Abstract
Background
As of 2014, there were approximately 8300 patients with a functioning liver transplant in the UK Transplant Registry, with 880 liver transplants performed in 2013–2014 alone. Tacrolimus, typically used in combination with steroids and mycophenolate mofetil, currently represents the cornerstone of post-transplant immunosuppression in liver transplant recipients.
Objectives
The objective of the present study was to evaluate the cost-effectiveness of prolonged-release (PR) tacrolimus (Advagraf®, Astellas Pharma Inc., Tokyo, Japan) versus branded immediate-release (IR) tacrolimus (Prograf®, Astellas Pharma Inc., Tokyo, Japan) in liver transplant recipients in the UK.
Methods
A model was developed in Microsoft Excel to estimate costs associated with immunosuppressive medications and retransplantation. Three-year patient and graft survival data were taken from a recent retrospective registry analysis and dose data were taken from prescribing information. Costs in 2014 pounds sterling were taken from the British National Formulary and the National Health Service National Tariff.
Results
Over a 3-year time horizon, the numbers needed to treat with PR tacrolimus relative to IR tacrolimus were 14 to avoid one graft loss and 18 to avoid one death. The model was sensitive to dosing assumptions, with incremental cost estimates varying between a saving of £1642 (standard deviation £885) per patient, assuming the same per-kilogram dosing of PR tacrolimus (Advagraf®) and IR tacrolimus (Prograf®) and an increase of £1350 (£964) using RCT dose data.
Conclusion
Data from a recent analysis of routine clinical practice data in liver transplant recipients on PR tacrolimus and IR tacrolimus showed significant differences in long-term graft survival in favor of PR tacrolimus. Modeling these data in the UK showed that, over a 3-year time horizon, one graft would be saved for every 14 patients treated with PR tacrolimus with minimal impact on costs when compared with branded IR tacrolimus (Prograf®).
Key Points
| Recent data from routine clinical practice shows that once-daily prolonged-release formulations of tacrolimus result in improved graft survival in liver transplant recipients relative to twice-daily immediate-release tacrolimus. |
| Based on these data, a model was constructed to estimate life expectancy, numbers needed to treat to avoid graft failure and death, and costs associated with immunosuppressive medications and graft failure over 3 years after transplantation. |
| While model outcomes were sensitive to tacrolimus dosing assumptions, prolonged-release tacrolimus (Advagraf®) resulted in improved patient and graft survival and reduced costs when compared with branded IR tacrolimus (Prograf®) in the base case analysis. |
Introduction
Liver transplantation is a highly effective treatment option for patients with end-stage liver disease, and as of 2014 there were approximately 8300 patients with a functioning liver transplant in the UK Transplant Registry, up from 7600 in 2009 [1]. One-year graft survival rates are now over 80 % and longer-term graft and patient survival have increased dramatically since the first liver transplants were conducted in the 1960s [2]. While these improvements are a result of changes to many aspects of operative and peri-operative treatment implemented since the early transplants, improvements in post-transplant immunosuppression are generally considered to be the most important innovation. Indeed, previous studies have divided large liver transplant populations into separate “eras” based on the availability of new immunosuppressive regimens and induction therapies at the time of transplant [3]. Jain et al. [3] selected the introduction of ciclosporin, muromonab-CD3 (OKT3), and tacrolimus as the cut-off points for three eras, corresponding to the periods spanning 1981–85, 1986–90, and 1991–98 [3]. Their study of 4000 liver transplant recipients showed that survival in the tacrolimus era was significantly improved relative to the previous eras, reporting 10-year survival of 60 % compared with 52 % and 53 % for the OKT3 and ciclosporin eras, respectively [3].
The tacrolimus registration trials for liver transplant, published in 1994, showed a significant reduction in the incidence of acute rejection relative to ciclosporin, although no significant differences in mortality or graft loss compared to ciclosporin were observed over 12 months [4, 5]. Specifically, the registration trials compared ciclosporin with twice-daily, immediate-release (IR) tacrolimus (Prograf®, Astellas Pharma Inc., Tokyo, Japan) that is now a cornerstone of immunosuppressive therapy in liver transplant recipients [6]. Since the publication of the registration trials, tacrolimus has been reformulated into a once-daily, prolonged-release (PR) formulation (Advagraf®, Astellas Pharma Inc., Tokyo, Japan), which received European Medicines Agency (EMA) marketing authorization in 2007 [7]. In the European public assessment report accompanying the marketing authorization, regarding Advagraf the EMA noted that “it is expected that it may help to improve compliance with dosing” and that the modified-release profile “would be expected to improve the variability in the exposure to tacrolimus” [8]. Several studies in liver transplant recipients have since confirmed that intra-patient variability is indeed reduced with PR tacrolimus relative to IR tacrolimus, and that the majority of patients prefer once-daily dosing over twice-daily dosing and are more adherent to the once-daily regimen [9–14]. One randomized controlled trial (RCT) of PR versus IR tacrolimus has been conducted to date, in which PR tacrolimus showed non-inferiority relative to IR tacrolimus in terms of the primary endpoint of biopsy-confirmed rejection at 24 weeks [15]. While the open-label 12-month extension of the RCT also showed non-inferiority in secondary endpoints of graft and patient survival, recent retrospective analyses of 3-year follow-up data from the European Liver Transplant Registry (ELTR) have been published demonstrating significant improvements in graft survival and numerical but not statistically significant improvements in patient survival with PR tacrolimus relative to IR tacrolimus [16]. Given these emerging data, the increasing size of the patient population with a functioning liver graft, and the concomitant increase in healthcare expenditure in these patients, the aim of the present analysis was to use data from the ELTR analysis to project treatment costs, patient and graft life expectancy, and numbers needed to treat to avoid graft loss or death with PR tacrolimus relative to IR tacrolimus.
Methods
Model
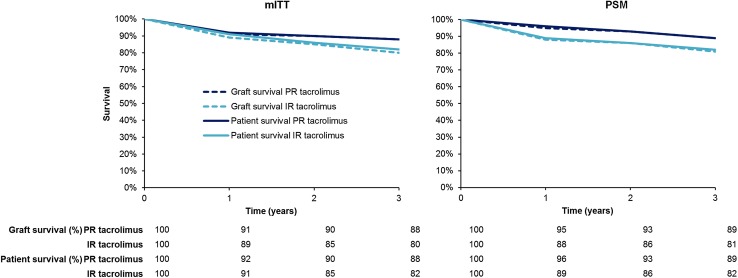
A model was constructed in Microsoft Excel (Microsoft Corporation, Redmond, WA USA) to project cost and effectiveness outcomes in de novo adult liver transplant recipients using PR tacrolimus (Advagraf®) or branded IR tacrolimus (Prograf®) as the primary immunosuppressive regimen in the UK setting. Patient and graft survival rates were based on a retrospective analysis of data from the ELTR [16]. The ELTR includes data on liver transplant recipients from 145 European transplant centers, 21 of which prescribed both PR and IR tacrolimus and were included in the analysis. In brief, Adam et al. [16] conducted a retrospective database analysis of primary liver transplant patients ≥18 years old who underwent their first liver transplant between January 2008 and December 2012 and received PR tacrolimus or IR tacrolimus, with or without concomitant immunosuppressive agents, within the first month after transplantation. Two analyses of the data were conducted. The first analysis was of a modified intent-to-treat (mITT) population that excluded all patients with less than 1 month of post-transplant follow-up (to avoid the confounding factors of post-operative complications). The second analysis looked at the same endpoints in a propensity-score matched (PSM) population, in which PR and IR tacrolimus patients were paired in a 1:2 ratio based on a propensity score. The propensity score was based on recipient age, recipient human immunodeficiency virus, hepatitis C and hepatocellular carcinoma status, United Network for Organ Sharing (UNOS) status, creatinine levels, donor age, date of transplantation, total ischemia time, and administration of other immunosuppressive medications early post-transplant [ciclosporin, mycophenolate mofetil (MMF), corticosteroids, daclizumab, and basiliximab].
The Kaplan–Meier analyses of patient and graft survival in the mITT cohort were used to calculate rates of graft loss and mortality in the base case analysis. Retransplantation rates were derived based on the assumption that retransplantation accounts for the entire difference between patient and graft survival. Since the ELTR analysis did not report tacrolimus dosing, data from the respective summaries of product characteristics (SPC) were used to establish the initial doses of IR and PR tacrolimus in the base case, both of which were taken to be the mid-point of the SPC-recommended starting dose range of 0.10–0.20 mg/kg/day. The initial dose was assumed to be maintained for 1 year after which the dose in both treatment arms was switched to match the end-of-study (EOS) IR tacrolimus dose (0.58 mg/kg/day) from the Trunečka et al. RCT [15]. Mean patient bodyweight was taken to be 77.2 kg based on the weighted average from Trunečka et al. [15].
The model was designed to evaluate the number needed to treat (NNT) to avoid one graft loss or one death with PR tacrolimus relative to IR tacrolimus, the life expectancy with PR relative to IR tacrolimus, and the number of graft years saved with PR relative to IR tacrolimus in addition to the costs associated with retransplantation and primary immunosuppressive therapy with each formulation.
Unit Costs
For the base case analysis, the per-milligram cost of PR tacrolimus (Advagraf®) and branded IR tacrolimus (Prograf®) were taken from the September 2014 British National Formulary (BNF; Table 1) [17]. The BNF was also used as the source of an alternative per-milligram cost of generic IR tacrolimus (Adoport®, Sandoz International GmbH, Holzkirchen, Germany) in one-way sensitivity analysis. The mean cost of liver retransplantation was assumed to be 1.84 times more costly than a first transplant based on the overall retransplant cost ratio reported by Azoulay et al. in a single-center study of 1038 first liver transplants and 139 retransplants [18].
Table 1.
Unit costs in cost-effectiveness analyses of prolonged-release (PR) tacrolimus versus branded and generic immediate-release (IR) tacrolimus as the primary immunosuppressive agents in renal transplant recipients
| Cost item | Cost | References |
|---|---|---|
| PR tacrolimus (Advagraf®) | 1.43 (£ per mg) | British National Formulary 68 [17] |
| IR tacrolimus (Prograf®) | 1.61 (£ per mg) | British National Formulary 68 [17] |
| IR tacrolimus (Adoport®), one-way sensitivity analysis only | 1.11 (£ per mg) | British National Formulary 68 [17] |
| Liver retransplantation | 35,164.23 (£) | NHS Tariff Information |
£ 2014 pounds sterling, IR immediate-release, NHS, National Health Service, PR prolonged-release
Perspective, Time Horizon, and Discounting
The base case analysis was performed over a 3-year time horizon to avoid extrapolation of the underlying graft and patient survival data from the ELTR. The model reported all outcomes annually and applied half-cycle correction to eliminate any systematic over- or underestimation of costs and effects. Cost and effectiveness outcomes were measured from the perspective of the UK healthcare payer, and future costs and effects were discounted at 3.5 % per annum in the base case. Sensitivity analyses were performed with a 1.5 % annual discount rate for both costs and effects in line with guidance from the National Institute for Health and Care Excellence [19]. All costs were reported in 2014 pounds sterling (£).
Threshold, Probabilistic, and One-Way Sensitivity Analyses
All analyses were run as probabilistic sensitivity analyses, in which uncertainty around patient body weight, the cost of liver retransplantation, and the Kaplan–Meier projections of mortality and graft loss were captured. Standard errors around the Kaplan–Meier curves were estimated based on binomial proportion 95 % confidence intervals around the percentage of patients and grafts surviving at each time point (Eq. 1), ensuring that both were monotonically decreasing functions and that patient survival always equaled or exceeded graft survival.
Equation 1: Assumed standard error around Kaplan–Meier projections of mortality and graft loss.
| 1 |
Patient body weight was sampled using the weighted standard deviation (SD) body weight from the Trunečka et al. [15] RCT and a confidence interval around the ratio of retransplantation costs to first transplant costs was approximated using Fieller’s theorem from standard deviations reported in the Azoulay et al. study [20].
The sensitivity of the model to changes in individual input parameters was explored in a series of one-way sensitivity analyses. Specifically, sensitivity analyses were conducted around the base case analysis in which the ELTR PSM population data were used in place of the mITT population. The sensitivity to dosing assumptions was investigated by using dosing data directly from the Trunečka et al. [15] study for the first year of simulation, followed by holding the dose steady at the final dose as reported by Trunečka et al. at day 365. A further dosing sensitivity analysis was conducted in which a rational model (i.e., a ratio of a first- and second-order polynomials) was fitted to the Trunečka dose curves for each arm of the simulation. The correlation coefficients (R2) of the rational model to the extracted data sets were 0.985 and 0.982 for PR tacrolimus and IR tacrolimus, respectively, and the models were used to extrapolate out to the full 3-year time horizon. Finally, four cost-centric sensitivity analyses were conducted; one in which the per-milligram cost of IR tacrolimus was set to the same as that for PR tacrolimus, a second in which the per-milligram cost of generic IR tacrolimus (Adoport®) was used in place of the branded IR tacrolimus (Prograf®) cost, and two analyses of retransplantation costs; one in which the cost of retransplantation was set to the same cost as a first transplant and a second in which the cost of retransplantation was abolished.
In line with guidance from the International Society for Pharmacoeonomics and Outcomes Research, a deterministic threshold analysis was conducted to establish the PR tacrolimus (Advagraf®) drug cost at which overall costs would be equivalent in the two treatment arms [21]. The threshold analysis was conducted using both the base case cost of branded IR tacrolimus (Prograf®) and the cost of generic IR tacrolimus (Adoport®).
Results
In the probabilistic base case analysis, graft and patient survival estimates matched those from the ELTR mITT analysis (Fig. 1). The mean NNT to avoid one graft loss with PR tacrolimus relative to IR tacrolimus over 3 years was 14 patients, while the corresponding NNT to avoid one death was 18. Mean (SD) patient life expectancy over the 3-year time horizon was 31.52 (0.22) months in the PR tacrolimus arm versus 30.62 (0.09) months with IR tacrolimus, representing an increase of 0.89 (0.23) months, while graft survival was 1.07 (0.21) months higher with PR tacrolimus at 31.2 (0.19) months versus 30.2 (0.09) months with IR tacrolimus (Table 2).
Fig. 1.
Patient and graft survival over time based on the propensity-score matched and modified intent-to-treat analyses of the European Liver Transplant Registry data. IR immediate-release, mITT modified intent-to-treat, PSM propensity-score matched, PR prolonged-release
Table 2.
Top-line probabilistic results from a 3-year analysis of the cost-effectiveness of prolonged-release (PR) versus immediate-release (IR) tacrolimus in liver transplant recipients in the UK
| IR tacrolimus (Prograf®) | PR tacrolimus (Advagraf®) | Difference | |
|---|---|---|---|
| Cost of immunosuppression, £ | 10,405 (2203) | 9469 (2006) | −937 (208) |
| Cost of retransplantation, £ | 1654 (443) | 949 (689) | −705 (820) |
| Total cost, £ | 12,062 (2245) | 10,420 (2130) | −1642 (885) |
| Life expectancy, months | 30.62 (0.09) | 31.52 (0.22) | +0.89 (0.23) |
| Graft life expectancy, months | 30.16 (0.09) | 31.23 (0.19) | +1.07 (0.21) |
| Annualized probability of graft loss | 0.064 | 0.039 | −0.025 |
| NNT to avoid graft loss with PR vs. IR tacrolimus | 14 | ||
| Annualized probability of death | 0.058 | 0.039 | −0.019 |
| NNT to avoid death with PR vs. IR tacrolimus | 18 | ||
Values are presented as mean (standard deviation)
£ 2014 pounds sterling, IR immediate-release, NNT number needed to treat, PR prolonged-release
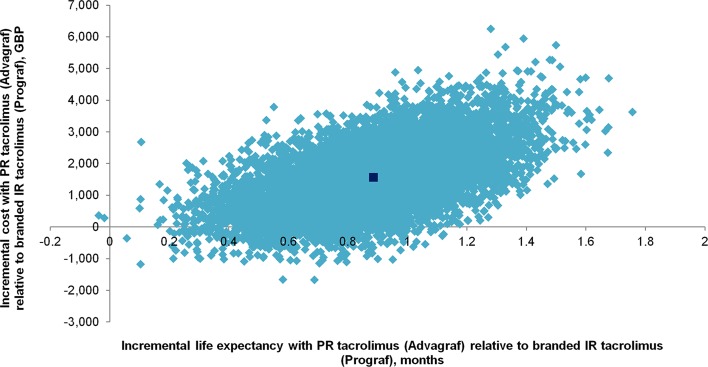
These increases in effectiveness were accompanied by mean (SD) per-patient cost savings with PR tacrolimus (Advagraf®) of £1642 (885) over 3 years, with PR tacrolimus (Advagraf®) thereby exhibiting dominance over the branded IR formulation (Prograf®). PR tacrolimus (Advagraf®) was less costly and more effective than branded IR tacrolimus (Prograf®) in 9559 (95.6 %) of 10,000 iterations (Fig. 2). PR tacrolimus (Advagraf®) was more costly and more effective than branded IR tacrolimus (Prograf®) in 439 analyses, in which the mean incremental cost-effectiveness ratio (ICER) was £4282 per life year gained. Two analyses (0.02 % of model iterations) showed reduced effectiveness and reduced costs with PR tacrolimus (Advagraf®) relative to branded IR tacrolimus (Prograf®).
Fig. 2.
Cost-effectiveness scatterplot showing incremental per-patient costs and life expectancy from 10,000 model iterations over a 3-year time horizon. IR immediate-release, PR prolonged-release
Findings of one-way sensitivity analyses are presented in Table 3. The largest effect on the incremental cost outcomes was observed when the dose data for each arm was based on the dose curves reported in the Trunečka et al. [15] RCT, in which PR tacrolimus (Advagraf®) was associated with an increase in costs of £1350 per patient over 3 years relative to branded IR tacrolimus (Prograf®), resulting in an ICER of £18,255 per life year gained. Switching the per-milligram IR tacrolimus cost to that of generic tacrolimus (Adoport®) resulted in incremental costs of £1556, yielding an ICER of £21,078 per life year gained for PR tacrolimus (Advagraf®) relative to generic IR tacrolimus (Adoport®). Using the PSM outcomes data from the ELTR had a large effect on both incremental costs and effects; incremental life expectancy increased to 2.00 months, while cost savings decreased to £763 as a result of the increased patient and graft survival with PR tacrolimus (Advagraf®). The rational model fit and extrapolation from the Trunečka dosing curves also had a relatively large effect on cost, reducing modelled cost savings with PR tacrolimus (Advagraf®) to £1237 per patient over 3 years.
Table 3.
Summary of one-way sensitivity analyses around the base case analysis
| Life expectancy (months) | Costs (£) | ICER (£ per life year gained) | |||||
|---|---|---|---|---|---|---|---|
| IR tacrolimus | PR tacrolimus | Difference | Branded IR tacrolimus (Prograf®) | PR tacrolimus (Advagraf®) | Difference | ||
| Base case | 30.62 (0.09) | 31.52 (0.22) | +0.89 (0.23) | 12,062 (2245) | 10,420 (2130) | −1642 (885) | PR dominant |
| 1.5 % discount rate | 31.48 (0.09) | 32.41 (0.22) | +0.93 (0.24) | 12,502 (2290) | 10,788 (2170) | −1714 (895) | PR dominant |
| Trunečka IR dosing in both arms, held at EOS dose [15] | 30.62 (0.09) | 31.52 (0.22) | +0.89 (0.23) | 10,102 (1824) | 8641 (1760) | −1461 (866) | PR dominant |
| Trunečka IR and PR dosing, held at EOS dose [15] | 30.62 (0.09) | 31.51 (0.22) | +0.89 (0.23) | 10,098 (1837) | 11,449 (2340) | +1350 (964) | 18,255 |
| Rational model fit to Trunečka IR and PR dose curves [15] | 30.62 (0.09) | 31.52 (0.22) | +0.89 (0.23) | 7445 (1298) | 6208 (1301) | −1237 (849) | PR dominant |
| ELTR PSM data used in place of mITT[15] | 30.05 (0.21) | 32.06 (0.21) | +2.00 (0.30) | 11,557 (2346) | 10,794 (2221) | −763(1220) | PR dominant |
| IR cost equivalence with PR tacrolimus | 30.62 (0.09) | 31.52 (0.22) | +0.89 (0.23) | 10,973 (2114) | 10,420 (2130) | −553 (855) | PR dominant |
| IR cost equivalence with generic IR tacrolimus (Adoport®) | 30.62 (0.09) | 31.52 (0.22) | +0.89 (0.23) | 8862 (1578) | 10,420 (2130) | +1556 (981) | 21,078 |
| Cost of retransplant same as first transplant | 30.62 (0.09) | 31.52 (0.22) | +0.89 (0.23) | 11,341 (2189) | 10,005 (2025) | −1336 (513) | PR dominant |
| Cost of retransplant abolished | 30.62 (0.09) | 31.52 (0.22) | +0.89 (0.23) | 10,476 (2186) | 9533 (1989) | −943 (207) | PR dominant |
Values are presented as mean (standard deviation)
£ 2014 pounds sterling, ELTR European Liver Transplant Registry, EOS end of study, IR immediate-release, mITT modified intent-to-treat, PR prolonged-release, PSM propensity-score matched
Deterministic threshold analysis showed that the PR tacrolimus (Advagraf®) breakeven price (the price at which the cost in both model arms is equivalent) would be £1.77 per milligram when branded IR tacrolimus (Prograf®) was used for the analysis, £0.34 per milligram higher than the current per-milligram cost of PR tacrolimus (Advagraf®) in the BNF. Threshold analysis using the cost of generic IR tacrolimus (AdoportR)) resulted in a breakeven price of £1.28 per milligram, £0.15 per milligram lower than the per-milligram cost of PR tacrolimus (Advagraf®) in the BNF. The additional £0.15 per milligram for Advagraf® yielded 0.89 additional months of life over the model time horizon (resulting in the ICER of £21,078 per life year gained as reported in one-way sensitivity analysis, Table 3).
Discussion
The present study showed that, based on a recent retrospective analysis of data from 4367 patients in the ELTR, PR tacrolimus would be expected to be associated with gains in life expectancy and graft survival relative to IR tacrolimus, while reducing costs borne by the healthcare payer (in comparison to branded IR tacrolimus (Prograf®).
As with any modeling analysis, the present study has a number of limitations that should be acknowledged. The largest limitation of the analysis was the use of heterogeneous data sources to model the clinical outcomes and dosing of the PR and IR tacrolimus regimens. Dose data were not recorded in the ELTR and as such did not form part of the retrospective analysis by Adam et al. [16] that underpinned the clinical aspects of the model. The most important consequence of this data heterogeneity was that clinical effectiveness outcomes were derived from a different dataset from the estimates of pharmacy dosing and hence also pharmacy costs. To establish the effect of dosing assumptions on model outcomes, an extensive series of sensitivity analyses were conducted around the base case analysis, including switching the model to use dosing data from the Trunečka et al. [15] study and either holding the projected dose flat at the EOS dose or projecting the dose out using a rational model fit to the Trunečka dose data.
The base case analysis used the BNF unit costs for PR tacrolimus (Advagraf®) and IR tacrolimus (Prograf®) to reflect the tacrolimus formulations used in the ELTR study on which the clinical outcomes were based. Other generic formulations of IR tacrolimus are listed in the BNF, including Adoport®, which is currently the cheapest twice-daily formulation at £1.11 per milligram. Sensitivity analysis using the Adoport® price showed that using PR tacrolimus (Advagraf®) in place of Adoport® would result in an ICER of £21,078 per life year gained based on an increase in life expectancy of 0.89 months, while threshold analysis showed the PR tacrolimus (Advagraf®) breakeven price to be £0.15 per milligram (10.5 %) lower than the current PR tacrolimus (Advagraf®) list price. Taken together, the analyses show that the additional £0.15 per milligram spend on PR tacrolimus (Advagraf®) resulted in an average of 0.89 additional months of life per patient over a 3-year time horizon.
Certain limitations should also be noted pertaining to the inclusion of retransplantation costs. The retransplantation cost estimate was based on the NHS tariff for an adult hepatobiliary transplant multiplied by a cost ratio (of second versus first liver transplant) derived from a single-center analysis [18]. While the size of the population analyzed was large enough (N = 1177) to capture a wide range of surgical complications and indications for transplant and retransplant, center-specific practices and protocols may have affected the cost estimates presented and the final cost estimate may not be applicable to other centers. Retransplantation was captured in the model as the difference between patient survival and graft survival. Given that retransplantation is the only treatment option for liver graft failure, this assumption is clinically realistic but, while the inclusion of retransplantation is also economically important given the high cost associated with the procedure, its role as a driver of incremental costs is challenging. Notably, local organ availability and center-specific ethical considerations such as outcomes-based versus urgency-based approaches to retransplant prioritization make the incidence of retransplantation less of a clinical consideration and more of a logistical and ethical issue [22]. To establish the extent to which retransplantation was driving cost outcomes, sensitivity analyses were conducted in which the cost of retransplantation was firstly set to the same cost as a first liver transplant and, in a separate analysis, abolished completely. Both analyses yielded cost savings with PR tacrolimus (Advagraf®), but the magnitude of the savings was reduced relative to the base case analysis.
As the main source of clinical data in the present analysis, the ELTR study design and its limitations should also be considered when interpreting the findings of the present analysis. An editorial that accompanied the original manuscript noted that the ELTR data is subject to reporting bias (in that it is collected on a voluntary basis) and that characteristics of the patients on IR and PR tacrolimus differed in terms of their age, concomitant mediation use, serum creatinine levels, hepatitis delta or hepatocellular carcinoma (HCC) as the primary indication, and donor age [23]. The PSM analysis attempted to address these known differences, but extraneous factors such as socioeconomic differences may have persisted and, as noted in the editorial, 49 % of patients on PR tacrolimus remained unmatched in the PSM analysis. The lack of randomization may have also resulted in bias arising from assignment of sicker patients to receive the longer established IR tacrolimus regimen and the effect of the choice of included ELTR centers should not be ignored. The authors of the ELTR data analysis noted that the 21 centers using PR tacrolimus and IR tacrolimus were selected “to prevent center bias,” [16] but it is conceivable that outcomes with IR tacrolimus in the 21 included centers may differ from those in the remaining 124 centers participating in the ELTR using IR tacrolimus exclusively.
While such criticisms of observational data are entirely valid, these issues are not unique to data from routine clinical practice, with small-scale RCTs suffering from many of the same methodological issues. In the present analysis, the mITT data were used in the base case and a sensitivity analysis was conducted with the PSM data to explore the extent to which the mortality and graft loss outcomes affected the analysis. PR tacrolimus (Advagraf®) remained cost saving in the PSM analysis, but the life expectancy benefit increased to 2.00 months over the 3-year time horizon, extending the dominance of PR tacrolimus (Advagraf®) over branded IR tacrolimus (Prograf®).
Certain drivers of costs were intentionally omitted from the present analysis, including surgical complications, new onset diabetes after transplantation, cytomegalovirus infection, and the myriad costs associated with various recurrent indications for liver transplant such as HCC and HCV. While these sequelae and complications contribute to the absolute cost of treating liver transplant recipients, differences in the incidence would not be anticipated to drive incremental cost or effectiveness outcomes between two tacrolimus formulations. Cost estimates in the present analysis should not therefore be considered instructive for the purposes of budget impact analysis. Based on the emerging data from the ELTR in concert with the previously established non-inferiority in terms of biopsy-confirmed acute rejection, we consider the model to be comprehensive in terms of its ability to capture drivers of incremental costs and effects between the two tacrolimus formulations.
Conclusion
Based on the present analysis, PR tacrolimus would be expected to prevent one graft loss for every 14 patients and one death for every 18 patients initiated on PR tacrolimus rather than IR tacrolimus. Furthermore, PR tacrolimus (Advagraf®) would be likely to reduce costs associated with immunosuppressive treatment and retransplantation by up to £ 1642 (885) per patient over 3 years versus branded IR tacrolimus (Prograf®). These findings, combined with the well established patient preference for once-daily over twice-daily dosing [10, 13], and the recent publication of clinical data showing a graft survival benefit with once-daily tacrolimus [16], provide a strong case for the preferential use of PR tacrolimus over IR tacrolimus in adult liver transplant recipients in the UK setting.
Compliance with Ethical Standards
Funding
This study was funded by Astellas Pharma EMEA Limited.
Conflicts of interest
RFP is a full-time employee of Ossian Health Economics and Communications GmbH, which received consultancy fees from Astellas Pharma EMEA Limited to construct the model and write the manuscript. GM and IO are full-time employees of Astellas Pharma EMEA Limited, a subsidiary of Astellas Pharma Inc., which manufactures prolonged-release and immediate-released tacrolimus.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Author contributions
RFP developed the cost-effectiveness model, ran the analyses, and drafted the manuscript. GM formulated the research question, reviewed the model, and provided critical revisions to the manuscript. IO reviewed and provided critical revisions to the manuscript.
References
- 1.NHS Blood and Transplant. Organ donation and transplantation activity report 2013/14. Available from: https://nhsbtmediaservices.blob.core.windows.net/organ-donation-assets/pdfs/activity_report_2013_14.pdf. Accessed 2 Mar 2015.
- 2.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Evolution of liver transplantation. Hepatology. 1982;2(5):614–636. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain A, Reyes J, Kashyap R, et al. Long-term survival after liver transplantation in 4,000 consecutive patients at a single center. Ann Surg. 2000;232(4):490–500. doi: 10.1097/00000658-200010000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European FK506 Multicentre Liver Study Group. Randomised trial comparing tacrolimus (FK506) and cyclosporin in prevention of liver allograft rejection. Lancet. 1994;344:423–428. [PubMed]
- 5.The US Multicentre FK506 Study Group A comparison of tacrolimus (FK 506) and ciclosporin for immunosuppression in liver transplantation. N Engl J Med. 1994;331:1110–1115. doi: 10.1056/NEJM199410273311702. [DOI] [PubMed] [Google Scholar]
- 6.Fischer L, Trunečka P, Gridelli B, et al. Pharmacokinetics for once-daily versus twice-daily tacrolimus formulations in de novo liver transplantation: a randomized, open-label trial. Liver Transpl. 2011;17(2):167–177. doi: 10.1002/lt.22211. [DOI] [PubMed] [Google Scholar]
- 7.European Medicines Agency. Advagraf Authorization Details. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000712/human_med_000629.jsp. Accessed 2 Mar 2015.
- 8.European Medicines Agency. Advagraf: EPAR—scientific discussion. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000712/WC500022237.pdf. Accessed 3 Mar 2015.
- 9.Florman S, Alloway R, Kalayoglu M, et al. Conversion of stable liver transplant recipients from a twice-daily Prograf-based regimen to a once-daily modified release tacrolimus-based regimen. Transplant Proc. 2005;37(2):1211–1213. doi: 10.1016/j.transproceed.2004.11.086. [DOI] [PubMed] [Google Scholar]
- 10.Sańko-Resmer J, Boillot O, Wolf P, Thorburn D. Renal function, efficacy and safety postconversion from twice- to once-daily tacrolimus in stable liver recipients: an open-label multicenter study. Transpl Int. 2012;25(3):283–293. doi: 10.1111/j.1432-2277.2011.01412.x. [DOI] [PubMed] [Google Scholar]
- 11.Lieber SR, Volk ML. Non-adherence and graft failure in adult liver transplant recipients. Dig Dis Sci. 2013;58(3):824–834. doi: 10.1007/s10620-012-2412-0. [DOI] [PubMed] [Google Scholar]
- 12.Valente G, Rinaldi L, Sgambato M, Piai G. Conversion from twice-daily to once-daily tacrolimus in stable liver transplant patients: effectiveness in a real-world setting. Transplant Proc. 2013;45(3):1273–1275. doi: 10.1016/j.transproceed.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Beckebaum S, Iacob S, Sweid D, et al. Efficacy, safety, and immunosuppressant adherence in stable liver transplant patients converted from a twice-daily tacrolimus-based regimen to once-daily tacrolimus extended-release formulation. Transpl Int. 2011;24(7):666–675. doi: 10.1111/j.1432-2277.2011.01254.x. [DOI] [PubMed] [Google Scholar]
- 14.Eberlin M, Otto G, Krämer I. Increased medication compliance of liver transplant patients switched from a twice-daily to a once-daily tacrolimus-based immunosuppressive regimen. Transplant Proc. 2013;45(6):2314–2320. doi: 10.1016/j.transproceed.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 15.Trunečka P, Boillot O, Seehofer D, Tacrolimus Prolonged Release Liver Study Group et al. Once-daily prolonged-release tacrolimus (ADVAGRAF) versus twice-daily tacrolimus (PROGRAF) in liver transplantation. Am J Transplant. 2010;10(10):2313–2323. doi: 10.1111/j.1600-6143.2010.03255.x. [DOI] [PubMed] [Google Scholar]
- 16.Adam R, Karam V, Delvart V, et al. All contributing centers (http://www.eltr.org) and the European Liver Intestine Transplant Association (ELITA). Improved Survival in Liver Transplant Recipients Receiving Prolonged-Release Tacrolimus in the European Liver Transplant Registry. Am J Transplant. 2015;15(5):1267–82. [DOI] [PubMed]
- 17.Joint Formulary Committee. British National Formulary (online) London: BMJ Group and Pharmaceutical Press. Available from: http://www.medicinescomplete.com. Accessed 2 Mar 2015.
- 18.Azoulay D, Linhares MM, Huguet E, et al. Decision for retransplantation of the liver: an experience- and cost-based analysis. Ann Surg. 2002;236(6):713–721. doi: 10.1097/00000658-200212000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. Available from: http://www.nice.org.uk/article/pmg9/resources/non-guidance-guide-to-the-methods-of-technology-appraisal-2013-pdf. Accessed 2 Mar 2015. [PubMed]
- 20.Fieller EC. The biological standardization of Insulin. J R Statist Soc. 1940;7:1–64. [Google Scholar]
- 21.Hay JW, Smeeding J, Carroll NV, et al. Good research practices for measuring drug costs in cost effectiveness analyses: issues and recommendations: the ISPOR Drug Cost Task Force report–Part I. Value Health. 2010;13(1):3–7. doi: 10.1111/j.1524-4733.2009.00663.x. [DOI] [PubMed] [Google Scholar]
- 22.Biggins SW, Beldecos A, Rabkin JM, Rosen HR. Retransplantation for hepatic allograft failure: prognostic modeling and ethical considerations. Liver Transpl. 2002;8(4):313–322. doi: 10.1053/jlts.2002.31746. [DOI] [PubMed] [Google Scholar]
- 23.Asrani SK, O’Leary JG. Can one pill a day keep rejection away? Am J Transplant. 2015;15(5):1135–1136. doi: 10.1111/ajt.13170. [DOI] [PubMed] [Google Scholar]