.21% average survival, and M. peregrinum showed the greatest survival at 32.47%. Unbuffered treatment solution pH values are as follows: 100 ppm solutions ranged from 11.67-14.10, 150 ppm ranged from 7.98-9.81, and control solutions ranged from 9.0-10.60. The difference in average survival between species was significant (Kruskal-Wallis; χ2(3)=35.5966, p<0.0001) with M. abscessus and M. peregrinum equivalent to each other, with M. peregrinum having significantly higher survival than M. chelonae and M. gordonae, but M. abscessus only having higher survival over M. chelonae. Mycobacterium gordonae was significantly different from M. chelonae as well as M. peregrinum (p<0.05). When comparing 100 ppm and 150 ppm chlorine bleach to one another by species, there was no significant difference between concentrations (p<0.05) (Figures 1-4).
Figure 1. Mycobacterium abscessus percent survival effect of disinfectant treatments.
(Clorox® bleach in Milli-Q®, hydrogen peroxide, and PVP-I). Group labels (A or B) identify treatments that differ significantly [χ2(4)=33.38, p < 0.05]. Treatments resulting in survival values less than 1% are indicated (<1).
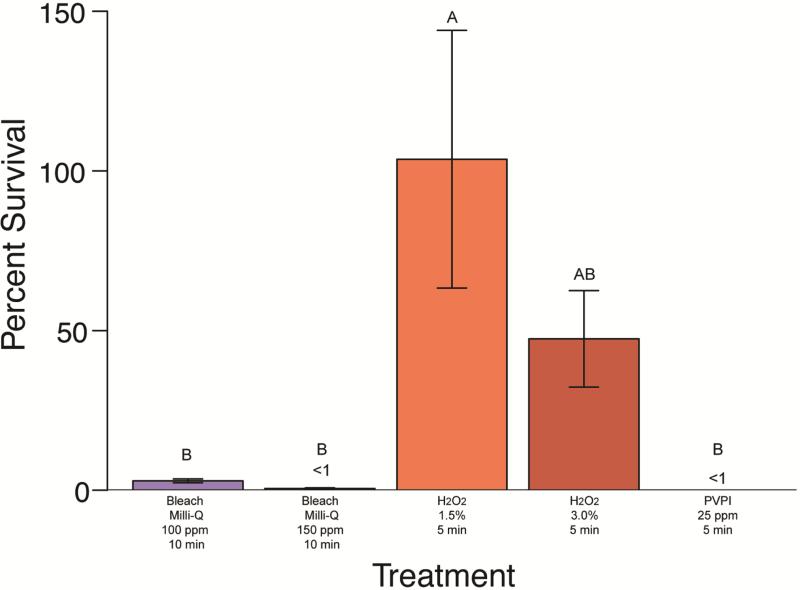
Figure 4. Mycobacterium peregrinum percent survival effect of disinfectant treatments.
(Clorox® bleach in Milli-Q®, hydrogen peroxide, and PVP-I). Group labels (A, B, or C) identify treatments that differ significantly [χ2(7)=67.23, p < 0.05]. Treatments resulting in survival values less than 1% are indicated (<1).
Additional Clorox® chlorine bleach treatment trials were completed using a CDFB as the diluent with M. chelonae and M. peregrinum, and compared to chlorine bleach diluted in Milli-Q® water. For M. chelonae bleach disinfection, there were significant differences between treatments (Kruskal-Wallis; χ2(7)=48.3911, p<0.0001), specifically, the 10 minute treatments in Milli-Q® were different from all others (Figure 2, significantly different groupings are indicated by group labels, p<0.05). Trials with CDFB resulted in less than 1% average survival for all concentration and treatment durations (not shown); whereas, only 150 ppm Clorox® chlorine bleach diluted in Milli-Q® resulted in this degree of knock-down (Figure 2). Trials with M. peregrinum resulted in similar outcomes (Figure 4); with less than 1% average survival for CDFB trails (not shown). For comparison, less than 1% average survival of M. peregrinum following unbuffered Clorox® chlorine bleach treatment was only observed for 5 minute, 100 ppm treatments (Figure 4). Buffered treatment solution pH values are as follows: 100 ppm solutions ranged from 5.07-5.1, 150 ppm ranged from 4.90-5.07, and control solutions ranged from 9.0-10.60.
Figure 2. Mycobacterium chelonae percent survival effect of disinfectant treatments.
(Clorox® bleach in Milli-Q®, hydrogen peroxide, and PVPI). Group labels (A or B) identify treatments that differ significantly [χ2(13)=99.55, p < 0.05]. Treatments resulting in survival values less than 1% are indicated (<1).
3.2 Hydrogen peroxide disinfection
Treatment of M. abscessus, M. chelonae, M. gordonae, and M. peregrinum with 15,000 ppm hydrogen peroxide resulted in a minimum average survival of 12.87% for M. abscessus, 45.26% survival for M. chelonae and 100% or more survival for M. gordonae and M. peregrinum (Figures 1-4). This treatment did differ significantly in its effectiveness as significant differences in mycobacterial survival are observed between species (Kruskal-Wallis; χ2(3)=12.0656, p<0.05). Post-hoc comparisons revealed that M. abscessus and M. chelonae had a significantly lower survival than M. peregrinum (p<0.01). Results for 30,000 ppm hydrogen peroxide were similar to the lower 15,000 ppm treatment (Figures 1-4). Mycobacterium abscessus had the lowest resulting average survival of 13.60%, followed by M. chelonae with 19.28% resulting average survival. Average survival of M. gordonae was 47.41%, and M. peregrinum had the highest resulting average survival of 76.01%. This treatment did differ significantly in its effectiveness between species (Kruskal-Wallis; χ2(3)=10.2262, p<0.01). Post-hoc comparisons revealed that M. abscessus had a significantly lower survival than M. peregrinum (p<0.01). When comparing these two treatment concentrations to one another by species, there was no significant difference between concentrations (p<0.05) (Figures 1-4).
3.3 Iodine disinfection
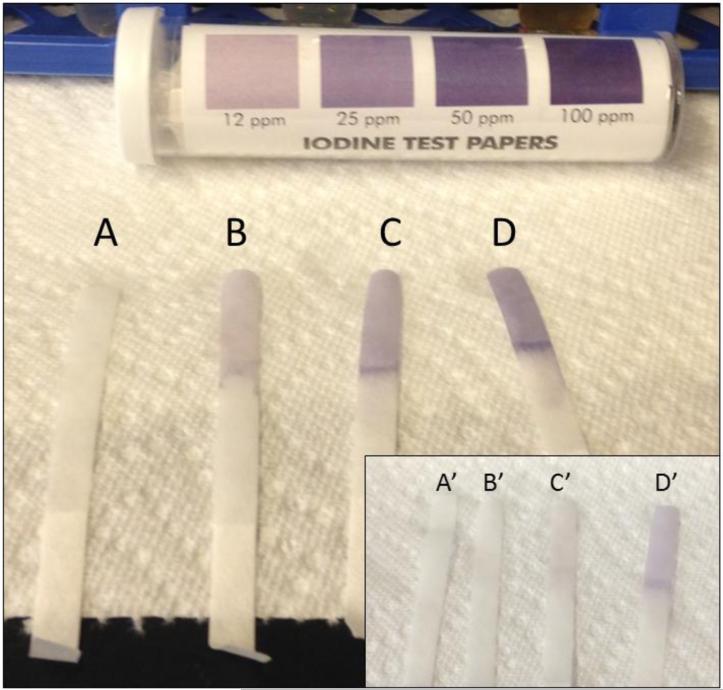
Initial PVPI disinfection treatments were tested on M. chelonae at a range of concentrations (12.5 ppm, 25 ppm, 50 ppm, and 100 ppm) for 5 minutes. Percent survival for treatments were significantly different between treatment concentration (Kruskal-Wallis; χ2(3)=32.5721, p<0.0001). Post-hoc comparisons revealed that there was no significant difference between the 25-100 ppm treatments (average survival less than 1% ); however, the 12.5 ppm concentration resulted in a significantly higher percent survival (average survival of 51.25%) of M. chelonae (p<0.05). Further PVPI testing was narrowed down to testing 25 ppm treatment as this was the lowest concentration found to be equally as effective as 50 and 100 ppm. Treatment of M. abscessus, M. chelonae, M. gordonae, and M. pergrinum with 25 ppm PVPI resulted in all species having less than 10% survival (Figures 1-4), with M. chelonae having the most survival at an average of 5.53%±18.79%. There was no significant difference found between species (Kruskal-Wallis; χ2(3)=8.25, p=0.05). Prepared iodine solutions were evaluated for iodine concentration using iodine test strips immediately after they were prepared and used in these trials, as well as 24 hours later. There was a noticeable decrease in concentration over time (Figure 5).
Figure 5.
Iodine test strips verifying the concentration of (A) 0 ppm, (B) 12.5 ppm, (C) 25 ppm, and (D) 50 ppm PVP-I solutions immediately following solution preparation. The concentration of the same solutions 24h later (A’, B’, C’, D’) has decreased and solutions are no longer usable.
4.0 Discussion
Our study has demonstrated that not all disinfectants are effective at preventing mycobacterial growth and that species-specific differences in susceptibility exist. The standard procedure for zebrafish embryo disinfection is currently bleaching in a 25-100 ppm bleach solution prepared in system water for a total of 10 minutes (Westerfield, 2000). We found that this method (100 ppm bleach in Milli-Q®) results in a decrease in mycobacterial survival; however, an average survival of 2.94-28.36% was observed and this was significantly variable between Mycobacterium spp. tested. This mycobacterial persistence post-bleaching emphasized the need for alternative disinfectant treatments to be considered. Additionally, because effectiveness varied between species, control and management recommendations are complicated as generally diagnostics are not performed prior to preventative measures. We did test bleaching further, this time using a CDFB as a diluent. We found that using a demand-free buffer does increase the effectiveness of bleach disinfection significantly, resulting in less than 1% mycobacterial survival. The difference between these two bleach treatments was not surprising as it has been previously shown that the germicidal properties of chlorine are reduced as pH increases above 7.5, and the toxicity of bleach to microorganisms doubles as pH changes from 9 to 7 (Clark et al. 1989; Health Canada 2004). A shift in pH from 7 to 9 results in a drastic decrease of the germicidal form of chlorine, hypochlorous acid (HOCl), as chlorine exists predominantly in the less-active hypochlorite (OCl−) form (Clark et al. 1989). Important considerations when assaying chlorine compounds is to make sure reaction buffers are rendered chlorine-demand free. Otherwise, HOCl can react with the buffer and subsequently decrease the amount available to reach with target molecules (Pizzi, 2002). For our experimental trials, the pH of the bleaching solution made in Milli-Q® water ranged from 7.98 to 14.10 during the treatment; whereas, the bleach solution prepared in CDFB was 4.9-5.1. This difference in pH and subsequent effect on chlorine availability explains the difference in germicidal activity on the Mycobacterium spp. tested. Despite this increased effectiveness, recommendations for using this buffered bleach treatment for embryos may not be ideal. First, this protocol for preparing the CDFB bleach solution would not be practical with the large-scale and frequent treatments used in many zebrafish facilities (Kent et al. 2014). Second, buffered chlorine bleach treatments on zebrafish embryos were previously shown to be more toxic to embryos corresponding to higher mortality and malformations than unbuffered (and currently utilized) protocols (Kent et al. 2014). Finally, throughout this study we experienced preparation of the bleaching solutions to be quite involved, requiring a chlorine meter to determine chlorine concentrations as calculations based on the concentration of chlorine in Clorox® resulted in solutions with varying amounts of actual measured chlorine. Due to this variability a more reliable and straight-forward treatment should be considered.
We then considered hydrogen peroxide disinfection as a candidate for preventing mycobacterial spread. This disinfectant is already used for controlling pathogens in other aquatic species (e.g., catfish) and is regularly used in bath and flow-through set-ups to treat eggs at a concentration of 250-500 ppm (Small, 2003). Recommendations for Mycobacterium spp. include the usage of a much more concentrated solution of 30,000 ppm hydrogen peroxide (Noga, 2010). We tested hydrogen peroxide at both 15,000 ppm and 30,000 for 5 minute treatments and found both treatments resulted in very little bacterial killing for all species of Mycobacterium tested (Figures 1-4). A longer duration of treatment may result in an increased germicidal effect, as bath treatments used in other fisheries are longer than five minutes (Small, 2003). However, germicidal effect was poor even at very high concentrations which are unlikely to be safe for fish, and we do not recommend hydrogen peroxide disinfection as an alternative to bleach.
Finally, we considered iodophor disinfection using PVPI. Iodine is already an established embryo disinfectant in salmonid culture (Wood 1979, Game 1983, Game 1988, Service 2004, Wagner, Arndt et al. 2008). We first tested PVPI disinfection on M. chelonae, a frequent zebrafish pathogen, at multiple concentrations (12.5 ppm, 25 ppm, 50 ppm, and 100 ppm) for a 5 minute duration. We found 25-100 ppm treatments resulted in a significant decrease of M. chelonae survival as well as no significant difference between these treatment concentrations. We then choose 25 ppm as the concentration to test additional Mycobacterium spp. as it was the lowest concentration with a significant effect on bacterial survival. This treatment was also effective for M. abscessus, M. gordonae and M. peregrinum resulting in less than 1% average survival for all of these species. This treatment is comparable to the buffered chlorine bleach treatment, but requires much less preparation. During these trials we found that preparing PVPI disinfection solutions was straight-forward and concentration calculated from the original solution consistently produced treatment solution concentrations, verified by iodine test strips. Importantly, we did find that these PVPI treatment solutions need to be prepared shortly before treatment, as the concentration of iodine in these solutions decreased over time (Figure 5). We recommend making stock solutions immediately prior to use and not to be stored longer than a day. Results from the PVPI disinfection experiments identify iodophor disinfection at 25 ppm for 5 minutes as an effetive alternative from chlorine bleach for killing mycobacteria from zebrafish. Toxicity of this disinfection treatment on zebrafish embryos is still unknown but is currently under investigation.
Additionally, many factors influence the effectiveness of disinfectants including temperature, time of contact, pH, concentration as well as the presence of organic matter (Mainous 2005). It is important to consider these factors. For example, embryos should be rinsed well to remove excess organic matter prior to disinfection. Rinsing solutions should be free of pathogens as rinsing with a contaminated solution following embryo disinfection could negate the efforts of disinfection. Adequate storage and preparation of disinfectants is important in order to ensure germicidal activity. Depending on the environmental conditions (e.g., temperature, lighting) within a zebrafish facility, long-term storage of disinfectants may not be appropriate and alternative storage is necessary (e.g., refrigeration) in order to ensure disinfectant integrity. Also, as previously discussed, working solutions of disinfectants should be prepared shortly prior to use and concentrations verified. As successful as these treatments are at preventing the spread of microorganisms, they will not inhibit intraovum pathogens (e.g., Pseudoloma neurophilia) (Sanders and Kent, 2013). Additional disease prevention and monitoring meaures should be used in addition to regular embryo disinfection.
The usage of disinfectants in zebrafish facilties is an important disease control measure that all facilities should consider, especially when introducing embryos from an outside faciltity. Here we showed that the germicidal effect of different disinfectants on Mycobacterium spp. varies by species, and that the currently used unbuffered chlorine bleach does have a germicidal effect, but 25 ppm PVPI for 5 min may be a improved alternative, once in vivo testing determines it is safe for embryos.
Figure 3. Mycobacterium gordonae percent survival effect of disinfectant treatments.
(Clorox® bleach in Milli-Q®, hydrogen peroxide, and PVP-I). Group labels (A or B) identify treatments that differ significantly [χ2(4)=17.71, p < 0.05]. Treatments resulting in survival values less than 1% are indicated (<1).
Acknowledgements
This research was funded in part by the Office of Research Infrastructure Programs of the National Institutes of Health (NIH) under a subcontract of the award number R24OD010998 to CMW. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We thank members of the Whipps Fish and Wildlife Disease Lab for their ongoing support, especially undergraduate research students, Team Chen, Kristen Doerr and Elle Palmer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alaska Department of Fish and Game . Fish culture manual. Juneau; Alaska: 1983. [Google Scholar]
- Alaska Department of Fish and Game . Safer chemical use in Alaskan aqauculture. Juneau; Alaska: 1988. [Google Scholar]
- Astrofsky KM, Schrenzel MD, Bullis RA, Smolowitz RM, Fox JG. Diagnosis and management of atypical Mycobacterium spp. infections in established laboratory zebrafish (Brachydanio rerio) facilities. Comparative Medicine. 2000;50:666–672. [PubMed] [Google Scholar]
- Bardouniotis E, Ceri H, Olson ME. Biofilm formation and biocide susceptibility testing of Mycobacterium fortuitum and Mycobacterium marinum. Current Microbiology. 2003;46:28–32. doi: 10.1007/s00284-002-3796-4. [DOI] [PubMed] [Google Scholar]
- Block SS. Disinfection, Sterilization, and Preservation. 5th ed. Lippincott Williams & Wilkins; Philadelphia: 2001. [Google Scholar]
- Clark RM, Eleanor RJ, Hoff J. Analysis of inactivation of Giardia lamblia by chlorine. J. Environ. Eng. 1989;115:80–90. [Google Scholar]
- Detrich HW, III, Westerfield M, Zon LI. The zebrafish: genetics genomics and informatics 3rd Ed. Methods in Cell Biology. 2011;104:471–473. [Google Scholar]
- Falkinham JO., 3rd Surrounded by mycobacteria: nontuberculous mycobacteria in the human environment. Journal of Applied Microbiology. 2009;107:356–367. doi: 10.1111/j.1365-2672.2009.04161.x. [DOI] [PubMed] [Google Scholar]
- Falkinham JO, 3rd, Norton CD, LeChevallier MW. Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other mycobacteria in drinking water distribution systems. Applied Environmental Microbiology. 2001;67:1225–1231. doi: 10.1128/AEM.67.3.1225-1231.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JA, Watral V, Schwindt AR, Kent ML. Spores of two fish microsporidia (Pseudoloma neurophilia and Glugea anomola) are highly resistant to chlorine. Diseases of Aquatic Organisms. 2007;76:205–214. doi: 10.3354/dao076205. [DOI] [PubMed] [Google Scholar]
- Fox J, Weisberg S. An {R} Companion to Applied Regression. Sage; Thousand Oaks, CA, USA.: 2011. [Google Scholar]
- Garner JS, Favero MS. Cdc Guideline for Handwashing and Hospital Environmental-Control, 1985. Infection Control and Hospital Epidemiology. 1986;7:231–243. doi: 10.1017/s0195941700084022. [DOI] [PubMed] [Google Scholar]
- Harper C, Lawerence C. The laboratory zebrafish. CRC Press; Boca Raton, FL.: 2011. [Google Scholar]
- Health Canada . Guidelines for Canadian drinking water quality: supporting documentation - Protozoa: Giardia and Cryptosporidium. Water Quality and Health Bureau, Healthy Environments and Consumer Safety Branch, Health Canada; Ottawa: 2004. [Google Scholar]
- Kent ML, Feist SW, Harper C, Hoogstraten-Miller S, Law JM, Sanchez-Morgado JM, Tanguay RL, Sanders GE, Spitsbergen JM, Whipps CM. Recommendations for control of pathogens and infectious diseases in fish research facilities. Comparative Biochemistry and Physiology. Toxicology & Pharmacology : CBP. 2009;149:240–248. doi: 10.1016/j.cbpc.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent ML, Whipps CM, Matthews JL, Florio D, Watral V, Bishop-Stewart JK, Poort M, Bermudez L. Mycobacteriosis in zebrafish (Danio rerio) research facilities. Comparative Biochemistry and Physiology. Toxicology & Pharmacology : CBP. 2004;138:383–390. doi: 10.1016/j.cca.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Kent MLS, J. M., Matthews JM, Fournie JW, Murray KN, Westerfield M. Diseases of Zebrafish in Research Facilities. Zebrafish International Resource Center; 2012. [Google Scholar]
- Kent MLS, Buchner C, Barton C, Tanguay RL. Toxicity of chlorine to zebrafish embryos. Diseases of Aquatic Organisms. 2014;107:235–240. doi: 10.3354/dao02683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C. The husbandry of zebrafish (Danio rerio): A review. Aquaculture. 2007;269:1–20. [Google Scholar]
- Mainous ME, Smith SA. Efficacy of Common Disinfectants against Mycobacterium marinum. Journal of Aquatic Animal Health. 2005;17:284–288. [Google Scholar]
- Milligan-Myhre K, Charette JR, Phennicie RT, Stephens WZ, Rawls JF, Guillemin K, Kim CH. Study of Host-Microbe Interactions in Zebrafish. Methods in Cell Biology. 2011;105:87–116. doi: 10.1016/B978-0-12-381320-6.00004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M. with code developed by the R Development Core Team, with general advice from the R-help listserv community and especially Duncan Murdoch, 2012. sciplot: Scientific Graphing Functions for Factorial Designs. R package version 1.1-0. ed [Google Scholar]
- Noga EJ. Fish Disease: Diagnosis and Treatment. 2 ed. Wiley-Blackwell; Iowa, USA: 2010. [Google Scholar]
- Pizzi N. Water Treatment Operator Handbook. 2nd. American Water Works Association. 2002.
- Pohlert T. PMCMR: Calculate Pairwise Multiple Comparisons of Mean Rank Sums., 1.1 ed. R package. 2015 [Google Scholar]
- Poort MJ, Whipps CM, Watral VG, Font WF, Kent ML. Molecular characterization of Mycobacterium species in non-native poeciliids in Hawaii using DNA sequences. Journal of Fish Diseases. 2006;29(3):181–185. doi: 10.1111/j.1365-2761.2006.00687.x. [DOI] [PubMed] [Google Scholar]
- R Core Team . R: A language and environment for statistical computing. . R Foundation for Statistical Computing. Vienna, Austria: 2013. [Google Scholar]
- R Studio . R Studio: Integrated development environment for R., 0.96.122 ed. R Studio; Boston, MA, USA: 2012. [Google Scholar]
- Revelle W. psych: Procedures for Personality and Psychological Research, Version = 1.4.8 ed. Northwestern University; Evanston, Illinois, USA: 2014. [Google Scholar]
- Russell AD. Activity of biocides against mycobacteria. Journal of Applied Bacteriology. 1996;81:S87–S101. [PubMed] [Google Scholar]
- Sanders JL, Kent ML. Verification of intraovum transmission of microsporidium of vertebrates: Pseudoloma neurophilia infecting zebrafish, Danio rerio. PLoS ONE. 2013;8:e76064. doi: 10.1371/journal.pone.0076064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small BC, Wolters WR. Hydrogen peroxide treatment during egg incubation improves channel catfish hatching success. North American Journal of Aquaculture. 2003;65:314–317. [Google Scholar]
- Steed KA, Falkinham JO., 3rd Effect of growth in biofilms on chlorine susceptibility of Mycobacterium avium and Mycobacterium intracellulare. Applied Environmental Microbiology. 2006;72:4007–4011. doi: 10.1128/AEM.02573-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Fish and Wildlife Service Iodophor Disinfection of Fish Eggs, Handbook of Aquatic Animal Health Procedures and Protocols. United States Fish and Wildlife Service. 2004:1–7. [Google Scholar]
- Wagner EJ, Arndt RE, Billman EJ, Forest A, Cavender W. Comparison of the efficacy of iodine, formalin, salt, and hydrogen peroxide for control of external bacteria on rainbow trout eggs. North American Journal of Aquaculture. 2008;70:118–127. [Google Scholar]
- Watral V, Kent ML. Pathogenesis of Mycobacterium spp. in zebrafish (Danio rerio) from research facilities. Comparative Biochemistry and Physiology. Toxicology & Pharmacology : CBP. 2007;145:55–60. doi: 10.1016/j.cbpc.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio) 4th ed. University of Oregon Press; Eugene: 2000. [Google Scholar]
- Westerfield M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio) 5th ed. University of Oregon Press; Eugene: 2007. [Google Scholar]
- Whipps CM, Butler WR, Pourahmad F, Watral VG, Kent ML. Molecular systematics support the revival of Mycobacterium salmoniphilum (ex Ross 1960) sp nov., nom. rev., a species closely related to Mycobacterium chelonae. International Journal of Systematic and Evolutionary Microbiology. 2007a;57:2525–2531. doi: 10.1099/ijs.0.64841-0. [DOI] [PubMed] [Google Scholar]
- Whipps CM, Dougan ST, Kent ML. Mycobacterium haemophilum infections of zebrafish (Danio rerio) in research facilities. FEMS microbiology letters. 2007b;270:21–26. doi: 10.1111/j.1574-6968.2007.00671.x. [DOI] [PubMed] [Google Scholar]
- Whipps CM, Lieggi C, Wagner R. Mycobacteriosis in Zebrafish Colonies. Ilar J. 2012;53:95–105. doi: 10.1093/ilar.53.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipps CM, Matthews JL, Kent ML. Distribution and genetic characterization of Mycobacterium chelonae in laboratory zebrafish Danio rerio. Diseases of Aquatic Organisms. 2008;82:45–54. doi: 10.3354/dao01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer AF, Frei R. Decontamination, Disinfection, and Sterilization. In: Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Yolken RH, editors. Manual of Clinical Microbiology. 8th ed. ASM Press; Washington, DC, USA: 2003. pp. 77–108. [Google Scholar]
- Wood JW. Diseases of Pacific Salmon: Their Prevention and Treatment. 3rd ed. State of Washington Department of Fisheries, Hatchery Division; 1979. [Google Scholar]