Abstract
Rationale
Working memory impairments in schizophrenia have been attributed to dysfunction of the dorsolateral prefrontal cortex (DLPFC) which in turn may be due to low DLPFC dopamine innervation. Conventional antipsychotic drugs block DLPFC D2 receptors, and this may lead to further dysfunction and working memory impairments. Aripiprazole is a D2 receptor partial agonist hypothesised to enhance PFC dopamine functioning, possibly improving working memory.
Objectives
We probed the implications of the partial D2 receptor agonist actions of aripiprazole within the DLPFC during working memory. Investigations were carried out in healthy volunteers to eliminate confounds of illness or medication status. Aripiprazole’s prefrontal actions were compared with the D2/5-HT2A blocker risperidone to separate aripiprazole’s unique prefrontal D2 agonist actions from its serotinergic and striatal D2 actions that it shares with risperidone.
Method
A double-blind, placebo-controlled, parallel design was implemented. Participants received a single dose of either 5 mg aripiprazole, 1 mg risperidone or placebo before performing the n-back task whilst undergoing fMRI scanning.
Results
Compared with placebo, the aripiprazole group demonstrated enhanced DLPFC activation associated with a trend for improved discriminability (d’) and speeded reaction times. In contrast to aripiprazole’s neural effects, the risperidone group demonstrated a trend for reduced DLPFC recruitment. Unexpectedly, the risperidone group demonstrated similar effects to aripiprazole on d’ and additionally had reduced errors of commission compared with placebo.
Conclusion
Aripiprazole has unique DLPFC actions attributed to its prefrontal D2 agonist action. Risperidone’s serotinergic action that results in prefrontal dopamine release may have protected against any impairing effects of its prefrontal D2 blockade.
Electronic supplementary material
The online version of this article (doi:10.1007/s00213-016-4234-9) contains supplementary material, which is available to authorized users.
Keywords: Working memory, Aripiprazole, Risperidone, n-Back, Dopamine, Dorsolateral prefrontal, Antipsychotic, Partial agonism, D2
Introduction
Aim and rationale
The aim of this study was to investigate the implications of the partial dopamine D2 receptor agonist properties of aripiprazole for the neural basis of working memory in healthy volunteers. Impaired working memory is a core feature of schizophrenia which is not ameliorated by conventional antipsychotic drugs (Forbes et al. 2009). The impairment has been attributed to an impaired dopamine innervation of the dorsolateral prefrontal cortex (DLPFC) (Akil et al. 1999; Davis et al. 1991; Pycock et al. 1980; Slifstein et al. 2015; Winterer and Weinberger 2004), a key component of the neural circuitry of working memory. There has been much clinical interest in the possibility that the partial agonist actions of aripiprazole might restore D2 neurotransmission to a low optimal level in prefrontal cortex in schizophrenia (Bolonna and Kerwin 2005). This would preserve or enhance working memory while antipsychotic effects are achieved by preventing overstimulation of D2 receptors in striatum. We investigated the effects of aripiprazole on the performance of a working memory task and its neural correlates using functional magnetic resonance imaging (fMRI). We compared the effects of aripiprazole with those of risperidone since both drugs have high affinity for the D2 receptor combined with serotonergic effects although risperidone lacks the intrinsic D2 agonist activity of aripiprazole.
Dopamine modulation of DLPFC and working memory
Working memory (WM) refers to the ability to hold and manage information ‘online’ over short periods of time, allowing its manipulation and usage in reasoning, comprehension and decision-making (Levy and Goldman-Rakic 2000). Numerous neuroimaging studies have shown that a network of cortical regions centred on the DLPFC is activated by performance of WM tasks such as the n-back task (see below). Impaired WM performance in patients with schizophrenia is associated with reduced DLPFC activation compared to controls (Carter et al. 1998; Perlstein et al. 2001; Weinberger et al. 1986). This is thought to reflect cortical inefficiency because greater DLPFC activation is elicited in patients when performance matches control levels (Callicott et al. 2000; Manoach et al. 1999, 2000).
Dopamine has an important role in WM performance in both non-human primates (Brozoski et al. 1979; Sawaguchi 2001; Sawaguchi et al. 1990) and in human volunteers (Egan et al. 2001). Dopamine modulates PFC activity by both D1 (Seamans and Yang 2004) and D2 receptor actions on pyramidal cells and interneurons (Wang et al. 2004), which influence different functional aspects of WM in experimental animals (Durstewitz and Seamans 2008; Seamans and Yang 2004). Studies in humans suggest a role for D2 receptors in working memory (Kimberg et al. 1997; Luciana et al. 1992; Luciana and Collins 1997; Mehta et al. 1999, 2001) although it is unknown whether these effects are driven by striatal or cortical D2 receptor actions given the systemic drug administration of these studies.
The foregoing evidence suggests that dysregulation of the DLPFC may contribute to the executive functioning impairments seen in schizophrenia and that impaired dopamine function could be a key mechanism. The actions of antipsychotic drugs on dopamine function within the dorsolateral prefrontal cortex are therefore of considerable interest.
Neurochemical profiles of aripiprazole and risperidone
Aripiprazole is an atypical antipsychotic with a very high affinity (0.34 nM) (Burris et al. 2002) for the D2 receptor. In contrast to all currently available antipsychotic drugs, aripiprazole has a partial agonist effect on the D2 receptor with a range of intrinsic efficacies dependent upon the G protein-coupled receptor system it is acting upon (Lawler et al. 1999; Shapiro et al. 2003). Because of its high D2 affinity, aripiprazole is thought to clamp synaptic dopamine function at a constant but sub-maximal level of activation regardless of local dopamine concentration. This results in functional antagonism in areas of high dopamine concentration such as within the striatum. However, in areas of low dopamine concentration such as prefrontal cortex, aripiprazole’s partial agonism would exceed the local action of dopamine (Bolonna and Kerwin 2005; Grunder et al. 2003) resulting in increased D2 function. In addition to direct effects at D2 receptors, aripiprazole has been shown to increase dopamine release into the mouse prefrontal cortex in in-vivo microdialysis studies (Li et al. 2004; Zocchi et al. 2005). This effect may be due to the 5-HT1A receptor partial agonism (Ki = 3.4 nM) and the 5-HT2A antagonism (Ki = 4.2 nM) that aripiprazole also possesses (Stark et al. 2007) as both of these actions have been shown to increase prefrontal dopamine release (Li et al. 2004). Therefore, aripiprazole may also indirectly increase D1 neurotransmission during working memory by increasing prefrontal dopamine levels.
Risperidone has a high affinity for D2 receptors but acts as a pure antagonist. Furthermore, risperidone, like aripiprazole, increases dopamine release in the prefrontal cortex (Kuroki et al. 1999) and is a high-affinity 5-HT2A receptor antagonist (Ki = 0.15–0.4 nM) (Leysen et al. 1994; Richelson and Souder 2000). By comparing the two drugs, we aimed to isolate aripiprazole’s D2 agonist effects from other actions that it shares with risperidone. This allowed us to separate out the functional importance of cortical versus striatal D2 receptors in working memory as both drugs reduce striatal D2 neurotransmission but only aripiprazole is hypothesised to have partial agonist effects at prefrontal D2 receptors. We hypothesised that aripiprazole would enhance or change prefrontal cortex WM activations in relation to improved performance compared to placebo and risperidone.
Methods
Participants
This study was approved by the NHS Research Ethics Committee and the Committee on the Ethics of Research on Human Beings of the University of Manchester. The study was therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. Male participants were recruited via e-mail and were paid for their participation. Participants had to meet the following criteria: healthy according to physical examination including blood pressure, kidney and liver function tests and blood glucose levels, general health questionnaire and electrocardiography. Participants had no current diagnosis, or history of, psychiatric illness or substance use disorder. Participants were not taking any medication, were non-smokers and were right handed. Participants consumed under 20 units of alcohol a week and were asked to remain abstinent from alcohol and caffeine for 24 h prior to the study; compliance was self-reported.
Screening procedures included the Quick Test (Ammon and Ammons 1962) to provide an estimate of IQ, Mini International Neuropsychiatric Interview (MINI) (Sheehan et al. 1998), and the Edinburgh Handedness Inventory (EHI) (Oldfield 1971). The mean age (SD) was 23 (4.96), mean IQ (SD) was 94 (7.96), and the mean weight (SD) was 68.2 kg (11.6). ANOVAs revealed that there were no significant differences in age (p = 0.32), IQ (p = 0.97) or weight (p = 0.39) of the participants assigned to the placebo, aripiprazole or risperidone drug group. Urine drug tests, electrocardiograms (ECG) and blood tests were carried out at screening and any participants with a positive result and/or abnormal ECG reading or blood results were excluded from the study. Written informed consent was obtained from the participants before enrolment to the study.
Experimental design and procedures
The study was performed following a randomised, double-blind, placebo-controlled, parallel group procedure. This parallel group design was chosen over a within-subject design to avoid practice effects on n-back performance and habituation effects to both the scanner environment and n-back task. Interactions between these effects and drug effects would make results difficult to interpret. Thirty-seven participants took part in the study in total: 13 participants received an acute oral dose of 5 mg aripiprazole, 12 received 1 mg risperidone, and 12 received placebo. Scanner technical problems resulted in loss of data from three participants (1 placebo, 1 aripiprazole, 1 risperidone). Therefore, three participants were excluded prior to any group analysis. The doses were subtherapeutic, chosen to minimise side effects in healthy volunteers. Recent studies have examined aripiprazole receptor occupancy in healthy volunteers after acute doses of aripiprazole; one study demonstrated that 5 mg of aripiprazole resulted in a mean striatal D2 occupancy of 55 % (Kim et al. 2012). Another study reported 74 % striatal and 51 % frontal D2 occupancy after 6 mg of aripiprazole (Takahata et al. 2012). A single 1 mg dose of risperidone results in striatal D2 occupancies of 50 % in healthy volunteers (Nyberg et al. 1993). Whilst this study did not measure extrastriatal D2 occupancy, PET studies assessing frontal occupancy after a single dose of 2 mg risperidone, and continuous dosing of 1 mg risperidone, suggest that frontal D2 occupancies of risperidone, whilst being slightly lower, do not differ significantly from striatal D2 occupancies (Agid et al. 2007; Ito et al. 2009). Taken together, it may be assumed that similar frontal D2 occupancies of slightly below 50 % were achieved with single doses of 5 mg aripiprazole and 1 mg risperidone.
Participants were scanned 3.5 h after taking the test drug as this represents the tmax for aripiprazole (Kubo et al. 2007). The tmax for risperidone and its equally potent metabolite is 1.5 and 3 h, respectively (Ereshefsky 1996; Huang et al. 1993). Participants were assessed for medication side effects by the on-site study clinician. Upon leaving the scanner, blood pressure was taken both standing and sitting and participants were assessed once more before being allowed to leave.
N-back task
The task was programmed using E-Prime v1.1.4.4. SP3 (Psychology Software Tools 2003). Stimuli were presented in a block design, with three different conditions presented in 26-s blocks. For the 0-back condition, participants viewed a series of 13 letters which were presented with an interstimulus interval of 2 s (letter presented for 1500 ms followed by a blank screen for 500 ms). Participants were asked to respond with a button press on a button box whenever they saw an ‘X’ appear on the screen, this condition does not require any working memory processing and therefore represents the control condition for the task. For the 1-back condition, participants were asked to respond when they saw a letter which was the same as the last one presented. For the 2-back condition, participants were asked to respond when they saw a letter on screen that was the same as the one presented before the last letter. To avoid potential ceiling effects, participants were not permitted to practise the task although they received detailed task instructions and it was ensured they understood each task condition before entering the scanner. Thirteen letters were presented in each test block and each block contained three target stimuli. The task comprised three 2-back blocks, three 1-back blocks and six 0-back blocks presented in a pseudo-randomised order with a block of the 0-back condition presented between each 1-back and 2-back block. Whilst in the scanner, participants were instructed on the type of block they were about to perform by verbal instructions presented on the screen for 9 s.
Missed targets were recorded as omission errors (OEs), whereas participants’ responses to non-targets were recorded as commission errors (CEs). There were a total of 9 target letters for the 1-back and 2-back condition and 18 target letters for the 0-back condition. The task lasted 7 min.
Statistical analysis of behavioural data
Participants made few errors of commission or omission. Chi-square tests (likelihood ratio; Lχ2) were used to assess whether there was an association between the drug taken and the frequency of OEs and CEs made for the 0- (baseline), 1- and 2-back conditions. Furthermore, discriminability or d prime (d’) was assessed. Hit rate was defined as the proportion of the targets responded to and the false alarm (FA) rate was the proportion of non-targets responded to. The hit rate and false alarm rate were transformed to Z-values using the NORMSINV function in excel (Haatveit et al. 2010). d’ is calculated with the following formula: Zhit − ZFA. Perfect hit rate or FA rates of 1 or 0 respectively were converted using the following formulas before the Z-transform was carried out; proportions of 1 were converted to 1 − 1/(2N) and proportions of 0 were converted to 1/(2N) (Haatveit et al. 2010). A mixed ANCOVA using SPSS was carried out (n-back level and drug group as the within- and between-subject factor respectively) with the d’ score for the 0-back condition (which does not measure working memory but indexes attentional processing) added as a mean centred covariate.
Statistical analyses were carried out using SPSS v22. A mixed ANCOVA (n-back level and drug group as the within and between group factor respectively) was used to investigate drug effects on reaction times for correct responses for the 1-back and 2-back levels. To control for individual differences in motor responses, the reaction times for correct responses on the 0-back condition were entered as a mean centred covariate in the analysis. For d’ and reaction time analyses, main effects of group were investigated with Sidak post hoc tests, and drug group by n-back level interactions were investigated with both paired and two-sample t tests. A one-way ANOVA was carried out on the 0-back condition to test for any effects of the drugs on attentional processing.
Due to technical problems with recording responses, response data was lost from 3 out of the 12 participants in the aripiprazole group and a further 1 from the 11 participants in the placebo group; therefore, behavioural data from 9 participants from the aripiprazole group, 10 from the placebo group and 11 from the risperidone group were entered into the behavioural analysis.
Image acquisition of functional data
Whole brain T2*-weighted images were acquired on a 3 T Philips Achieva scanner with single shot, gradient echo-planar imaging with the following parameters: FOV = 230 mm, acquisition matrix = 128 × 128, TR/TE 2000/35 ms, voxel size = 1.8 × 1.8 × 3.0 mm, 0.5-mm slice gap, and 34 slices. T1-weighted structural images were acquired for each participant in order to exclude patients with any anatomical abnormalities and for use during the preprocessing of functional images.
Preprocessing and analysis of fMRI data
Final group size for fMRI data analysis was 12 for aripiprazole, 11 for risperidone and 11 for placebo. The functional images were converted from the PARREC format to the analyse format using MRIcro (version 1.40). Functional data were analysed using Statistical Parametric Mapping (SPM12; The Wellcome Department of Cognitive Neurology, London, England) implemented in Matlab (R2013a).
Images were realigned to correct for motion artefacts, and the T1 structural image was coregistered with the mean image of the realigned functional images. The T1 structural image was segmented and normalised using tissue probability templates supplied by SPM. Normalisation parameters were then applied to the functional images. Images were smoothed using a Gaussian kernel of the following dimensions 5.4 mm × 5.4 mm × 10.5 (three times the voxel size).
First-level analysis
Data were fitted via general linear modelling in SPM (0-back, 1-back and 2-back conditions). The input model was convolved with the haemodynamic response function (HRF) in order to provide a better fit of the data (Smith 2001). The low-frequency drifts in the data were modelled out using a high pass filter set to 140 s, equivalent to two times the main repetition time of the task. The following contrasts were specified: 1-back minus 0-back, 2-back minus 0-back, 1-back&2-back minus 0-back and 2-back minus 1-back. Movement outliers (scan to scan displacement of >2 mm or volumes with global brain activation >3 standard deviations away from the mean) were detected using Artifact Detection Tools (ART) (http://www.nitrc.org/projects/artifact_detect/), and these outliers were added as nuisance regressors to the first-level model. All participants had less than 10 % of volumes detected as outliers.
Second level analysis
As fMRI packages only produce one error term during statistical analysis, statistical tests containing both within- and between-subject factors within a single model are not validly performed (McLaren DG et al. 2011). For this reason, separate models were used for both within- and between-subject factors. To assess the effect of the n-back task and main effect of drug group, an ANOVA was specified with the average of the 1-back and 2-back condition (compared to 0-back) as a factor. To assess main effect of n-back level and n-back level by drug group interactions, an ANOVA was specified containing the 2-back minus 1-back contrast as a factor. Cluster thresholding was used with a cluster forming thresholded of p < 0.005. For the main effects of task and n-back level in all 34 participants, a whole-brain family-wise error cluster threshold of p < 0.05 was applied (pFWEc < 0.05). Assessments of drug group effects and n-back level by drug group interactions were confined to a DLPFC region of interest and thresholded at pFWEc < 0.05 small volume corrected (SVC). The DLPFC region of interest (ROI) comprised of a sphere of 10-mm radius centred around the Talairach coordinates (42, 32, 30) representing the peak DLPFC activation generated from a meta-analysis of verbal, identity-monitoring n-back task data in healthy controls (Owen et al. 2005). Furthermore, ROI analysis was carried out with and without a mask of the positive main effect of the n-back task (pFWEc < 0.05) to determine whether the drug effects occurred within regions activated by the task. The average of the beta-estimates were extracted from clusters significant for drug group effects (main effects or interactions) and plotted. Effects significant in the ANOVA were investigated with post-hoc two-sample t test comparisons.
To determine if any of the drug effects on brain activation influenced the behavioural measures, correlational analyses were carried out between BOLD signal change within the 1-back and 2-back conditions compared to 0-back and the behavioural measures (CE, OE, d’ and reaction time for correct responses), using any clusters showing significant drug effects as regions of interest. This was carried out within each drug group individually and with all participants from all three drug groups together.
Results
Tolerability
The medications were well tolerated. Mild nausea was reported by one participant taking aripiprazole although this was temporary and the participant was able to carry out the scan. There were no other adverse reactions to the medications, and no participants were excluded on the basis of lack of tolerability.
Behavioural
Omission and commission errors
Most participants made no CEs or OEs in the 0-back and 1-back condition. This pattern was not modified by drug treatment (Lχ2 not shown). More errors were made in the 2-back condition but OEs remained unaffected by drug treatment (Lχ2(6) = 9.92, p = 0.16; see supplementary materials for figure). In contrast, CEs at 2-back were affected by drug treatment (Lχ2(4) = 13.723, p = 0.013); only 3 of 11 participants treated with risperidone made CEs compared with 9/10 placebo and 7/9 aripiprazole treated participants.
Discriminability d’
There were no significant effects of drug group on the 0-back condition, F(2,27) = 0.60, p = 0.55. The mixed ANCOVA revealed a significant effect of n-back, F(1,26) = 31.95, p < 0.001, and a significant drug by n-back level interaction, F(2,26) = 3.45, p = 0.047. From Fig. 1, it can be seen that the main effect is driven by a general lowering of d’scores in the 2-back condition compared to the 1-back condition, reflecting the greater task difficulty. However. post hoc paired t tests revealed the interaction to be caused by d’ scores being significantly different for the 2-back compared to 1-back for placebo (p < 0.001) and risperidone (p = 0.027), but not for the aripiprazole condition (p = 0.223). Furthermore, drug group t test comparisons at each of the two n-back levels (with 0-back covariate) revealed a trend for a significant difference between placebo and aripiprazole (p = 0.057) and risperidone and placebo (p = 0.054) for the 2-back condition but not for the 1-back condition (p > 0.1).
Fig. 1.
N-back d' scores. Scores shown for the 0-back (baseline condition), 1-back and 2-back condition for the placebo, aripiprazole and risperidone groups. Error bars indicate standard error of the mean
Reaction times
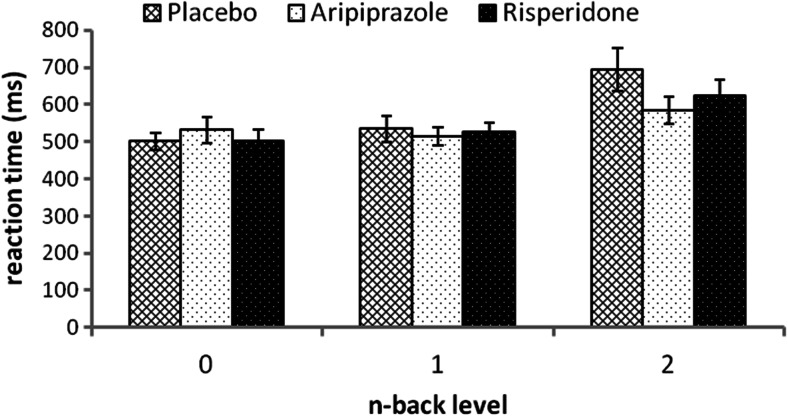
There was no significant effect of drug group on reaction times for correct responses for the 0-back condition, F(2,27) = 0.31, p = 0.74, indicating that there were no drug effects on motor speed or attention. The mixed ANCOVA revealed an overall significant effect of the 0-back reaction time covariate, F(1,26) = 25.407, p < 0.001, but no covariate by n-back level interaction, F(1,26) = 0.063, p = 0.804, indicating that reaction times for the 0-back condition were significantly related to reaction times for both the 1-back and the 2-back condition. The mixed ANCOVA revealed a significant effect of n-back level F(1,26) = 25.4, p < 0.001, but no n-back level by drug interaction, F(2,26) = 1.511, p = 0.24, indicating that reaction times slowed with increasing task difficulty although this occurred to the same degree across all treatment groups. Reaction times were faster after both drugs (notably aripiprazole) (Fig. 2), but this fell short of full statistical significance (F(2,26) = 3.006, p = 0.07). Sidak post hoc tests revealed a trend for faster reaction time with aripiprazole (p = 0.06) compared with placebo. No trends were found for the risperidone placebo comparison (p = 0.59) nor were there any trends for significant differences in the aripiprazole versus risperidone comparison (p = 0.44).
Fig. 2.
Reaction time for correct responses. Reactions times shown for 0-back (baseline condition), 1-back and 2-back condition of the n-back task for the placebo, aripiprazole and risperidone groups. Error bars indicate standard error of the mean
fMRI
Positive effect of n-back task and n-back level (cluster forming threshold of p < 0.005, pFWEc < 0.05)
The n-back task (1&2back across all groups) produced significant activations in the expected cortical areas for working memory tasks as identified in the normative meta-analysis of n-back task (Owen et al. 2005). These included the bilateral DLPFC, the bilateral ventrolateral prefrontal cortex (VLPFC), the bilateral premotor cortex, the bilateral rostral PFC, the bilateral cingulate gyri and the bilateral inferior and superior parietal cortices (Fig. S1 included in supplementary materials). Furthermore, all regions were recruited to a greater degree in the 2-back condition compared to the 1-back condition, reflecting the greater task difficulty.
Main effects of drug and drug by n-back level interactions (cluster forming threshold of p < 0.005, pFWEc < 0.05, SVC)
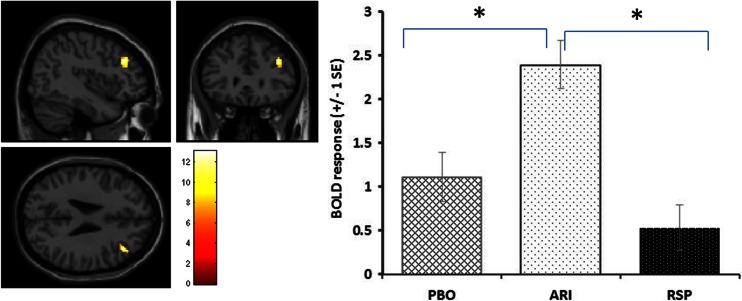
Main effect of drug group was found within the DLPFC with an extent of 64 voxels at Talairach coordinates (42, 32, 28), pFWEc = 0.008 SVC for the DLPFC ROI. Masking with the positive effect of task revealed this drug effect to occur entirely within regions activated by the task. Post hoc two-sample t tests with the DLPFC ROI revealed that this effect was due to aripiprazole increasing activation within the DLPFC compared to both the risperidone group, pFWEc = 0.004 SVC, and the placebo group, pFWEc = 0.027. Compared to the placebo, there was a trend for a significant decrease with risperidone although this fell short of statistical significance, pFWEc = 0.07 SVC (also see Fig. 3)
Fig. 3.
Brain images show the sagittal, coronal and axial views of the significant DLPFC cluster demonstrating a main effect of drug group (F-contrast image thresholded at p < 0.005). Histograms demonstrate the mean BOLD response within this cluster for the different drug groups. * = comparisons significant at pFWEc < 0.05 SVC for DLPFC in post hoc tests
No n-back level by drug interactions were found indicating that the drugs had similar effects during the 1-back and the 2-back conditions.
Correlational analyses
None of the areas produced by the main effect of drug were found to be positively or negatively correlated with any of the behavioural measures for the 1-back minus 0-back or the 2-back minus the 0-back conditions.
Discussion
The aim of this study was to determine whether partial agonist effects of aripiprazole on dopamine D2 receptors in the prefrontal cortex would improve performance of a working memory task in healthy volunteers in comparison with neutral or adverse effects of the full D2 receptor antagonist risperidone. As hypothesised, aripiprazole enhanced activation in the DLPFC and demonstrated a trend for improved reaction times and discriminability (d’) compared to placebo. Risperidone reduced activation within the right DLPFC compared to aripiprazole with a trend for reduced activation compared to placebo. However, against our prediction, the risperidone group had reduced errors of commission and had similar effects to aripiprazole on d’. Aripiprazole and risperidone have direct actions at D2 receptors and serotonin receptors, but they are also expected to have indirect actions at D1 receptors due to their serotonin receptor–mediated increases in prefrontal dopamine release (Kuroki et al. 1999; Li et al. 2004). Therefore, the fMRI and behavioural effects of the study drugs are discussed in terms of actions at D2, D1, and 5-HT receptors.
Dopamine modulation of the prefrontal cortex during working memory
Dopamine is released in the prefrontal cortex during the performance of working memory tasks (Watanabe et al. 1997) where it acts to modulate the activity of PFC neurons (Durstewitz et al. 2000; Williams and Goldman-Rakic 1995). Studies have demonstrated that direct application of D1 (Sawaguchi 2001; Sawaguchi and Goldman-Rakic 1991, 1994) and D2 drugs (Wang et al. 2004) modulate prefrontal neuronal activity during working memory performance in non-human primates. Studies in humans demonstrate that D2 receptors (Kimberg et al. 1997; Luciana and Collins 1997; Luciana et al. 1992; Mehta et al. 1999, 2001; Tarantino et al. 2011) modulate working memory performance and prefrontal activation (although the striatal vs prefrontal contribution to these is unknown). Electrophysiological studies demonstrate that D1 and D2 receptors influence prefrontal cortex pyramidal neurons in different ways (Seamans and Yang 2004) which may explain the behavioural and fMRI effects of aripiprazole and risperidone. Both antipsychotic drugs cause the release of dopamine into the prefrontal cortex (Li et al. 2004) therefore, both drugs are expected to have indirect D1 receptor effects. Given the D2 partial agonist effects of aripiprazole versus the D2 antagonism of risperidone, we expect aripiprazole to have both D2- and D1-mediated agonist PFC actions whereas we expect risperidone’s dopaminergic actions to be mediated by D1 receptor agonism.
D2-mediated effects within the prefrontal cortex during performance of the n-back task
The n-back task requires participants to attend to newly presented letters whilst maintaining a representation of the letter that was presented ‘n’ letters back. The letter presented n trials back quickly becomes irrelevant as the next letter is presented, and the representation of this letter must be quickly replaced with newly presented letters. In contrast to delayed response tasks whereby a single stimulus needs to be maintained over a delay, items maintained in the n-back task are constantly changing, and for successful performance, new information needs to be readily incorporated into the PFC. D2 agonists have been shown to increase working memory capacity (the number of new items than can be encoded) whereas D1 effects are crucial for tasks involving the maintenance of a stimulus over a delay (Tarantino et al. 2011). D2 and D1 receptors have opposing effects on excitatory and inhibitory neurotransmission within the PFC. Studies have shown that D2 receptors decrease, and D1 receptors increase, NMDA (Zheng et al. 1999) and GABA currents (Kroener and Lavin 2010; Seamans et al. 2001). According to computational models, D2 receptors decrease the competition between groups of neurons within the PFC. These actions facilitate movement along representations, aiding the fast switching of attention and incorporation of new information into PFC networks (Durstewitz and Seamans 2008). Thus, aripiprazole’s partial D2 agonism may have contributed to the trends for speeded reaction time and improved d’. In a study examining executive functioning in healthy volunteers, the D2 agonist bromocriptine speeded reaction time in a Stroop task (i.e. decreased interference) (Roesch-Ely et al. 2005). The authors explain this effect in terms of D2-receptor-mediated facilitation of the switching from reading the colour of words to naming them. Too much D2 to D1 receptor stimulation can be detrimental to cognitive performance by increasing distractibility (Durstewitz and Seamans 2008) as demonstrated by the cognitive impairing effect of the D2 agonist bromocriptine in individuals with a high baseline working capacity who are hypothesised to have optimum dopamine levels at baseline but are ‘overdosed’ after bromocriptine administration (Gibbs and D’Esposito 2005; Kimberg et al. 1997). Therefore, a combination of aripiprazole’s partial, rather than full, D2 agonism, along with its secondary D1 receptor effects, as aripiprazole has been shown to reverse cognitive deficits in rodents via D1 receptor agonism (Nagai et al. 2009), may contribute to its favourable effects on reaction time. Notably, however, a very recent study assessing the acute effects of 10 mg aripiprazole on n-back performance in healthy volunteers found contradictory results to the present study in that they reported increased reaction times with aripiprazole and no change in neural activation compared to placebo (Goozee et al. 2015). We believe that dose differences underlie these contradictory findings. Kim et al. compared resting brain metabolism, receptor occupancy and n-back performance in healthy volunteers taking various doses of aripiprazole (Kim et al. 2013). They demonstrated that both 10- and 30-mg doses significantly decreased resting brain metabolism whereas 2 and 5 mg of aripiprazole did not. Furthermore, frontal brain metabolism was found to be inversely correlated with striatal D2 receptor occupancy and n-back reaction time. These and our findings suggest a dose dependency of the cognitive effects of aripiprazole in healthy volunteers. Doses above 10 mg may have detrimental effects related to excessive striatal D2 blockade and reduced frontal metabolism. Doses of 5 mg may have beneficial effects which we hypothesise to be due to a combination of D2 and D1 prefrontal agonism and insufficient striatal D2 blockade to cause impairment.
Studies in schizophrenia patients support a cognitive enhancing role of higher doses (10 mg and above) of aripiprazole during working memory and verbal cognitive functioning (Bervoets et al. 2012; Kern et al. 2006; Maat et al. 2014; Schlagenhauf et al. 2010), although not all studies support a working memory enhancing role of aripiprazole (Riedel et al. 2010). Given that schizophrenia is associated with excessive striatal dopamine transmission (Howes et al. 2012), higher doses of aripiprazole may be tolerated before striatal D2 neurotransmission is reduced to detrimental levels.
The increased DLPFC activation with aripiprazole may be due to D2 receptor actions to reduce GABAA inhibition of pyramidal neurons (Seamans et al. 2001). Whilst D1 receptor actions can spatially tune activation within the prefrontal cortex by increasing the inhibition of pyramidal neurons (Williams and Goldman-Rakic 1995), D2 receptor activation will reduce this inhibition, possibly allowing a greater extent of DLPFC to be recruited by the task. However, this explanation may be somewhat speculative when considering the BOLD signal reflects neuronal mass action (Logothetis 2008), whereas the above studies of Williams and Goldman Rakic and those of Seamans and colleagues report effects in individual neurons. Furthermore, D2 enhancements of prefrontal GABA neurotransmission have also been reported (Tseng and O’Donnell 2007). The increased BOLD signal in the DLPFC with aripiprazole was associated with a trend for faster reaction times and an improvement in d’, arguing against the suggestion that aripiprazole reduces neuronal efficiency, but rather that this enhancement had a functionally beneficial effect. This is in agreement with a recent finding in schizophrenia patients demonstrating greater PFC activation and improved performance on the n-back task after switching from typical antipsychotic medications to aripiprazole (Schlagenhauf et al. 2010).
D1 receptor effects during the n-back task
Against our hypotheses, risperidone had similar effects to aripiprazole on ‘d, although unlike the aripiprazole group, this was driven by a reduced tendency to make commission errors, with minimal effects on reaction time. The lack of cognitive impairment after risperidone in this study is likely due to the low dose used (with predicted D2 occupancy of 50 %). We and others have reported that single doses of risperidone at 2-mg impair reaction time and performance in healthy volunteers (Koychev et al. 2012). However, studies in clinical populations have also failed to demonstrate a superiority of aripiprazole over risperidone for cognitive functioning (Khanna et al. 2013, 2014). This suggests that there are protective factors associated with risperidone’s mode of action that compensate for its high-affinity D2 blockade. Although both drugs release PFC dopamine (due to their serotinergic actions), risperidone may do this to a greater degree given its higher 5-HT2A receptor affinity. Reduced CEs after risperidone may reflect a combination of increased dopamine release acting on D1 receptors with insufficient D2 blockade to interfere with performance. Computational accounts suggest that D1 receptors increase signal and reduce noise through simultaneously enhancing glutamatergic maintenance of representations while enhancing GABA surround inhibition (Durstewitz and Seamans 2002; Williams and Castner 2006). D1-mediated improved signal to noise could thus account for the improved performance and the trend for reduced cortical BOLD response after risperidone—the latter reflecting more efficient cortical processing. Indeed, dopamine-releasing drugs reduce PFC metabolic demand and BOLD signal in healthy volunteers during the performance of cognitive tasks, both in the context of improved performance and in the absence of a performance effect (Mattay et al. 2003; Mehta et al. 2000; Tipper et al. 2005; Volkow et al. 2008).
Direct actions at serotonin receptors
A reason for the lack of performance differences between aripiprazole and risperidone (and the lack of differences in cognitive effects found clinically) could be the serotonergic actions of risperidone. The effects of 5-HT2A antagonists on neuronal activity appear to be similar to that of D2 agonism of reducing pyramidal and interneuron activity to decrease signal to noise during delayed response tasks (Williams et al. 2002). Direct application of a 5-HT2A receptor antagonist into the PFC reduces impulsive responding in rats (Winstanley et al. 2003); therefore, risperidone may be reducing commission errors via a reduction in impulsivity. A recent computational model suggests that 5-HT1A receptor activation has functional actions within the PFC during working memory, again similar to effects expected from D2 activation (Cano-Colino et al. 2013). Therefore, some of aripiprazole’s fMRI and behavioural effects may be the result of a combination of D2 agonism, 5-HT2A antagonism and 5-HT1A agonism.
Correlations between behavioural data and BOLD signals
No positive or negative correlations were found between task performance and activity within the DLPFC. A likely reason is that each drug affects several neural and cognitive processes that influence performance which lack a final common, performance-related effect on DLPFC neuronal activity and resultant BOLD signal. Risperidone appeared to increase neural efficiency in the DLPFC since reduced activation was associated with enhanced performance, whereas aripiprazole appeared to increase the recruitment of DLPFC neurons to aid performance. The n-back task requires the execution of numerous cognitive operations, and therefore different strategies are likely to be used by different individuals to complete the task, which could explain why correlations were not found (Rypma et al. 2006). Furthermore, ceiling effects may have resulted in insufficient performance variations to allow for correlations to be found.
Limitations
The main limitation of this study is the low sample size which resulted in low statistical power. This may have contributed to the majority of the performance findings being of only trend-level significance. Ceiling effects may also have constrained detection of performance-enhancing effects of aripiprazole. Indeed, other studies have reported drug-induced behavioural differences in healthy volunteers only in the 3-back version of the task (Mattay et al. 2000, 2003). Similarly, near-perfect accuracy in the controls reduced the possibility of detecting improved accuracy after drug treatment. Nevertheless, the data suggest the drugs may improve performance in different ways—aripiprazole increased arousal (faster reaction times), whereas risperidone reduced commission errors but had a reduced benefit on arousal.
Another limitation associated with the low statistical power of the study may be that effects meeting statistical significance (such as the fMRI effects in this study) may represent an overestimation of the ‘true’ effect (Button et al. 2013).
The use of healthy volunteers rather than schizophrenia patients could be seen as a limitation. However, risperidone has been shown to both improve (Green et al. 1997) and impair (Reilly et al. 2006, 2007) performance of a working memory task in schizophrenia patients. The contrasting results are likely to have been due to differences, possibly unknown, in illness and medication status of the patients. Such confounds are by-passed in experiments in healthy volunteers.
Another potential limitation is that low doses of the study drugs were used to minimise side effects, and therefore study findings may not be applicable to the recommended doses for the treatment of schizophrenia. As discussed, there appears to be markedly different effects of aripiprazole and risperidone in healthy volunteers, depending upon the dose used. Therefore, it is quite possible that any beneficial effects of these drugs at the doses used in this study may disappear at recommended therapeutic doses, possibly explaining the limited effects of these drugs on working memory in a study of schizophrenia patients (Riedel et al. 2010). However, 5 mg of aripiprazole was shown to significantly reduce negative symptoms in a double-blind placebo controlled study in acutely relapsing schizophrenia patients (Cutler et al. 2006). Furthermore, low doses of aripiprazole and risperidone are efficacious as adjunct treatments for major depressive disorder, an indication for which aripiprazole is FDA-approved (Berman et al. 2007; Mahmoud et al. 2007; Marcus et al. 2008; Terao 2008). Therefore, the doses used in the study have clinically significant effects that may be mediated by the prefrontal actions demonstrated in this study. Although the degree to which the single dose used in the current study can be applicable to effects observed after repeated dosing is unknown, we feel that the finding that acute low doses of antipsychotic drugs do not impair but may potentially enhance cognitive function is of clinical interest.
Conclusion
Aripiprazole increased DLPFC activation during the n-back task, and this was associated with a trend for faster performance and improved discriminability compared to placebo. Risperidone also tended to improve discriminability and reduced incorrect responding which was associated with a trend for reduced recruitment of the DLPFC compared to placebo. The results are compatible with computational models according to which (i) partial D2 agonist effects of aripiprazole would enhance working memory capacity and thus processing speed whereas (ii) D2 receptor blockade after risperidone together with increased dopamine release onto D1 receptors would enhance signal to noise and thus cortical efficiency and performance accuracy. Probably more complex interactions between dopamine and serotonin play a role in the contrasting actions of the drugs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(RTF 13397 kb)
Acknowledgments
The authors would like to thank Patrick Horgan, Gabriella Juhasz, Tolga Turgut and Ivan Koychev for providing medical cover for the study.
Compliance with ethical standards
Funding
The research study was funded by Bristol Myers-Squibb.
Bristol Myers-Squibb was not involved in the study design, collection, analysis and interpretation of data, writing or approval of the report.
A Murphy and JFW Deakin have full control of all primary data and they agree to allow the journal to review their data if requested.
Conflict of interest
A Murphy’s PhD studentship was part funded by Bristol Myers-Squibb.
JFW Deakin currently advises or carries out research funded by Autifony, Sunovion, Lundbeck, AstraZeneca and Servier. All payment is to the University of Manchester.
No other authors declared any conflicts of interest.
References
- Agid O, Mamo D, Ginovart N, Vitcu I, Wilson AA, Zipursky RB, Kapur S. Striatal vs extrastriatal dopamine D2 receptors in antipsychotic response--a double-blind PET study in schizophrenia. Neuropsychopharmacology. 2007;32:1209–1215. doi: 10.1038/sj.npp.1301242. [DOI] [PubMed] [Google Scholar]
- Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, Lewis DA. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry. 1999;156:1580–1589. doi: 10.1176/ajp.156.10.1580. [DOI] [PubMed] [Google Scholar]
- Ammons, RB, Ammons, CH (1962) The Quick Test (QT): provisional manual. Psychological Reports, 11:111–162.
- Berman RM, Marcus RN, Swanink R, McQuade RD, Carson WH, Corey-Lisle PK, Khan A. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68:843–853. doi: 10.4088/JCP.v68n0604. [DOI] [PubMed] [Google Scholar]
- Bervoets C, Morrens M, Vansteelandt K, Kok F, de Patoul A, Halkin V, Pitsi D, Constant E, Peuskens J, Sabbe B. Effect of aripiprazole on verbal memory and fluency in schizophrenic patients: results from the ESCAPE study. CNS Drugs. 2012;26:975–982. doi: 10.1007/s40263-012-0003-4. [DOI] [PubMed] [Google Scholar]
- Bolonna AA, Kerwin RW. Partial agonism and schizophrenia. Br J Psychiatry. 2005;186:7–10. doi: 10.1192/bjp.186.1.7. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Burris KD, Molski TF, Xu C, Ryan E, Tottori K, Kikuchi T, Yocca FD, Molinoff PB. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther. 2002;302:381–389. doi: 10.1124/jpet.102.033175. [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafo MR. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Cano-Colino M, Almeida R, Compte A. Serotonergic modulation of spatial working memory: predictions from a computational network model. Front Integr Neurosci. 2013;7:71. doi: 10.3389/fnint.2013.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD. Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry. 1998;155:1285–1287. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- Cutler AJ, Marcus RN, Hardy SA, O’Donnell A, Carson WH, McQuade RD. The efficacy and safety of lower doses of aripiprazole for the treatment of patients with acute exacerbation of schizophrenia. CNS Spectr. 2006;11:691–702. doi: 10.1017/s1092852900014784. [DOI] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK. The computational role of dopamine D1 receptors in working memory. Neural Netw. 2002;15:561–572. doi: 10.1016/S0893-6080(02)00049-7. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK. The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-o-methyltransferase genotypes and schizophrenia. Biol Psychiatry. 2008;64:739–749. doi: 10.1016/j.biopsych.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. J Neurophysiol. 2000;83:1733–1750. doi: 10.1152/jn.2000.83.3.1733. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ereshefsky L. Pharmacokinetics and drug interactions: update for new antipsychotics. J Clin Psychiatry. 1996;57(Suppl 11):12–25. [PubMed] [Google Scholar]
- Forbes NF, Carrick LA, McIntosh AM, Lawrie SM. Working memory in schizophrenia: a meta-analysis. Psychol Med. 2009;39:889–905. doi: 10.1017/S0033291708004558. [DOI] [PubMed] [Google Scholar]
- Gibbs SE, D’Esposito M. A functional MRI study of the effects of bromocriptine, a dopamine receptor agonist, on component processes of working memory. Psychopharmacology. 2005;180:644–653. doi: 10.1007/s00213-005-0077-5. [DOI] [PubMed] [Google Scholar]
- Goozee R, Reinders AA, Handley R, Marques T, Taylor H, O’Daly O, McQueen G, Hubbard K, Mondelli V, Pariante C, Dazzan P. Effects of aripiprazole and haloperidol on neural activation during the n-back in healthy individuals: a functional MRI study. Schizophr Res. 2015 doi: 10.1016/j.schres.2015.02.023. [DOI] [PubMed] [Google Scholar]
- Green MF, Marshall BD, Jr, Wirshing WC, Ames D, Marder SR, McGurk S, Kern RS, Mintz J. Does risperidone improve verbal working memory in treatment-resistant schizophrenia? Am J Psychiatry. 1997;154:799–804. doi: 10.1176/ajp.154.6.799. [DOI] [PubMed] [Google Scholar]
- Grunder G, Carlsson A, Wong DF. Mechanism of new antipsychotic medications: occupancy is not just antagonism. Arch Gen Psychiatry. 2003;60:974–977. doi: 10.1001/archpsyc.60.10.974. [DOI] [PubMed] [Google Scholar]
- Haatveit BC, Sundet K, Hugdahl K, Ueland T, Melle I, Andreassen OA. The validity of d prime as a working memory index: results from the “Bergen n-back task”. J Clin Exp Neuropsychol. 2010;32:871–880. doi: 10.1080/13803391003596421. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, Kapur S. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–786. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ML, Van Peer A, Woestenborghs R, De Coster R, Heykants J, Jansen AA, Zylicz Z, Visscher HW, Jonkman JH. Pharmacokinetics of the novel antipsychotic agent risperidone and the prolactin response in healthy subjects. Clin Pharmacol Ther. 1993;54:257–268. doi: 10.1038/clpt.1993.146. [DOI] [PubMed] [Google Scholar]
- Ito H, Arakawa R, Takahashi H, Takano H, Okumura M, Otsuka T, Ikoma Y, Shidahara M, Suhara T. No regional difference in dopamine D2 receptor occupancy by the second-generation antipsychotic drug risperidone in humans: a positron emission tomography study. Int J Neuropsychopharmacol. 2009;12:667–675. doi: 10.1017/S1461145708009577. [DOI] [PubMed] [Google Scholar]
- Kern RS, Green MF, Cornblatt BA, Owen JR, McQuade RD, Carson WH, Ali M, Marcus R. The neurocognitive effects of aripiprazole: an open-label comparison with olanzapine. Psychopharmacology (Berlin) 2006;187:312–320. doi: 10.1007/s00213-006-0428-x. [DOI] [PubMed] [Google Scholar]
- Khanna P, Komossa K, Rummel-Kluge C, Hunger H, Schwarz S, El-Sayeh HG, Leucht S. Aripiprazole versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2013;2:CD006569. doi: 10.1002/14651858.CD006569.pub4. [DOI] [PubMed] [Google Scholar]
- Khanna P, Suo T, Komossa K, Ma H, Rummel-Kluge C, El-Sayeh HG, Leucht S, Xia J. Aripiprazole versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2014;1:CD006569. doi: 10.1002/14651858.CD006569.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Howes OD, Kim BH, Jeong JM, Lee JS, Jang IJ, Shin SG, Turkheimer FE, Kapur S, Kwon JS. Predicting brain occupancy from plasma levels using PET: superiority of combining pharmacokinetics with pharmacodynamics while modeling the relationship. J Cereb Blood Flow Metab. 2012;32:759–768. doi: 10.1038/jcbfm.2011.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Howes OD, Turkheimer FE, Kim BH, Jeong JM, Kim JW, Lee JS, Jang IJ, Shin SG, Kapur S, Kwon JS. The relationship between antipsychotic D2 occupancy and change in frontal metabolism and working memory: a dual [(11)C]raclopride and [(18) F]FDG imaging study with aripiprazole. Psychopharmacology. 2013;227:221–229. doi: 10.1007/s00213-012-2953-0. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, D’Esposito M, Farah MJ. Effects of bromocriptine on human subjects depend on working memory capacity. Neuroreport. 1997;8:3581–3585. doi: 10.1097/00001756-199711100-00032. [DOI] [PubMed] [Google Scholar]
- Koychev I, McMullen K, Lees J, Dadhiwala R, Grayson L, Perry C, Schmechtig A, Walters J, Craig KJ, Dawson GR, Dourish CT, Ettinger U, Wilkinson L, Williams S, Deakin JF, Barkus E. A validation of cognitive biomarkers for the early identification of cognitive enhancing agents in schizotypy: a three-center double-blind placebo-controlled study. Eur Neuropsychopharmacol. 2012;22:469–481. doi: 10.1016/j.euroneuro.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Kroener S, Lavin A. Altered dopamine modulation of inhibition in the prefrontal cortex of cocaine-sensitized rats. Neuropsychopharmacology. 2010;35:2292–2304. doi: 10.1038/npp.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo M, Koue T, Maune H, Fukuda T, Azuma J. Pharmacokinetics of aripiprazole, a new antipsychotic, following oral dosing in healthy adult Japanese volunteers: influence of CYP2D6 polymorphism. Drug Metab Pharmacokinet. 2007;22:358–366. doi: 10.2133/dmpk.22.358. [DOI] [PubMed] [Google Scholar]
- Kuroki T, Meltzer HY, Ichikawa J. Effects of antipsychotic drugs on extracellular dopamine levels in rat medial prefrontal cortex and nucleus accumbens. J Pharmacol Exp Ther. 1999;288:774–781. [PubMed] [Google Scholar]
- Lawler CP, Prioleau C, Lewis MM, Mak C, Jiang D, Schetz JA, Gonzalez AM, Sibley DR, Mailman RB. Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology. 1999;20:612–627. doi: 10.1016/S0893-133X(98)00099-2. [DOI] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp Brain Res. 2000;133:23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- Leysen JE, Janssen PM, Megens AA, Schotte A. Risperidone: a novel antipsychotic with balanced serotonin-dopamine antagonism, receptor occupancy profile, and pharmacologic activity. J Clin Psychiatry. 1994;55(Suppl):5–12. [PubMed] [Google Scholar]
- Li Z, Ichikawa J, Dai J, Meltzer HY. Aripiprazole, a novel antipsychotic drug, preferentially increases dopamine release in the prefrontal cortex and hippocampus in rat brain. Eur J Pharmacol. 2004;493:75–83. doi: 10.1016/j.ejphar.2004.04.028. [DOI] [PubMed] [Google Scholar]
- Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Luciana M, Collins PF. Dopaminergic modulation of working memory for spatial but not object cues in normal humans. J Cogn Neurosci. 1997;9:330–347. doi: 10.1162/jocn.1997.9.3.330. [DOI] [PubMed] [Google Scholar]
- Luciana M, Depue RA, Arbisi P, Leon A. Facilitation of working memory in humans by a d2 dopamine receptor agonist. J Cogn Neurosci. 1992;4:58–68. doi: 10.1162/jocn.1992.4.1.58. [DOI] [PubMed] [Google Scholar]
- Maat A, Cahn W, Gijsman HJ, Hovens JE, Kahn RS, Aleman A. Open, randomized trial of the effects of aripiprazole versus risperidone on social cognition in schizophrenia. Eur Neuropsychopharmacol. 2014;24:575–584. doi: 10.1016/j.euroneuro.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Mahmoud RA, Pandina GJ, Turkoz I, Kosik-Gonzalez C, Canuso CM, Kujawa MJ, Gharabawi-Garibaldi GM. Risperidone for treatment-refractory major depressive disorder: a randomized trial. Ann Intern Med. 2007;147:593–602. doi: 10.7326/0003-4819-147-9-200711060-00003. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Press DZ, Thangaraj V, Searl MM, Goff DC, Halpern E, Saper CB, Warach S. Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biol Psychiatry. 1999;45:1128–1137. doi: 10.1016/S0006-3223(98)00318-7. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E, Saper CB, Rauch SL. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry. 2000;48:99–109. doi: 10.1016/S0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- Marcus RN, McQuade RD, Carson WH, Hennicken D, Fava M, Simon JS, Trivedi MH, Thase ME, Berman RM. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2008;28:156–165. doi: 10.1097/JCP.0b013e31816774f9. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Callicott JH, Bertolino A, Heaton I, Frank JA, Coppola R, Berman KF, Goldberg TE, Weinberger DR. Effects of dextroamphetamine on cognitive performance and cortical activation. NeuroImage. 2000;12:268–275. doi: 10.1006/nimg.2000.0610. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG SA, Locascio JJ, Sperling RA, Atri A. Repeated-measures designs overestimate between-subject effects in fMRI packages using one error term. Quebec City: 17th Annual Meeting of Organization for Human Brain Mapping; 2011. [Google Scholar]
- Mehta MA, Sahakian BJ, McKenna PJ, Robbins TW. Systemic sulpiride in young adult volunteers simulates the profile of cognitive deficits in Parkinson’s disease. Psychopharmacology (Berlin) 1999;146:162–174. doi: 10.1007/s002130051102. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 2000;20:RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Swainson R, Ogilvie AD, Sahakian J, Robbins TW. Improved short-term spatial memory but impaired reversal learning following the dopamine D(2) agonist bromocriptine in human volunteers. Psychopharmacology (Berlin) 2001;159:10–20. doi: 10.1007/s002130100851. [DOI] [PubMed] [Google Scholar]
- Nagai T, Murai R, Matsui K, Kamei H, Noda Y, Furukawa H, Nabeshima T. Aripiprazole ameliorates phencyclidine-induced impairment of recognition memory through dopamine D1 and serotonin 5-HT1A receptors. Psychopharmacology. 2009;202:315–328. doi: 10.1007/s00213-008-1240-6. [DOI] [PubMed] [Google Scholar]
- Nyberg S, Farde L, Eriksson L, Halldin C, Eriksson B. 5-HT2 and D2 dopamine receptor occupancy in the living human brain. A PET study with risperidone. Psychopharmacology (Berlin) 1993;110:265–272. doi: 10.1007/BF02251280. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 9(1):97–113 [DOI] [PubMed]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Pycock CJ, Carter CJ, Kerwin RW. Effect of 6-hydroxydopamine lesions of the medial prefrontal cortex on neurotransmitter systems in subcortical sites in the rat. J Neurochem. 1980;34:91–99. doi: 10.1111/j.1471-4159.1980.tb04625.x. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Harris MS, Keshavan MS, Sweeney JA. Adverse effects of risperidone on spatial working memory in first-episode schizophrenia. Arch Gen Psychiatry. 2006;63:1189–1197. doi: 10.1001/archpsyc.63.11.1189. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Harris MS, Khine TT, Keshavan MS, Sweeney JA. Antipsychotic drugs exacerbate impairment on a working memory task in first-episode schizophrenia. Biol Psychiatry. 2007;62:818–821. doi: 10.1016/j.biopsych.2006.10.031. [DOI] [PubMed] [Google Scholar]
- Richelson E, Souder T. Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci. 2000;68:29–39. doi: 10.1016/S0024-3205(00)00911-5. [DOI] [PubMed] [Google Scholar]
- Riedel M, Schennach-Wolff R, Musil R, Dehning S, Cerovecki A, Opgen-Rhein M, Matz J, Seemuller F, Obermeier M, Engel RR, Muller N, Moller HJ, Spellmann I. Neurocognition and its influencing factors in the treatment of schizophrenia-effects of aripiprazole, olanzapine, quetiapine and risperidone. Human Psychopharmacol. 2010;25:116–125. doi: 10.1002/hup.1101. [DOI] [PubMed] [Google Scholar]
- Roesch-Ely D, Scheffel H, Weiland S, Schwaninger M, Hundemer HP, Kolter T, Weisbrod M. Differential dopaminergic modulation of executive control in healthy subjects. Psychopharmacology (Berlin) 2005;178:420–430. doi: 10.1007/s00213-004-2027-z. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, Prabhakaran V, Bly BM, Kimberg DY, Biswal BB, D’Esposito M. Neural correlates of cognitive efficiency. Neuroimage. 2006;33:969–979. doi: 10.1016/j.neuroimage.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T. The effects of dopamine and its antagonists on directional delay-period activity of prefrontal neurons in monkeys during an oculomotor delayed-response task. Neurosci Res. 2001;41:115–128. doi: 10.1016/S0168-0102(01)00270-X. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. The role of D1-dopamine receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. J Neurophysiol. 1994;71:515–528. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Matsumura M, Kubota K. Effects of dopamine antagonists on neuronal activity related to a delayed response task in monkey prefrontal cortex. J Neurophysiol. 1990;63:1401–1412. doi: 10.1152/jn.1990.63.6.1401. [DOI] [PubMed] [Google Scholar]
- Schlagenhauf F, Dinges M, Beck A, Wustenberg T, Friedel E, Dembler T, Sarkar R, Wrase J, Gallinat J, Juckel G, Heinz A. Switching schizophrenia patients from typical neuroleptics to aripiprazole: effects on working memory dependent functional activation. Schizophr Res. 2010;118:189–200. doi: 10.1016/j.schres.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 2001;21:3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, Roth BL, Mailman R. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28:1400–1411. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH et al (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of clinical psychiatry 59(2):22–33. [PubMed]
- Slifstein M, van de Giessen E, Van Snellenberg J, Thompson JL, Narendran R, Gil R, Hackett E, Girgis R, Ojeil N, Moore H, D’Souza D, Malison RT, Huang Y, Lim K, Nabulsi N, Carson RE, Lieberman JA, Abi-Dargham A. Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: a positron emission tomographic functional magnetic resonance imaging study. JAMA Psychiatry. 2015;72:316–324. doi: 10.1001/jamapsychiatry.2014.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. Overview of fMRI analysis. In: Jezzard P, Matthews P, Smith S, editors. Functional MRI an introduction to methods. Oxford: Oxford University Press; 2001. pp. 217–218. [Google Scholar]
- Stark AD, Jordan S, Allers KA, Bertekap RL, Chen R, Mistry Kannan T, Molski TF, Yocca FD, Sharp T, Kikuchi T, Burris KD. Interaction of the novel antipsychotic aripiprazole with 5-HT1A and 5-HT 2A receptors: functional receptor-binding and in vivo electrophysiological studies. Psychopharmacology (Berlin) 2007;190:373–382. doi: 10.1007/s00213-006-0621-y. [DOI] [PubMed] [Google Scholar]
- Takahata K, Ito H, Takano H, Arakawa R, Fujiwara H, Kimura Y, Kodaka F, Sasaki T, Nogami T, Suzuki M, Nagashima T, Shimada H, Kato M, Mimura M, Suhara T. Striatal and extrastriatal dopamine D(2) receptor occupancy by the partial agonist antipsychotic drug aripiprazole in the human brain: a positron emission tomography study with [(1)(1)C]raclopride and [(1)(1)C]FLB457. Psychopharmacology (Berlin) 2012;222:165–172. doi: 10.1007/s00213-011-2633-5. [DOI] [PubMed] [Google Scholar]
- Tarantino IS, Sharp RF, Geyer MA, Meves JM, Young JW. Working memory span capacity improved by a D2 but not D1 receptor family agonist. Behav Brain Res. 2011;219:181–188. doi: 10.1016/j.bbr.2010.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao T. Small doses of aripiprazole augmentation of antidepressant treatment: a report of 3 cases. Prim Care Companion J Clin Psychiatry. 2008;10:252–253. doi: 10.4088/PCC.v10n0312e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper CM, Cairo TA, Woodward TS, Phillips AG, Liddle PF, Ngan ET. Processing efficiency of a verbal working memory system is modulated by amphetamine: an fMRI investigation. Psychopharmacology (Berlin) 2005;180:634–643. doi: 10.1007/s00213-005-0025-4. [DOI] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. D2 dopamine receptors recruit a GABA component for their attenuation of excitatory synaptic transmission in the adult rat prefrontal cortex. Synapse. 2007;61:843–850. doi: 10.1002/syn.20432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Wong C, Ma J, Pradhan K, Benveniste H, Swanson JM. Methylphenidate decreased the amount of glucose needed by the brain to perform a cognitive task. PLoS One. 2008;3:e2017. doi: 10.1371/journal.pone.0002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303:853–856. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Kodama T, Hikosaka K. Increase of extracellular dopamine in primate prefrontal cortex during a working memory task. J Neurophysiol. 1997;78:2795–2798. doi: 10.1152/jn.1997.78.5.2795. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Williams GV, Castner SA. Under the curve: critical issues for elucidating D1 receptor function in working memory. Neuroscience. 2006;139:263–276. doi: 10.1016/j.neuroscience.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Williams GV, Rao SG, Goldman-Rakic PS. The physiological role of 5-HT2A receptors in working memory. J Neurosci. 2002;22:2843–2854. doi: 10.1523/JNEUROSCI.22-07-02843.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Chudasama Y, Dalley JW, Theobald DE, Glennon JC, Robbins TW. Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharmacology. 2003;167:304–314. doi: 10.1007/s00213-003-1398-x. [DOI] [PubMed] [Google Scholar]
- Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27:683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Zheng P, Zhang XX, Bunney BS, Shi WX. Opposite modulation of cortical N-methyl-D-aspartate receptor-mediated responses by low and high concentrations of dopamine. Neuroscience. 1999;91:527–535. doi: 10.1016/S0306-4522(98)00604-6. [DOI] [PubMed] [Google Scholar]
- Zocchi A, Fabbri D, Heidbreder CA. Aripiprazole increases dopamine but not noradrenaline and serotonin levels in the mouse prefrontal cortex. Neurosci Lett. 2005;387:157–161. doi: 10.1016/j.neulet.2005.06.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(RTF 13397 kb)