Abstract
Unlike other aspects of language comprehension, the ability to process complex sentences develops rather late in life. Brain maturation as well as verbal working memory (vWM) expansion have been discussed as possible reasons. To determine the factors contributing to this functional development, we assessed three aspects in different age-groups (5–6 years, 7–8 years, and adults): first, functional brain activity during the processing of increasingly complex sentences; second, brain structure in language-related ROIs; and third, the behavioral comprehension performance on complex sentences and the performance on an independent vWM test. At the whole-brain level, brain functional data revealed a qualitatively similar neural network in children and adults including the left pars opercularis (PO), the left inferior parietal lobe together with the posterior superior temporal gyrus (IPL/pSTG), the supplementary motor area, and the cerebellum. While functional activation of the language-related ROIs PO and IPL/pSTG predicted sentence comprehension performance for all age-groups, only adults showed a functional selectivity in these brain regions with increased activation for more complex sentences. The attunement of both the PO and IPL/pSTG toward a functional selectivity for complex sentences is predicted by region-specific gray matter reduction while that of the IPL/pSTG is additionally predicted by vWM span. Thus, both structural brain maturation and vWM expansion provide the basis for the emergence of functional selectivity in language-related brain regions leading to more efficient sentence processing during development.
Keywords: Brain development, Functional selectivity, Language network, Sentence processing, Verbal working memory
Introduction
Language acquisition rests on coherent developmental trajectories on cognitive behavioral, brain structural, and brain functional levels. Children typically acquire their native language spontaneously and without conscious effort. Newborns are equipped with amazing implicit mechanisms to acquire each language they are exposed to, but their ability to discriminate non-native sound contrasts (Werker and Tees, 2002) or to automatically extract rules from the auditory input (Mueller et al., 2012) is dramatically reduced as the underlying brain systems mature (Citron et al., 2011). For languages learned after childhood, high proficiency can only be achieved via more explicit learning mechanisms (for a review, see Zevin et al., 2012), suggesting that native language acquisition is confined to a certain period (Lenneberg, 1967). Within this period, acquisition of native phonetic and prosodic processing skills take place during the first year of life (for a review, see Kuhl and Rivera-Gaxiola, 2008, Kuhl, 2004), whereas grammar acquisition, although starting before the age of 3 years (Hamburger and Crain, 1982, Weissenborn, 1994), extends until the age of 7 years (Dittmar et al., 2008, Johnson and Newport, 1989, Meisel, 2011, Zevin et al., 2012).
The observed developmental periods for specific aspects of language acquisition suggest fundamental time windows for neural plasticity in language-relevant brain regions. Seminal studies on the relationship between structural brain maturation and language development found that receptive and productive phonological skills of children between 5 and 11 years correlate with measurements of gray matter probability (GMP) in the left inferior frontal gyrus (IFG; Lu et al., 2007), and that gray matter of the left supramarginal gyrus and left posterior temporal regions correlate with vocabulary knowledge in teenagers between 12 and 17 years of age (Richardson et al., 2010). In general, gray matter density decreases along development, with higher-order association regions decreasing later than lower-order sensorimotor regions (Brain Development Cooperative Group, 2012, Gogtay et al., 2004). Specifically, gray matter in those frontal and parietal brain regions that are involved in sentence processing in adults (for a review, see Friederici, 2011) only appears to decrease between 7 and 12 years (Giedd et al., 1999, Sowell et al., 2003), and recent structural imaging data have shown that the structural integrity of these regions correlates with the crucial cognitive abilities underlying complex sentence comprehension (Fengler et al., 2015).
However, it is unclear whether the maturation of cortical gray matter constrains the functional attunement of language-relevant brain areas to sentence processing. In the fully matured adult brain, a functional dissociation of syntactic and semantic processing has been shown within the IFG (Goucha and Friederici, 2015, Newman et al., 2010), with the left PO being involved in syntactic processing, and semantic processes involving more anterior parts of the IFG (Friederici, 2011). Children, around the age of 6 years, however, do not yet show a similar functional segregation in the IFG (Brauer and Friederici, 2007, Skeide et al., 2014). Moreover, in adults, the left PO has been found to increase its activation with the complexity of sentences (Friederici, 2011, Kinno et al., 2008, Makuuchi et al., 2009, Newman et al., 2010, Röder et al., 2002), whereas a functional selectivity for sentence complexity in this region only emerges around the age of 6 years (Knoll et al., 2012). In adults, the processing of complex sentences, however, is not supported by the IFG alone, but rather by a frontotemporal network (Friederici, 2011). This network also includes the pSTG, which is thought to subserve the integration of syntactic and semantic information; in addition, the network involves the IPL, which is proposed to subserve verbal working memory (vWM) during sentence comprehension (Meyer et al., 2012). In children, the pSTG and the IPL are also part of the network active during sentence processing—at least in 6-years-olds (Brauer and Friederici, 2007, Knoll et al., 2012). Thus, there are indications that the functional language network develops toward an adult-like system around the age of 6 years. While developmental trajectories from children-like to adult-like functional activation patterns within this network have been described with respect to both brain functional changes and brain structural changes of the gray matter, descriptions of the tripartite relationship between brain structure, brain function, and behavioral performance are rare. From the two studies investigating the tripartite relationship at school age, one used orthographic naming (Lu et al., 2009) and the other a sentence comparison paradigm (Nuñez et al., 2011).
Here, we investigate three age-groups: children aged 5–6 years, aged 7–8 years and adults. We hypothesized that gray matter maturation of the language-relevant brain regions in the left hemisphere across age-groups may lead to adult-like brain activation patterns for complex sentence processing, and that more mature activation patterns are associated with better performance. In addition, a hypothesis concerning the vWM was formulated, based on the findings that the processing of complex sentences is memory-demanding (Felser et al., 2003a), and vWM expansion has been proposed as a crucial predictor of children’s sentence processing skills between 6 and 8 years of age (Felser et al., 2003b, Montgomery et al., 2008, Weighall and Altmann, 2011). We hypothesized that the activation pattern for complex sentence processing may partially be predicted by activation in the IPL; in turn, the activation of the IPL should correlate with an increase of vWM span. To test these hypotheses, a number of different analyses were performed. First, to assess functional brain activation, we conducted functional magnetic resonance imaging (fMRI) during the processing of syntactically complex as compared to simple sentences in all three age-groups. Sentence complexity was operationalized by varying the number of embedded relative clauses with increasing levels of hierarchy systematically leading to more complex sentence structures (see Fig. 1). Previous studies attributed children’s difficulties in processing relative clauses to limitations in the cognitive capacities such as vWM (e.g., Kidd and Bavin, 2002, Kidd et al., 2007), which result in a non-adult-like processing strategy (Felser et al., 2003b, Sheldon, 1977, Tavakolian, 1981). Behavioral performance on these sentences was assessed during fMRI scanning for each participant using a sentence–picture-matching paradigm (see Fig. 1 and Method Section). In addition to these sentence comprehension tests, the vWM capacity of each participant was measured by a standardized Digit Span test (Tewes, 1994). Second, to evaluate whether brain structural maturation underlies brain functional maturation, we conducted a voxel-based morphometry (VBM) analysis extracting the GMP for those regions of interests (ROIs) that are reported in the literature to support sentence processing (Friederici, 2011) and which in the present data showed increased functional activation during sentence processing in the whole-brain functional analysis. Based on prior studies, we expected functional activation in PO and STG as core parts of the language network and in IPL as a region supporting vWM during the processing of complex sentences. Third, to investigate which age-related changes (GMP, performance differences, and digit span) contribute to changes in functional activation patterns, we computed correlational analyses and explorative multiple regressions. Finally, activation of different regions activated during sentence processing were used as predictors for the functional brain results in PO.
Fig. 1.
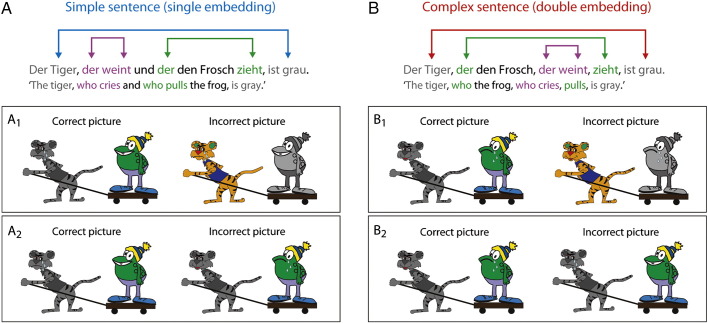
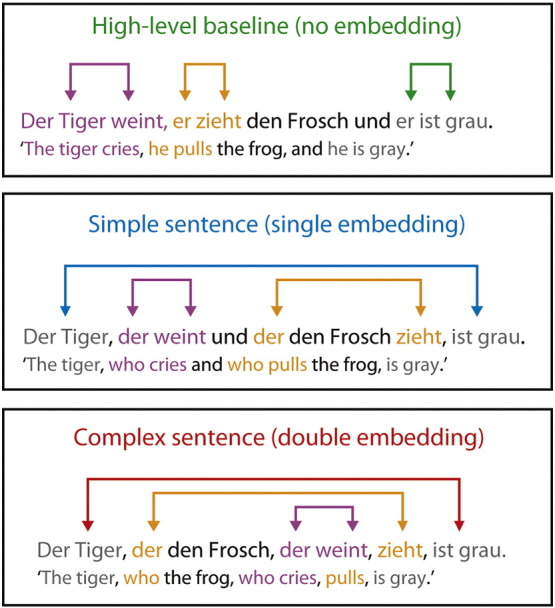
Exemplary sentence materials and picture sets. Sentence complexity was manipulated by the number of embeddings. (A) Simple sentences contained a single relative clause and (B) complex sentences contained two relative clauses. In parallel to the auditory presentation of the simple and complex sentences, participants were presented with two pictures, one matching the stimulus sentence and one not matching the stimulus sentence. Picture set A1 and B1 illustrate stimuli of the experimental condition which tested the comprehension of the long-distance dependencies, picture set A2 and B2 are filler items which were included in order to prevent the application of processing strategies. Via button press participants indicated which of these pictures was the correct one.
Methods
Participants
Children were recruited from local kindergartens and schools. Before participation, parents were invited for an informative meeting about the experiment and scanning procedure. Parental consent and children’s verbal assent was obtained prior to data acquisition. In total, 59 children and 21 adults (mean age: 27 years, standard deviation (SD): 44 months) took part in the experiment. According to questionnaires filled out by our participants or their parents, all of them were monolingual German speakers, right-handed (abridged version of Oldfield, 1971), and had no neurological, medical, or psychological disorders. Twenty-one children had to be excluded after fMRI scanning due to excessive movement in more than 50% of the trials and/or quitting (see Procedure section; n = 9), no baseline brain activation in response to sound (n = 4), performance accuracy below 50% (see Procedure section; n = 4), performance below average on the standardized test for the reception of grammar (n = 1), constantly responding after the response time window (see Procedure section; n = 1), or brain anomalies as verified by trained clinicians (n = 2). The final children sample consisted of eighteen children between 5 and 6 years (mean age: 72.0 months, SD: 6 months) and twenty children between 7 and 8 (mean age: 95.5 months, SD: 7 months). All procedures were approved by the Research Ethics Committee of the University of Leipzig.
Materials
Sentence comprehension test
Sentence materials (see Fig. 1) were sentences containing three clauses, arranged into different levels of syntactic complexity: sentences with coordinated clauses (baseline condition, see Inline Supplementary Fig. S1), sentences with one embedded relative clause (simple sentences), and sentences with two embedded relative clauses (complex sentences).
Inline Supplementary Figure S1.
Fig. S1.
Sentence materials. Sentences with coordinated clauses (high-level baseline), sentences with one embedded relative clause (simple sentences), and sentences with two embedded relative clauses (complex sentences). Brain activity for simple and complex sentences was assessed relative to the high-level baseline. Sentences of the high-level baseline controlled for brain activations with respect to pronoun and verb processing.
Twenty-two sets in the three conditions were constructed, yielding a full set of 66 sentences. Sentences were recorded by a trained female speaker, digitized (44.1 kHz/16 bit sampling rate, mono), and normalized according to the root-mean-square amplitude of all files. Average sentence duration was 5.2 s (SD: 0.5 s). Sentences across conditions contained an equal number of clauses (3), pronouns (2), and verbs (3). Due to the coordination, simple sentences contained one additional word (altogether 11 words) in comparison to complex sentences (altogether 10 words). To avoid semantic complexity effects, the content of the sentences of the different conditions was held as constant as possible: Each sentence described a scene involving two interacting animals. One of the three clauses described the color of one of the animals using a copula, the second clause described the action involving the two animals by a reversible transitive verb, and the third clause described the emotional expression (laughing/crying) of one of the animals by an intransitive verb.
The coordinated sentences contained the same amount of sub-clauses, words, verbs, and pronouns as embedded sentences. They were constructed as a high-level baseline that controlled for brain activations with respect to pronoun and verb processing. By embedding relative clauses into superordinate clauses, we increased the number of long-distance dependencies between the subject and the verb of a sentence and the sentence’s level of hierarchy.
Corresponding to each sentence, picture sets of two pictures were created, focusing on the comprehension of the long-distance dependency between the sentence-initial subject and sentence-final verb (see Fig. 1A1 and B1). To avoid the development of strategies, we included 18 filler items (picture sets), 6 for each sentence structure, that tested comprehension performance of the other two sub-clauses (see Fig. 1A2 and B2). Altogether, an experimental list contained 66 trials and 11 null events (6 s of a blank screen), from which an individual list was pseudo-randomized for each participant.
Working memory test
For the assessment of participants’ vWM abilities, we employed the German-abridged version of the digit span test (Tewes, 1994) from the Wechsler Intelligence Scale for children (WISC IV; Wechsler, 2003). Although vWM not only includes the maintenance of information, but also attentional processes, we chose to use digit span as representative measurement for our study because it measures the ability to store verbal material and successful comprehension of embedded sentences crucially relies on this capacity. Functional brain imaging in adults has observed previously that brain activity during the comprehension of sentences with working memory intensive long-distance dependencies correlates substantially with participants’ forward digit span, unlike executive functions required to track the positioning of subjects and objects inside a sentence (Meyer et al., 2012).
Procedure
In a separate session prior to fMRI scanning, digit span was assessed (see Materials section). During the experiment, sentences and pictures were presented using the Presentation® software package (Neurobehavioral Systems, Inc., Albany, CA, USA). Auditory stimuli were presented via air-conduction headphones; visual stimuli were presented via LCD display glasses (both VisuaStim XGA, Resonance Technology Inc., Northridge, CA, USA). To familiarize children with the scanning procedure, they were invited for a training session 1 week prior to scanning. The training session involved stimuli of the same syntactic structure as the experimental items. However, to prevent training effects, sentence structures contained different words compared to the experimental stimuli. In this session, children watched a movie before and after the experiment in a model scanner to simulate the time needed for anatomical data collection. Motion was controlled by a motion sensor, and verbal and visual motion feedback was given. Before actual scanning, the experimenter presented printed examples of stimuli that were not presented during the experiment to remind children of and to familiarize adults with the procedure. The experimental procedure (see below) during actual scanning was the same as in the training session. Both training session and actual experiment started with an introduction, during which volume acquisition was already started to allow for magnetic saturation effects to establish. An experimental trial started with a random onset jitter of 0, 400, 800, 1200, or 1600 ms length, after which an auditory sentence stimulus was presented. In parallel, two pictures were presented on the left and right of the display glasses, one matching and one mismatching the auditory stimulus. Participants held a button in each hand and were instructed to indicate via button press whether the left or right picture matched the sentence heard, whereby the response time window was limited to 4 s after stimulus offset. Presentation side of the correct picture was counterbalanced across conditions and participants. Each trial lasted 12 s, resulting in a total scanning time of approximately 22 minutes.
Data acquisition
During fMRI scanning, we obtained the response accuracy (i.e., correct versus incorrect response) and the reaction time (RT) for each trial. Anatomical and functional brain data were acquired with a whole-body 3-T Magnetom TIM TRIO scanner (Siemens Healthcare, Erlangen, Germany) with a 12-channel head coil. Functional data were acquired with a gradient-echo EPI sequence (repetition time (TR) = 2 s; echo time (TE) = 30 ms; flip angle = 90°; 26 slices; data matrix = 64 × 64 voxels; voxel size = 3 × 3 × 3 mm3; 1 mm gap; field of view (FOV) = 192 mm; 484 volumes). Structural data were obtained with a T1-weighted magnetization-prepared rapid gradient-echo 3D sequence with selective water excitation and linear phase encoding. The magnetization preparation consisted of a non-selective inversion pulse. To avoid aliasing, oversampling was performed in the read direction (inversion time = 740 ms; TR = 1480 ms; TR of the gradient-echo kernel (snapshot FLASH) = 10 ms; TE = 3.46 ms; flip angle = 10°; data matrix = 256 × 240 voxels; voxel size = 1 × 1 × 1.5 mm3; FOV = 256 × 240 mm2; 128 partitions; slab thickness = 192 mm).
Data analysis
Behavioral data analysis
We quantified sentence comprehension performance for each participant by calculating mean response accuracies and mean reaction times. To exclude that children performed at chance level, we performed a one-sample t-test between the mean response accuracy and chance level performance (50% correct responses) for each age-group. In order to rule out that participants only focus on relevant parts of the sentence, paired-samples t-tests between mean response accuracy for test and filler items of each sentence structure in each age-group were computed. To determine potential performance differences between simple and complex sentences as well as the influence of age, we entered the performance data into a 2 (COMPLEXITY) × 3 (AGE) analyses of variance (ANOVA). A comparison of performance data between experimental conditions and baseline sentences can be found in the supplementary material (see Inline Supplementary Fig. S2).
Inline Supplementary Figure S2.
Fig. S2.
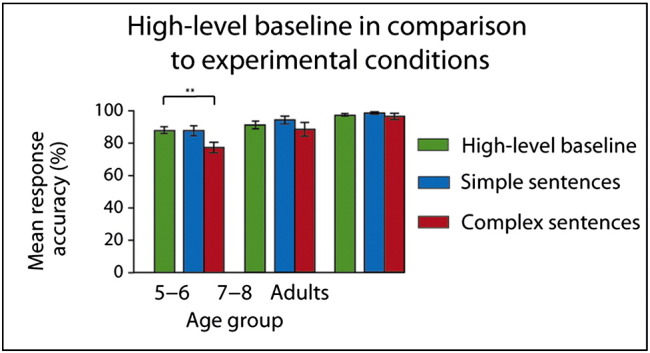
Comparison of performance for experimental conditions to performance for the baseline condition. Paired-samples t-tests between mean accuracy rates for experimental (simple or complex sentences) and baseline conditions were computed for each age-group. Only the comparison between baseline condition and complex sentences in 5- to 6-year-olds was significant (t(17) = 3.97, p < 0.01). High-level baseline sentences in green; simple sentences in blue; complex sentences in red; **p < 0.01.
Analysis of MRI data
Functional MRI data
Analysis of functional MRI data was performed using the SPM8 software package (Wellcome Department of Imaging Neurosciences, UCL, London, UK). Images were corrected for slice timing, and the time series was realigned to the first image. Trials with excessive movement (> 3 mm in any direction) were excluded from statistical analysis (5- to 6-year-olds: 4.4% of trials; 7- to 8-year-olds: 1.6% of trials; adults: 0% of trials). This also resulted in the exclusion of four participants from further analyses (see Participants section). Before image normalization (gray matter segmentation-based procedure), functional images were co-registered to participants’ anatomical image, then to a template appropriate from early to advanced puberty (Fonov et al., 2011) to keep normalization bias equal across age-groups. Previous studies have shown that normalization to a standard adults’ MR template is valid only from 7 years of age (Burgund et al., 2002, Kang et al., 2003) but can drive spurious differences between age-groups (Wilke et al., 2002) and increased variance in brain contours (Muzik et al., 2000) in younger age-groups. Functional data were resampled to 2 × 2 × 2 mm3 voxel size. A spatial smoothing filter with a kernel of 8.0 mm3 FWHM was applied. A temporal high-pass filter with a cutoff frequency of 1/100 Hz was used to remove low-frequency signal changes and baseline drifts. For statistical analysis, experimental epochs were modeled starting at the last word of each sentence, where the relationship between the initial subject and the sentence-final verb is established. For each participant, these events were passed into a general linear model, creating a design matrix on the basis of a convolution with a canonical hemodynamic response function, yielding statistical parametric maps. Excluded error and movement trials as well as the six movement parameters for each scan were modeled as covariates of no interest.
Whole-brain analysis
Two contrast images were generated to capture brain activity during the successful processing of the simple sentences and complex sentences, respectively, compared to the high-level baseline. Group statistics were computed from the two contrast images per participant, using a 2 (COMPLEXITY) × 3 (AGE) random-effects model, as well as gender and the lateralization quotient from the handedness assessment as covariates of no interest. Statistical maps were thresholded at peak level p < 0.001 (uncorrected) with a cluster-level false discovery rate (FDR) correction of q < 0.05. In order to avoid circular analyses, post hoc comparisons were not computed on the whole-brain level, but restricted to ROIs.
ROI analyses
To explore the underlying signal behind the interaction effects on the whole-brain level (Poldrack, 2007; see Results section), we calculated percentage signal change values using MarsBar (available at http://marsbar.sourceforge.net) inside four different ROIs as defined by the group-peak activation clusters in the whole-brain analysis (see Results section). Results of repeated-measures GLMs can be found in the supplementary material (see Inline Supplementary Fig. S4). To quantify the functional selectivity of our ROIs, which are language-relevant for complex sentence processing (PO and IPL/pSTG), we subtracted the percent signal change of simple sentences from complex sentences—henceforth referred to as Brain Functional Complexity Score. Values above zero indicate complexity sensitivity, and values at or below zero point to complexity insensitivity. To further investigate age-related changes of the functional selectivity for syntactic complexity in each ROI, we computed between-group planned comparisons with Bonferroni-corrected significance thresholds.
Inline Supplementary Figure S4.
Fig. S4.
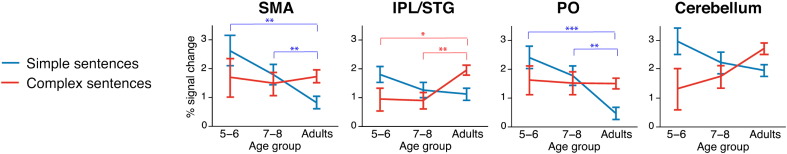
Developmental changes for the percent signal change in each condition. Multivariate analyses were computed. PO: activation for simple sentences decreases with age: F(2,56) = 11.44, p < 0.001); IPL/pSTG: activation for complex sentences increases with age (F(2,56) = 5.15, p < 0.01); SMA: activation for simple sentences decreases with age (F(2,56) = 6.69, p < 0.01); Cerebellum: no significant age effects could be found. PO = pars opercularis; IPL = inferior parietal lobe; pSTG = posterior superior temporal gyrus; SMA = supplementary motor area; simple sentences in blue; complex sentences in red. Post hoc between-group analyses adjusted to α-level = 0.017; **p < 0.01; ***p < 0.001.
Voxel-based morphometry analyses
Structural brain data were analyzed using VBM to quantify region-specific cortical maturation. Images were resampled to 1 × 1 × 1 mm3 and segmented into gray matter, white matter, and cerebro-spinal fluid based on intensity values and tissue probability maps. Because tissue probability maps generated from adult images can misclassify children’s data (Altaye et al., 2008), we used different maps for our adult (ICBM atlas) and children (age-appropriate maps form the NIHPD-database; Fonov et al., 2011) groups. The gray and white matter segments were then iteratively matched onto a template generated from their own mean by employing diffeomorphic anatomical registration using exponentiated lie algebra (DARTEL; Ashburner, 2007). To avoid the non-linear warping to obscure regional GMPs, GMP values were corrected for the relative amount of warping then resampled to 1.5 × 1.5 × 1.5 mm3 voxel size and smoothed using an isotropic Gaussian kernel of 8 mm3 at FWHM. Each participant’s GMP was averaged across each of the language-related ROIs derived from the functional analysis (see Results section): PO and pSTG/IPL.
Between-group comparisons
Previous studies indicate age-related changes in GMP of frontal and parietal areas (Giedd et al., 1999, Sowell et al., 2003), digit span (e.g., Camos and Barrouillet, 2011), and sentence comprehension performance (e.g., Kidd and Bavin, 2002, Kidd et al., 2007) along the trajectory outlined by our age-groups. Thus, we performed univariate analyses for each of these factors to check for significant age effects.
Correlational analyses
First, to determine whether functional activation is related to more efficient processing, we ran partial correlational analyses between the percent signal change and performance data (mean response accuracy and mean reaction time) for each condition in these ROIs, controlling for the mean percent signal change. In order to identify whether these effects are subject-specific or developmentally driven, we included age as additional controlling variable into a further analysis. Since selectivity of brain regions can only be achieved by identical processing strategies and previous developmental studies indicate that insufficient vWM may alter sentence processing strategies (Felser et al., 2003b, Kidd and Bavin, 2002, Kidd et al., 2007, Sheldon, 1977, Tavakolian, 1981), we computed correlations between Brain Functional Complexity Scores and digit span. This was done to test whether the functional selectivity of our brain regions which are language-relevant to complex sentence processing is associated with the vWM of our participants.
Second, to analyze the relationship between brain function and brain structure, we correlated Brain Functional Complexity Scores and GMP in each ROI. Again, age was included to test for specific age effects. All Pearson’s correlational analyses were Bonferroni-corrected for multiple comparisons.
Finally, we sought to address the question which age-related changes (see Inline Supplementary Fig. S6) may contribute to the functional attunement of our language-related regions. To assess the differential relationship between an area’s functional selectivity, structural maturation of the underlying gray matter, changes in performance differences between conditions, and the expansion of vWM, we ran a multiple regression analysis in each ROI. Here we treated Brain Functional Complexity Scores as dependent variable; age, GMP, performance differences between conditions, and digit span were used as predictors. Due to small variance in response accuracies, only reaction time differences were included into the model. However, fairly high correlation coefficients (see Inline Supplementary Table S1, Inline Supplementary Table S2) indicate that multicollinearity between our predictors might be present in the model. Following Kraha et al. (2012), further steps involved techniques addressing the problem of multicollinearity. Since beta weights are affected by covariances of predictors, we computed structure coefficients to determine how much each predictor directly contributes to the explanation of the Brain Functional Complexity Scores (see Table 4). In addition, we ran commonality analyses (Nimon et al., 2008) to asses shared and unique contributions of our predictors to explain the Brain Functional Complexity Score in each ROI. By means of testing against a random baseline, which contained the mean of variance contribution from 5000 commonality analyses computed with 5000 random permutations for each predictor, the significance of each contribution was tested. Finally, relative importance weights (Braun and Oswald, 2011) were computed to determine proportionate contributions of each predictor to the model, after correcting for the effects of intercorrelations among each other.
Inline Supplementary Figure S6.
Fig. S6.
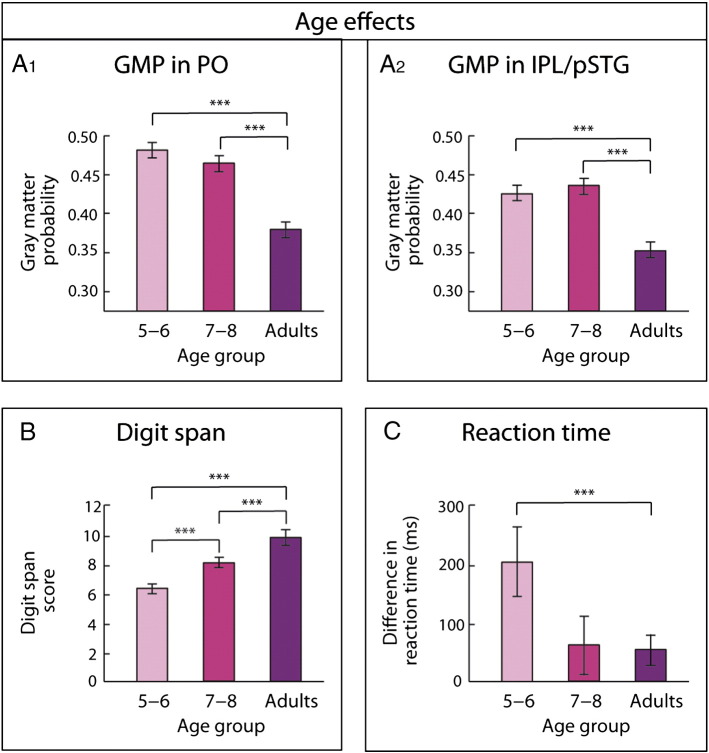
Age-related changes. (A1) Decrease of gray matter probability (GMP) in the left pars opercularis (PO). (A2) Decrease of GMP in the left inferior parietal lobe extending to the posterior superior temporal gyrus (IPL/pSTG). (B) Increase of digit span. (C) Decrease of reaction time differences (reaction time for simple sentences subtracted from reaction time for complex sentences); ***p < 0.001.
Inline Supplementary Table S1.
Table S1.
Matrix of correlations for PO.
| Age | GMP | RTdiff | DS | BFCS of PO | |
|---|---|---|---|---|---|
| Age | 1 | − 0.68*** | − 0.18 | 0.53*** | 0.47*** |
| GMP | 1 | 0.33* | − 0.42** | − 0.40** | |
| RTdiff | 1 | − 0.19 | − 0.27* | ||
| DS | 1 | 0.33* | |||
| BFCS | 1 |
PO = pars opercularis; GMP = gray matter probability; RTdiff = reaction for complex sentences − reaction time for simple sentences; DS = digit span; BFCS = Brain Functional Complexity Score.
p < 0.05.
p < 0.01.
p < 0.001.
Inline Supplementary Table S2.
Table S2.
Matrix of correlations for IPL/pSTG.
| Age | GMP | RTdiff | DS | BFCS of IPL/pSTG | |
|---|---|---|---|---|---|
| Age | 1 | − 0.64*** | − 0.18 | 0.53*** | 0.54*** |
| GMP | 1 | 0.26* | − 0.29* | − 0.43** | |
| RTdiff | 1 | − 0.19 | − 0.26* | ||
| DS | 1 | 0.41** | |||
| BFCS | 1 |
IPL = inferior parietal lobe; pSTG = posterior superior temporal gyrus; GMP = gray matter probability; RTdiff = reaction for complex sentences − reaction time for simple sentences; DS = digit span; BFCS = Brain Functional Complexity Score.
p < 0.05.
p < 0.01.
p < 0.001.
Table 4.
Explorative multiple regression analyses.
| β | r | R2 | rs | rs2 | ||
|---|---|---|---|---|---|---|
| PO | Age | 0.334 | 0.465 | 0.216 | 0.908 | 0.825 |
| GMP | − 0.080 | − 0.400 | 0.160 | − 0.781 | 0.611 | |
| RTdiff | − 0.167 | − 0.269 | 0.072 | − 0.525 | 0.276 | |
| DS | 0.089 | 0.331 | 0.110 | 0.646 | 0.418 | |
| PL/pSTG | Age | 0.365 | 0.544 | 0.296 | 0.927 | 0.858 |
| GMP | − 0.116 | − 0.427 | 0.182 | − 0.727 | 0.528 | |
| RTdiff | − 0.134 | − 0.258 | 0.067 | − 0.440 | 0.193 | |
| DS | 0.153 | 0.406 | 0.165 | 0.692 | 0.478 |
Dependent variable: Brain Functional Complexity Score; PO = pars opercularis; IPL = inferior parietel lobe; pSTG = posterior superior temporal gyrus; GMP = gray matter probability; RTdiff = reaction for complex sentences − reaction time for simple sentences; DS = digit span.
Results
Behavioral results
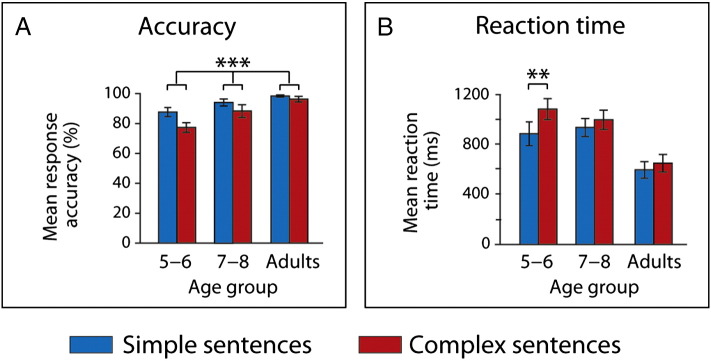
Performance accuracy on sentence comprehension was above chance across conditions and age-groups (all p < 0.001; see Fig. 2). However, mean response accuracy for test and filler items significantly differed for 5- to 6-year-olds (t(17) = 4.51, p < 0.001; see Inline Supplementary Fig. S3). Filler items, which tested the comprehension of the other sub-clauses, were included to prevent that our participants develop the strategy to selectively focus on the final sub-clause. While this strategy would increase performance for test items, it would result in poor performance for filler items at the same time. Since significant differences between test and filler items, which have been found for the 5- to 6-year-olds, may indicate the application of this strategy, we tested if this age-group performs above chance with respect to filler items. As the performance of 5- to 6-year-olds for filler items significantly differs from chance (t(17) = 3.28; p < 0.01), we can conclude that the 5- to 6-year-olds children paid attention to the sub-clauses tested by the filler items. The 2 (complexity) × 3 (age) ANOVAs on the performance data (see Fig. 2) yielded main effects of sentence complexity (F(1,56) = 19.03, p < 0.001) and age (F(2,56) = 8.96, p < 0.001) for mean response accuracy and an interaction between sentence complexity and age (F(2,56) = 3.33, p < 0.05) for mean reaction time. Post hoc comparisons with Bonferroni adjustments (α = 0.02) reveal that while mean response accuracy was better for simple than for complex sentence structures across age-groups (t(58) = 4.09, p < 0.001), only 5- to 6-year-olds show significant differences in mean reaction time (t(17) = − 3.57, p < 0.01; see Fig. 2).
Fig. 2.
Behavioral results. All age-groups performed above chance. (A) Response accuracy (%) and (B) reaction times (ms). Across age-groups, participants performed significantly better for simple sentences. Reaction time differences could be only found for the youngest age-group. **p < 0.01; ***p < 0.001.
Inline Supplementary Figure S3.
Fig. S3.
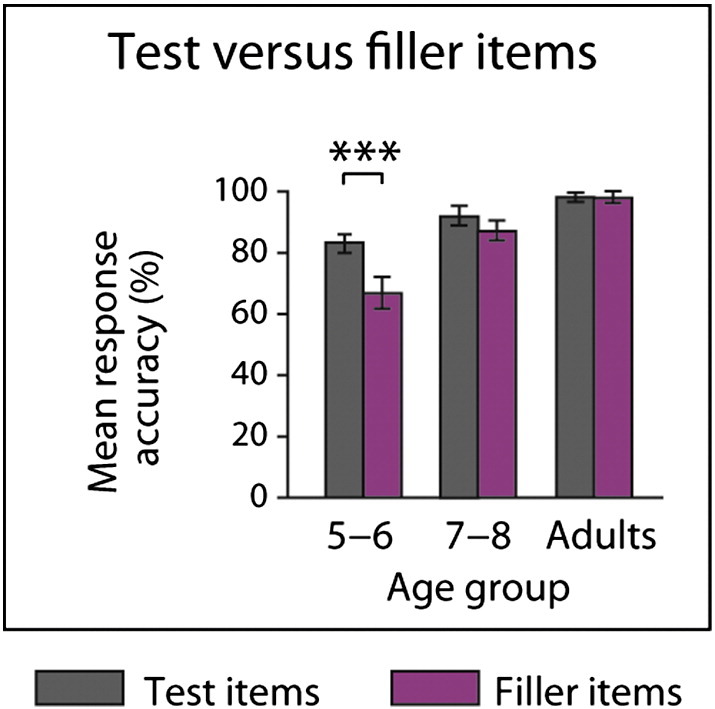
Comparison of test and filler items. Mean accuracy of all test items depicted in gray; mean accuracy of all filler items depicted in purple. Although mean response accuracy for test and filler items significantly differed for 5- to 6-year-olds (t(17) = 4.51, p < 0.001), a selective focus on the final sub-clause in this age-group can be excluded since performance for test and filler items is positively correlated (r = 0.74, p < 0.001), which suggests that 5- to 6-year-old children who show high accuracy for test items also show high accuracy for filler items.
Functional MRI results
Whole-brain analysis
The whole-brain analysis revealed a sentence complexity × age interaction in the language-related left PO (main peak at x = –32, y = 12, z = 28) and left IPL extending into the left pSTG (main peak at x = –36, y = –46, z = 38), as well as bilaterally in the cerebellum (main peak at x = 14, y = –78, z = –28), and the supplementary motor area (SMA; main peak at x = 0, y = 24, z = 48). No significant main effects were obtained (see Fig. 3 and Table 1).
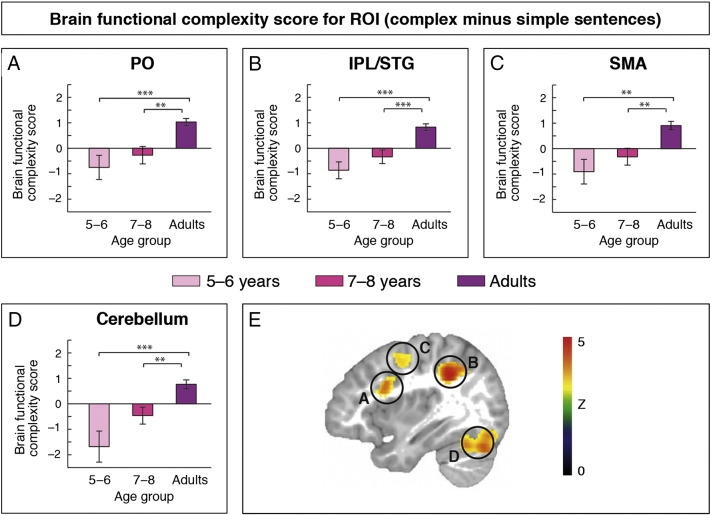
Fig. 3.
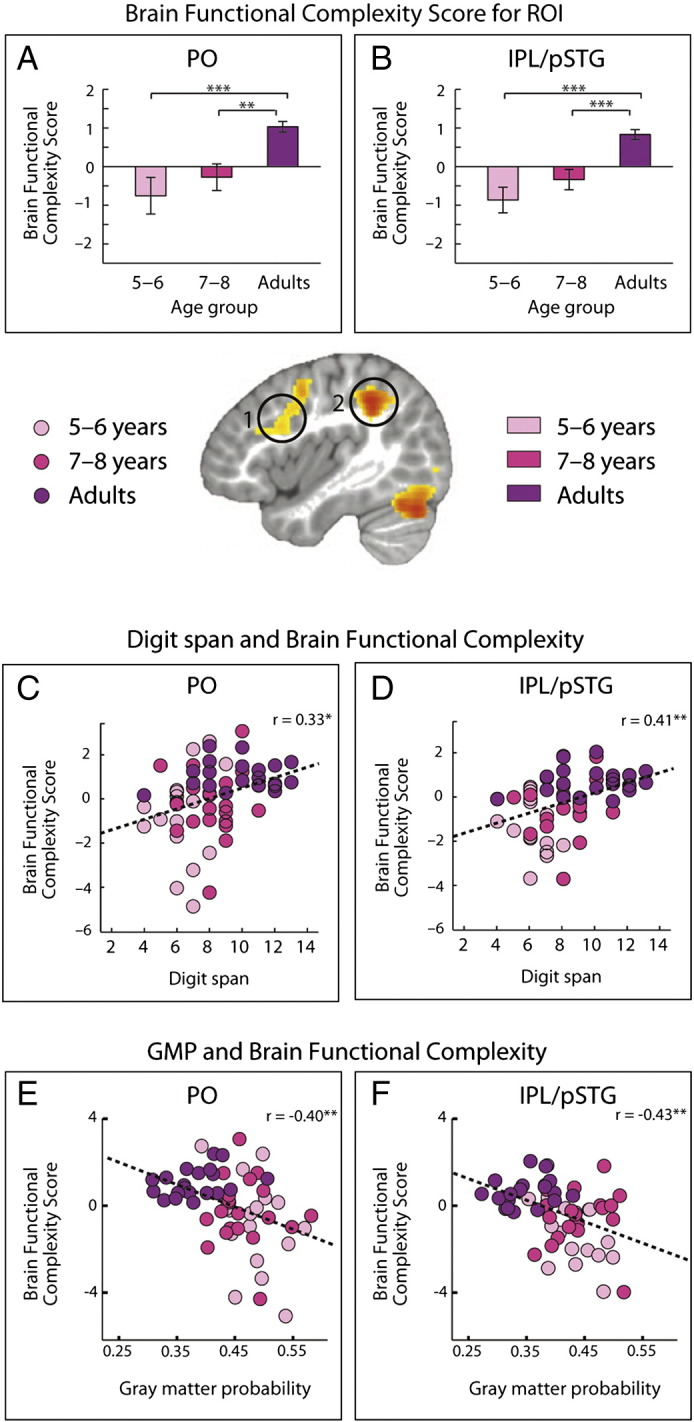
Functional magnetic resonance imaging results and correlations. Whole-brain results (p < 0.001, corrected) reveal interactions between age and sentence complexity in left pars opercularis (PO), left inferior parietal lobe together with the posterior superior temporal gyrus (IPL/STG), bilateral supplementary motor area (SMA), and bilateral cerebellum. (A, B) Planned comparisons for language-related ROIs on Brain Functional Complexity Scores (i.e., percent single change for simple sentences subtracted from percent signal change for complex sentences; p < 0.01, corrected) indicate increased functional selectivity for syntactic complexity with age. (C, D) Brain Functional Complexity Scores of our ROIs are positively related to digit span. (E, F) Brain Functional Complexity Scores of our ROIs are negatively related to gray matter probability. *< 0.013 (adjusted to α-level); **< 0.01; ***< 0.001.
Table 1.
Significant clusters in the interaction between sentence complexity and age-group in whole-brain analysis.
| Hemisphere | Region | BA | MNI coordinate |
Cluster size (number of voxels) | Z-value | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Left | IFG | 44 | –32 | 12 | 28 | 1146 | 4.03 |
| Left | IFG | 44 | –48 | 6 | 38 | 4.03 | |
| Left | IFG | 44 | –52 | 10 | 24 | 3.95 | |
| Left | IPL | 40 | –36 | –46 | 38 | 1781 | 4.78 |
| Left | pSTG | 22 | –58 | –48 | 12 | 4.01 | |
| Left | pSTG | 22 | –56 | –36 | 6 | 3.96 | |
| Left | SMA | 6 | 0 | 24 | 48 | 629 | 4.15 |
| Right | SMA | 6 | 8 | 4 | 46 | 3.64 | |
| Right | MCC | 6 | 4 | -20 | 46 | 3.43 | |
| Left | CB | 14 | –78 | –28 | 4621 | 4.70 | |
| Left | CB | –44 | –72 | –28 | 4.63 | ||
| Left | CB | –12 | –66 | –32 | 4.53 | ||
Peak level p < 0.001 uncorrected, FDR cluster corrected at q < 0.05; BA = Brodmann area; MNI = Montreal Neurological Institute; IFG = inferior frontal gyrus; IPL = inferior parietal lobe; pSTG = posterior superior temporal gyrus; SMA = supplementary motor area; MMC = middle cingulate cortex, CB = Cerebellum.
ROI analysis
The exploration of age-related changes in the percent signal change for each condition in each ROI (see Inline Supplementary Fig. S4) as well as results for SMA and the cerebellum can be found in the supplementary material (see Inline Supplementary Figure S4, Inline Supplementary Figure S5).
Inline Supplementary Figure S5.
Fig. S5.
Functional magnetic resonance imaging results. Whole-brain results (p < 0.001, corrected) reveal interactions between age and sentence complexity in left pars opercularis (PO), left inferior parietal lobe extending into the posterior superior temporal gyrus (IPL/STG), bilateral supplementary motor area (SMA), and bilateral cerebellum. Planned comparisons in each region of interest (ROI; A–D) on functional complexity scores (i.e., percent single change for simple sentences subtracted from percent signal change for complex sentences; p < 0.01, corrected) indicate increased functional selectivity for syntactic complexity with age for all ROIs. (E) Neuroanatomical location of the ROIs.
Brain Functional Complexity Scores increased with age (see Table 2) only between adults and both children groups, but not among children groups in left PO (5- to 6-year-olds versus adults: t(37) = − 3.85, p < 0.001; 7- to 8-year-olds versus adults: t(39) = − 3.58, p < 0.008) and left IPL/pSTG (5- to 6-year-olds versus adults: t(37) = − 5.06, p < 0.001; 7- to 8-year-olds versus adults: t(39) = − 4.03, p < 0.001), which are depicted in Fig. 3A and B, as well as in the Cerebellum (5- to 6-year-olds versus adults: t(37) = − 4.10, p < 0.001; 7- to 8-year-olds versus adults: t(39) = − 3.30, p < 0.008) and SMA (5- to 6-year-olds versus adults: t(37) = − 3.74, p < 0.008; 7- to 8-year-olds versus adults: t(39) = − 3.36, p < 0.008; Inline Supplementary Fig. S5C and D).
Table 2.
Comparison of Brain Functional Complexity Scores between age-groups for ROIs.
| Older children versus younger children | Adults versus younger children | Adults versus older children | |
|---|---|---|---|
| Left PO | t(36) = 0.83 | t(37) = 3.85*** | t(39) = 3.58** |
| Left IPL/pSTG | t(36) = 1.26 | t(37) = 5.06*** | t(39) = 4.03*** |
Brain Functional Complexity Scores: percent signal change for simple sentences subtracted from percent signal change for complex sentences; PO = pars opercularis; IPL = inferior parietal lobe; pSTG = posterior superior temporal gyrus; results are corrected for multiple comparisons; **p < 0.008; ***p < 0.001.
Age-related changes
As shown in other developmental studies, age-related changes of the GMP could be found for the left PO (F(2,56)) = 27.53, p < 0.001) and the left IPL/pSTG (F(2,56)) = 27.53, p < 0.001). These effects are driven by a particular decrease of GMP between childhood and adulthood whereas children of different age-groups do not differ (see Inline Supplementary Fig. S6A1 and A2). Behaviorally, we predicted age-related changes in digit span and performance. Since previous analyses only indicate an interaction between performance for the sentence structures and age in reaction time, we focused our analysis on the age-related differences in reaction time. An increase of digit span (F(2,56) = 18.83, p < 0.001; see Inline Supplementary Fig. S6B) as well as a decrease of performance differences based on reaction time could be shown (F(2,56) = 3.33, p < 0.05; see Inline Supplementary Fig. S6C).
Correlational results
Brain function—behavior
With respect to accuracy, we found that partial correlational analyses indicate a negative relationship between performance for simple sentences and percent signal change in both the left PO (rp = − 0.43, p < 0.01) and the left IPL/pSTG (rp = − 0.54, p < 0.001). Thus, the more accurate performance for simple sentences is associated with less activation in our ROIs. Whereas correlations in PO are developmentally driven across age-groups, the correlation in the left IPL/pSTG also remains if controlled for the influence of age (rp = − 0.46, p < 0.001). While increased activation of the left IPL/pSTG for complex sentences is also clearly correlated with increased response accuracy (rp = 0.47, p < 0.001), the left PO only shows a tendency toward a correlation (rp = 0.32, p = 0.015; Bonferroni adjustment of α = 0.013). Decreased reaction time and increased percent signal change is only related for complex sentences in the left PO (rp = − 0.39; p < 0.01) and the left IPL/pSTG (rp = − 0.45, p < 0.001), but not for simple sentences (PO: rp = 0.26, p = 0.05; IPL/pSTG: rp = 0.31, p = 0.02). These results are summarized in Table 3.
Table 3.
Partial correlations between performance and percent signal change for each condition.
| Accuracy | Reaction time | ||||
|---|---|---|---|---|---|
| PO | Simple | r = − 0.43 | p = 0.001 | r = 0.26 | p = 0.05 |
| Complex | r = 0.32 | p = 0.015 | r = − 0.39 | p = 0.002 | |
| IPL/pSTG | Simple | r = − 0.54 | p < 0.001a | r = 0.31 | p = 0.02 |
| Complex | r = 0.47 | p < 0.001 | r = − 0.45 | p < 0.001 | |
Can be controlled for age (rp = − 0.46, p < 0.001); PO = pars opercularis; IPL = inferior parietal lobe; pSTG = posterior superior temporal gyrus; bold = significant results controlled for multiple comparison (p < 0.013).
Furthermore, we found correlations between the Brain Functional Complexity Scores and digit span for both ROIs. As increased Brain Functional Complexity Scores represent a higher functional selectivity of a brain region, this correlation reveals a positive relationship between the areas’ functional selectivity and vWM (PO: r = 0.33, p < 0.05; IPL/pSTG: r = 0.41, p < 0.01; see Fig. 3C and D).
Brain function—brain structure
Correlational analyses indicate that in the left PO and in the left IPL/pSTG, decreased GMP was accompanied by increased Brain Functional Complexity Scores (PO: r = –0.40, p < 0.01, IPL/pSTG: β = –0.43, p < 0.01; see Fig. 3E and F). Partial correlations with age are not significant.
The tripartite relation between brain function, brain structure, and behavior
Multiple regression analyses reveal that the predictors age, GMP, reaction time differences, and digit span explain 26.2% of the Brain Functional Complexity Score of PO (R = 0.512, p < 0.01) and 34.5% of IPL/pSTG (R = 0.587, p < 0.001). Beta weights indicate a high impact of age on the Brain Functional Complexity Score in both ROIs (see Table 4). However, since there exist high correlations among our predictors (see Inline Supplementary Table S1, Inline Supplementary Table S2), and since beta weights are highly influenced by covariance, we computed structure coefficients for each predictor which represent correlations between the predictor and the predicted criterion scores. Therefore, structure coefficients are correlational coefficients that are not influenced by other predictors. The squared structure coefficients reveal that GMP can account for 61% of the explained variance in the Brain Functional Complexity Scores in PO and 52% of the explained variance in IPL/pSTG. Digit span can account for 42% of the obtained effect in PO and 48% of the obtained effect in IPL/pSTG (see Table 4). Furthermore, the sum of squared structure coefficients greater than 1 for PO (2.14) and IPL/pSTG (2.6) confirms considerable amount of multicollinearity between predictors.
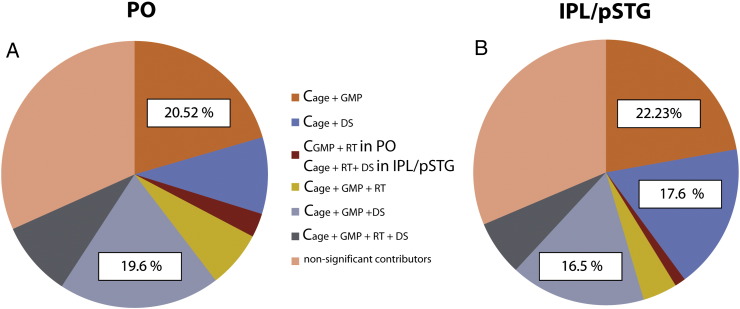
The commonality analyses (see Inline Supplementary Table S3) address the problem of multicollinearity by providing separate measures for unique and shared variance of predictors. Brain Functional Complexity Scores of both ROIs were best explained by the shared variance components of age and GMP (PO: 20.52%; IPL/pSTG: 22.23%) as well as age, GMP, and digit span (PO: 19.6%, IPL/pSTG: 16.5; see Fig. 4). In the left IPL/pSTG, Brain Functional Complexity Scores were also optimally predicted by shared variance of age and digit span (17.6%; see Fig. 4B).
Inline Supplementary Table S3.
Table S3.
Commonality analysis.
| PO |
IPL/pSTG |
|||
|---|---|---|---|---|
| Commonality coefficient | Percent | Commonality coefficient | Percent | |
| Uage | 0.0509 | 19.4624 | 0.0618 | 17.9204 |
| UGMP | 0.0032 | 1.2044 | 0.0077 | 2.2235 |
| URT | 0.0244 | 9.3347 | 0.0165 | 4.7746 |
| UDS | 0.0056 | 2.1425 | 0.0164 | 4.7528 |
| Cage + GMP | 0.0537 | 20.5243 | 0.0766 | 22.2184 |
| Cage + RT | − 0.0062 | − 2.3724 | − 0.0029 | − 0.8438 |
| Cage + DS | 0.0243 | 9.2804 | 0.061 | 17.6868 |
| CGMP + RT | 0.0077 | 2.9456 | 0.0059 | 1.6994 |
| CGMP + DS | 0.0005 | 0.1989 | − 0.002 | − 0.5741 |
| C RT + DS | 0.0024 | 0.9343 | 0.0049 | 1.4328 |
| Cage + GMP + RT | 0.0181 | 6.9202 | 0.0146 | 4.2251 |
| Cage + GMP + DS | 0.0513 | 19.6157 | 0.057 | 16.5267 |
| Cage + RT + DS | 0.0006 | 0.218 | 0.0045 | 1.3055 |
| CDS + GMP + RT | 0.0013 | 0.4919 | − 0.0003 | − 0.0955 |
| Cage + GMP + RT + DS | 0.0238 | 9.0992 | 0.0233 | 6.7476 |
U = unique variance; C = common variance; GMP = gray matter probability; RT = reaction time for complex sentences − reaction time for simple sentences; DS = digit span; PO = pars opercularis; IPL = inferior parietal lobe; pSTG = posterior superior temporal gyrus; bold = significant results (95% confidence level).
Fig. 4.
Commonality analysis of four predictors. Predictors = age, gray matter probability (GMP), reaction time differences between conditions (RTdiff), and digit span (DS). (A) Total variance of Brain Functional Complexity Score in pars opercularis (PO) is best explained by shared variance (C = common) of age and GMP (20.52%) and by shared variance of age, GMP, and DS (19.62%). (B) Total variance of Brain Functional Complexity Score of the inferior parietal lobe together with the posterior superior temporal gyrus (IPL/pSTG) is best explained by shared variance of age and GMP (22.23%), by shared variance of age and DS (17.69%), and by shared variance of age, GMP, and DS (16.53%).
Relative importance weights constitute the proportionate contribution from each predictor to R2 after correcting the effect of intercorrelation between predictors. As age has the highest relative importance weights across different models (see Table 5), it can be considered to be the most important variable predicting the Brain Functional Complexity Score in both ROIs. If age is excluded from the model, GMP becomes the most important predictor in PO, whereas GMP and digit span compete for the relative importance in IPL/pSTG (see Table 5).
Table 5.
Relative importance weights.
| PO |
IPL/pSTG |
|||||||
|---|---|---|---|---|---|---|---|---|
| Age | GMP | RTdiff | Digit span | Age | GMP | RTdiff | Digit span | |
| Cage + GMP | 0.143 | 0.086 | 0.210 | 0.097 | ||||
| Cage + RT | 0.198 | 0.054 | 0.276 | 0.047 | ||||
| Cage + DS | 0.166 | 0.060 | 0.223 | 0.092 | ||||
| CGMP + RT | 0.134 | 0.047 | 0.161 | 0.045 | ||||
| CGMP + DS | 0.122 | 0.071 | 0.143 | 0.126 | ||||
| CRT + DS | 0.058 | 0.095 | 0.050 | 0.149 | ||||
| Cage + GMP + RT | 0.140 | 0.072 | 0.040 | 0.204 | 0.086 | 0.039 | ||
| Cage + GMP + DS | 0.115 | 0.074 | 0.048 | 0.158 | 0.087 | 0.083 | ||
| Cage + RT + DS | 0.156 | 0.049 | 0.053 | 0.212 | 0.041 | 0.084 | ||
| CGMP + RT + DS | 0.103 | 0.042 | 0.066 | 0.129 | 0.036 | 0.118 | ||
| Cage + GMP + RT + DS | 0.114 | 0.062 | 0.041 | 0.044 | 0.155 | 0.078 | 0.034 | 0.078 |
C = common variance; GMP = gray matter probability; RT = reaction for complex sentences − reaction time for simple sentences; DS = digit span; PO = pars opercularis; IPL = inferior parietal lobe; pSTG = posterior superior temporal gyrus.
Brain functional predictors for activation in PO
As our functional ROIs have been discussed as being part of different functional networks underlying sentence processing across development (Brauer et al., 2013), we computed multiple regression analyses in each age-group. More specifically, this was done to assess changes of the relationship between the activation patterns inside the PO and the other functional brain regions as revealed by the whole-brain analysis across age-groups. For this analysis, the Brain Functional Complexity Score of PO was used as dependent variable and the Brain Functional Complexity Scores of the IPL/pSTG, the cerebellum, and the SMA as predictors. Results revealed different correlational patterns within the language network across development. In the youngest age-group (5–6 years), the activation difference between complex and simple sentences was predicted by the activation in the SMA (β = 0.93, p < 0.001; R2 = 0.864, p < 0.001). In the older children (7–8 years), it was partly predicted by activation in the SMA (β = 0.55, p < 0.01) and by activation in the IPL/pSTG (β = 0.43, p < 0.05, R2 = 0.864, p < 0.001). In adults, activation in PO was completely predicted by activation in IPL/pSTG (β = 0.91, p < 0.001, R2 = 0.831).
Discussion
The present study’s goal was to establish the missing link between the brain functional attunement of language-relevant areas to sentence processing and the underlying gray matter brain structural changes from childhood to adulthood. Furthermore, we aimed at characterizing the emergence of accurate processing of complex sentences, by considering the relation between behavioral performance during sentence processing, vWM, and the functional brain activation during complex sentence processing. For adults, a well-described network in the left hemisphere including the PO, the IPL, and the pSTG was found to support sentence processing. For all these regions of interest, a functional attunement toward an adult-like selectivity for complex sentences across development was observed. Shared variances of age and GMP, shared variances of age and digit span, and shared variances of age, GMP, and digit span explain most of the variance in Brain Functional Complexity Scores of both language-related ROIs (see Fig. 4). However, relative importance weights indicate that the functional attunement of the left PO activation was clearly related to the region’s GMP maturation whereas the functional attunement of the IPL/pSTG rather reflects an interplay between gray matter maturation and increasing vWM span (see Table 5). The former finding is in line with previous work providing evidence for the relevance of the left PO in the ability to process complex sentences (Friederici, 2011, Friederici et al., 2006, Kinno et al., 2008, Makuuchi et al., 2009, Newman et al., 2010, Röder et al., 2002). The latter finding can be related to the observation that the IPL supports vWM processes in adults (Leff et al., 2009, Owen et al., 2005), especially during sentence processing (Buchsbaum et al., 2005, Meyer et al., 2012, Novais-Santos et al., 2007). Age-related functional changes were also observed in the SMA and the cerebellum bilaterally.
The development toward an adult-like language processing system is reflected in changes of the relation between the different brain regions within the language network. In adults, the activation in PO is fully predicted by the functional activation of the IPL/pSTG. In the older children, PO activation is partially predicted by functional activation in IPL/pSTG and partially by activation of SMA, whereas in the 5- to 6-year-olds it is fully predicted by the functional activation of SMA. These correlations indicate a stepwise progression toward the functional adult frontotemporal language network. This finding could be related to the developmental changes in the white matter structural connectivity between these regions (Brauer et al., 2013, Skeide et al., 2015). In adults, the left PO, IPL, and pSTG are structurally connected via a dorsally located pathway consisting of the superior longitudinal and arcuate fasciculi (Friederici and Gierhan, 2013). These fiber tracts have been proposed to play a major role in the processing of complex sentences in adults (Friederici, 2011, Meyer et al., 2012, Meyer et al., 2014, Wilson et al., 2011). However, this connection develops slowly. Diffusion-weighted imaging studies reveal that the dorsal pathway fiber tract connecting pSTG to PO are not observable in newborns (Perani et al., 2011), and that although they start to develop after the first weeks of life (Dubois et al., 2015), the fiber bundles are still not fully matured by the age of 7 (Brauer et al., 2011, Brauer et al., 2013, Skeide et al., 2015). In contrast, the ventrally located white matter pathways connecting the temporal lobe to the frontal lobe are well established early in life, with one sub-component terminating in the prefrontal cortex, the premotor cortex, and the superior frontal gyrus reaching out to the SMA (Brauer et al., 2013).
As expected, the development toward a brain functional selectivity for syntactic complexity within the observed neural network is correlated with sentence processing performance: increased performance for simple sentences is associated with decreased activation in PO and IPL/pSTG. For complex sentence processing, more accurate processing is associated with increased activation in the IPL/pSTG, whereas faster processing is paralleled by increased activation in PO and IPL/pSTG. These findings indicate that the functional attunement toward complex sentence processing of these brain regions is related the improvement of behavioral performance. We will discuss the different brain regions separately below.
The PO
The current fMRI data indicate that only adults show complexity-sensitive activation in the PO, which is a crucial ROI in the language network. The data are in line with earlier work reporting the left PO to be involved in the processing of syntactically complex structures in adults (Friederici et al., 2006, Makuuchi et al., 2009, Meyer et al., 2012). The functional activation observed in children can be related to earlier studies showing that sentence processing in children, compared to adults, generally leads to higher and more diffuse activation in the prefrontal cortex (Brauer and Friederici, 2007, Durston et al., 2006, Lidzba et al., 2011). The absence of a functional selectivity of the left PO for children in the present study appears to be due to the demanding center-embedded sentences used here. In support of this interpretation, Knoll et al. (2012) using short object-first sentences, reported a developmental change toward a functional selectivity of the left PO for complex sentences in 6-year-olds, but only in a high-performing subgroup.
In addition to these age-dependent changes, our analyses show that the establishment of the adult-like functional selectivity for complex sentences is predicted by a reduction of the PO’s GMP across age-groups (see Fig. 3C). The apparently immature brain morphology in children’s PO (see Inline Supplementary Fig. S6A) suggests a fundamental role of cortical maturation in the functional attunement to complex sentence processing. The exact neurophysiological substrate of GMP changes in the cortex is still unclear because GMP depends on the extent of the cortical surface, cortical thickness, and myelination in adjacent white matter (for a review, see Mechelli et al., 2005). In general, brain maturation during childhood is characterized by progressive changes of a number of parameters: overgrowth of cell bodies (Petanjek et al., 2008), dendritic sprouting (Simonds and Scheibel, 1989), overproduction of dendritic spines (Petanjek et al., 2011), and overgrowth of synaptic connections (for a review, see Huttenlocher and Dabholkar, 1997, Huttenlocher, 1979). All of these are assumed to provide the basis for maximal learning opportunities (e.g., Petanjek et al., 2011, Rakic et al., 1994) especially in language-related areas (Johnson, 2011, Judas and Cepanec, 2007, Simonds and Scheibel, 1989). These progressive changes are accompanied by concurrent regressive changes such as synaptic pruning (Rakic et al., 1994). During this process, some synapses become incorporated into larger networks, while redundant synapses are eliminated to increase transmission efficiency. These structural changes have been related to functional changes, namely, a decline in metabolic activity (Chugani, 1998, Chugani et al., 1987). In frontal areas, GMP starts to decline between 9 and 12 years of age (Giedd et al., 1999, Tanaka et al., 2012), that is when language learning becomes more effortful (Hensch and Bilimoria, 2012, Lenneberg, 1967). In sum, while our data clearly suggest that neural plasticity of the PO plays a crucial role in the region’s functional attunement to complex sentence processing, more work is necessary to better understand the underlying neurophysiological mechanisms.
The IPL/pSTG
In the left IPL/pSTG, which is another crucial ROI of the language network, the functional selectivity for syntactic complexity is also only evident in adults. While the IPL is assumed to support vWM (Leff et al., 2009, Meyer et al., 2012, Novais-Santos et al., 2007), the left pSTG has been proposed to be involved in integrating semantic and syntactic information into an overall sentence meaning (for a review, see Friederici, 2011). The latter argument is based on the finding that the pSTG only becomes active in experimental paradigms using sentence material, but not in artificial grammar paradigms (Bahlmann et al., 2008, Friederici et al., 2009, Opitz and Friederici, 2004, Opitz and Friederici, 2007). The higher contribution of vWM span—as approximated by the forward digit span measure—to the variance in brain functional selectivity in the left IPL/pSTG in our study (see Fig. 3F, Table 5) provides support that both IPL and pSTG support the processing of vWM-intensive sentences—possibly with the pSTG responsible for integration processes and IPL supporting vWM as such.
Our interpretation is in line with the view that for sentences with double embeddings, vWM processing demands are high (Gibson, 1998). It also fits the proposal that children’s difficulties in processing complex sentences including relative clauses can be attributed to vWM limitations (Felser et al., 2003b).
As in the left PO, GMP reduction in the left IPL/pSTG is associated with the attunement toward the adult-like activation pattern (see Fig. 3D). Structural maturation appears to provide the basis for a specific engagement of the IPL/pSTG during the processing of sentences with high vWM demands. Gray matter development has been found to follow similar trajectories in frontal and parietal regions (Giedd et al., 1999; see also Inline Supplementary Fig. S6A and B). The late onset of gray matter decrease in both regions occurs simultaneously with the maturational increase of the dorsal white matter fiber tracts (Brauer et al., 2011, Brauer et al., 2013) connecting the pSTG with the PO passing through the IPL (Catani et al., 2005). Our correlational data suggest that the development of the cortical language network for complex sentence processing depends not only on specific brain regions but, moreover, on interregional processes between the left PO and the left IPL/pSTG.
Other regions
In the whole-brain analysis, the SMA and the cerebellum also showed significant signal changes.
The SMA shows complexity-sensitive activation in adults (see Inline Supplementary Fig. S5C). Interestingly, there is a developmental change in the relation of activation in the SMA and the PO. In children, but not in adults, the activation of the SMA predicts the activation of the PO and thus appears to be part of the language network in children. Functionally, the SMA has been suggested to be involved in temporal sequencing in adults (Coull et al., 2011), more specifically in the prediction of dynamic sequence processing (Schubotz, 2007). In children, increased pre-SMA activity is observed for the extraction of statistical regularities from sequential structures in speech perception, when prosodic cues are provided (McNealy et al., 2010).
The peak of the current interaction in the SMA is located in the pre-SMA, that is, rostral to the vertical line intersecting the anterior commissure, which has been suggested to separate the rostral pre-SMA from the caudal SMA-proper (Lehéricy et al., 2004, Picard and Strick, 2001; for a review, see Schwartze et al., 2012). The present activity of the pre-SMA may be related to the sequential ordering of the hierarchical sentence structure, as pre-SMA activation has also been found during the processing of embedded structured sentences in adults (Makuuchi et al., 2012), processing of abstract item sequences in the non-language domain (Bahlmann et al., 2009, Hanakawa et al., 2002), processing of abstract rules in the verbal domain (Bunge et al., 2003), and for working memory tasks (Honey et al., 2000, LaBar et al., 1999, Ravizza et al., 2011).
The cerebellum also showed a complexity-sensitive activation in adults (see Inline Supplementary Fig. S5D). Structurally this region is connected to the prefrontal, parietal, and temporal cortices via cortico-ponto-cerebellar and dentate-thalamo-cortical pathways (Schmahmann, 1996). Functionally, the cerebellum has been shown to be involved in the modulation of a broad spectrum of linguistic functions (for a review, see Murdoch, 2010), more specifically in the generation of internal representations of the temporal structure of spoken sentences (Kotz and Schwartze, 2010). This is of particular interest in the context of the present results, as in German, it has been found that the embeddedness of spoken sentences entails the embeddedness of prosodic domains as marked by clausal boundary tones (Féry and Schubö, 2010). The increased cerebellar activity in the current study might thus reflect the application of timing and sequencing schemes provided by prosodic features to facilitate vWM storage of embedded clauses. In line with this, it has been reported that the interaction between the right inferior posterior cerebellum and the left temporo-parietal cortex contributes to vWM (Chen and Desmond, 2005a, Chen and Desmond, 2005b, Desmond et al., 1997).
Thus, the cerebellum may be essential for generating timing and sequencing schemes, which in turn facilitate the sequential ordering of hierarchical sentence structures. The activation pattern in the cerebellum might be mirrored by the SMA which in turn is related to the activation in PO in the younger children. Further research manipulating prosodic features in the sentence presentation can help to clarify the relation of the activation in the cerebellum and the SMA.
Conclusion
The present data reveal that the functional selectivity of language-relevant brain regions develops across childhood, and that activation in language-related areas, that is the left PO and IPL/pSTG, correlates with behavioral performance in sentence processing. The attunement of PO and IPL/pSTG toward an adult-like activation pattern during sentence processing is differentially predicted by region-specific gray matter changes and partly by inter-individual differences in vWM span. In sum, our results suggest that both the maturational changes in brain structure and the developmental increase of vWM span facilitate the emergence of brain functional specificity in language-related areas.
Funding
This work was supported by a grant from the European Research Council (ERC-2010-360 AdG 20100407 awarded to A.D.F.).
Acknowledgments
We thank all the participating children and parents, M. Makuuchi, K. Mueller, J. Lepsien, I. Henseler, R. Roggenhofer, C. Beese, P. Stempel, R. Schwartz, M. Jochemko, A. Wiedemann, S. Wipper, A. Gast-Sandmann, K. Flake, and E. Kelly.
Conflict of interest
The authors declare no conflicts of interest.
References
- Altaye M., Holland S.K., Wilke M., Gaser C. Infant brain probability templates for MRI segmentation and normalization. NeuroImage. 2008;43:721–730. doi: 10.1016/j.neuroimage.2008.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Bahlmann J., Schubotz R.I., Friederici A.D. Hierarchical artificial grammar processing engages Broca’s area. NeuroImage. 2008;42:525–534. doi: 10.1016/j.neuroimage.2008.04.249. [DOI] [PubMed] [Google Scholar]
- Bahlmann J., Schubotz R.I., Mueller J.L., Koester D., Friederici A.D. Neural circuits of hierarchical visuo-spatial sequence processing. Brain Res. 2009;1298:161–170. doi: 10.1016/j.brainres.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Brain Development Cooperative Group Total and regional brain volumes in a population-based normative sample from 4 to 18 years: the NIH MRI study of normal brain development. Cereb. Cortex. 2012;22:1–12. doi: 10.1093/cercor/bhr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer J., Friederici A.D. Functional neural networks of semantic and syntactic processes in the developing brain. J. Cogn. Neurosci. 2007;19:1609–1623. doi: 10.1162/jocn.2007.19.10.1609. [DOI] [PubMed] [Google Scholar]
- Brauer J., Anwander A., Friederici A.D. Neuroanatomical prerequisites for language functions in the maturing brain. Cereb. Cortex. 2011;21:459–466. doi: 10.1093/cercor/bhq108. [DOI] [PubMed] [Google Scholar]
- Brauer J., Anwander A., Perani D., Friederici A.D. Dorsal and ventral pathways in language development. Brain Lang. 2013;127:289–295. doi: 10.1016/j.bandl.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Braun M.T., Oswald F.L. Exploratory regression analysis: a tool for selecting models and determining predictor importance. Behav. Res. Methods. 2011;43:331–339. doi: 10.3758/s13428-010-0046-8. [DOI] [PubMed] [Google Scholar]
- Buchsbaum B.R., Olsen R.K., Koch P., Berman K.F. Human dorsal and ventral auditory streams subserve rehearsal-based and echoic processes during verbal working memory. Neuron. 2005;48:687–697. doi: 10.1016/j.neuron.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Bunge S.A., Kahn I., Wallis J.D., Miller E.K., Wagner A.D. Neural circuits subserving the retrieval and maintenance of abstract rules. J. Neurophysiol. 2003;90:3419–3428. doi: 10.1152/jn.00910.2002. [DOI] [PubMed] [Google Scholar]
- Burgund E.D., Kang H.C., Kelly J.E., Buckner R.L., Snyder A.Z., Petersen S.E., Schlaggar B.L. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. NeuroImage. 2002;17:184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Camos V., Barrouillet P. Developmental change in working memory strategies: from passive maintenance to active refreshing. Dev. Psychol. 2011;47:898–904. doi: 10.1037/a0023193. [DOI] [PubMed] [Google Scholar]
- Catani M., Jones D.K., Ffytche D.H. Perisylvian language networks of the human brain. Ann. Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Chen S.H.A., Desmond J.E. Temporal dynamics of cerebro-cerebellar network recruitment during a cognitive task. Neuropsychologia. 2005;43:1227–1237. doi: 10.1016/j.neuropsychologia.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Chen S.H.A., Desmond J.E. Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. NeuroImage. 2005;24:332–338. doi: 10.1016/j.neuroimage.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Chugani H.T. A critical period of brain development: studies of cerebral glucose utilization with PET. Prev. Med. (Baltim) 1998;27:184–188. doi: 10.1006/pmed.1998.0274. [DOI] [PubMed] [Google Scholar]
- Chugani H.T., Phelps M.E., Mazziotta J.C. Positron emission tomography study of human brain functional development. Ann. Neurol. 1987;22:487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- Citron F.M.M., Oberecker R., Friederici A.D., Mueller J.L. Mass counts: ERP correlates of non-adjacent dependency learning under different exposure conditions. Neurosci. Lett. 2011;487:282–286. doi: 10.1016/j.neulet.2010.10.038. [DOI] [PubMed] [Google Scholar]
- Coull J.T., Cheng R.-K., Meck W.H. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology. 2011;36:3–25. doi: 10.1038/npp.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond J.E., Gabrieli J.D., Wagner A.D., Ginier B.L., Glover G.H. Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. J. Neurosci. 1997;17:9675–9685. doi: 10.1523/JNEUROSCI.17-24-09675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar M., Abbot-Smith K., Lieven E., Tomasello M. German children’s comprehension of word order and case marking in causative sentences. Child Dev. 2008;79:1152–1167. doi: 10.1111/j.1467-8624.2008.01181.x. [DOI] [PubMed] [Google Scholar]
- Dubois J., Poupon C., Thirion B., Simonnet H., Kulikova S., Leroy F., Hertz-Pannier L., Dehaene-Lambertz G. Exploring the early organization and maturation of linguistic pathways in the human infant brain. Cereb. Cortex. 2015 doi: 10.1093/cercor/bhv082. [DOI] [PubMed] [Google Scholar]
- Durston S., Davidson M.C., Tottenham N., Galvan A., Spicer J., Fossella J.A., Casey B.J. A shift from diffuse to focal cortical activity with development. Dev. Sci. 2006;1:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Felser C., Clahsen H., Münte T.F. Storage and integration in the processing of filler-gap dependencies: an ERP study of topicalization and wh-movement in German. Brain Lang. 2003;87:345–354. doi: 10.1016/s0093-934x(03)00135-4. [DOI] [PubMed] [Google Scholar]
- Felser C., Marinis T., Clahsen H., Taylor P. Children’s processing of ambiguous sentences: a study of relative clause attachment. Lang. Acquis. 2003;11:127–163. [Google Scholar]
- Fengler A., Meyer L., Friederici A.D. Brain structural correlates of complex sentence comprehension in children. Dev. Cogn. Neurosci. 2015;15:48–57. doi: 10.1016/j.dcn.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Féry C., Schubö F. Hierarchical prosodic structures in the intonation of center-embedded relative clauses. Linguist. Rev. 2010;27:293–317. [Google Scholar]
- Fonov V., Evans A.C., Botteron K., Almli C.R., McKinstry R.C., Collins D.L. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage. 2011;54:313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici A.D. The brain basis of language processing: from structure to function. Physiol. Rev. 2011;91:1357–1392. doi: 10.1152/physrev.00006.2011. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Gierhan S.M.E. The language network. Curr. Opin. Neurobiol. 2013;23:250–254. doi: 10.1016/j.conb.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Fiebach C.J., Schlesewsky M., Bornkessel I.D., Von Cramon D.Y. Processing linguistic complexity and grammaticality in the left frontal cortex. Cereb. Cortex. 2006;16:1709–1717. doi: 10.1093/cercor/bhj106. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Makuuchi M., Bahlmann J. The role of the posterior superior temporal cortex in sentence comprehension. Neuroreport. 2009;20:563–568. doi: 10.1097/WNR.0b013e3283297dee. [DOI] [PubMed] [Google Scholar]
- Gibson E. Linguistic complexity: locality of syntactic dependencies. Cognition. 1998;68:1–76. doi: 10.1016/s0010-0277(98)00034-1. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Paus T., Evans A.C., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Nugent T.F., Herman D.H., Clasen L.S., Toga A.W., Rapoport J.L., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goucha T., Friederici A.D. The language skeleton after dissecting meaning: a functional segregation within Broca’s Area. NeuroImage. 2015;114:294–302. doi: 10.1016/j.neuroimage.2015.04.011. [DOI] [PubMed] [Google Scholar]
- Hamburger H., Crain S. Relative acquisition. In: Kuczaj S., editor. Language Development. vol. 1. Erlbaum; Hillsdale, NJ: 1982. pp. 245–274. (Syntax and Semantics). [Google Scholar]
- Hanakawa T., Honda M., Sawamoto N., Okada T., Yonekura Y., Fukuyama H., Shibasaki H. The role of rostral Brodmann area 6 in mental-operation tasks: an integrative neuroimaging approach. Cereb. Cortex. 2002;12:1157–1170. doi: 10.1093/cercor/12.11.1157. [DOI] [PubMed] [Google Scholar]
- Hensch T.K., Bilimoria P.M. Cerebrum Dana forum brain Sci. 2012. Re-opening windows: manipulating critical periods for brain development. [PMC free article] [PubMed] [Google Scholar]
- Honey G.D., Bullmore E.T., Sharma T. Prolonged reaction time to a verbal working memory task predicts increased power of posterior parietal cortical activation. NeuroImage. 2000;12:495–503. doi: 10.1006/nimg.2000.0624. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P.R. Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P.R., Dabholkar A.S. Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Johnson M.H. Interactive specialization: a domain-general framework for human functional brain development? Dev. Cogn. Neurosci. 2011;1:7–21. doi: 10.1016/j.dcn.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.S., Newport E.L. Critical period effects in second language learning: the influence of maturational state on the acquisition of English as a second language. Cogn. Psychol. 1989;21:60–99. doi: 10.1016/0010-0285(89)90003-0. [DOI] [PubMed] [Google Scholar]
- Judas M., Cepanec M. Adult structure and development of the human fronto-opercular cerebral cortex (Broca’s region) Clin. Linguist. Phon. 2007;21:975–989. doi: 10.1080/02699200701617175. [DOI] [PubMed] [Google Scholar]
- Kang H.C., Burgund E.D., Lugar H.M., Petersen S.E., Schlaggar B.L. Comparison of functional activation foci in children and adults using a common stereotactic space. NeuroImage. 2003;19:16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Kidd E., Bavin E.L. English-speaking children’s comprehension of relative clauses: evidence for constraints on development. J. Psycholinguist. Res. 2002;31:599–617. doi: 10.1023/a:1021265021141. [DOI] [PubMed] [Google Scholar]
- Kidd E., Brandt S., Lieven E., Tomasello M. Object relatives made easy: a cross-linguistic comparison of the constraints influencing young children’s processing of relative clauses. Lang. Cogn. Process. 2007;22:860–897. [Google Scholar]
- Kinno R., Kawamura M., Shioda S., Sakai K.L. Neural correlates of noncanonical syntactic processing revealed by a picture-sentence matching task. Hum. Brain Mapp. 2008;29:1015–1027. doi: 10.1002/hbm.20441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll L.J., Obleser J., Schipke C.S., Friederici A.D., Brauer J. Left prefrontal cortex activation during sentence comprehension covaries with grammatical knowledge in children. NeuroImage. 2012;62:207–216. doi: 10.1016/j.neuroimage.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Kotz S.A., Schwartze M. Cortical speech processing unplugged: a timely subcortico-cortical framework. Trends Cogn. Sci. 2010;14:392–399. doi: 10.1016/j.tics.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Kraha A., Turner H., Nimon K., Zientek L.R., Henson R.K. Tools to support interpreting multiple regression in the face of multicollinearity. Front. Psychol. 2012;3:1–16. doi: 10.3389/fpsyg.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl P.K. Early language acquisition: cracking the speech code. Nat. Rev. Neurosci. 2004;5:831–843. doi: 10.1038/nrn1533. [DOI] [PubMed] [Google Scholar]
- Kuhl P., Rivera-Gaxiola M. Neural substrates of language acquisition. Annu. Rev. Neurosci. 2008;31:511–534. doi: 10.1146/annurev.neuro.30.051606.094321. [DOI] [PubMed] [Google Scholar]
- LaBar K.S., Gitelman D.R., Parrish T.B., Mesulam M. Neuroanatomic overlap of working memory and spatial attention networks: a functional MRI comparison within subjects. NeuroImage. 1999;10:695–704. doi: 10.1006/nimg.1999.0503. [DOI] [PubMed] [Google Scholar]
- Leff A.P., Schofield T.M., Crinion J.T., Seghier M.L., Grogan A., Green D.W., Price C.J. The left superior temporal gyrus is a shared substrate for auditory short-term memory and speech comprehension: evidence from 210 patients with stroke. Brain. 2009;132:3401–3410. doi: 10.1093/brain/awp273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehéricy S., Ducros M., Krainik A., Francois C., Van De Moortele P.F., Ugurbil K., Kim D.S. 3-D diffusion tensor axonal tracking shows distinct SMA and pre-SMA projections to the human striatum. Cereb. Cortex. 2004;14:1302–1309. doi: 10.1093/cercor/bhh091. [DOI] [PubMed] [Google Scholar]
- Lenneberg E.H. Wiley; New York: 1967. Biological foundations of language. [Google Scholar]
- Lidzba K., Schwilling E., Grodd W., Krägeloh-Mann I., Wilke M. Language comprehension vs. language production: age effects on fMRI activation. Brain Lang. 2011;119:6–15. doi: 10.1016/j.bandl.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Lu L., Leonard C., Thompson P., Kan E., Jolley J., Welcome S., Toga A., Sowell E. Normal developmental changes in inferior frontal gray matter are associated with improvement in phonological processing: a longitudinal MRI analysis. Cereb. Cortex. 2007;17:1092–1099. doi: 10.1093/cercor/bhl019. [DOI] [PubMed] [Google Scholar]
- Lu L.H., Dapretto M., O’Hare E.D., Kan E., McCourt S.T., Thompson P.M., Toga A.W., Bookheimer S.Y., Sowell E.R. Relationships between brain activation and brain structure in normally developing children. Cereb. Cortex. 2009;19:2595–2604. doi: 10.1093/cercor/bhp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makuuchi M., Bahlmann J., Anwander A., Friederici A.D. Segregating the core computational faculty of human language from working memory. Proc. Natl. Acad. Sci. U. S. A. 2009;106:8362–8367. doi: 10.1073/pnas.0810928106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makuuchi M., Bahlmann J., Friederici A.D. An approach to separating the levels of hierarchical structure building in language and mathematics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367:2033–2045. doi: 10.1098/rstb.2012.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNealy K., Mazziotta J.C., Dapretto M. The neural basis of speech parsing in children and adults. Dev. Sci. 2010;13:385–406. doi: 10.1111/j.1467-7687.2009.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A., Price C.J., Friston K.J., Ashburner J. Voxel-based morphometry of the human brain: methods and applications. Curr. Med. Imaging Rev. 2005;1:105–113. [Google Scholar]
- Meisel J.M. Cambridge University Press; Cambridge: 2011. First and second language acquisition: parallels and differences. [Google Scholar]
- Meyer L., Obleser J., Anwander A., Friederici A.D. Linking ordering in Broca's area to storage in left temporo-parietal regions: the case of sentence processing. NeuroImage. 2012;62:1987–1998. doi: 10.1016/j.neuroimage.2012.05.052. [DOI] [PubMed] [Google Scholar]
- Meyer L., Cunitz K., Obleser J., Friederici A.D. Sentence processing and verbal working memory in a white-matter-disconnection patient. Neuropsychologia. 2014;61:190–196. doi: 10.1016/j.neuropsychologia.2014.06.014. [DOI] [PubMed] [Google Scholar]
- Montgomery J.W., Magimairaj B.M., O’Malley M.H. Role of working memory in typically developing children’s complex sentence comprehension. J. Psycholinguist. Res. 2008;37:331–354. doi: 10.1007/s10936-008-9077-z. [DOI] [PubMed] [Google Scholar]
- Mueller J.L., Friederici A.D., Männel C. Auditory perception at the root of language learning. Proc. Natl. Acad. Sci. U. S. A. 2012;109:15953–15958. doi: 10.1073/pnas.1204319109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch B.E. The cerebellum and language: historical perspective and review. Cortex. 2010;46:858–868. doi: 10.1016/j.cortex.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Muzik O., Chugani D.C., Juhász C., Shen C., Chugani H.T. Statistical parametric mapping: assessment of application in children. NeuroImage. 2000;12:538–549. doi: 10.1006/nimg.2000.0651. [DOI] [PubMed] [Google Scholar]
- Newman S.D., Ikuta T., Burns T. The effect of semantic relatedness on syntactic analysis: an fMRI study. Brain Lang. 2010;113:51–58. doi: 10.1016/j.bandl.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimon K., Lewis M., Kane R., Haynes R.M. An R package to compute commonality coefficients in the multiple regression case: an introduction to the package and a practical example. Behav. Res. Methods. 2008;40:457–466. doi: 10.3758/brm.40.2.457. [DOI] [PubMed] [Google Scholar]
- Novais-Santos S., Gee J., Shah M., Troiani V., Work M., Grossman M. Resolving sentence ambiguity with planning and working memory resources: evidence from fMRI. NeuroImage. 2007;37:361–678. doi: 10.1016/j.neuroimage.2007.03.077. [DOI] [PubMed] [Google Scholar]
- Nuñez S.C., Dapretto M., Katzir T., Starr A., Bramen J., Kan E., Bookheimer S., Sowell E.R. fMRI of syntactic processing in typically developing children: structural correlates in the inferior frontal gyrus. Dev. Cogn. Neurosci. 2011;1:313–323. doi: 10.1016/j.dcn.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Opitz B., Friederici A.D. Brain correlates of language learning: the neuronal dissociation of rule-based versus similarity-based learning. J. Neurosci. 2004;24:8436–8440. doi: 10.1523/JNEUROSCI.2220-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz B., Friederici A.D. Neural basis of processing sequential and hierarchical syntactic structures. Hum. Brain Mapp. 2007;28:585–592. doi: 10.1002/hbm.20287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen A.M., McMillan K.M., Laird A.R., Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani D., Saccuman M.C., Scifo P., Anwander A., Spada D., Baldoli C. Neural language networks at birth. Proc. Natl. Acad. Sci. U. S. A. 2011;108:16056–16061. doi: 10.1073/pnas.1102991108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z., Judas M., Kostović I., Uylings H.B.M. Lifespan alterations of basal dendritic trees of pyramidal neurons in the human prefrontal cortex: a layer-specific pattern. Cereb. Cortex. 2008;18:915–929. doi: 10.1093/cercor/bhm124. [DOI] [PubMed] [Google Scholar]
- Petanjek Z., Judaš M., Šimic G., Rasin M.R., Uylings H.B.M., Rakic P., Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N., Strick P.L. Imaging the premotor areas. Curr. Opin. Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Poldrack R.A. Region of interest analysis for fMRI. Soc. Cogn. Affect. Neurosci. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P., Bourgeois J.P., Goldman-Rakic P.S. Synaptic development of the cerebral cortex: implications for learning, memory, and mental illness. Prog. Brain Res. 1994;102:227–243. doi: 10.1016/S0079-6123(08)60543-9. [DOI] [PubMed] [Google Scholar]
- Ravizza S.M., Hazeltine E., Ruiz S., Zhu D.C. Left TPJ activity in verbal working memory: implications for storage- and sensory-specific models of short term memory. NeuroImage. 2011;55:1836–1846. doi: 10.1016/j.neuroimage.2010.12.021. [DOI] [PubMed] [Google Scholar]
- Richardson F.M., Thomas M.S.C., Filippi R., Harth H., Price C.J. Contrasting effects of vocabulary knowledge on temporal and parietal brain structure across lifespan. J. Cogn. Neurosci. 2010;22:943–954. doi: 10.1162/jocn.2009.21238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röder B., Stock O., Neville H., Bien S., Rösler F. Brain activation modulated by the comprehension of normal and pseudo-word sentences of different processing demands: a functional magnetic resonance imaging study. NeuroImage. 2002;15:1003–1014. doi: 10.1006/nimg.2001.1026. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum. Brain Mapp. 1996;4:174–198. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Schubotz R.I. Prediction of external events with our motor system: towards a new framework. Trends Cogn. Sci. 2007;11:211–218. doi: 10.1016/j.tics.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Schwartze M., Rothermich K., Kotz S. a. Functional dissociation of pre-SMA and SMA-proper in temporal processing. NeuroImage. 2012;60:290–298. doi: 10.1016/j.neuroimage.2011.11.089. [DOI] [PubMed] [Google Scholar]
- Sheldon A. On strategies for processing relative clauses: a comparison of children and adults. J. Psycholinguist. Res. 1977;6:305–318. [Google Scholar]
- Simonds R.J., Scheibel A.B. The postnatal development of the motor speech area: a preliminary study. Brain Lang. 1989;37:42–58. doi: 10.1016/0093-934x(89)90100-4. [DOI] [PubMed] [Google Scholar]
- Skeide M.A., Brauer J., Friederici A.D. Syntax gradually segregates from semantics in the developing brain. NeuroImage. 2014;100C:106–111. doi: 10.1016/j.neuroimage.2014.05.080. [DOI] [PubMed] [Google Scholar]
- Skeide M.A., Brauer J., Friederici A.D. Brain functional and structural predictors of language performance. Cereb. Cortex. 2015 doi: 10.1093/cercor/bhv042. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Peterson B.S., Thompson P.M., Welcome S.E., Henkenius A.L., Toga A.W. Mapping cortical change across the human life span. Nat. Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Tanaka C., Matsui M., Uematsu A., Noguchi K., Miyawaki T. Developmental trajectories of the fronto-temporal lobes from infancy to early adulthood in healthy individuals. Dev. Neurosci. 2012;34:477–487. doi: 10.1159/000345152. [DOI] [PubMed] [Google Scholar]
- Tavakolian S.L. The conjoined-clause analysis of relative clauses. In: Tavakolian S.L., editor. Language Acquisition and Linguistic Theory. MIT Press; Cambridge, Mass: 1981. pp. 167–187. [Google Scholar]
- Tewes U. Huber; Bern: 1994. Hamburg-Wechsler-Intelligenztest für Erwachsene. [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio: 2003. Wechsler Intelligence Scale for Children (WISC-IV) [Google Scholar]
- Weighall A.R., Altmann G.T.M. The role of working memory and contextual constraints in children’s processing of relative clauses. J. Child Lang. 2011;38:579–605. doi: 10.1017/S0305000910000267. [DOI] [PubMed] [Google Scholar]
- Weissenborn J. Constraining the child’s grammar. Local wellformedness in the development of verb movement in German and French. In: Lust B., Suner M., Whitman J., editors. Syntactic theory and language acquisition: crosslinguistic perspectives. vol. 1. Lawrence Erlbaum; Hillsdale, NJ: 1994. pp. 215–247. (Phrase Structure). [Google Scholar]
- Werker J.F., Tees R.C. Cross-language speech perception: evidence for perceptual reorganization during the first year of life. Infant Behav. Dev. 2002;25:121–133. [Google Scholar]
- Wilke M., Schmithorst V.J., Holland S.K. Assessment of spatial normalization of whole-brain magnetic resonance images in children. Hum. Brain Mapp. 2002;17:48–60. doi: 10.1002/hbm.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S.M., Galantucci S., Tartaglia M.C., Rising K., Patterson D.K., Henry M.L., Ogar J.M., DeLeon J., Miller B.L., Gorno-Tempini M.L. Syntactic processing depends on dorsal language tracts. Neuron. 2011;72:397–403. doi: 10.1016/j.neuron.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zevin J.D., Datta H., Skipper J.I. Sensitive periods for language and recovery from stroke: conceptual and practical parallels. Dev. Psychobiol. 2012;54:332–342. doi: 10.1002/dev.20626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.