Abstract
Objectives. To evaluate oral tofacitinib versus placebo for treatment of active rheumatoid arthritis in Japanese patients with inadequate response to disease-modifying antirheumatic drugs.
Methods. In this double-blind, placebo-controlled, randomized, parallel-group, 12-week, phase 2 study (clinicaltrials.gov NCT00687193), 317 patients received tofacitinib: 1, 3, 5, 10, or 15 mg as monotherapy or placebo twice daily (BID). Primary endpoint: response rate by American College of Rheumatology (ACR) ≥ 20% improvement criteria (ACR20) at week 12.
Results. ACR20 response rates: 37.7% (20/53), 67.9% (36/53), 73.1% (38/52), 84.9% (45/53), and 90.7% (49/54) with tofacitinib: 1, 3, 5, 10, and 15 mg BID, respectively, versus 15.4% (8/52) with placebo (p < 0.01; all doses). Dose-dependent ACR20 responses with tofacitinib versus placebo occurred from week 2 onward (p < 0.05). Changes from baseline in 28-joint disease activity score using erythrocyte sedimentation rate improved with tofacitinib versus placebo from week 4 (p < 0.01; all doses). Six tofacitinib patients experienced treatment-related serious adverse events (AEs). Most common treatment-emergent AEs: nasopharyngitis (10% vs 12%) and hyperlipidemia (5% vs 0%). Serum creatinine, hemoglobin, and total-, low-, and high-density lipoprotein-cholesterol levels increased with tofacitinib.
Conclusions. Tofacitinib produced dose-dependent ACR20 responses and reduced disease activity. The safety profile was consistent with that reported from global monotherapy trials.
Keywords: Japan, Monotherapy, Randomized controlled trial, Rheumatoid arthritis, Tofacitinib
Introduction
Rheumatoid arthritis (RA) is a chronic, debilitating disease that negatively impacts on patient quality of life. Treatment options are based on disease-modifying antirheumatic drugs (DMARDs), typically starting with methotrexate [1–3]. Biologic DMARDs, such as tumor necrosis factor inhibitors (TNFis), are often used in patients with inadequate response to methotrexate (or other synthetic DMARDs). In Japan, the biologic DMARDs, infliximab, etanercept, adalimumab, golimumab, certolizumab pegol (TNFi), tocilizumab and abatacept, have been approved for use in patients with active RA and an inadequate response to existing therapies [4–6]. However, not all patients achieve an adequate response with available synthetic or biologic DMARDs [7–11]. Therefore, there remains an unmet need for additional therapeutic options with alternative mechanisms of action.
Tofacitinib is an oral Janus kinase (JAK) inhibitor for the treatment of RA [12,13]. Tofacitinib preferentially inhibits signaling by heterodimeric receptors associated with JAK3 and/or JAK1 with functional selectivity over receptors that signal via pairs of JAK2, blocking signaling for several cytokines, including interleukin (IL)-2, -4, -7, -9, -15, and -21 [14]. These cytokines are integral to lymphocyte activation and function, and inhibition of their signaling may modulate multiple aspects of the immune response [15].
Tofacitinib has demonstrated efficacy as monotherapy or in combination with DMARDs (mostly methotrexate) for the treatment of active RA in 6 phase 3 randomized controlled trials (RCTs) in various patient populations [16–21]. Furthermore, a phase 2 RCT in Japanese patients who had an inadequate response to methotrexate reported tofacitinib efficacy and safety in combination with stably dosed methotrexate [22]. Here, we evaluated multiple doses of tofacitinib monotherapy versus placebo for the treatment of RA in Japanese patients who have had an inadequate response to synthetic or biologic DMARDs.
Materials and methods
Patients
Key inclusion criteria included a diagnosis of RA based on the American College of Rheumatology (ACR) 1987 revised criteria [23]. Patients were required to have an active disease defined as ≥ 6 tender/painful joints and ≥ 6 swollen joints, and either erythrocyte sedimentation rate (ESR) above upper limit of normal (ULN) or C-reactive protein (CRP) > 7 mg/L. Patients were also required to have had an inadequate response to at least one synthetic or biologic DMARD, which had washed out ≥ 4 weeks prior to the first dose.
Key exclusion criteria included: cytopenias; estimated glomerular filtration rate < 50 mL/min (Cockcroft–Gault calculation); total bilirubin, aspartate transaminase (AST), or alanine transaminase (ALT) > 2 × ULN; and evidence of active infection, including latent tuberculosis.
Study design and treatment
This was a 12-week, randomized, double-blind, placebo-controlled, parallel-group phase 2 study conducted at 47 centers in Japan from March 2009 to July 2010 (clinicaltrials.gov NCT00687193; Pfizer protocol A3921040). The study was performed in compliance with the 2008 update of the Declaration of Helsinki and the International Conference on Harmonisation's Good Clinical Practice Guidelines, and approved by the Institutional Review Boards at each study center. All patients provided written informed consent.
Based on a randomization table pre-prepared by the study sponsor's Global Clinical Data Service Department, patients were randomized (1:1:1:1:1:1) to tofacitinib: 1, 3, 5, 10, or 15 mg, or placebo, given orally twice daily (BID) for 12 weeks. The study sponsor, investigators, and patients were blinded to the identity of study medications.
Concomitant medications
No DMARDs were permitted as concomitant therapy during the study. Cytochrome P450 (CYP) 3A inducers and CYP3A4, 5, and 7 inhibitors were not permitted due to the potential for drug interactions, as tofacitinib metabolism is primarily mediated by CYP3A4 [24]. Stable doses of non-steroidal anti-inflammatory drugs, selective cyclo-oxygenase-2 inhibitors, or glucocorticoids (≤ 10 mg/day prednisone or equivalent) were permitted, provided they were stably dosed for ≥ 4 weeks before the first study drug dose.
Study assessments
The primary objective was to evaluate the dose–response relationship of the 5 tofacitinib doses compared with placebo; the primary efficacy endpoint was the response rate according to the ACR 20% improvement criteria (ACR20) compared with placebo at week 12. Secondary efficacy endpoints included ACR20 at all other timepoints, ACR50 and ACR70 response rates, change from baseline in 28-joint disease activity score using ESR (DAS28-4[ESR]), and proportions of patients achieving DAS-defined remission (DAS28-4[ESR] < 2.6) [25]. Other secondary efficacy endpoints included selected patient-reported outcomes, namely the Health Assessment Questionnaire-Disability Index (HAQ-DI) [26], Medical Outcomes Study Short-Form (36-item) Health Survey (SF-36), and the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) scale [27]. ACR, DAS, and HAQ-DI assessments were performed at screening, baseline, and weeks 2, 4, 8, and 12. SF-36 was assessed at baseline and week 12, or early termination. FACIT-F was assessed at baseline, week 2, and week 12, or early termination.
Safety endpoints included the incidence and severity of adverse events (AEs), serious AEs, laboratory tests, and vital sign assessments. AEs were recorded at every visit from the first dose through the last visit, and mapped to preferred terms according to the Medical Dictionary for Regulatory Activities (version 13.0). Laboratory parameters were measured at a central laboratory, except ESR, which was measured locally. Hematologic, lipid, hepatic, and renal function tests were performed at baseline, weeks 2, 4, 8, and 12, or early termination.
Statistical analysis
The planned sample size of 50 patients per group (6 groups; total 300) had ≥ 80% power, with a significance level of 5%, to detect a 30% difference from placebo in the ACR20 response rate, assuming the placebo response rate was 35%.
The primary analysis population and the safety analysis set for this study was the full analysis set (FAS; all patients who were randomized to the study and received ≥ 1 dose of study medication).
For the primary endpoint, pairwise comparisons of the tofacitinib doses versus placebo were conducted using a chi-squared test with two-sided significance level of 0.05. The type I error rate for the pairwise comparisons was protected using a step-down procedure. When the pairwise comparison of the higher tofacitinib dose group versus placebo was statistically significant, the step-down procedure continued to the next lower tofacitinib dose group versus placebo comparison.
For the secondary endpoints, response rates from binary endpoints were analyzed using the normal approximation to the binomial, comparing each dose of study treatment with placebo. For continuous measures, a longitudinal, mixed-effect, repeated-measures model was employed. Treatment, week, and treatment-by-week interaction were included as fixed effects, along with patients as a random effect. Estimates of mean and mean difference from placebo were derived from the model and contrasts with placebo were formed.
To address missing data, 3 types of imputation were employed: baseline observation carried forward, also known as non-responder imputation (NRI); last observation carried forward (LOCF); and data as is (no imputation). LOCF was used for the ACR20 primary analysis at week 12; additional analyses were performed as a measure of robustness of the results.
Post-hoc exploratory subanalyses were performed on the primary endpoint, ACR20 at week 12, and DAS28-4(ESR) remission rates, with respect to patient age, baseline DAS28-4(ESR), and RA disease duration.
Results
Patients
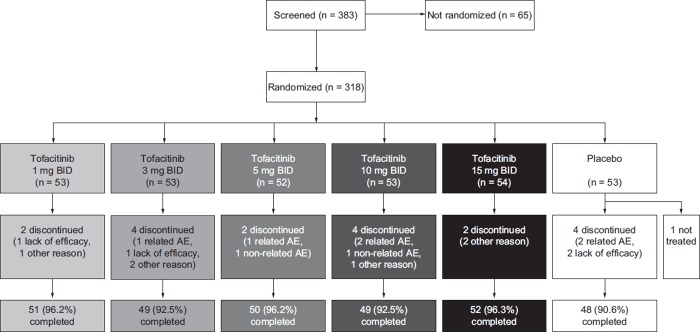
Of 383 patients screened, 318 were randomized; 317 patients received ≥ 1 dose of study medication and 299 patients (94.0%) completed the study (Figure 1). The patient demographics and disease characteristics at baseline were similar across treatment groups (Table 1).
Figure 1. Patient disposition. AEs were categorized according to whether they were considered related to study drug or not. AE adverse event, BID twice daily.
Table 1. Patient baseline demographics and disease characteristics.
| Characteristic, Mean | Tofacitinib | |||||
|---|---|---|---|---|---|---|
| 1 mg BID (n = 53) | 3 mg BID (n = 53) | 5 mg BID (n = 52) | 10 mg BID (n = 53) | 15 mg BID (n = 54) | Placebo (n = 52) | |
| Age, years (SD) | 53.3 (9.9) | 52.8 (11.6) | 52.6 (10.9) | 54.7 (10.8) | 53.6 (12.5) | 53.3 (11.4) |
| Male, n (%) | 11 (20.8) | 6 (11.3) | 8 (15.4) | 9 (17.0) | 10 (18.5) | 9 (17.3) |
| Weight, kg (SD) | 52.9 (9.4) | 54.1 (10.2) | 54.2 (6.6) | 54.1 (10.0) | 53.8 (9.9) | 57.4 (11.7) |
| BMI, kg/m2 (SD) | 21.5 (3.2) | 21.9 (3.8) | 22.2 (2.9) | 21.9 (3.9) | 22.1 (3.2) | 22.8 (3.8) |
| Duration of RA, years (range) | 8.1 (0.5–39.0) | 6.8 (0.6–28.0) | 11.0 (0.4–34.0) | 7.3 (0.5–45.0) | 7.4 (0.5–38.3) | 6.4 (0.5–38.0) |
| Tender joint count (SD) | 13.55 (7.98) | 17.26 (11.44) | 18.58 (13.02) | 17.13 (10.27) | 17.35 (8.96) | 15.10 (8.76) |
| Swollen joint count (SD) | 11.30 (6.49) | 14.64 (10.09) | 15.31 (10.83) | 13.77 (7.66) | 14.48 (8.99) | 11.96 (5.69) |
| PGA, mm (SD) | 60.30 (22.40) | 60.28 (19.92) | 68.77 (22.28) | 64.91 (21.25) | 68.33 (18.79) | 58.13 (25.27) |
| PtGA, mm (SD) | 60.62 (22.19) | 59.57 (18.83) | 70.44 (19.85) | 64.53 (22.51) | 67.00 (19.97) | 58.38 (21.83) |
| Patient pain assessment, mm (SD) | 61.57 (17.37) | 62.13 (18.09) | 71.13 (17.54) | 69.85 (15.21) | 66.93 (17.60) | 61.08 (16.79) |
| HAQ-DI (SD) | 1.25 (0.59) | 1.19 (0.64) | 1.50 (0.69) | 1.20 (0.65) | 1.20 (0.69) | 1.21 (0.69) |
| CRP, mg/L (SD) | 30.21 (28.40) | 25.65 (24.54) | 35.61 (34.15) | 26.88 (27.81) | 27.37 (35.69) | 24.01 (23.01) |
| DAS28-4(ESR) (SD) | 6.04 (0.89) | 6.08 (1.04) | 6.41 (1.05) | 6.06 (0.92) | 6.20 (1.02) | 5.83 (0.93) |
BID twice daily, BMI body mass index, CRP C-reactive protein, DAS28-4(ESR) 28-joint disease activity score using erythrocyte sedimentation rate, HAQ-DI health assessment questionnaire-disability index, PGA physician global assessment, PtGA patient global assessment, RA rheumatoid arthritis, SD standard deviation.
Efficacy
The ACR20 response rates (FAS, LOCF) at week 12 (primary endpoint) were 20/53 (37.7%), 36/53 (67.9%), 38/52 (73.1%), 45/53 (84.9%), and 49/54 (90.7%) patients receiving tofacitinib: 1, 3, 5, 10, and 15 mg BID, respectively, and 8/52 (15.4%) patients receiving placebo (p < 0.0001 vs placebo for all doses of tofacitinib except 1 mg BID, where p < 0.01). The 12-week ACR response rates were similar when NRI was applied (Supplementary Table 1 to be found online at http://informahealthcare.com/doi/abs/10.3109/14397595.2014.995875).
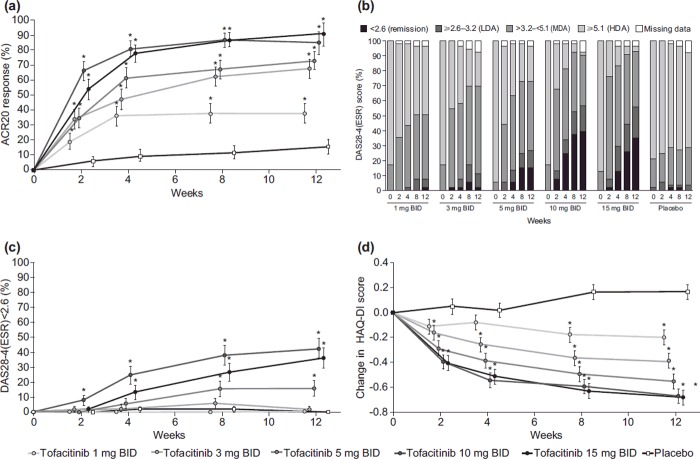
Dose-dependent and statistically significant ACR20 responses were observed in all tofacitinib groups versus placebo from week 2, and were maintained throughout the 12-week period (p < 0.05; Figure 2a). A dose-dependent relationship was also observed for ACR50 response rates over the course of 12 weeks, with significant improvements versus placebo for tofacitinib doses of ≥ 3 mg BID at all timepoints (p < 0.05; Supplementary Figure 1a to be found online at http://informahealthcare.com/doi/abs/10.3109/14397595.2014.995875). In addition, a dose-dependent relationship was seen for ACR70 response rates, with significant improvements versus placebo for tofacitinib doses of ≥ 5 mg BID at all timepoints, except at week 2 with tofacitinib: 5 mg BID; significant improvements in ACR70 were observed with tofacitinib: 3 mg BID at weeks 8 and 12 (Supplementary Figure 1b to be found online at http://informahealthcare.com/doi/abs/10.3109/14397595.2014.995875). For the 1-mg BID dose, significant improvement versus placebo was only seen for ACR 50 response at week 8.
Figure 2. Response rates for patients receiving tofacitinib monotherapy or placebo over time. (a) ACR20 response (± SE), FAS, LOCF. (b) DAS28-4(ESR) < 2.6 (remission), 2.6–3.2 (LDA), > 3.2–< 5.1 (MDA), and ≥ 5.1 (HDA), FAS, no imputation. (c) DAS28-4(ESR) < 2.6 (remission) (± SE), FAS, no imputation. (d) Mean HAQ-DI (± SE) change from baseline, FAS. *p < 0.05 versus placebo. ACR20 American College of Rheumatology 20% improvement criteria, BID twice daily, DAS28-4(ESR) 28-joint disease activity score using erythrocyte sedimentation rate, FAS full analysis set, HAQ-DI Health Assessment Questionnaire-Disability Index, HDA high disease activity, LDA low disease activity, LOCF last observation carried forward, MDA medium disease activity, SE standard error.
Disease activity decreased in a dose-dependent manner over the 12 weeks of treatment (Figure 2b). Mean changes from baseline in DAS28-4(ESR) and ESR showed significant improvement versus placebo from week 2 for all tofacitinib doses (p < 0.01), except 1 mg BID, which showed a statistical difference from placebo at week 4 for DAS28-4(ESR) and week 8 for ESR (p < 0.01; Supplementary Figures 2a and b to be found online at http://informahealthcare.com/doi/abs/10.3109/14397595.2014.995875). The proportion of patients achieving DAS28-defined remission, DAS28-4(ESR) < 2.6, was significantly greater for patients receiving tofacitinib: ≥ 5 mg BID compared with placebo at weeks 8 and 12 (p < 0.05; Figure 2c). The proportion of patients achieving low disease activity, defined as DAS28-4(ESR) ≤ 3.2, was significantly greater than placebo at weeks 4, 8, and 12 for those receiving tofacitinib: ≥ 5 mg BID (p < 0.05).
HAQ-DI values significantly improved from baseline compared with placebo from week 2 onward with tofacitinib doses of ≥ 3 mg BID (p < 0.05), and from week 8 onward with tofacitinib: 1 mg BID (p < 0.0001; Figure 2d). At week 12, a dose-dependent response was seen in the percentage of patients achieving a clinically meaningful decrease (≥ 0.22 units) in HAQ-DI from baseline (p < 0.01 vs placebo; Supplementary Table 2 to be found online at http://informahealthcare.com/doi/abs/10.3109/14397595.2014.995875).
SF-36 scores were significantly improved in patients receiving tofacitinib versus those receiving placebo. The mean change in SF-36 domain scores for physical function, role physical, bodily pain, and role-emotional were significantly higher for all tofacitinib doses versus placebo (p < 0.05). Mean changes in general health, vitality, social function, and mental health domain scores were significantly higher with tofacitinib: ≥ 3 mg BID versus placebo (p < 0.05). The proportion of patients achieving a clinically meaningful increase (≥ 2.5 points) from baseline was higher with all tofacitinib doses versus placebo in the SF-36 physical component score (p < 0.001), and was higher with ≥ 3 mg of tofacitinib BID versus placebo in the SF-36 mental component score (p < 0.05; Supplementary Table 2 to be found online at http://informahealthcare.com/doi/abs/10.3109/14397595.2014.995875).
Change in FACIT-F scores from baseline to week 12 were significantly greater (improved) in all tofacitinib groups versus placebo; mean score change (standard error): 2.51 (0.97), 5.44 (1.00), 7.52 (1.00), 8.51 (1.00), and 8.03 (0.97), with tofacitinib: 1, 3, 5, 10, and 15 mg BID, respectively, versus placebo –1.39 (1.01) (p < 0.01 for all tofacitinib doses vs placebo).
Exploratory subanalyses revealed significantly greater ACR20 response rates in patients receiving tofacitinib: ≥ 3 mg BID versus placebo at week 12 regardless of patient's age (18–44, 45–64, and ≥ 65 years), baseline DAS28-4(ESR) (score ≤ 5.1, > 5.1), and RA disease duration (< 2, ≥ 2 to < 7, ≥ 7 years). However, owing to low patient numbers in these subgroups, results must be interpreted with caution.
Safety
The incidence of treatment-emergent AEs was similar across treatment groups, with a slight trend toward higher incidence with increasing tofacitinib dose (Table 2). Eight patients discontinued due to AEs. There were no deaths in the study.
Table 2. Summary of safety data; all-causality TEAEs.
| Tofacitinib | ||||||
|---|---|---|---|---|---|---|
| 1 mg BID (n = 53) | 3 mg BID (n = 53) | 5 mg BID (n = 52) | 10 mg BID (n = 53) | 15 mg BID (n = 54) | Placebo (n = 52) | |
| TEAEs, n | 37 | 31 | 42 | 62 | 50 | 41 |
| Patients with ≥ 1 TEAE, n (%) | 21 (39.6) | 23 (43.4) | 29 (55.8) | 32 (60.4) | 28 (51.9) | 23 (44.2) |
| Patients with ≥ 1 TESAE, n (%) | 0 | 3 (5.7) | 2 (3.8) | 2 (3.8) | 1 (1.9) | 1 (1.9) |
| Discontinuations due to AEs, n (%) | 0 | 1 (1.9) | 2 (3.8) | 3 (5.7) | 0 | 2 (3.8) |
| Deaths | 0 | 0 | 0 | 0 | 0 | 0 |
AE adverse event, BID twice daily, TEAE treatment-emergent adverse event, TESAE treatment-emergent serious adverse event.
The most common all-causality AEs (in ≥ 10% of patients) were nasopharyngitis and abnormalities in laboratory tests including hyperlipidemia and increased low-density lipoprotein cholesterol (LDL-C); most AEs (Table 3) were mild in severity. In 4 patients, 6 severe AEs were reported: gastric ulcer perforation and rheumatoid vasculitis in the tofacitinib: 3 mg BID group (1 patient each); a fall and fracture of fibula and tibia in 1 patient receiving tofacitinib: 5 mg BID; herpes zoster in 1 patient receiving tofacitinib: 10 mg BID (the multidermatomal rash was widespread and was recorded as a serious AE, see below). No opportunistic infections were reported, including tuberculosis. Nine patients had serious AEs, of whom six had serious events considered treatment-related. The serious AEs were elevated creatine kinase, AST, and ALT levels leading to hospitalization for evaluation (3 mg BID); gastric ulcer perforation (3 mg BID); rheumatoid vasculitis (3 mg BID); and herpes zoster and post-herpetic nerve paralysis considered by the investigator to be attributed to the herpes zoster (5 mg BID), herpes zoster (10 mg BID), and herpes zoster oticus/Ramsay Hunt syndrome (15 mg BID). All patients with serious AEs were withdrawn from the study, and all serious AEs resolved, apart from the herpes zoster oticus and rheumatoid vasculitis; both patients were still recovering at the last follow-up date (98 days and 163 days after study withdrawal, respectively; Supplementary Text to be found online at http://informahealthcare.com/doi/abs/10.3109/14397595.2014.995875).
Table 3. Most common all-causality treatment-emergent AEs occurring in ≥ 2% patients in any of the treatment groups.
| AE, n (%)* | Tofacitinib | |||||
|---|---|---|---|---|---|---|
| 1 mg BID (n = 53) | 3 mg BID (n = 53) | 5 mg BID (n = 52) | 10 mg BID (n = 53) | 15 mg BID (n = 54) | Placebo (n = 52) | |
| Nasopharyngitis | 6 (11.3) | 4 (7.5) | 6 (11.5) | 3 (5.7) | 8 (14.8) | 6 (11.5) |
| Hyperlipidemia | 1 (1.9) | 0 | 2 (3.8) | 6 (11.3) | 3 (5.6) | 0 |
| Headache | 1 (1.9) | 3 (5.7) | 2 (3.8) | 1 (1.9) | 0 | 1 (1.9) |
| LDL-C increased | 0 | 1 (1.9) | 0 | 1 (1.9) | 6 (11.1) | 0 |
| ALT increased | 0 | 2 (3.8) | 0 | 1 (1.9) | 1 (1.9) | 3 (5.8) |
| Constipation | 1 (1.9) | 0 | 1 (1.9) | 0 | 3 (5.6) | 2 (3.8) |
| Pharyngitis | 0 | 0 | 0 | 3 (5.7) | 2 (3.7) | 1 (1.9) |
| Stomatitis | 1 (1.9) | 1 (1.9) | 2 (3.8) | 1 (1.9) | 0 | 1 (1.9) |
| Abdominal discomfort | 2 (3.8) | 1 (1.9) | 0 | 1 (1.9) | 0 | 1 (1.9) |
| AST increased | 0 | 1 (1.9) | 0 | 1 (1.9) | 0 | 3 (5.8) |
| Blood cholesterol increased | 0 | 1 (1.9) | 1 (1.9) | 1 (1.9) | 2 (3.7) | 0 |
| Fall | 3 (5.7) | 0 | 1 (1.9) | 1 (1.9) | 0 | 0 |
| Herpes zoster | 0 | 0 | 1 (1.9) | 3 (5.7) | 1 (1.9) | 0 |
| Hypercholesterolemia | 0 | 2 (3.8) | 0 | 3 (5.7) | 0 | 0 |
| Bronchitis | 1 (1.9) | 1 (1.9) | 0 | 2 (3.8) | 0 | 0 |
| Contusion | 2 (3.8) | 1 (1.9) | 0 | 0 | 1 (1.9) | 0 |
| Dental caries | 0 | 0 | 2 (3.8) | 1 (1.9) | 0 | 1 (1.9) |
| Gingivitis | 0 | 1 (1.9) | 0 | 0 | 2 (3.7) | 1 (1.9) |
| Hypertension | 0 | 0 | 3 (5.8) | 1 (1.9) | 0 | 0 |
| Upper respiratory tract infection | 0 | 0 | 1 (1.9) | 3 (5.7) | 0 | 0 |
| Diarrhea | 1 (1.9) | 0 | 0 | 0 | 0 | 2 (3.8) |
| Gastritis | 0 | 1 (1.9) | 0 | 0 | 2 (3.7) | 0 |
| RA | 0 | 0 | 0 | 0 | 0 | 2 (3.8) |
| Upper respiratory tract inflammation | 0 | 0 | 0 | 0 | 0 | 2 (3.8) |
AE adverse event, ALT alanine transaminase, AST aspartate transaminase, BID twice daily, LDL-C low-density lipoprotein cholesterol.
*Preferred terms according to Medical Dictionary for Regulatory Activities version 13.0.
Significant dose-dependent mean decreases in neutrophil and platelet counts, dose-dependent mean increases in LDL-C, high-density lipoprotein cholesterol (HDLC), and total cholesterol (TC) levels, and mean increases in hemoglobin and serum creatinine levels were observed with tofacitinib versus placebo (Table 4; Supplementary Figure 3 to be found online at http://informahealthcare.com/doi/abs/10.3109/14397595.2014.995875). Moderate-to-severe neutropenia (absolute neutrophil count [ANC] 500–1500/mm3) occurred in 2 patients receiving tofacitinib: 3 mg BID and 5 patients receiving 15 mg BID. None of the patients had potentially life-threatening neutropenia (ANC < 500/mm3). None of the patients had a platelet count < 75000/mm3. Across all groups, 74 patients (23.3%) had their absolute lymphocyte counts decrease to < 1000/mm3 (Table 4). Three patients in the 10 mg BID group had more severe reductions (< 500/mm3; Table 4); none of these patients experienced severe infections, and all three had low lymphocyte counts at baseline. One patient receiving tofacitinib: 1 mg BID had mild iron deficiency anemia that was deemed to be treatment-related. The patient receiving tofacitinib: 3 mg BID who had a gastric ulcer perforation was first discontinued from the study due to a decrease of > 30% from baseline in hematocrit level. The hemoglobin level recorded for this patient at discontinuation was 7.4 g/dL. No trends were seen in changes to hepatic enzymes. ALT or AST levels > 3 × ULN were observed in 3 patients receiving tofacitinib and 2 patients receiving placebo (Table 4). None of the patients were neutropenic at the last study date (Supplementary Text to be found online at http://informahealthcare.com/doi/abs/10.3109/14397595.2014.995875).
Table 4. Mean changes in laboratory parameters from baseline at week 12.
| Parameter Mean change | Tofacitinib | |||||
|---|---|---|---|---|---|---|
| 1 mg BID | 3 mg BID | 5 mg BID | 10 mg BID | 15 mg BID | Placebo | |
| n = 51 | n = 49 | n = 50 | n = 49 | n = 52 | n = 48 | |
| Neutrophils, × 103/mm3 | 0.06 | –0.98‡ | –1.44‡§ | –2.10‡ | –1.66‡ | 0.47 |
| HDL-C, mg/dL | 5.04† | 10.81‡ | 17.73‡ | 21.94‡ | 21.11‡ | –0.94 |
| LDL-C, mg/dL | 3.21 | 11.77† | 16.43‡ | 21.45‡ | 24.69‡ | –0.24 |
| TC, mg/dL | 11.52† | 25.44‡ | 35.83‡ | 50.35‡ | 51.31‡ | –0.96 |
| Hemoglobin, g/dL | 0.15* | 0.25† | 0.48‡§ | 0.56‡ | 0.19* | –0.15 |
| Serum creatinine, mg/dL | 0.01 | 0.02* | 0.04‡ | 0.05‡ | 0.06‡ | –0.01 |
| No. of patients (%) | n = 53 | n = 53 | n = 52 | n = 53 | n = 54 | n = 52 |
|---|---|---|---|---|---|---|
| Lymphocytes, < 1000/mm3 | 11 (20.8) | 6 (11.3) | 11 (21.2) | 12 (22.6) | 16 (29.6) | 18 (34.6) |
| Lymphocytes, < 500/mm3 | 0 | 0 | 0 | 3 (5.7) | 0 | 0 |
| Neutrophils, < 500/mm3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hemoglobin, decrease of 2–3 g/dL from baseline or hemoglobin < 8 g/dL | 0 | 0 | 0 | 0 | 0 | 0 |
| AST or ALT > 3 × ULN | 1 (1.9) | 1 (1.9) | 0 | 0 | 1 (1.9) | 2 (3.8) |
ALT alanine transaminase, AST aspartate transaminase, BID twice daily, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, TC total cholesterol, ULN upper limit of normal.
*p < 0.05; † p < 0.01; ‡ p < 0.001 versus placebo. § n = 49.
Discussion
In Japanese patients with active RA, of mean duration: 6.4–11.0 years across groups, and inadequate response to DMARDs, tofacitinib doses from 1 to 15 mg BID for 12 weeks demonstrated significant improvement in signs and symptoms of RA compared with placebo. Dose-related efficacy was demonstrated for all tofacitinib doses versus placebo by statistically significantly greater rates of patients achieving ACR20, ACR50, ACR70 (at tofacitinib: ≥ 5 mg BID), and DAS28-defined remission (at tofacitinib: ≥ 5 mg BID). Moreover, significant improvements in physical function and other patient-reported outcomes, such as HAQ-DI, SF-36 physical component scores, and FACIT-F scores, represent improvements in function and quality of life measures among patients receiving tofacitinib at all doses.
The incidence of patients experiencing treatment-emergent AEs was similar between treatment groups, with a trend toward higher incidence with increasing tofacitinib dose. Dose-dependent mean changes in laboratory parameters, including decreases in ANC and increases in hemoglobin, cholesterol, and serum creatinine, were also observed with tofacitinib. There were 3 serious AEs of herpes zoster/herpes zoster oticus, and 1 serious AE of rheumatoid vasculitis. Some of the cytokines that are inhibited by tofacitinib play an important role in lymphocyte development and function [28], which suggests that immunosurveillance may be negatively impacted by tofacitinib; however, in most patients no decrease in lymphocyte levels was seen and none of the patients were neutropenic.
The efficacy of tofacitinib monotherapy was similar to a previously published clinical trial of tofacitinib with background methotrexate in 136 Japanese patients with active RA [22], in which 12-week ACR20 response rates (NRI) were 60.7–88.9% with 1–10 mg BID versus 14.3% with placebo (p < 0.0001 for all doses). Similar to the present study, nasopharyngitis was the most common AE. In that study, increases in serum creatinine were reported with tofacitinib versus placebo; the serum creatinine increases in the Tanaka et al.'s study and the present study were not considered clinically meaningful. That study also reported elevated levels of AST and ALT in all groups (including placebo), suggesting that the background methotrexate may have contributed to the elevated transaminase levels.
Tofacitinib efficacy was also similar to previously published RCTs of tofacitinib in global studies of patients with RA [29–31]. The global phase 2b study of tofacitinib monotherapy in 384 treated patients reported significantly greater 12-week ACR20 response rates of 39.2–71.9% (NRI) with doses of 315 mg BID versus 22.0% with placebo [29]. In addition, a global phase 3 study of tofacitinib monotherapy in 610 treated patients reported significantly greater 3-month ACR20 response rates of 59.8% and 65.7% with tofacitinib: 5 mg BID and 10 mg BID, respectively, versus 26.7% with placebo [16]. The above studies reported similar AEs to the present study, including headache, diarrhea, nausea, nasopharyngitis, upper respiratory tract infection, and urinary tract infection. One case of disseminated herpes zoster was reported in the global phase 3 study [16]. A similar percentage of patients in the global monotherapy studies discontinued due to an AE (0–10.8%) [16,29] compared with the present study (0–5.7%). The studies above also reported elevated lipid levels and a slight increase in mean serum creatinine levels with tofacitinib, similar to the present study.
In this study, tofacitinib was associated with increased levels of LDL-C, HDL-C, and TC. IL-6 signaling is implicated in the regulation of cholesterol levels, and an anti-IL-6 receptor monoclonal antibody, tocilizumab, has also been associated with elevated lipid levels in patients with RA [32–34]. As the mode of action of tofacitinib includes inhibition of IL-6 signaling [28], the changes in cholesterol levels in response to tofacitinib in patients with RA may involve a degree of IL-6 inhibition; however, further studies will be required to confirm the mechanism behind these lipid changes.
This study was limited by the 12-week study period; a long-term extension study including patients from the present study has recently been completed to evaluate longer-term safety and efficacy of tofacitinib in Japanese patients (NCT00661661). In addition, this study did not assess radiographic progression, which could have provided further insight into the effect of treatment on the disease process.
In conclusion, tofacitinib produced dose-dependent improvements in signs and symptoms, and disease activity in Japanese patients with RA. The safety profile was consistent with that reported from global monotherapy trials and other studies in Japanese patients.
Supplementary Material
Acknowledgments
This study was sponsored by Pfizer Inc. The authors would like to thank the patients who were involved in this study, and the A3921040 investigators and study team. The authors would like to acknowledge Makoto Suzuki and Keiko Yazawa for their valuable contribution to the study, and So Miyoshi who supported design-operating characteristics work on this study. Medical writing support was provided by Jonny Miller and Kate Silverthorne at Complete Medical Communications and was funded by Pfizer Inc.
Appendix
Principal study investigators
Ryutaro Matsumura, Shigeto Tohma, Masato Matsushita, Yoshiya Tanaka, Hisashi Yamanaka, Nobuyuki Miyasaka, Kouichi Amano, Hajime Yamagata, Shunsuke Mori, Eiichi Suematsu, Yasuhiko Munakata, Hide Yoshida, Katsumi Chiba, Yasushi Nawata, Akihiro Yamaguchi, Shigeru Honjyo, Yuji Yamanishi, Yoshinobu Koyama, Eisuke Shono, Tomomi Tsuru, Kiyoshi Migita, Yukitaka Ueki, Motohiro Oribe, Takao Sugiyama, Yojiro Kawabe, Shigenori Tamaki, Masakazu Kondo, Masaya Mukai, Atsushi Kaneko, Naoki Ishiguro, Tatsuya Atsumi, Kazuhide Tanimura, Kou Katayama, Takayuki Sumida, Mitchishi Tsukano, Mitsuru Sakaguchi, Seizo Yamana, Daisuke Kawabata, Atsushi Kawakami, Hajime Sano, Hiroshi Inoue, Tatsuo Hirose, Junji Chiba, Kenshi Higami, Teruaki Nakano, Yasuhiko Hirabayashi, Masato Yagita, Yutaka Kawahito, Takuya Sawabe.
Disclosure: Data from this study were reported at congresses:
ACR 2011, Chicago, IL, USA, November 9, 2011: Tanaka Y, Takeuchi T, Yamanaka H, Suzuki M, Nakamura H, Toyoizumi S, et al. Tofacitinib (CP-690,550), an oral Janus kinase inhibitor, as monotherapy in Japanese patients with active rheumatoid arthritis: A 12-week phase 2b study. [abstract]. Arthritis Rheum 2011;63(Suppl 10):2192.
55th Annual Meeting of the Japan College of Rheumatology, Kobe, Japan, July 19, 2011: Tanaka Y, Yamanaka H, Nakamura H, Takeuchi T. Study results of oral Jak inhibitor tasocitinib in pat
Conflict of interest
Y Tanaka has received consultancy fees, speaking fees, and honoraria from Abbvie, Chugai, Astellas, Takeda, Santen, MitsubishiTanabe, Pfizer, Janssen, Eisai, Daiichi-Sankyo, UCB Japan, GlaxoSmithKline, and Bristol-Myers-Squib.
T Takeuchi has received consultancy fees, speaking fees, and honoraria from Abbott Japan, AbbVie, Asahi Kasei Medical, Astellas Pharma, AstraZeneca, Bristol-Myers Squibb, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly Japan, Janssen Pharma, Mitsubishi Tanabe Pharma Corporation, Novartis, Pfizer, Symbio, Takeda, and UCB Japan.
H Yamanaka has received consultancy fees, speaking fees, and honoraria from AbbVie, AstraZeneca, Bristol-Myers Squibb, Chugai, Daiichi-Sankyo, Eisai, Janssen Pharma, Mitsubishi Tanabe Pharma Corporation, Pfizer, and Takeda.
H Nakamura and S. Toyoizumi are employees of Pfizer Japan Inc, Tokyo, Japan.
S Zwillich is an employee of Pfizer Inc, Groton, USA.
Supplementary material available online
Supplementary Tables 1–2, Figures 1–3 and Supplementary Text.
References
- Smolen JS, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas D, Burmester G, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69((4)):631–7. doi: 10.1136/ard.2009.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59((6)):762–84. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- Strand V, Singh JA. Improved health-related quality of life with effective disease-modifying antirheumatic drugs: evidence from randomized controlled trials. Am J Manag Care. 2007;13((Suppl 9)):S237–51. [PubMed] [Google Scholar]
- Koike R, Harigai M, Atsumi T, Amano K, Kawai S, Saito K, et al. Japan College of Rheumatology 2009 guidelines for the use of tocilizumab, a humanized anti-interleukin-6 receptor monoclonal antibody, in rheumatoid arthritis. Mod Rheumatol. 2009;19((4)):351–7. doi: 10.1007/s10165-009-0197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike R, Takeuchi T, Eguchi K, Miyasaka N. Update on the Japanese guidelines for the use of infliximab and etanercept in rheumatoid arthritis. Mod Rheumatol. 2007;17((6)):451–8. doi: 10.1007/s10165-007-0626-3. [DOI] [PubMed] [Google Scholar]
- Miyasaka N, Takeuchi T, Eguchi K. Guidelines for the proper use of etanercept in Japan. Mod Rheumatol. 2006;16((2)):63–7. doi: 10.1007/s10165-006-0457-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study group. N Engl J Med. 2000;343((22)):1594–602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor: Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340((4)):253–9. doi: 10.1056/NEJM199901283400401. [DOI] [PubMed] [Google Scholar]
- Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48((1)):35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- Finckh A, Simard JF, Gabay C, Guerne PA. Evidence for differential acquired drug resistance to anti-tumour necrosis factor agents in rheumatoid arthritis. Ann Rheum Dis. 2006;65((6)):746–52. doi: 10.1136/ard.2005.045062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka H, Seto Y, Tanaka E, Furuya T, Nakajima A, Ikari K, et al. Management of rheumatoid arthritis: the 2012 perspective. Mod Rheumatol. 2013;23((1)):1–7. doi: 10.1007/s10165-012-0702-1. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Yamaoka K. JAK inhibitor tofacitinib for treating rheumatoid arthritis: from basic to clinical. Mod Rheumatol. 2012;23:415–24. doi: 10.1007/s10165-012-0799-2. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Maeshima K, Yamaoka K. In vitro and in vivo analysis of a JAK inhibitor in rheumatoid arthritis. Ann Rheum Dis. 2012;71((Suppl 2)):i70–4. doi: 10.1136/annrheumdis-2011-200595. [DOI] [PubMed] [Google Scholar]
- Meyer DM, Jesson MI, Li X, Elrick MM, Funckes-Shippy CL, Warner JD, et al. Anti-inflammatory activity and neutrophil reductions mediated by the JAK1/JAK3 inhibitor, CP-690,550, in rat adjuvant-induced arthritis. J Inflamm (Lond) 2010;7((1)):41. doi: 10.1186/1476-9255-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by γc family cytokines. Nat Immunol Rev. 2009;9((7)):480–90. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367((6)):495–507. doi: 10.1056/NEJMoa1109071. [DOI] [PubMed] [Google Scholar]
- Kremer JM, Li ZG, Hall S, Fleischmann R, Genovese M, Martin-Mola E, et al. Tofacitinib (CP-690,550), an oral JAK inhibitor, in combination with traditional DMARDS: Phase 3 study in patients with active rheumatoid arthritis with inadequate response to DMARDs. Ann Rheum Dis. 2011;70((Suppl 3)):170. [Google Scholar]
- Lee EB, Fleischmann RM, Hall S, van Vollenhoven RF, Bradley J, Gruben D, et al. Radiographic, clinical and functional comparison of tofacitinib monotherapy versus methotrexate in methotrexate-nave patients with rheumatoid arthritis. Arthritis Rheum. 2012;64((Suppl 10)):S1049. [Google Scholar]
- Burmester GR, Blanco R, Charles-Schoeman C, Wollenhaupt J, Zerbini C, Benda B, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381((9865)):451–60. doi: 10.1016/S0140-6736(12)61424-X. [DOI] [PubMed] [Google Scholar]
- van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, García Meijide JA, Wagner S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367((6)):508–19. doi: 10.1056/NEJMoa1112072. [DOI] [PubMed] [Google Scholar]
- van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 2013;65((3)):559–70. doi: 10.1002/art.37816. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Suzuki M, Nakamura H, Toyoizumi S, Zwillich SH. Phase II study of tofacitinib (CP-690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Care Res (Hoboken) 2011;63((8)):1150–8. doi: 10.1002/acr.20494. [DOI] [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31((3)):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Gupta P, Alvey C, Wang R, Dowty ME, Fahmi OA, Walsky RL, et al. Lack of effect of tofacitinib (CP-690,550) on the pharmacokinetics of the CYP3A4 substrate midazolam in healthy volunteers: confirmation of in vitro data. Br J Clin Pharmacol. 2012;74((1)):109–15. doi: 10.1111/j.1365-2125.2012.04168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radboud University Nijmegen Medical Centre DAS-score.nl: Disease activity score in rheumatoid arthritis. http://www.das-score.nl [Google Scholar]
- Fries JF, Spitz PW, Young DY. The dimensions of health outcomes: the health assessment questionnaire, disability and pain scales. J Rheumatol. 1982;9((5)):789–93. [PubMed] [Google Scholar]
- Cella D, Yount S, Sorensen M, Chartash E, Sengupta N, Grober J. Validation of the functional assessment of chronic illness therapy fatigue scale relative to other instrumentation in patients with rheumatoid arthritis. J Rheumatol. 2005;32((5)):811–9. [PubMed] [Google Scholar]
- Ghoreschi K, Jesson MI, Li X, Lee JL, Ghosh S, Alsup JW, et al. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550) J Immunol. 2011;186((7)):4234–43. doi: 10.4049/jimmunol.1003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann R, Cutolo M, Genovese MC, Lee EB, Kanik KS, Sadis S, et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum. 2012;64((3)):617–29. doi: 10.1002/art.33383. [DOI] [PubMed] [Google Scholar]
- Kremer JM, Cohen S, Wilkinson BE, Connell CA, French JL, Gomez-Reino J, et al. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum. 2012;64((4)):970–81. doi: 10.1002/art.33419. [DOI] [PubMed] [Google Scholar]
- Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009;60((7)):1895–905. doi: 10.1002/art.24567. [DOI] [PubMed] [Google Scholar]
- Kawashiri SY, Kawakami A, Yamasaki S, Imazato T, Iwamoto N, Fujikawa K, et al. Effects of the anti-interleukin-6 receptor antibody, tocilizumab, on serum lipid levels in patients with rheumatoid arthritis. Rheumatol Int. 2011;31((4)):451–6. doi: 10.1007/s00296-009-1303-y. [DOI] [PubMed] [Google Scholar]
- Koike T, Harigai M, Inokuma S, Ishiguro N, Ryu J, Takeuchi T, et al. Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann Rheum Dis. 2011;70((12)):2148–51. doi: 10.1136/ard.2011.151092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto N, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Azuma J, Kishimoto T. Study of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): significant reduction in disease activity and serum vascular endothelial growth factor by IL-6 receptor inhibition therapy. Mod Rheumatol. 2009;19((1)):12–19. doi: 10.1007/s10165-008-0125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.