Abstract
Background:
Allergan’s Natrelle round silicone-filled breast implants were approved by the U.S. Food and Drug Administration in 2006 based on interim results from the Core Study; final 10-year study results are now available.
Methods:
Seven hundred fifteen subjects were implanted with smooth and Biocell textured Natrelle round silicone implants and attended clinic visits at 0 to 4 weeks, 6 months, 1 year, and annually through 10 years. Approximately one-third of subjects underwent magnetic resonance imaging at years 1, 3, 5, 7, and 9 to assess rupture.
Results:
Complication rates showed modest increases over the previously published 6-year rates. The Kaplan-Meier capsular contracture rate was 18.9 percent for augmentation, 28.7 percent for revision-augmentation, and 24.6 percent for reconstruction. Among augmentation subjects, capsular contracture was significantly lower (p = 0.023) for submuscular (15.7 percent) versus subglandular (26.3 percent) placement. The overall rupture rate in the magnetic resonance imaging cohort was 13.0 percent for subjects and 7.7 percent for implants. By the end of the study, 81.8 percent of augmentation subjects still had an original implant in place. Using a five-point scale, 94.2 percent of augmentation, 83.8 percent of revision-augmentation, and 90.7 percent of reconstruction subjects reported being satisfied or definitely satisfied with their implants. Significant improvement over baseline was also seen in overall breast satisfaction and satisfaction with breast size, shape, feel, and how well they matched.
Conclusion:
The 10-year data from the Natrelle Core Study, which can guide surgeons and patients in decision-making, demonstrate safety and high levels of patient satisfaction.
CLINICAL QUESTION/LEVEL OF EVIDENCE:
Therapeutic, III.

It is now 50 years since silicone gel–filled breast implants first became commercially available.1 Over the past five decades, these devices have gone through a number of modifications that have ultimately led to improvements in terms of safety, quality, performance, and manufacturing.
In the United States, before 1991, there were many versions of silicone breast implants available from several different manufacturers. There were double-lumen, reverse double-lumen, adjustable, smooth shell, and textured shell implants manufactured by Dow Corning, Surgitek, Bristol-Myers, McGhan Medical, Silimed, and Mentor corporations among others.
With the U.S. Food and Drug Administration voluntary moratorium in January of 1992 that effectively removed silicone breast implants from the U.S. market, there was a great winnowing of options for patients and surgeons. Many doubted that silicone gel–filled breast implants would ever again be available in the United States. As the dust from the U.S. Food and Drug Administration panel meetings of 1991 and 1992 settled, some things eventually did become clear. Future silicone gel–filled breast implants would have to be designed and manufactured to meet substantial performance and safety benchmarks that would be significantly higher than demonstrated by earlier versions, which would come to be known as first-, second-, and third-generation silicone gel–filled breast implants. Also, the next generation of silicone gel–filled breast implants (fourth generation) would have to undergo rigorous preclinical and premarket clinical testing before U.S. Food and Drug Administration approval for distribution in the United States.
Initially, two companies [Mentor Corp. (Santa Barbara, Calif.) and McGhan Medical Corp. (Santa Barbara, Calif.), later known as Inamed and then Allergan] began clinical studies in preparation for an application to the U.S. Food and Drug Administration. More recently, Sientra began studies and was ultimately the third company to file an application. As a result of U.S. Food and Drug Administration plastic surgery panel meetings in 2003 and 2005, on November 17, 2006, the U.S. Food and Drug Administration approved both Mentor’s and Allergan’s applications to market silicone gel–filled breast implants (fourth generation). In Allergan’s case, that approval was based largely on the 3-year data reported for the Core Study of Allergan’s Natrelle round cohesive gel implants.
In 2007, the 6-year results of the Core Study were published in Plastic and Reconstructive Surgery.2 We now have the final data from this Core clinical study, and the following is a synopsis of the key 10-year data. Importantly, this is the first publication of 10-year data from a U.S. Food and Drug Administration–regulated trial of silicone gel–filled breast implants.
Since the 2007 publication, there has been increased interest in three issues surrounding capsular contracture, the most common complication following breast implantation: first, the relative advantages, if any, of the Biocell textured surface compared with the smooth surface; second, the comparative advantages of a submuscular over a subglandular pocket; and third, any measurable outcome differences between incision sites. Thus, although the Core Study was not designed to investigate those questions, the 10-year data were analyzed to examine those issues, and these results should be interpreted accordingly.
PATIENTS AND METHODS
The detailed study design and inclusion/exclusion criteria were published in the 2007 article along with the 6-year results.2 Quality-of-life data3 and early subject satisfaction data4 have also been published, so the focus here is on safety and satisfaction results at 10 years.
Briefly, subjects were implanted with Natrelle round silicone-filled breast implants (smooth styles 40 and 45, and Biocell textured styles 110 and 120) and attended clinic visits at 0 to 4 weeks, 6 months, 1 year, and annually through 10 years. Approximately one-third of subjects (n = 264) underwent magnetic resonance imaging at years 1, 3, 5, 7, and 9 to assess rupture. All subjects provided written informed consent, and the study was approved by institutional review boards and registered at www.clinicaltrials.gov.
RESULTS
Subjects and Surgical Characteristics
Study enrollment took place between January of 1999 and June of 2000 at 33 U.S. sites, and operations were performed according to each site’s standard technique (i.e., there was no standardization of surgical techniques across sites). Of 715 subjects reported here, 455 were augmentation, 98 were reconstruction (all postmastectomy), 147 were revision-augmentation, and 15 were revision-reconstruction patients. For augmentation subjects, the indication for implant placement was dissatisfaction with breast size/shape for 54.9 percent, asymmetry for 23.7 percent, ptosis for 15.8 percent, and aplasia for 5.5 percent.
The majority of subjects were Caucasian, with a median age ranging from 34 years in the augmentation cohort to 54 years in the revision-reconstruction cohort. Average body mass index was in the ideal range (20.7 for augmentation, 21.0 for revision-augmentation, 23.4 for reconstruction, and 23.6 for revision-reconstruction). Across all cohorts 56.2 percent of implants were smooth and 43.8 percent were textured (59.0, 57.0, 36.2, and 44.0 percent were smooth for each cohort, respectively); 69.1 percent were placed submuscularly. Partial submuscular placement (subpectoral only) was much more common than full submuscular placement, which included subpectoral and subserratus (60.2, 46.9, 62.2, and 68.0 percent for each of the four cohorts versus 9.5, 13.5, 20.5, and 8.0 percent). The most common implant size in each cohort was 300 cc.
The predominant incision sites for augmentation and revision-augmentation were inframammary (45.9 and 63.5 percent, respectively) and periareolar (39.2 and 31.3 percent). For reconstruction and revision-reconstruction, the implants were most often placed through the mastectomy scar (59.1 and 52.0 percent, respectively) or inframammary incision (29.9 and 44.0 percent, respectively). Drains were placed for the majority of reconstruction implants (63.8 percent) but not for the majority of augmentation (17.8 percent), revision-augmentation (34.4 percent), or revision-reconstruction (32.0 percent) implants.
Concurrent procedures were performed at implantation for 15.5 percent of augmentation, 91.3 percent of revision-augmentation, 74.8 percent of reconstruction, and 96.0 percent of revision-reconstruction operations. The most common procedures were mastopexy for augmentation (13.3 percent) and capsulotomy or capsulectomy for the other cohorts plus tissue expander removal for reconstruction. Pocket irrigation with medications was performed for the majority of implantations in each cohort (92.1, 87.5, 78.7, and 100 percent, respectively), and antibiotics were the medication most often introduced (79.0, 76.4, 52.0, and 72.0 percent of pockets were irrigated with antibiotics). Most subjects also received parenteral antibiotics (87.9, 95.2, 91.8, and 80.0 percent).
As expected in a study with such a long duration, follow-up compliance declined over the course of the 10 years from 100 percent at the first visit to 76.1 percent overall at 7 years. By 10 years, the overall compliance rate was still 66.6 percent (66.7 percent for augmentation, 62.2 percent for revision-augmentation, 73.0 percent for reconstruction, and 72.7 percent for revision-reconstruction). Magnetic resonance imaging compliance for the final 9-year magnetic resonance imaging was even higher at 74.4, 77.8, 78.9, and 100 percent.
Safety
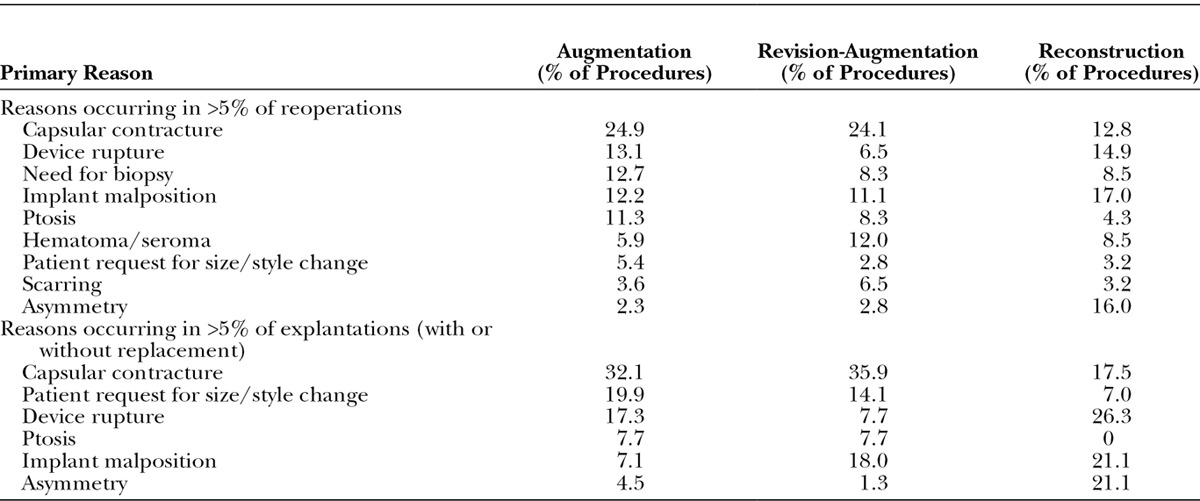
Consistent with the findings at 6 years,2 reoperation (any secondary procedure to the breast area) was least common in augmentation subjects (with a Kaplan-Meier risk rate of 36.1 percent), followed by revision-augmentation at 46.0 percent, and then by reconstruction at 71.5 percent (Table 1). Because of the small sample size of the revision-reconstruction cohort (n = 15), the safety results presentation will focus on the other cohorts. The most common primary reason for reoperation was capsular contracture for augmentation and revision-augmentation, and implant malposition closely followed by asymmetry for reconstruction (Table 2). The most common primary procedure performed for all three cohorts was implant removal with replacement; other common procedures were biopsy, capsulotomy, mastopexy, and aspiration of a hematoma or seroma.
Table 1.
Kaplan-Meier Complication Rates by Subject through 10 Years
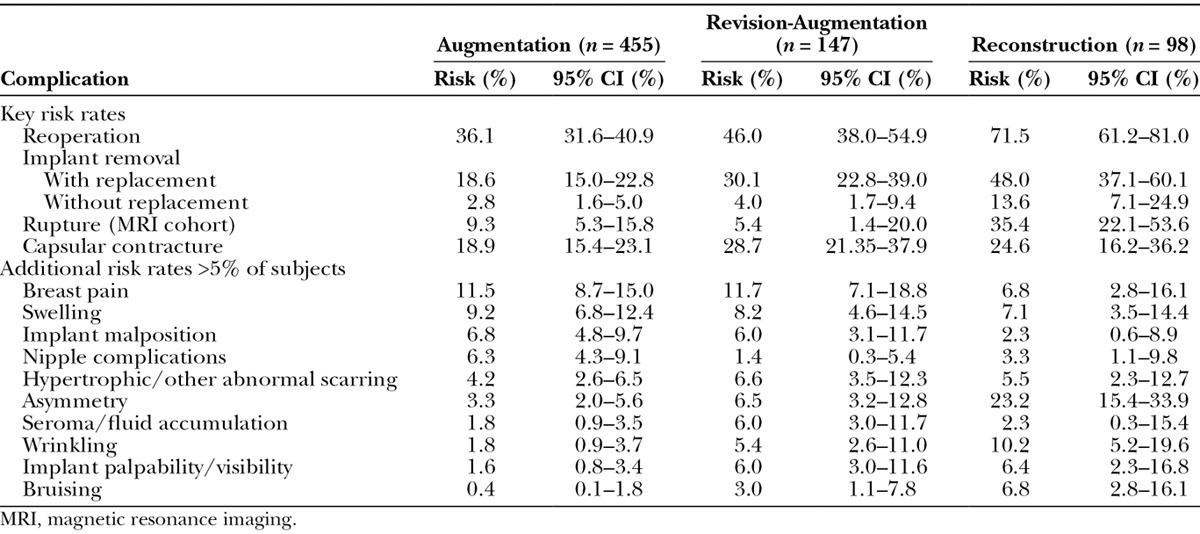
Table 2.
Reasons for Reoperation and Explantation
For all cohorts, implant removal with replacement was much more common than removal without replacement (Table 1). Replacement implants were more likely to be a larger size for augmentation (56.7 percent) and revision-augmentation (59.3 percent) and a smaller size for reconstruction (57.1 percent). The Kaplan-Meier risk rate of having an implant replaced with a different size/style implant is 14.1 percent for augmentation, 20.3 percent for revision-augmentation, and 31.5 percent for reconstruction. By the end of the study, the majority of women in each cohort had an implant in place (91.9, 78.9, and 63.3 percent, respectively), and many still had an original implant (81.8, 69.4, and 40.8 percent, respectively).
By 10 years, the overall Kaplan-Meier rupture rate was 13.0 percent for subjects and 7.7 percent for implants as measured in all subjects undergoing serial magnetic resonance imaging (Fig. 1). Given the size of the magnetic resonance imaging group within each indication cohort, pooling the results across cohorts provides a more accurate estimate of the rupture rate. The rupture rate includes both ruptures confirmed by means of explantation (71.4 percent of the suspected ruptures) and those that are unconfirmed (28.6 percent). Of the confirmed ruptures, 64.4 percent were identified by means of magnetic resonance imaging, with the remainder identified by means of physician examination (15.6 percent) or ultrasound (8.9 percent), or discovered during a secondary operation (11.1 percent). For the confirmed ruptures, 71.1 percent were partial submuscular, 17.8 percent were subglandular, 6.7 percent were complete submuscular, and 4.4 percent were below a tissue flap; 82.2 percent were textured, and 17.8 percent were smooth implants.
Fig. 1.
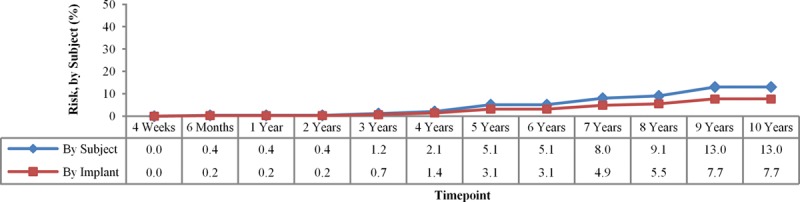
Magnetic resonance imaging cohort Kaplan-Meier rupture rates through 10 years.
Like rupture, capsular contracture (Baker grade III and IV) also showed a steady increase over the course of 10 years, with a final rate of 18.9 percent for augmentation, 28.7 percent for revision-augmentation, and 24.6 percent for reconstruction (Fig. 2). These capsular contracture rates were stratified by implant texture, which did not result in significant differences for smooth versus textured devices:
Fig. 2.
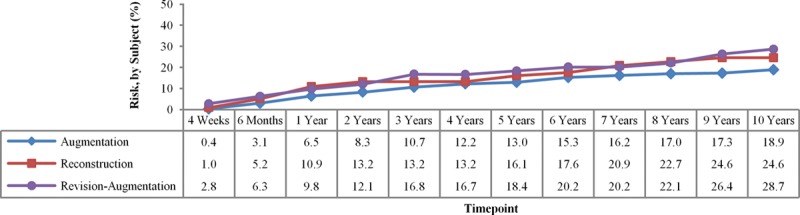
Capsular contracture Kaplan-Meier rates through 10 years.
Augmentation: smooth, 19.9 percent (95 percent CI, 15.4 to 25.7 percent); textured, 17.2 percent (95 percent CI, 12.2 to 23.8 percent).
Revision-augmentation: smooth, 23.7 percent (95 percent CI, 15.0 to 36.2 percent); textured, 34.9 percent (95 percent CI, 23.6 to 49.6 percent).
Reconstruction: smooth, 25.8 percent (95 percent CI, 12.9 to 47.5 percent); textured, 23.7 percent (95 percent CI, 13.9 to 38.6 percent).
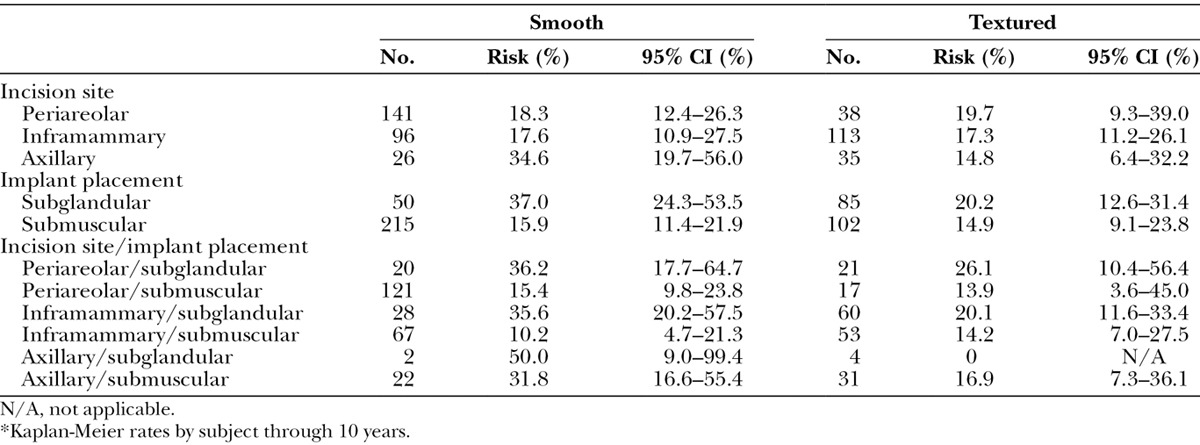
To better inform surgical decision-making, we further characterized the augmentation capsular contracture rate by implant texture, incision site, and implant placement (Tables 3 and 4). Using a Z test with Greenwood estimate for variance,5 the significant differences were for subglandular (26.3 percent) versus submuscular (15.7 percent) (p = 0.023), and smooth implants subglandular (37.0 percent) versus smooth implants submuscular (15.9 percent) (p = 0.008). Subglandular placement with smooth implants (37.0 percent) versus subglandular placement with textured implants (20.2 percent) was borderline significant (p = 0.058), and axillary incision with smooth implants (34.6 percent) versus axillary incision with textured implants (14.8 percent) trended toward significance (p = 0.077).
Table 3.
Capsular Contracture for Augmentation Subjects by Implant Texture, Incision Site, and Implant Placement*
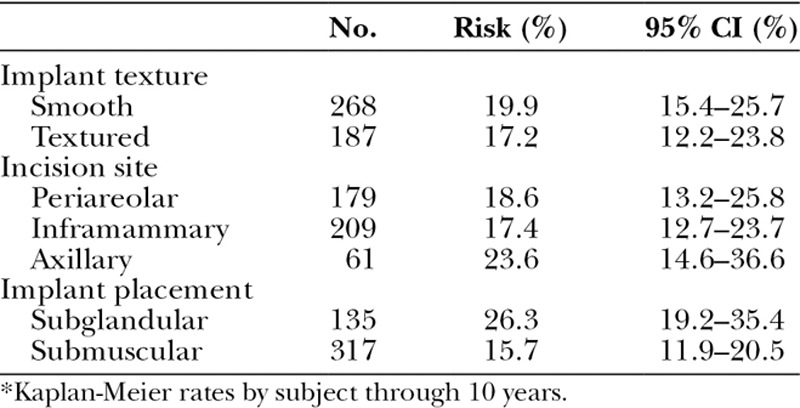
Table 4.
Capsular Contracture for Augmentation Subjects with Smooth versus Textured Implants*
The risk of seroma was low (Table 1), and there were just five late-occurring seromas (beyond 1 year): one augmentation (subglandular, inframammary, textured implant), three revision-augmentations (partial submuscular, periareolar, textured implants, two bilaterally in the same subject, and all three at the same investigational site), and one reconstruction (complete submuscular, mastectomy scar placement, textured implant). Thus, late seromas were found in 0.37 percent of 1348 implants overall and in 0.86 percent of 584 textured implants.
New diagnoses of breast cancer were less than 1 percent (augmentation, 0.9 percent and revision-augmentation, 0.7 percent), and there were no instances of the implant interfering with mammography. The only non–breast cancer reported in the study through 10 years was brain cancer in one augmentation subject (0.2 percent). Anaplastic large cell lymphoma was not reported in any subjects. Connective tissue disease rates were also low (1.1 percent for augmentation, 1.4 percent for revision-augmentation, and 2.0 percent for reconstruction), with rheumatoid arthritis and fibromyalgia being those most commonly reported.
Satisfaction
At 10 years, augmentation subjects rated their satisfaction with implants as a mean of 4.7 on a five-point scale where 1 is definitely dissatisfied and 5 is definitely satisfied. The mean satisfaction was 4.4 for revision-augmentation and 4.6 for reconstruction subjects, and the physician assessments were 4.8, 4.4, and 4.7, respectively. Using the results from this same scale, 94.2 percent of augmentation, 83.8 percent of revision-augmentation, and 90.7 percent of reconstruction subjects reported being satisfied or definitely satisfied with their implants.
Before implantation and at years 2, 4, 6, 8, and 10, subjects rated their satisfaction with their breasts overall and satisfaction with breast size, shape, feel, and how well they matched. For each cohort, there was a significant improvement (p < 0.001) in all of these measures from baseline to year 1, and the results remained significantly improved at every time point thereafter. For augmentation subjects, 4.1 percent of subjects were satisfied or very satisfied at baseline (on a five-point scale ranging from very dissatisfied to very satisfied), and that increased to 92.9 percent at 10 years. Satisfaction with breast size increased from 2.2 percent to 89.1 percent; shape, from 20.1 percent to 86.8 percent; and feel, from 40.2 percent to 87.4 percent. Subjects rated how well their breasts matched on a six-point scale (excellent, very good, good, fair, poor, and very poor), and augmentation ratings of good to excellent increased from 62.9 percent before implantation to 94.2 percent at 10 years.
Large increases in breast satisfaction ratings were also seen in the other cohorts. Revision-augmentation overall satisfaction increased from 35.1 percent to 78.3 percent at 10 years, satisfaction with size increased from 40.5 percent to 91.9 percent, satisfaction with shape increased from 40.5 percent to 75.6 percent, satisfaction with feel increased from 37.8 percent to 70.2 percent, and satisfaction with breast matching increased from 48.6 percent to 88.6 percent. For reconstruction subjects, overall breast satisfaction increased from 33.3 percent to 81.9 percent; satisfaction with size increased from 27.3 percent to 66.7 percent; satisfaction with shape increased from 27.2 percent to 60.7 percent; satisfaction with feel increased from 21.2 percent to 75.8 percent; and satisfaction with breast matching increased from 31.0 percent to 65.5 percent.
DISCUSSION
As we assess the significance of the 10-year data in this prospective, multicenter, U.S. Food and Drug Administration–regulated clinical trial, it is important to start with a discussion of the statistical tool used here, which is the Kaplan-Meier method. The risk that is shown at 10 years is the cumulative risk over the 10-year period. Thus, breast pain of 11.5 percent or swelling of 9.2 percent does not mean that subjects had breast pain or swelling at 10 years, but rather that those percentages of subjects had those symptoms at some point in time during the preceding 10 years. The symptoms could have all been in the first 30 days after surgery, but they would still be carried out to 10 years and included in the 10-year cumulative data. For example, augmentation breast pain was 9.6 percent at 6 years and 11.5 percent at 10 years, suggesting that only 2 percent of subjects developed pain over the past 4 years. Similarly, swelling went from 8.3 percent of augmented subjects at 6 years to 9.2 percent of subjects at 10 years.2
In looking at the data overall, the slope and shape of the data curve are more interesting than the absolute number at 10 years. The slope of the capsular contracture curve is steepest in the first year, where it goes from 0 percent to 6.5 percent in augmentation but only rises an additional 12 percent to 18.9 percent over the next 9 years (Fig. 2). The slope of the rupture curve is different. It is virtually 0 percent through the first 3 years and then gradually climbs to 7.7 percent of implants at 10 years (Fig. 1).
Each of the tracked complications will have its unique curve and slope. The hope and reality of most of these events is that, although the total percentage is increasing over time, the slope is flat and the rate of increase is slow. For example, removal without replacement was 2.8 percent for augmentation at 6 years and remained 2.8 percent at 10 years—a flat slope with no additional women having their implants removed without replacement between 6 and 10 years. Augmentation reoperations were modestly increased from 28.0 percent at 6 years to 36.1 percent at 10 years. Augmentation capsular contracture was relatively unchanged from 14.8 percent at 6 years to 18.9 percent at 10 years. The incremental increases in the other less common complications were all very small between 6 and 10 years.2
Because of the increased interest in the effect of incision, pocket, and texture on outcomes, the data were further analyzed to look at those variables as well. As the Core Study was not designed to specifically capture that information, one needs to be careful in drawing definitive conclusions from this analysis. Nevertheless, this additional data mining did yield some interesting findings that either support previous studies or encourage additional investigations.
Regarding implant shell texture, we find that augmentation capsular contracture rates were overall relatively comparable between textured (17.2 percent) and smooth (19.9 percent). There was, however, a more notable though not statistically significant difference when looking at the rates of capsular contracture between the subglandular texture (20.2 percent) and subglandular smooth groups (37.0 percent). This is a finding similar to other published series that specifically looked at that question.6–12
Looking at implant pocket, submuscular (15.7 percent) was significantly better (p = 0.023) than subglandular (26.3 percent). This is consistent with previous research, which has shown lower capsular contracture rates for implants placed in the submuscular plane.8,13 However, a recent study examining risk factors for capsular contracture in augmentation patients found that implant pocket was not a significant risk factor, and only implant type (silicone), antibiotic pocket irrigation, and patients being nonsmokers contributed significantly to reducing capsular contracture rates.14
Regarding the possible effect of different augmentation incisions on capsular contracture, inframammary (17.4 percent) and periareolar (18.6 percent) incisions had lower rates than axillary (23.6 percent) incisions and trended toward significance (p = 0.077) when the axillary incision was further stratified by smooth (34.6 percent) and textured (14.8 percent). The lowest capsular contracture rates at 10 years were seen with inframammary submuscular smooth (10.2 percent) or textured (14.2 percent) implants and periareolar submuscular textured implants (13.9 percent). The highest rates were with transaxillary subglandular smooth (50 percent, albeit with an n of 2), periareolar subglandular smooth (36.2 percent), and inframammary subglandular smooth implants (35.6 percent). This finding of possible correlation between incision choice and capsular contracture rates is in agreement with other studies that have looked at this issue.15,16
Silicone-filled breast implants have now been back on the U.S. market for 7 years since approval by the U.S. Food and Drug Administration in November of 2006, and this generation of implants has been widely available around the world for over 20 years. Since our report on the 6-year data from the Core Study,2 not much has changed in terms of safety information or health concerns as reviewed by the National Academy of Science’s Institute of Medicine, Judge Pointer’s federal court panel of independent experts, and multiple independent professional societies and governmental oversight agencies.17,18
There are two recent events that are peripherally related and relevant to this topic. The first is the recent report of a problem associated with one specific European manufacturer of implants where there were frequent early ruptures associated with poorly performing implant shells and release of non–medical-grade silicone.19 The second is the report of anaplastic large cell lymphoma identified in the breasts of a very small number of women with breast implants, with the possibility that this number is higher than expected and possibly associated with their breast implants.20 This topic is extraordinarily complex and outside the scope of this report but is a reminder of the wider context in which these data are presented. This 10-year prospective study was designed to acquire specific information and events that could be captured and interpreted in a study looking at 715 women. It was not designed for, nor could it be expected to provide, meaningful data on very rare events.
Careful review of the tables will provide insight into the likelihood of certain events occurring over the span of 10 years following primary breast augmentation, revision breast augmentation, and breast reconstruction. Of particular interest is the 10-year risk after augmentation of magnetic resonance imaging–diagnosed rupture in 9.3 percent of subjects and capsular contracture in 18.9 percent. Also interesting was the permanent removal of implants without replacement in only 2.8 percent of augmented women by 10 years. Surgeons and patients now have 10-year outcome data to guide them in decision-making and can be reassured by the safety and high satisfaction levels associated with Natrelle silicone-filled breast implants at 10 years.
Footnotes
This study is registered under the name “Safety and Effectiveness of Natrelle(TM) Cohesive Round Silicone-Filled Breast Implants,” ClinicalTrials.gov identification number NCT00689871 (http://www.clinicaltrials.gov/ct2/show/NCT00689871?term=NCT00689871&rank=1).
Disclosure: Allergan designed and funded the study, and statistical analysis of the data was performed by James Robinson, M.S., and Ramkumar Krish, M.S., of Allergan. Scott L. Spear, M.D., is an Allergan consultant and received research support for conducting this study. Diane K. Murphy, M.B.A., is an Allergan employee and stockholder.
REFERENCES
- 1.Jewell ML. Silicone gel breast implants at 50: The state of the science. Aesthet Surg J. 2012;32:1031–1034. doi: 10.1177/1090820X12461649. [DOI] [PubMed] [Google Scholar]
- 2.Spear SL, Murphy DK, Slicton A, Walker PS Inamed Silicone Breast Implant U.S. Study Group. Inamed silicone breast implant core study results at 6 years. Plast Reconstr Surg. 2007;120(Suppl 1):8S–16S; discussion 17S. doi: 10.1097/01.prs.0000286580.93214.df. [DOI] [PubMed] [Google Scholar]
- 3.Murphy DK, Beckstrand M, Sarwer DB. A prospective, multi-center study of psychosocial outcomes after augmentation with natrelle silicone-filled breast implants. Ann Plast Surg. 2009;62:118–121. doi: 10.1097/SAP.0b013e31817f01f8. [DOI] [PubMed] [Google Scholar]
- 4.Gladfelter J, Murphy D. Breast augmentation motivations and satisfaction: A prospective study of more than 3,000 silicone implantations. Plast Surg Nurs. 2008;28:170–174; quiz 175. doi: 10.1097/PSN.0b013e31818ea7e0. [DOI] [PubMed] [Google Scholar]
- 5.Com-Nougue C, Rodary C, Patte C. How to establish equivalence when data are censored: A randomized trial of treatments for B non-Hodgkin lymphoma. Stat Med. 1993;12:1353–1364. doi: 10.1002/sim.4780121407. [DOI] [PubMed] [Google Scholar]
- 6.Burkhardt BR, Demas CP. The effect of Siltex texturing and povidone-iodine irrigation on capsular contracture around saline inflatable breast implants. Plast Reconstr Surg. 1994;93:123–128; discussion 129. [PubMed] [Google Scholar]
- 7.Asplund O, Gylbert L, Jurell G, Ward C. Textured or smooth implants for submuscular breast augmentation: A controlled study. Plast Reconstr Surg. 1996;97:1200–1206. doi: 10.1097/00006534-199605000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Barnsley GP, Sigurdson LJ, Barnsley SE. Textured surface breast implants in the prevention of capsular contracture among breast augmentation patients: A meta-analysis of randomized controlled trials. Plast Reconstr Surg. 2006;117:2182–2190. doi: 10.1097/01.prs.0000218184.47372.d5. [DOI] [PubMed] [Google Scholar]
- 9.Wong CH, Samuel M, Tan BK, Song C. Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: A systematic review. Plast Reconstr Surg. 2006;118:1224–1236. doi: 10.1097/01.prs.0000237013.50283.d2. [DOI] [PubMed] [Google Scholar]
- 10.Hakelius L, Ohlsén L. Tendency to capsular contracture around smooth and textured gel-filled silicone mammary implants: A five-year follow-up. Plast Reconstr Surg. 1997;100:1566–1569. doi: 10.1097/00006534-199711000-00030. [DOI] [PubMed] [Google Scholar]
- 11.Spear SL, Elmaraghy M, Hess C. Textured-surface saline-filled silicone breast implants for augmentation mammaplasty. Plast Reconstr Surg. 2000;105:1542–1552; discussion 1553. [PubMed] [Google Scholar]
- 12.Collis N, Coleman D, Foo IT, Sharpe DT. Ten-year review of a prospective randomized controlled trial of textured versus smooth subglandular silicone gel breast implants. Plast Reconstr Surg. 2000;106:786–791. doi: 10.1097/00006534-200009040-00005. [DOI] [PubMed] [Google Scholar]
- 13.Henriksen TF, Fryzek JP, Hölmich LR, et al. Surgical intervention and capsular contracture after breast augmentation: A prospective study of risk factors. Ann Plast Surg. 2005;54:343–351. doi: 10.1097/01.sap.0000151459.07978.fa. [DOI] [PubMed] [Google Scholar]
- 14.Blount AL, Martin MD, Lineberry KD, Kettaneh N, Alfonso DR. Capsular contracture rate in a low-risk population after primary augmentation mammaplasty. Aesthet Surg J. 2013;33:516–521. doi: 10.1177/1090820X13484465. [DOI] [PubMed] [Google Scholar]
- 15.Wiener TC. Relationship of incision choice to capsular contracture. Aesthetic Plast Surg. 2008;32:303–306. doi: 10.1007/s00266-007-9061-2. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson JM, Gatti ME, Schaffner AD, Hill LM, Spear SL. Effect of incision choice on outcomes in primary breast augmentation. Aesthet Surg J. 2012;32:456–462. doi: 10.1177/1090820X12444267. [DOI] [PubMed] [Google Scholar]
- 17.Bondurant S, Ernster V, Herdman R Committee on the Safety of Silicone Breast Implants, Division of Health Promotion and Disease Prevention, Institute of Medicine. Safety of Silicone Implants. Washington, DC: Institute of Medicine; 2000. [Google Scholar]
- 18.Tugwell P, Wells G, Peterson J, et al. Do silicone breast implants cause rheumatologic disorders? A systematic review for a court-appointed national science panel. Arthritis Rheum. 2001;44:2477–2484. doi: 10.1002/1529-0131(200111)44:11<2477::aid-art427>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 19.Maijers MC, Niessen FB. Prevalence of rupture in poly implant Prothèse silicone breast implants, recalled from the European market in 2010. Plast Reconstr Surg. 2012;129:1372–1378. doi: 10.1097/PRS.0b013e31824f0108. [DOI] [PubMed] [Google Scholar]
- 20.Eaves FF, Haeck PC, Rohrich RJ. Breast implants and anaplastic large cell lymphoma: Using science to guide our patients and plastic surgeons worldwide. Plast Reconstr Surg. 2011;127:2501–2503. doi: 10.1097/PRS.0b013e31821787e0. [DOI] [PubMed] [Google Scholar]


