Abstract
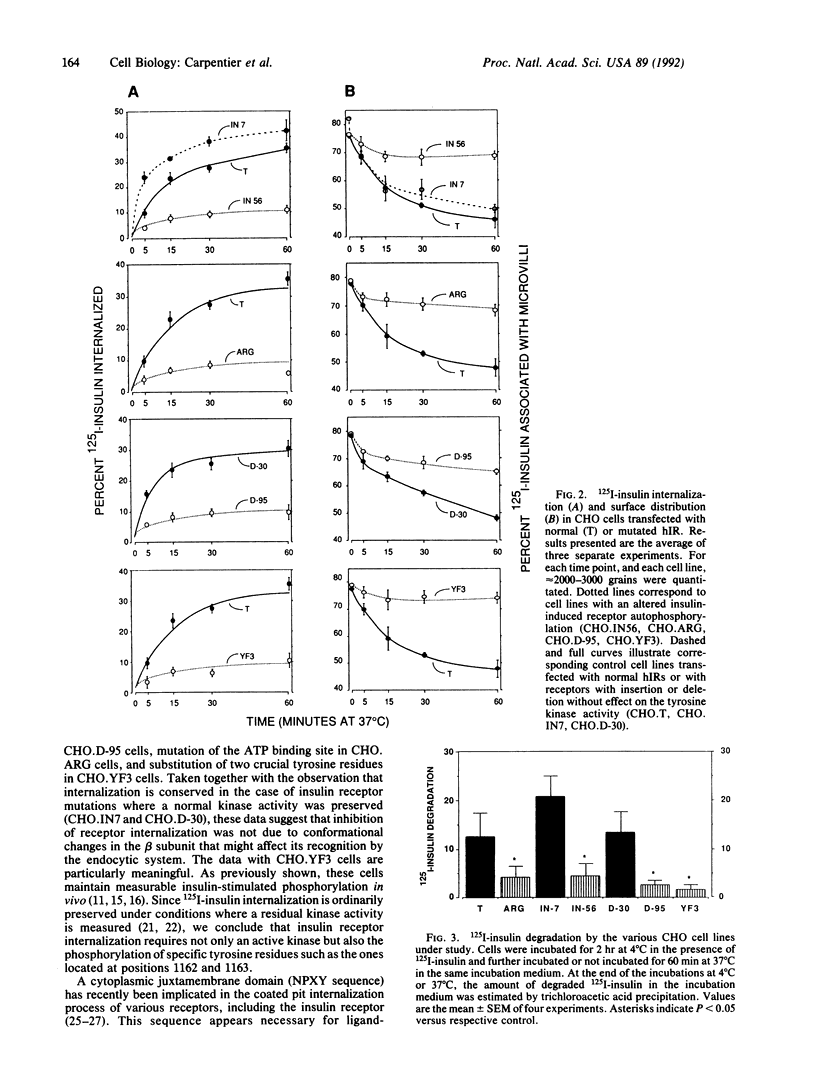
The role of insulin-induced receptor autophosphorylation in its internalization was analyzed by comparing 125I-labeled insulin (125I-insulin) internalization in Chinese hamster ovary (CHO) cell lines transfected with normal (CHO.T) or mutated insulin receptors. In four cell lines with a defect of insulin-induced autophosphorylation, 125I-insulin internalization was impaired. By contrast, in CHO.T cells and in two other CHO cell lines with amino acid deletions or insertions that do not perturb autophosphorylation, 125I-insulin internalization was not affected. A morphological analysis showed that the inhibition is linked to the ligand-specific surface redistribution in which the insulin-receptor complexes leave microvilli and concentrate on nonvillous segments of the membrane where endocytosis occurs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backer J. M., Kahn C. R., Cahill D. A., Ullrich A., White M. F. Receptor-mediated internalization of insulin requires a 12-amino acid sequence in the juxtamembrane region of the insulin receptor beta-subunit. J Biol Chem. 1990 Sep 25;265(27):16450–16454. [PubMed] [Google Scholar]
- Backer J. M., Kahn C. R., White M. F. Tyrosine phosphorylation of the insulin receptor is not required for receptor internalization: studies in 2,4-dinitrophenol-treated cells. Proc Natl Acad Sci U S A. 1989 May;86(9):3209–3213. doi: 10.1073/pnas.86.9.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Fehlmann M., Van Obberghen E., Gorden P., Orci L. Redistribution of 125I-insulin on the surface of rat hepatocytes as a function of dissociation time. Diabetes. 1985 Oct;34(10):1002–1007. doi: 10.2337/diab.34.10.1002. [DOI] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Amherdt M., Van Obberghen E., Kahn C. R., Orci L. 125I-insulin binding to cultured human lymphocytes. Initial localization and fate of hormone determined by quantitative electron microscopic autoradiography. J Clin Invest. 1978 Apr;61(4):1057–1070. doi: 10.1172/JCI109005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Anderson R. G., Goldstein J. L., Brown M. S., Cohen S., Orci L. Co-localization of 125I-epidermal growth factor and ferritin-low density lipoprotein in coated pits: a quantitative electron microscopic study in normal and mutant human fibroblasts. J Cell Biol. 1982 Oct;95(1):73–77. doi: 10.1083/jcb.95.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L. The cell biology of the insulin receptor. Diabetologia. 1989 Sep;32(9):627–635. doi: 10.1007/BF00274248. [DOI] [PubMed] [Google Scholar]
- Carpentier J. L., Van Obberghen E., Gorden P., Orci L. Surface redistribution of 125I-insulin in cultured human lymphocytes. J Cell Biol. 1981 Oct;91(1):17–25. doi: 10.1083/jcb.91.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. J., Goldstein J. L., Brown M. S. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990 Feb 25;265(6):3116–3123. [PubMed] [Google Scholar]
- De Diego J. G., Gorden P., Carpentier J. L. The relationship of ligand receptor mobility to internalization of polypeptide hormones and growth factors. Endocrinology. 1991 Apr;128(4):2136–2140. doi: 10.1210/endo-128-4-2136. [DOI] [PubMed] [Google Scholar]
- Debant A., Ponzio G., Clauser E., Contreres J. O., Rossi B. Receptor cross-linking restores an insulin metabolic effect altered by mutation on tyrosine 1162 and tyrosine 1163. Biochemistry. 1989 Jan 10;28(1):14–17. doi: 10.1021/bi00427a003. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Araki E., Taira M., Shimada F., Mori M., Craik C. S., Siddle K., Pierce S. B., Roth R. A., Rutter W. J. Replacement of lysine residue 1030 in the putative ATP-binding region of the insulin receptor abolishes insulin- and antibody-stimulated glucose uptake and receptor kinase activity. Proc Natl Acad Sci U S A. 1987 Feb;84(3):704–708. doi: 10.1073/pnas.84.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Edery M., Ellis L., Standring D., Beaudoin J., Roth R. A., Rutter W. J. Expression of a functional human insulin receptor from a cloned cDNA in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8014–8018. doi: 10.1073/pnas.82.23.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Fan J. Y., Carpentier J. L., Gorden P., Van Obberghen E., Blackett N. M., Grunfeld C., Orci L. Receptor-mediated endocytosis of insulin: role of microvilli, coated pits, and coated vesicles. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7788–7791. doi: 10.1073/pnas.79.24.7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari J., Roth R. A. Defective internalization of insulin and its receptor in cells expressing mutated insulin receptors lacking kinase activity. J Biol Chem. 1987 Nov 15;262(32):15341–15344. [PubMed] [Google Scholar]
- Kahn C. R., White M. F. The insulin receptor and the molecular mechanism of insulin action. J Clin Invest. 1988 Oct;82(4):1151–1156. doi: 10.1172/JCI113711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Karlsson F. A., Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science. 1982 Jan 8;215(4529):185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- McClain D. A., Maegawa H., Lee J., Dull T. J., Ulrich A., Olefsky J. M. A mutant insulin receptor with defective tyrosine kinase displays no biologic activity and does not undergo endocytosis. J Biol Chem. 1987 Oct 25;262(30):14663–14671. [PubMed] [Google Scholar]
- Reddy S. S., Lauris V., Kahn C. R. Insulin receptor function in fibroblasts from patients with leprechaunism. Differential alterations in binding, autophosphorylation, kinase activity, and receptor-mediated internalization. J Clin Invest. 1988 Oct;82(4):1359–1365. doi: 10.1172/JCI113739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynet C., Caron M., Magré J., Cherqui G., Clauser E., Picard J., Capeau J. Mutation of tyrosine residues 1162 and 1163 of the insulin receptor affects hormone and receptor internalization. Mol Endocrinol. 1990 Feb;4(2):304–311. doi: 10.1210/mend-4-2-304. [DOI] [PubMed] [Google Scholar]
- Rosen O. M. After insulin binds. Science. 1987 Sep 18;237(4821):1452–1458. doi: 10.1126/science.2442814. [DOI] [PubMed] [Google Scholar]
- Russell D. S., Gherzi R., Johnson E. L., Chou C. K., Rosen O. M. The protein-tyrosine kinase activity of the insulin receptor is necessary for insulin-mediated receptor down-regulation. J Biol Chem. 1987 Aug 25;262(24):11833–11840. [PubMed] [Google Scholar]
- Soos M. A., O'Brien R. M., Brindle N. P., Stigter J. M., Okamoto A. K., Whittaker J., Siddle K. Monoclonal antibodies to the insulin receptor mimic metabolic effects of insulin but do not stimulate receptor autophosphorylation in transfected NIH 3T3 fibroblasts. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5217–5221. doi: 10.1073/pnas.86.14.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies R. S., Webster N. J., McClain D. A. A domain of the insulin receptor required for endocytosis in rat fibroblasts. J Biol Chem. 1990 Jun 15;265(17):10132–10137. [PubMed] [Google Scholar]
- Trischitta V., Wong K. Y., Brunetti A., Scalisi R., Vigneri R., Goldfine I. D. Endocytosis, recycling, and degradation of the insulin receptor. Studies with monoclonal antireceptor antibodies that do not activate receptor kinase. J Biol Chem. 1989 Mar 25;264(9):5041–5046. [PubMed] [Google Scholar]