European Society of Cardiology (ESC) curriculum section and guidelines referenced.
2.4 Invasive imaging: cardiac catheterisation and angiography.
ESC: updated contrast-induced nephropathy (CIN) prevention guidelines 2014.
European Society of Urogenital Radiology: updated contrast media safety committee guidelines 2011.
Learning objectives.
Define CIN and recognise this as a common and serious complication in susceptible patients receiving intravascular contrast media.
Understand the possible pathological mechanisms underlying CIN.
Describe the clinical and periprocedural risk factors for CIN and perform a risk assessment for patients receiving contrast media.
Appreciate the established strategies used to prevent CIN and be aware of novel therapies.
Recognise the onset of CIN and manage this complication appropriately.
Introduction
Contrast-induced nephropathy (CIN), also known as contrast-induced acute kidney injury, is an iatrogenic renal injury that follows intravascular administration of radio-opaque contrast media (CM) in susceptible individuals. CIN was first described during the 1950s in case reports of fatal acute renal failure that had occurred following intravenous pyelography in patients with renal disease arising from multiple myeloma.1 2 Despite technological advances, CIN remains responsible for a third of all hospital-acquired acute kidney injury (AKI)3 4 and affects between 1% and 2% of the general population and up to 50% of high-risk subgroups following coronary angiography (CA) or percutaneous coronary intervention (PCI).5
The proliferation of imaging methods and interventional procedures involving administration of intravascular CM in both non-cardiac modalities (eg, vascular CT angiography and interventional vascular angiography) and in established (eg, CA and PCI) and emerging cardiac modalities (eg, CT coronary angiography (CTCA) and transcatheter aortic valve implantation (TAVI)) has significantly increased the number of patients exposed to CM and thus the number at risk of CIN. The widespread adoption of primary PCI for the treatment of acute myocardial infarction (AMI), despite significantly improving cardiovascular outcomes, has increased the incidence of CIN due to the inherent difficulties in rapidly assessing CIN risk, instigating prophylactic measures, attendant haemodynamic compromise and higher contrast volumes, all known risk factors for the development of CIN.6 Despite several therapeutic approaches, the rising age and incidence of comorbidity within the broad cohort of cardiac patients receiving CM has ensured that the prevention of CIN remains a significant clinical challenge.7
As will be discussed in the following sections, the estimated risk of an individual developing CIN can be calculated using known pre-existent clinical and periprocedural factors, which are consistent with the proposed pathological mechanisms of CIN. Pre-existent stage III chronic kidney disease (CKD), defined as an estimated glomerular filtration rate (eGFR)<60 mL/min/1.73 m2 for greater than 3 months, is the most commonly identified risk factor for CIN; however, CIN can occur in the absence of underlying CKD if a number of other risk factors are also present.5 Risk scoring systems have been developed from cohort studies8 9 that have enabled clinicians to predict the likelihood of CIN occurrence and have allowed targeted use of preventative therapies. The wholly iatrogenic and predictable nature of CIN makes it a particularly well-suited area for ongoing cardiovascular and nephrology research, with focus on pathophysiological mechanisms as well as novel risk assessment, preventative, diagnostic and therapeutic measures.
Definition and diagnostic criteria of CIN
The generally accepted definition of CIN is a 25% relative increase, or a 0.5 mg/dL (44 µmol/L) absolute increase, in serum creatinine (SCr) within 72 h of contrast exposure, in the absence of an alternative explanation.10 Criticisms of this definition include the lack of sensitivity to minor increases in SCr that have been shown to correlate with adverse events,11 12 the combination of both relative and absolute SCr changes and the absence of any functional assessment such as changes in urine output, as used in the RIFLE,13 AKIN14 and KDIGO15 classification systems. However, this definition has the advantage of being widely used as the end point in most CIN studies and it correlates well with adverse clinical end points.
An alternative definition proposed by Harjai et al16 aims to classify CIN according to three grades corresponding to three relative and absolute creatinine rise cut-offs, including a group with only minor rise (<25% or 0.5 mg/dL), which also correlates with long-term adverse outcomes (table 1).
Table 1.
CIN severity grading system (adapted from Harjai el al16)
| CIN grade | Change in serum creatinine | 6 month outcomes |
|---|---|---|
| Grade 0 | SCr increase <25% and <0.5 mg/dL above baseline | MACE 12.4% Mortality 10.2% |
| Grade 1 | SCr increase ≥25% and <0.5 mg/dL above baseline | MACE 19.4% Mortality 10.4% |
| Grade 2 | SCr increase ≥0.5 mg/dL above baseline | MACE 28.6% Mortality 40.9% |
CIN, contrast-induced nephropathy; MACE, major adverse cardiovascular event; SCr, serum creatinine.
A significant problem with SCr is that it is relatively insensitive to the rapid GFR changes seen in AKI, particularly in patients with normal baseline renal function.17 Elevations in SCr typically take 2–3 days to reach the current diagnostic threshold following an acute renal insult, thus reducing its usefulness as a marker of AKI. However early and minor incremental changes in SCr may be a useful marker of CIN; a recent clinical trial performed by Ribichini et al,18 which included 216 at-risk patients undergoing CA, demonstrated that at 12 h a 5% increase in SCr from baseline was a sensitive (75%) and specific (72%) marker of CIN at 48 h and persistent worsening of renal function at 30 days.
Several novel renal biomarkers, including NGAL,19 Cystatin C,20 urinary Kim-121 and interleukin-1822 have been proposed to specifically detect CIN within minutes to hours of the renal insult. Unfortunately, to date, these biomarkers have yet to progress beyond the realms of clinical research; there is a clear clinical need for large trial validation of their use in the early detection and intervention in AKI.
Adverse outcomes following CIN
CIN is often regarded in clinical practice as a transient event; in up to 80% of cases, SCr levels normalise after approximately 1–3 weeks.23 However, CIN is of clinical importance as a number of clinical trials have revealed that it portends a multitude of short-term and long-term adverse events.24 After adjusting for comorbidities, observational studies have demonstrated that in-hospital mortality is approximately five times higher in patients who suffer CIN compared with patients receiving CM who do not,25 and mortality rates at 1 and 5 years are approximately four times higher,26 with some demonstrating a 1-year mortality rates of between 20%12 and 38%.27 In other observational studies, as many as 20% of those developing CIN suffer persistent worsening renal function after CM exposure,28 with renal replacement therapy occurring in between 0.7%25 and 7%27 of patients with CIN (table 2). As such the additional healthcare costs associated with CIN are thought to be considerable.12
Table 2.
Cardiovascular adverse outcomes following CIN
| Adverse event | Outcome: CIN group vs no CIN group |
|---|---|
| In-hospital mortality | 7.1% vs 1.1% (p<0.0000001) McCullough et al,25 n=1826 |
| 1 year mortality | 37.7% vs 19.4% (p=0.001) Gruberg et al,27 n=439 |
| Persistent worsening of renal function (eGFR>25% baseline at 3/12) | 18.6% vs 0.9% (p=0.0001) Maioli et al,28 n=1490 |
| Haemodialysis | 0.7% McCullough et al25 7% Gruberg et al27 |
CIN, contrast-induced nephropathy; CM, contrast media; eGFR, estimated glomerular filtration rate.
However, it is important to recognise that a direct causal relationship between CIN and mortality has not been established in these observational studies. The onset of CIN is more likely to occur in the presence of severe cardiac injury or disease, which alone conveys a poor prognosis. As such CIN may be a marker of adverse cardiovascular outcomes rather than an independent risk factor. A recent meta-analysis by James et al,29 reviewed 39 observational studies that investigated cardiovascular outcomes in those with CIN and demonstrated an increased risk of mortality, cardiovascular events, renal failure and prolonged hospitalisation. However, it was found that baseline clinical characteristics that simultaneously predispose to both CIN and mortality were strong confounders, especially so in unadjusted studies. Even with appropriate adjustment for comorbidity, the authors recommend that any firm conclusions about causality should be interpreted with caution.
Nonetheless, a number of plausible pathological mechanisms exist that might explain a direct link between CIN and major adverse cardiac events (MACE). In the short term these include acute volume overload, electrolyte disturbance, uraemia or haemodialysis (HD) in cases of severe CIN. Longer term MACE in those who suffer persistent renal injuries may be increased secondary to the cardiovascular risk associated with progressive CKD and its many pathological manifestations, including accelerated atherosclerosis, vascular calcification and left ventricular (LV) hypertrophy.30 However, it is less conceivable how minor or transient changes in kidney function might increase MACE risk.
In order to demonstrate a definite causal link between CIN and MACE, large randomised controlled trials (RCTs) are needed to demonstrate that effective CIN prevention strategies are also able to reduce short-term and long-term MACE, specifically using therapies that offer no additional cardiovascular risk reduction benefits. Given the similarities that exist between renal and cardiovascular disease and their respective treatments, this is unlikely to be feasible. In view of the many complex confounders associated with MACE, CIN clinical trials that focus exclusively on clinically important renal outcomes, such as persistent worsening of renal function, new onset proteinuria and progression to end-stage renal failure (ESRF), may to some degree circumvent any spurious relationship that is seen with MACE correlates.
Despite the lack of evidence supporting any direct causality effect on MACE, the onset of CIN following cardiac procedures remains an ominous event which should prompt additional clinical vigilance and early intervention.
Proposed mechanisms underlying CIN
Intravascular CM are concentrated tri-iodinated benzene compounds that are radio-opaque as a result of their associated iodine moieties. All CM agents are cytotoxic and this may be compounded by the ionic strength, osmolality or viscosity of each specific agent.31 Ionic ‘hyper-osmolar’ solutions were the first to be used; however, these agents were found to be highly nephrotoxic and are now rarely administered.32 As such safer agents, including non-ionic ‘low-osmolar’ (LOCM) or ‘iso-osmolar’ (IOCM) solutions were developed. These formulations are significantly more viscous than blood plasma, with viscosity inversely related to osmolality33 (table 3). These two physicochemical properties of CM are thought to be implicated in the pathogenesis of CIN in addition to direct vasoactive and cytotoxic effects.34
Table 3.
Comparison of CM agents by osmolality and viscosity
| Blood plasma | Iso-osmolar eg, Visipaque |
Low-osmolar eg, Omnipaque |
High-osmolar eg, Hypaque |
|
|---|---|---|---|---|
| Osmolality | 290 mosmol/L | 290 mosmol/L | 890 mosmol/L | 2100 mosmol/L |
| Viscosity | 3–4 mPa s | 8.8 mPa s | 6.8 m mPa s | 4.1 mPa s |
| CIN risk | N/A | Low | Low | High |
CIN, contrast-induced nephropathy; CM, contrast media.
The kidney is particularly vulnerable to ischaemic injury as it is subjected to high metabolic and osmotic stress and it is supplied by an intricate microvascular circulation susceptible to local and systemic hypoperfusion. This is most evident in the outer medullary region of the kidney where oxygen requirements are high due to active sodium resorption in the ascending loop of Henle and the partial pressure of oxygen is low at approximately 20 mmHg.35 This relative ischaemia is related to the delicate blood supply provided for by the descending vasa recta (DVR), which is a long and narrow diameter vessel with high vascular resistance, and which may be further compounded by arteriovenous shunting.36 Patients with CKD are at an additional risk of renal ischaemia due to the increased metabolic demands placed on a reduced nephron bed which is often coupled with a compromised microvascular and macrovascular circulation.37
Although understanding of the complex pathogenesis of CIN is incomplete, the primary model identifies ischaemia in the vulnerable outer medullary region of the kidney as being pivotal.36 Following intravascular administration of CM, a prolonged period of renal vasoconstriction occurs due to an imbalance of local vasoactive mediators, such as nitrous oxide,38 adenosine, endothelin,39 prostaglandin and reactive oxygen species (ROS)40 which are released by the vascular endothelium in direct response to CM cytotoxicity. The resulting ischaemic tissue releases further noxious vasoactive mediators including ROS, thus prolonging the duration of vasoconstriction.
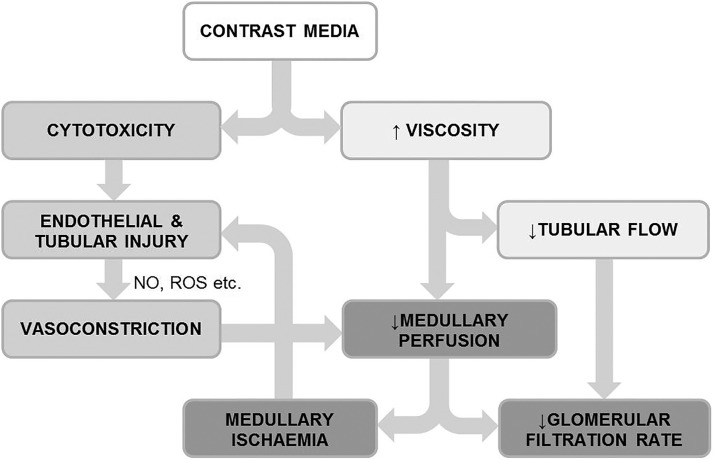
The increased viscosity of the admixture of CM and blood plasma within the DVR results in a further reduction in medullary blood flow41 and the hyperosmolality of CM in plasma has been shown to cause red cell distortion and aggregation which may also contribute to reduced perfusion due to capillary obstruction.42 As CM is filtered and concentrated within the tubules, the resulting increase in viscosity causes tubular obstruction which, coupled with ROS release, induces an acute tubular injury.43 Thus, the combination of cytotoxicity, vasoconstriction and viscosity present a potent combination for the induction of medullary ischaemia/reperfusion injury (figure 1).
Figure 1.
Pathophysiological mechanism underlying CIN is shown. CIN, contrast-induced nephropathy; NO, nitric oxide, ROS, reactive oxygen species. Adapted from Seeliger et al.89
The risk of CIN is increased in elderly patients and those with diabetes or CKD, which may be due to the presence of endothelial dysfunction and an exaggerated vasoconstrictive response to CM.44 Equally, patients with poor pre-renal perfusion, such as those with congestive cardiac failure (CCF), renovascular disease or intravascular volume depletion, are also at increased risk of CIN due to the deleterious effect of renal vasoconstriction coupled with low preload. As observed in clinical studies, the presence of anaemia and thus reduced oxygen carrying capacity of blood would be expected to worsen ischaemia in the outer medullary region of the kidney.45
Risk factors and risk assessment
In order to reduce the chance of CIN occurrence it is important to review the risk factors and indications for CM administration prior to any CM procedure. Most CIN risk factors can be assessed from the clinical history, physical examination and common laboratory investigations. Further risk factors may become apparent periprocedurally.
Pre-existent CKD is probably the most important pre-procedural risk factor for CIN. The European Society of Urogenital Radiology Consensus Working Panel46 in 1999 stated that CIN risk becomes clinically significant when baseline SCr concentration is ≥1.3 mg/dL (≥115 mmol/L) in men and ≥1.0 mg/dL (≥88.4 mmol/L) in women. These figures approximate to an eGFR <60 mL/min/1.73 m2, which defines CKD stages 3–5 and which is now generally recognised as the threshold for CIN risk.47 This simple biochemical assessment is the most widely adopted screening tool; however, it is important to recognise that SCr is an insensitive measure of renal function and that other risk factors are highly contributory to CIN, which can occur in patients without pre-existent CKD.5
Based on clinical experience, dehydration is a major risk factor for CIN; however, as it is largely a clinical diagnosis and is challenging to quantify, it has never been formally investigated in clinical trials. Hypotension, defined as a systolic blood pressure of <80 mm Hg for more than 60 min, is a recognised risk factor for CIN, attributable to intravascular volume depletion (eg, severe dehydration, haemorrhage or sepsis), cardiogenic shock (eg, AMI) or excessive vasodilation (eg, anaphylaxis) which results in renal hypoperfusion and thus increased sensitivity to CM-induced renal ischaemia. The presence of CCF classified according to New York Heart Failure Association III or IV, a recent history of pulmonary oedema,9 AMI,6 or LV ejection fraction of less than 45% have all been shown to be independent risk factors.48 49 Diabetes mellitus is also an independent CIN risk factor as demonstrated in a number of clinical trials,9 48 especially so when coexistent with CKD.50 Advanced age, usually quantified as over 75 years,9 is also associated with CIN. Anaemia is another important risk factor, usually defined as a haematocrit (HCT) of less than 0.39 in males or 0.36 in females.45 The coadministration of nephrotoxic agents (see table 6), are thought to increase the risk of CIN, although this is not well documented in clinical studies.51 There is conflicting evidence regarding the risk of concurrent treatment with ACE inhibitors and angiotensin receptor blocker’s (ARB's), however if part of established therapy, continuation is considered safer than the risk of withdrawal.47
Table 6.
European Society of Cardiology CIN prevention guidelines, 2014
| Recommendation | Detail | Class | Level |
|---|---|---|---|
| Intravenous hydration with isotonic saline is recommended | I | A | |
| Use of either LOCM or IOCM is recommended | <350 mL or <4 mL/kg or V/CrCl <3.7:1 | I | A |
| IOCM use should be considered over LOCM | IIa | A | |
| Short term, high-dose statin therapy should be considered | Rosuvastatin 20/40 mg or atorvastatin 80 mg or simvastatin 80 mg | IIa | A |
| Volume of CM should be minimised | IIa | B | |
| A CIN risk assessment should be performed | IIa | C | |
| In patients at very high CIN risk or when prophylactic hydration is impossible, furosemide with matched hydration may be considered over standard hydration | 250 mL 0.9% saline intravenously over 30 min (or ≤150 mL in LV dysfunction) with 0.25–0.5 mg/kg of furosemide intravenous bolus. Adjust intravenous fluid rate to match urine output until >300 mL/h then perform CM procedure. Continue matched fluid replacement for 4 h post procedure | IIb | A |
| In severe CKD, prophylactic haemofiltration prior to complex PCI may be considered | Fluid replacement rate 1 L/h without negative loss, 0.9% sodium chloride intravenous hydration for 24 h post procedure | IIb | B |
| N-acetyl-cysteine instead of intravenous hydration is not recommended | III | A | |
| Infusion of 8.4% sodium bicarbonate instead of 0.9% sodium chloride is not recommended | III | A | |
| In severe CKD prophylactic renal replacement therapy is not routinely recommended | III | B |
CIN, contrast-induced nephropathy; CKD, chronic kidney disease; CM, contrast medium; IOCM, iso-osmolar contrast medium; LOCM, low-osmolar contrast medium; LV, left ventricular; PCI, percutaneous coronary intervention; V/CrCl, volume of contrast media to creatinine clearance.
Adapted from Windecker et al.59
Procedural factors such as the total volume of CM9 (>350 mL or >4 mL/kg) and previous CM exposure within 72 h8 are directly related to the development of CIN. A specific method for quantifying the maximum safe volume of contrast has been proposed by Laskey et al52 who demonstrated that a ratio of the volume of contrast media to creatinine clearance (V/CrCl) greater than 3.7:1 correlates strongly with the risk of developing CIN in patients with moderate CKD undergoing CA. In addition, the presence of periprocedural haemodynamic instability requiring the use of inotropic agents or intra-arterial balloon pump9 therapy is particularly high-risk feature. A number of these risk factors have been integrated into a well-known post-procedure risk scoring system and validated in a large cohort study by Mehran et al9 (table 4).
Table 4.
The Mehran risk score for the prediction of CIN9
| Mehran score periprocedural CIN risk factor | Score | |||
|---|---|---|---|---|
| Hypotension (SBP <80 mm Hg or >1 h of inotropic support) | 5 | |||
| Intra-arterial balloon pump therapy | 5 | |||
| Chronic heart failure, (NYHA III/IV or recent pulmonary oedema) | 5 | |||
| Age >75 years | 4 | |||
| Diabetes mellitus | 3 | |||
| Anaemia (male: HCT<0.39, female: HCT<0.36) | 3 | |||
| Estimated glomerular filtration rate <20 mL/min | 6 | |||
| Estimated glomerular filtration rate 20–40 mL/min | 4 | |||
| Estimated glomerular filtration rate 40–60 mL/min | 2 | |||
| Contrast media volume | 1 per cc | |||
| Score | <5 | 6–10 | 11–16 | >16 |
| CIN risk | Low 7.5% | Moderate 14% | High 26.1% | Very high 57.3% |
| Dialysis risk | 0.04% | 0.12% | 1.09% | 12.6% |
CIN, contrast-induced nephropathy; HCT, haematocrit; NYHA, New York Heart Failure Association; SBP, systolic blood pressure.
A similar scoring system has also been proposed by Tziakas et al53 who found that pre-existing renal disease, metformin use, history of previous PCI, peripheral arterial disease and ≥300 mL of contrast volume were also independent predictors of CIN. A limitation of these scoring systems is that calculation is only possible after CM has been administered. However, it is clinically desirable to be able to predict the risk of CIN before the patient is exposed to CM allowing appropriate precautionary measures to be taken. Such a pre-procedural CIN risk score has been proposed by Maioli et al,8 following validation in a prospective cohort study (table 5).
Table 5.
A pre-procedural risk score for CIN (adapted from Maioli et al8)
| Pre-procedural risk factor | Score | |||
|---|---|---|---|---|
| Prior CM exposure within 72 h | 3 | |||
| Left ventricular ejection fraction <45% | 2 | |||
| Pre-procedure SCr >baseline SCr | 2 | |||
| Baseline SCr >1.5 mg/dL | 2 | |||
| Diabetes mellitus | 2 | |||
| Creatinine clearance (eGFR) <44 mL/min | 2 | |||
| Age >73 years | 1 | |||
| Score | 0–3 | 4–6 | 7–8 | >9 |
| CIN risk | Low 1.1% | Moderate 7.5% | High 22.3% | Very high 52.1% |
CIN, contrast-induced nephropathy; CM, contrast media; eGFR, estimated glomerular filtration rate; SCr, serum creatinine.
A number of other novel CIN risk factors have been identified, including pre-procedure glucose levels54 55 and low-density lipoprotein cholesterol;56 however, these have yet to be integrated into risk scoring systems. It may be possible to use commonly used cardiovascular risk scoring methods to approximate CIN risk, for example, a Global Registry of Acute Coronary Events score of >140 in patients with AMI having normal baseline renal function has been shown to predict risk of CIN in a small cohort study.57 A novel fluid status assessment method using Bio-Impedance Vector Analysis has also been demonstrated to independently predict CIN58 in a small clinical trial; however, it has not yet been translated into a CIN risk scoring system or guided volume repletion strategy.
Established preventative measures
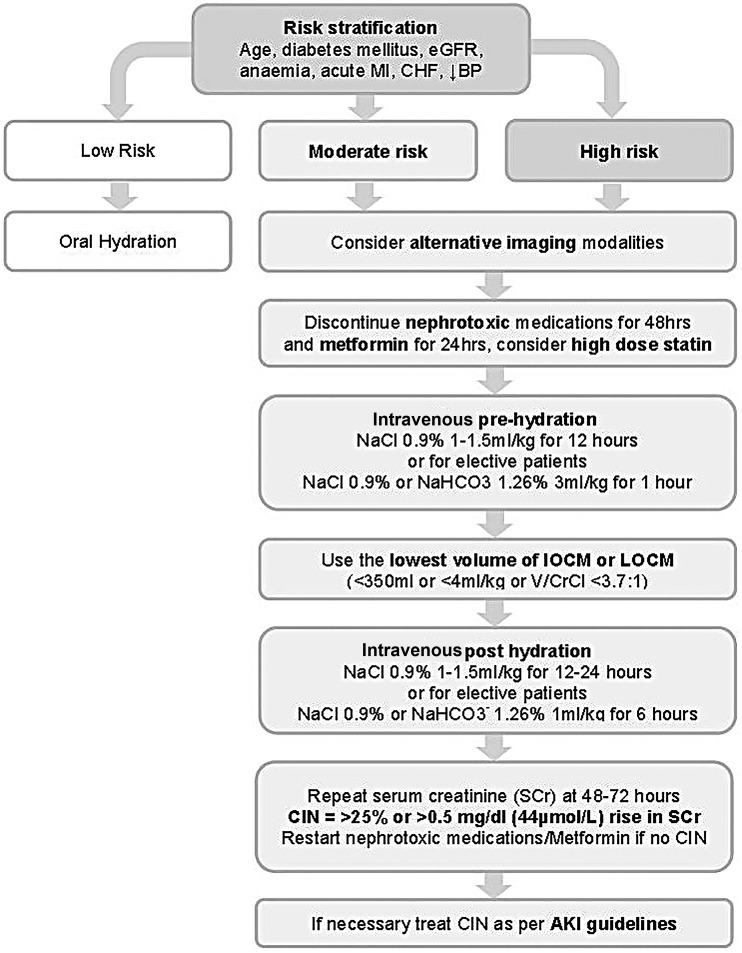
In 2014, the European Society of Cardiology published updated guidelines on CIN59 prevention which provides a framework for the use of the following evidence-based strategies (figure 2 and table 6). For all patients referred for CM procedures, a CIN risk assessment should be performed which includes baseline measurement of SCr and calculation of eGFR using a suitable formula, for example, Modification of Diet in Renal Disease or Chronic Kidney Disease–Epidemiology. If patients are identified as being at risk of CIN, particularly if eGFR is <40 mL/min, clinical indications for the CM procedure should be reviewed and preventative measures instigated. A single invasive approach should ideally be adopted, with CA followed by ad hoc PCI to reduce the risk of atheroembolic complications while minimising contrast volumes to <4 mL/kg or V/CrCl <3.7:1. However if a second CM procedure is necessitated, it is advisable to delay until adequate clearance of CM and recovery from any renal injury has occurred, which may be up to 2 weeks or as long as is clinically acceptable. It should be acknowledged that due to the overlap of common risk factors, patients at risk for CIN are also at significantly higher risk of cardiovascular events and therefore should not be excluded or unnecessarily delayed from receiving prognostic CM procedures unless the risks are considered to be excessive.
Figure 2.
Algorithm for the prevention of CIN is shown. AKI, acute kidney injury; BP, blood pressure; CHF, chronic heart failure; CIN, contrast-induced nephropathy; eGFR, estimated glomerular filtration rate; IOCM, iso-osmolar contrast medium; LOCM, low-osmolar contrast medium; MI, myocardial infarction; NaCl, sodium chloride; NaHCO3−, sodium bicarbonate; Scr, serum creatinine; V/CrCl, volume of contrast media to creatinine clearance.
Patients should be advised to stop all non-essential nephrotoxic medications (table 7) for 24 h prior to and for 48 h following the CM procedure pending SCr measurement. It is also recommended that patients receiving intra-arterial CM with an eGFR of <60 mL/min/1.73 m2, or those receiving intravenous CM with an eGFR of <45 mL/min/1.73 m2, discontinue metformin for 48 h prior to CM exposure and restart once a 48 h SCr measurement excludes CIN. This is to mitigate the risk of lactic acidosis due to reduced renal clearance of metformin that may occur following a potential CIN episode, rather than metformin nephrotoxicity per se.
Table 7.
Nephrotoxic medications requiring withdrawal 24 h pre-procedure
| Drug class | Examples |
|---|---|
| Non-steroidal anti-inflammatory | Naproxen, Ibuprofen, Diclofenac, Celecoxib90 |
| Antibiotics | Aminoglycosides: (Gentamycin, Tobramycin, Amikacin)91 |
| Antifungals | Amphotericin B92 |
| Antivirals | Acyclovir, Tenofovir, Foscarnet93 |
| Immunomodulatory | Ciclosporin A94 |
| Antineoplastic | Cisplatin, Ifosfamide, Mitomycin95 |
Recent studies have investigated whether IOCM formulations, which are thought to induce less osmotic stress despite generally having higher viscosity, are preferable over LOCM. A number of meta-analyses have been performed, some of which suggest the superiority of IOCM; however, others have shown no benefit.60 61 The current guidelines recommend the use of either IOCM or LOCM, although a preference for IOCM is reasonable, with the more important proviso that the minimum amount of CM required for diagnostic accuracy is used. During CA or PCI, the use of biplane imaging by experienced operators may reduce the amount of contrast required as simultaneous orthogonal views can be acquired following each CM injection.62
The most effective prophylactic intervention is provision of adequate hydration prior to CM exposure. Supplementing intravascular volume ensures renal blood flow is maintained and acts to dilute CM in both blood plasma and tubular filtrate. In lower risk ambulant patients, the oral route may be appropriate if adequate fluid intake is assured. However, in moderate/higher risk or in hospitalised patients intravenous hydration with a crystalloid fluid is preferred over oral hydration as it guarantees delivery of appropriate fluid volumes and has been demonstrated as superior in clinical trials.63 The choice of which crystalloid to use is, however, less clear; when compared with isotonic (normal) saline (0.9%), intravenous sodium bicarbonate (1.26%) may have additional ROS scavenging properties mediated through urine alkalinisation64 and lacks chloride ions that are thought to exacerbate renal vasoconstriction.65 Two recent meta-analyses have demonstrated a modest reduction in CIN when using intravenous sodium bicarbonate 1.26% as compared with isotonic saline,66 67 although no significant mortality benefit has been demonstrated. In view of the current lack of evidence supporting the use of sodium bicarbonate 1.26% and with some studies offering conflicting evidence,68 the current ESC guidelines recommend pre-hydration with sodium chloride 0.9% at 1–1.5 mL/kg/h for 12 h pre-procedure and up to 24 h post procedure. For elective day case patients and for those with CCF in whom large volumes of intravenous fluid may provoke pulmonary oedema,69 it is also reasonable to use an alternative protocol delivering a shorter duration and volume of sodium chloride 0.9% (table 8).
Table 8.
Intravenous pre-hydration regimes, Updated ESUR guidelines 201147
| Intravenous fluid | Pre-hydration | Post-hydration |
|---|---|---|
| Isotonic saline (0.9%) | 12 h, 1–1.5 mL/kg/h | 12–24 h, 1–1.5 mL/kg/h |
| Isotonic saline (0.9%) or sodium bicarbonate (1.26%) | 1 h at 3 mL/kg/h | 6 h at 1 mL/kg/h |
ESUR, European Society of Urogenital Radiology.
All patients determined as being at risk of CIN should have SCr levels measured between 48 and 72 h following CM exposure. If CIN is diagnosed, then it should be managed using recommended AKI guidelines, such as the recent European Best Practice position statement on AKI.70 This includes follow-up SCr measurements, withdrawal of nephrotoxic medications and unnecessary loop diuretics, electrolyte and hydration optimisation, nutritional advice and, if severe AKI occurs, early hospitalisation with referral to a specialist nephrologist.
Novel preventative measures
A number of prophylactic pharmacological agents have been investigated; however, at present the evidence for benefit in CIN prevention is limited. Originally one of the most promising agents, N-acetyl-cysteine (NAC), is inexpensive, well tolerated and has both antioxidant and vasodilatory properties. Several large RCTs have shown oral NAC at dose of 600 mg twice a day for 24 h pre-procedure and post procedure reduces the incidence of CIN.71 72 However meta-analyses73 74 have failed to reach consensus, most likely due to clinical heterogeneity, variable reporting and publication bias in the included studies.75 As such the ESC guidelines recommend that NAC is not to be used alone, although it may be used in addition to standard intravenous hydration regimes.70 More recently high-dose statin therapy (eg, rosuvastatin 40/20 mg, atorvastatin 80 mg or simvastatin 80 mg) has shown efficacy in preventing CIN in statin-naïve patients in several clinical studies76 77 and as such is regarded as reasonable preventative therapy in the current ESC guidelines. Other pharmaceutical agents with antioxidant (eg, ascorbic acid) and vasodilatory properties have also been investigated and although some have shown promise, further evaluation is required (table 9).
Table 9.
Potential pharmacological prophylactic agents
| Drug name | Study | Outcome: treatment vs control |
|---|---|---|
| High-dose statins | PRATO-ACS study76 n=504 Marenzi et al77 n=1134 |
CIN 6.7% vs 15.1% OR 0.38; 95% CI 0.20 to 0.71; p=0.003 CIN 5.5% vs 15% RR=0.37; 95% CI 0.25 to 0.55; p<0.0001 |
| N-acetyl cysteine | Gonzales et al73 Meta-analysis n=2746 |
High-quality RCTs—no CIN benefit RR=0.87; 95% CI 0.68 to 1.12, p=0.28 Low-quality RCTs—high CIN benefit RR=0.15; 95% CI 0.07 to 0.33, p<0.0001 |
| Ascorbic acid | Spargias et al,96 RCT n=231 |
CIN 9% vs 20% OR 0.38 95% CI 0.17 to 0.85; P=0.02 |
| Theophylline | Ix et al97 Meta-analysis, n=480 |
Difference in mean SCr 11.5 µmol/L 95% CI 5.3 to 19.4 µmol/L, p=0.004 |
| Iloprost | Spargias et al98 RCT n=208 |
CIN 8% vs 20% OR 0.29 95% CI 0.12 to 0.69; p=0.005 |
| Prostaglandin E1 | Li et al99 n=163, RCT |
CIN 3.7 vs 11.1% p<0.05 |
| Trimetazidine | Shehata et al,100 RCT n=100 |
CIN 12% vs 28% (p<0.05) (lower Troponin-T in Trimetazidine group) |
| Atrial natriuretic peptide | Morikawa et al,101 RCT n=254 |
CIN 3.2% vs 11.7% OR 0.24; p=0.016 |
Conflicting or negative evidence.
CIN, contrast-induced nephropathy; RCT, randomised controlled trial; RR, relative risk; SCr, serum creatinine.
There has also been considerable interest in novel interventional therapies that may offer additional protection when combined with conventional therapy. These can be characterised into three categories: direct renal protection, hydration optimisation and CM delivery and extraction technologies. The prototypical renal protection approach is exemplified by remote ischaemic preconditioning, which has been shown in many models to be cytoprotective against ischaemia/reperfusion injury, believed to be characteristic of CIN.78 Several small RCTs have shown promise using cycles of brief, non-injurious remote tissue ischaemia to trigger renal protection. Both blood pressure cuff inflation on the upper arm prior to CM exposure (preconditioning)79 and catheter balloon inflation in the target coronary artery following PCI (post-conditioning)80 81 have demonstrated a 60%–70% reduction in the incidence of CIN. Although encouraging, larger Phase II/III studies are required to confirm efficacy of this safe and cost–effective intervention.
Two hydration optimisation strategies have been trialled in CIN: LV end diastolic pressure (LVEDP) guided volume expansion and high urine output matched fluid replacement (RENALGUARD). The optimum rate and volume of intravenous fluid delivery represents a significant challenge: under-hydration increases CIN risk, whereas overhydration may precipitate acute pulmonary oedema in vulnerable patients with severe CKD and CCF. The recent POSEIDON RCT82 demonstrated that LVEDP-guided volume expansion in at-risk patients was safe and significantly reduced the incidence of CIN from 16.3% (28/172) in controls to 6.7% (12/178) in the fluid-guided group. An alternative fluid management system is delivered by the RENALGUARD system, which maintains a high urine output (>300 mL/h) using balanced intravenous isotonic saline (0.9%) delivery and intravenous furosemide infusion (0.25 mg/kg). The REMEDIAL II study83 demonstrated superiority of the RENALGUARD system plus oral NAC in preventing CIN (11%, 16/146) against a control group receiving sodium bicarbonate (1.26%) regimen plus oral NAC (20.5%, 30/146; OR 0.47; 95% CI 0.24 to 0.92). As such these novel therapies hold promise for higher risk patients especially those who are physiologically unable to tolerate large intravenous fluid volumes.
In order to minimise CM load, novel automated contrast injection devices have been developed which decrease the volume of CM used and which have been shown to reduce the incidence of CIN.84 It has been proposed that rapid removal of CM from the blood pool may have benefit in preventing CIN; although prophylactic HD has not been shown attenuate the incidence of CIN,85 some benefit has been observed with both pre-procedural and post-procedural86 haemofiltration (HF) and simultaneous HF,87 which may be partially explained through optimisation of periprocedural intravascular volumes. However, HF is a resource-intensive therapy that should be reserved for very-high-risk patients, such as for those with pre-dialysis ESRF88 or those with severe CKD undergoing complex PCI. Direct extraction of CM in coronary venous blood, captured using a coronary sinus catheter, is an interesting experimental intervention89 which has yet to be proven in clinical trials.
Summary and conclusion
CIN represents a significant clinical and health economic problem that may be under-recognised through limitations in the currently available biomarkers. Although often a transient injury, CIN may progress to significant persistent renal impairment, ESRF and adverse cardiovascular outcomes. There are a number of recognised risk factors, although the prediction of CIN, particularly prior to contrast administration, remains challenging. Current interventions are largely centred on the avoidance of dehydration, the withdrawal of nephrotoxic agents and minimisation of contrast load, which has limited efficacy in preventing CIN in vulnerable patients. The unmet clinical need in CIN therefore resides in accurate prediction, effective intervention and rapid detection to prevent adverse cardiorenal outcomes. Each of these areas, particularly predictive risk scoring systems, innovative pharmacological and mechanical interventions and novel biomarkers are currently the subject of intensive research and development that may lead to the future development effective strategies to mitigate the risk of CIN.
You can get CPD/CME credits for Education in Heart.
Education in Heart articles are accredited by both the UK Royal College of Physicians (London) and the European Board for Accreditation in Cardiology—you need to answer the accompanying multiple choice questions (MCQs). To access the questions, click on BMJ Learning: Take this module on BMJ Learning from the content box at the top right and bottom left of the online article. For more information please go to: http://heart.bmj.com/misc/education.dtl
RCP credits: Log your activity in your CPD diary online (http://www.rcplondon.ac.uk/members/CPDdiary/index.asp)—pass mark is 80%.
EBAC credits: Print out and retain the BMJ Learning certificate once you have completed the MCQs—pass mark is 60%. EBAC/ EACCME Credits can now be converted to AMA PRA Category 1 CME Credits and are recognised by all National Accreditation Authorities in Europe (http://www.ebac-cme.org/newsite/?hit=men02).
Please note: The MCQs are hosted on BMJ Learning—the best available learning website for medical professionals from the BMJ Group. If prompted, subscribers must sign into Heart with their journal's username and password. All users must also complete a one-time registration on BMJ Learning and subsequently log in (with a BMJ Learning username and password) on every visit.
Footnotes
Contributors: All contributors meet the criteria for authorship.
Funding: DJH is funded by the British Heart Foundation (grant number FS/10/039/28270), the Rosetrees Trust, and is supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
Competing interests: None declared.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1.Bartels ED, Brun GC, Gammeltoft A, et al. Acute anuria following intravenous pyelography in a patient with myelomatosis. Acta Med Scand 1954;150:297–302. 10.1111/j.0954-6820.1954.tb18632.x [DOI] [PubMed] [Google Scholar]
- 2.Killmann SA, Gjorup S, Thaysen JH. Fatal acute renal failure following intravenous pyelography in a patient with multiple myeloma. Acta Med Scand 1957;158:43–6. 10.1111/j.0954-6820.1957.tb15742.x [DOI] [PubMed] [Google Scholar]
- 3.Hou SH, Bushinsky DA, Wish JB, et al. Hospital-acquired renal insufficiency: a prospective study. Am J Med 1983;74:243–8. 10.1016/0002-9343(83)90618-6 [DOI] [PubMed] [Google Scholar]
- 4.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis 2002;39:930–6. 10.1053/ajkd.2002.32766 [DOI] [PubMed] [Google Scholar]
- 5.Mehran R, Nikolsky E. Contrast-induced nephropathy: definition, epidemiology, and patients at risk. Kidney Int Suppl 2006:S11–15. 10.1038/sj.ki.5000368 [DOI] [PubMed] [Google Scholar]
- 6.Marenzi G, Lauri G, Assanelli E, et al. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol 2004;44:1780–5. 10.1016/j.jacc.2004.07.043 [DOI] [PubMed] [Google Scholar]
- 7.Solomon R. Contrast-medium-induced acute renal failure. Kidney Int 1998;53:230–42. 10.1038/sj.ki.4495510 [DOI] [PubMed] [Google Scholar]
- 8.Maioli M, Toso A, Gallopin M, et al. Preprocedural score for risk of contrast-induced nephropathy in elective coronary angiography and intervention. J Cardiovasc Med (Hagerstown) 2010;11:444–9. 10.2459/JCM.0b013e328335227c [DOI] [PubMed] [Google Scholar]
- 9.Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol 2004;44:1393–9. 10.1016/j.jacc.2004.06.068 [DOI] [PubMed] [Google Scholar]
- 10.Morcos SK, Thomsen HS, Webb JA. Contrast-media-induced nephrotoxicity: a consensus report. Contrast media safety committee, European society of urogenital radiology (ESUR). Eur Radiol 1999;9:1602–13. 10.1007/s003300050894 [DOI] [PubMed] [Google Scholar]
- 11.Solomon RJ, Mehran R, Natarajan MK, et al. Contrast-induced nephropathy and long-term adverse events: cause and effect? Clin J Am Soc Nephrol 2009;4:1162–9. 10.2215/CJN.00550109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weisbord SD, Chen H, Stone RA, et al. Associations of increases in serum creatinine with mortality and length of hospital stay after coronary angiography. J Am Soc Nephrol 2006;17:2871–7. 10.1681/ASN.2006030301 [DOI] [PubMed] [Google Scholar]
- 13.Bellomo R, Ronco C, Kellum JA, et al. , Acute Dialysis Quality Initiative w. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit Care 2004;8:R204–12. 10.1186/cc2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta RL, Kellum JA, Shah SV, et al. , Acute Kidney Injury N. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31 10.1186/cc5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kidney Disease: Improving Global Outcomes (KDIGO) AKI Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Inter Suppl 2012;2:1–138. 10.1038/kisup.2012.1 [DOI] [Google Scholar]
- 16.Harjai KJ, Raizada A, Shenoy C, et al. A comparison of contemporary definitions of contrast nephropathy in patients undergoing percutaneous coronary intervention and a proposal for a novel nephropathy grading system. Am J Cardiol 2008;101:812–19. 10.1016/j.amjcard.2007.10.051 [DOI] [PubMed] [Google Scholar]
- 17.Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol 2009;20:672–9. 10.1681/ASN.2008070669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ribichini F, Graziani M, Gambaro G, et al. Early creatinine shifts predict contrast-induced nephropathy and persistent renal damage after angiography. Am J Med 2010;123: 755–63. 10.1016/j.amjmed.2010.02.026 [DOI] [PubMed] [Google Scholar]
- 19.Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, et al. NGAL (neutrophil gelatinase-associated lipocalin) and cystatin C: are they good predictors of contrast nephropathy after percutaneous coronary interventions in patients with stable angina and normal serum creatinine? Int J Cardiol 2008;127:290–1. 10.1016/j.ijcard.2007.04.048 [DOI] [PubMed] [Google Scholar]
- 20.Briguori C, Visconti G, Rivera NV, et al. Cystatin c and contrast-induced acute kidney injury. Circulation 2010;121:2117–22. 10.1161/CIRCULATIONAHA.109.919639 [DOI] [PubMed] [Google Scholar]
- 21.Duan SB, Liu GL, Yu ZQ, et al. Urinary KIM-1, IL-18 and Cys-c as early predictive biomarkers in gadolinium-based contrast-induced nephropathy in the elderly patients. Clin Nephrol 2013;80:349–54. 10.5414/CN107829 [DOI] [PubMed] [Google Scholar]
- 22.Bulent Gul CB, Gullulu M, Oral B, et al. Urinary IL-18: a marker of contrast-induced nephropathy following percutaneous coronary intervention? Clin Biochem 2008;41:544–7. 10.1016/j.clinbiochem.2008.01.002 [DOI] [PubMed] [Google Scholar]
- 23.McCullough PA, Sandberg KR. Epidemiology of contrast-induced nephropathy. Rev Cardiovasc Med 2003;4(Suppl 5):S3–9. [PubMed] [Google Scholar]
- 24.Finn WF. The clinical and renal consequences of contrast-induced nephropathy. Nephrol Dial Transplant 2006;21:i2–10. 10.1093/ndt/gfl213 [DOI] [PubMed] [Google Scholar]
- 25.McCullough PA, Wolyn R, Rocher LL, et al. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med 1997;103:368–75. 10.1016/S0002-9343(97)00150-2 [DOI] [PubMed] [Google Scholar]
- 26.Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA 1996;275:1489–94. 10.1001/jama.1996.03530430033035 [DOI] [PubMed] [Google Scholar]
- 27.Gruberg L, Mintz GS, Mehran R, et al. The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol 2000;36:1542–8. 10.1016/S0735-1097(00)00917-7 [DOI] [PubMed] [Google Scholar]
- 28.Maioli M, Toso A, Leoncini M, et al. Persistent renal damage after contrast-induced acute kidney injury: Incidence, evolution, risk factors, and prognosis. Circulation 2012;125:3099–107. 10.1161/CIRCULATIONAHA.111.085290 [DOI] [PubMed] [Google Scholar]
- 29.James MT, Samuel SM, Manning MA, et al. Contrast-induced acute kidney injury and risk of adverse clinical outcomes after coronary angiography: a systematic review and meta-analysis. Circ Cardiovasc Interv 2013;6:37–43. 10.1161/CIRCINTERVENTIONS.112.974493 [DOI] [PubMed] [Google Scholar]
- 30.Vanholder R, Massy Z, Argiles A, et al. , European Uremic Toxin Work G. Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant 2005;20:1048–56. 10.1093/ndt/gfh813 [DOI] [PubMed] [Google Scholar]
- 31.Heinrich MC, Kuhlmann MK, Grgic A, et al. Cytotoxic effects of ionic high-osmolar, nonionic monomeric, and nonionic iso-osmolar dimeric iodinated contrast media on renal tubular cells in vitro. Radiology 2005;235:843–9. 10.1148/radiol.2353040726 [DOI] [PubMed] [Google Scholar]
- 32.Barrett BJ, Carlisle EJ. Metaanalysis of the relative nephrotoxicity of high- and low-osmolality iodinated contrast media. Radiology 1993;188:171–8. 10.1148/radiology.188.1.8511292 [DOI] [PubMed] [Google Scholar]
- 33.Pannu N, Wiebe N, Tonelli M, Alberta Kidney Disease N. Prophylaxis strategies for contrast-induced nephropathy. JAMA 2006;295:2765–79. 10.1001/jama.295.23.2765 [DOI] [PubMed] [Google Scholar]
- 34.Sendeski MM. Pathophysiology of renal tissue damage by iodinated contrast media. Clin Exp Pharmacol Physiol 2011;38:292–9. 10.1111/j.1440-1681.2011.05503.x [DOI] [PubMed] [Google Scholar]
- 35.Brezis M, Rosen S. Hypoxia of the renal medulla—its implications for disease. N Engl J Med 1995;332:647–55. 10.1056/NEJM199503093321006 [DOI] [PubMed] [Google Scholar]
- 36.Tumlin J, Stacul F, Adam A, et al. , Panel CINCW. Pathophysiology of contrast-induced nephropathy. Am J Cardiol 2006;98:14K–20K. 10.1016/j.amjcard.2006.01.020 [DOI] [PubMed] [Google Scholar]
- 37.Pruijm M, Hofmann L, Vogt B, et al. Renal tissue oxygenation in essential hypertension and chronic kidney disease. Int J Hypertens 2013;2013:696598 10.1155/2013/696598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz D, Blum M, Peer G, et al. Role of nitric oxide (EDRF) in radiocontrast acute renal failure in rats. Am J Physiol 1994;267:F374–9. [DOI] [PubMed] [Google Scholar]
- 39.Heyman SN, Clark BA, Kaiser N, et al. Radiocontrast agents induce endothelin release in vivo and in vitro. J Am Soc Nephrol 1992;3:58–65. [DOI] [PubMed] [Google Scholar]
- 40.Heyman SN, Rosen S, Khamaisi M, et al. Reactive oxygen species and the pathogenesis of radiocontrast-induced nephropathy. Invest Radiol 2010;45:188–95. 10.1097/RLI.0b013e3181d2eed8 [DOI] [PubMed] [Google Scholar]
- 41.Seeliger E, Flemming B, Wronski T, et al. Viscosity of contrast media perturbs renal hemodynamics. J Am Soc Nephrol 2007;18:2912–20. 10.1681/ASN.2006111216 [DOI] [PubMed] [Google Scholar]
- 42.Liss P, Nygren A, Olsson U, et al. Effects of contrast media and mannitol on renal medullary blood flow and red cell aggregation in the rat kidney. Kidney Int 1996;49:1268–75. 10.1038/ki.1996.181 [DOI] [PubMed] [Google Scholar]
- 43.Heyman SN, Rosen S, Rosenberger C. Renal parenchymal hypoxia, hypoxia adaptation, and the pathogenesis of radiocontrast nephropathy. Clin J Am Soc Nephrol 2008;3:288–96. 10.2215/CJN.02600607 [DOI] [PubMed] [Google Scholar]
- 44.Weisberg LS, Kurnik PB, Kurnik BR. Risk of radiocontrast nephropathy in patients with and without diabetes mellitus. Kidney Int 1994;45:259–65. 10.1038/ki.1994.32 [DOI] [PubMed] [Google Scholar]
- 45.Nikolsky E, Mehran R, Lasic Z, et al. Low hematocrit predicts contrast-induced nephropathy after percutaneous coronary interventions. Kidney Int 2005;67:706–13. 10.1111/j.1523-1755.2005.67131.x [DOI] [PubMed] [Google Scholar]
- 46.Lameire N, Adam A, Becker CR, et al. , Panel CINCW. Baseline renal function screening. Am J Cardiol 2006;98:21K–6K. 10.1016/j.amjcard.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 47.Stacul F, van der Molen AJ, Reimer P, et al. , Contrast Media Safety Committee of European Society of Urogenital R. Contrast induced nephropathy: updated ESUR contrast media safety committee guidelines. Eur Radiol 2011;21:2527–41. 10.1007/s00330-011-2225-0 [DOI] [PubMed] [Google Scholar]
- 48.Rihal CS, Textor SC, Grill DE, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 2002;105:2259–64. 10.1161/01.CIR.0000016043.87291.33 [DOI] [PubMed] [Google Scholar]
- 49.Dangas G, Iakovou I, Nikolsky E, et al. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol 2005;95:13–19. 10.1016/j.amjcard.2004.08.056 [DOI] [PubMed] [Google Scholar]
- 50.Parfrey PS, Griffiths SM, Barrett BJ, et al. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. N Engl J Med 1989;320:143–9. 10.1056/NEJM198901193200303 [DOI] [PubMed] [Google Scholar]
- 51.McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol 2008;51:1419–28. 10.1016/j.jacc.2007.12.035 [DOI] [PubMed] [Google Scholar]
- 52.Laskey WK, Jenkins C, Selzer F, et al. Volume-to-creatinine clearance ratio: a pharmacokinetically based risk factor for prediction of early creatinine increase after percutaneous coronary intervention. J Am Coll Cardiol 2007;50:584–90. 10.1016/j.jacc.2007.03.058 [DOI] [PubMed] [Google Scholar]
- 53.Tziakas D, Chalikias G, Stakos D, et al. Development of an easily applicable risk score model for contrast-induced nephropathy prediction after percutaneous coronary intervention: a novel approach tailored to current practice. Int J Cardiol 2013;163:46–55. 10.1016/j.ijcard.2011.05.079 [DOI] [PubMed] [Google Scholar]
- 54.Stolker JM, McCullough PA, Rao S, et al. Pre-procedural glucose levels and the risk for contrast-induced acute kidney injury in patients undergoing coronary angiography. J Am Coll Cardiol 2010;55:1433–40. 10.1016/j.jacc.2009.09.072 [DOI] [PubMed] [Google Scholar]
- 55.Marenzi G, De Metrio M, Rubino M, et al. Acute hyperglycemia and contrast-induced nephropathy in primary percutaneous coronary intervention. Am Heart J 2010;160:1170–7. 10.1016/j.ahj.2010.09.022 [DOI] [PubMed] [Google Scholar]
- 56.Liu YH, Liu Y, Chen JY, et al. LDL cholesterol as a novel risk factor for contrast-induced acute kidney injury in patients undergoing percutaneous coronary intervention. Atherosclerosis 2014;237:453–9. 10.1016/j.atherosclerosis.2014.10.022 [DOI] [PubMed] [Google Scholar]
- 57.Raposeiras-Roubín S, Aguiar-Souto P, Barreiro-Pardal C, et al. Grace risk score predicts contrast-induced nephropathy in patients with acute coronary syndrome and normal renal function. Angiology 2013;64:31–9. 10.1177/0003319711434800 [DOI] [PubMed] [Google Scholar]
- 58.Maioli M, Toso A, Leoncini M, et al. Pre-procedural bioimpedance vectorial analysis of fluid status and prediction of contrast-induced acute kidney injury. J Am Coll Cardiol 2014;63:1387–94. 10.1016/j.jacc.2014.01.025 [DOI] [PubMed] [Google Scholar]
- 59.Windecker S, Kolh P, Alfonso F, et al. , Authors/Task Force members. 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541–619. 10.1093/eurheartj/ehu278 [DOI] [PubMed] [Google Scholar]
- 60.McCullough PA, Bertrand ME, Brinker JA, et al. A meta-analysis of the renal safety of isosmolar iodixanol compared with low-osmolar contrast media. J Am Coll Cardiol 2006;48:692–9. 10.1016/j.jacc.2006.02.073 [DOI] [PubMed] [Google Scholar]
- 61.Reed M, Meier P, Tamhane UU, et al. The relative renal safety of iodixanol compared with low-osmolar contrast media: a meta-analysis of randomized controlled trials. JACC Cardiovasc Interv 2009;2:645–54. 10.1016/j.jcin.2009.05.002 [DOI] [PubMed] [Google Scholar]
- 62.Heintzen PH, Bürsch HJ, Hahne HJ, et al. Assessment of cardiovascular function by digital angiocardiography. J Am Coll Cardiol 1985;5:150S–7S. 10.1016/S0735-1097(85)80156-X [DOI] [PubMed] [Google Scholar]
- 63.Trivedi HS, Moore H, Nasr S, et al. A randomized prospective trial to assess the role of saline hydration on the development of contrast nephrotoxicity. Nephron Clin Pract 2003;93:C29–34. 10.1159/000066641 [DOI] [PubMed] [Google Scholar]
- 64.Efrati S, Berman S, Ilgiyeav I, et al. Differential effects of n-acetylcysteine, theophylline or bicarbonate on contrast-induced rat renal vasoconstriction. Am J Nephrol 2009;29:181–91. 10.1159/000154471 [DOI] [PubMed] [Google Scholar]
- 65.Quilley CP, Lin YS, McGiff JC. Chloride anion concentration as a determinant of renal vascular responsiveness to vasoconstrictor agents. Br J Pharmacol 1993;108:106–10. 10.1111/j.1476-5381.1993.tb13447.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trivedi H, Nadella R, Szabo A. Hydration with sodium bicarbonate for the prevention of contrast-induced nephropathy: a meta-analysis of randomized controlled trials. Clin Nephrol 2010;74:288–96. 10.5414/CNP74288 [DOI] [PubMed] [Google Scholar]
- 67.Meier P, Ko DT, Tamura A, et al. Sodium bicarbonate-based hydration prevents contrast-induced nephropathy: a meta-analysis. BMC Med 2009;7:23 10.1186/1741-7015-7-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brar SS, Shen AY, Jorgensen MB, et al. Sodium bicarbonate vs sodium chloride for the prevention of contrast medium-induced nephropathy in patients undergoing coronary angiography: a randomized trial. JAMA 2008;300:1038–46. 10.1001/jama.300.9.1038 [DOI] [PubMed] [Google Scholar]
- 69.Navaneethan SD, Singh S, Appasamy S, et al. Sodium bicarbonate therapy for prevention of contrast-induced nephropathy: a systematic review and meta-analysis. Am J Kidney Dis 2009;53:617–27. 10.1053/j.ajkd.2008.08.033 [DOI] [PubMed] [Google Scholar]
- 70.Fliser D, Laville M, Covic A, et al. , ERBP A-HWG. A European renal best practice (ERBP) position statement on the kidney disease improving global outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transpl 2012;27:4263–72. 10.1093/ndt/gfs375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tepel M, van der Giet M, Schwarzfeld C, et al. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med 2000;343:180–4. 10.1056/NEJM200007203430304 [DOI] [PubMed] [Google Scholar]
- 72.Briguori C, Manganelli F, Scarpato P, et al. Acetylcysteine and contrast agent-associated nephrotoxicity. J Am Coll Cardiol 2002;40:298–303. 10.1016/S0735-1097(02)01958-7 [DOI] [PubMed] [Google Scholar]
- 73.Gonzales DA, Norsworthy KJ, Kern SJ, et al. A meta-analysis of n-acetylcysteine in contrast-induced nephrotoxicity: unsupervised clustering to resolve heterogeneity. BMC Med 2007;5:32 10.1186/1741-7015-5-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kelly AM, Dwamena B, Cronin P, et al. Meta-analysis: effectiveness of drugs for preventing contrast-induced nephropathy. Ann Intern Med 2008;148:284–94. 10.7326/0003-4819-148-4-200802190-00007 [DOI] [PubMed] [Google Scholar]
- 75.Biondi-Zoccai GG, Lotrionte M, Abbate A, et al. Compliance with quorom and quality of reporting of overlapping meta-analyses on the role of acetylcysteine in the prevention of contrast associated nephropathy: case study. BMJ 2006;332:202–9. 10.1136/bmj.38693.516782.7C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leoncini M, Toso A, Maioli M, et al. Early high-dose rosuvastatin for contrast-induced nephropathy prevention in acute coronary syndrome: results from the prato-ACS study (protective effect of rosuvastatin and antiplatelet therapy on contrast-induced acute kidney injury and myocardial damage in patients with acute coronary syndrome). J Am Coll Cardiol 2014;63:71–9. 10.1016/j.jacc.2013.04.105 [DOI] [PubMed] [Google Scholar]
- 77.Marenzi G, Cosentino N, Werba JP, et al. A meta-analysis of randomized controlled trials on statins for the prevention of contrast-induced acute kidney injury in patients with and without acute coronary syndromes. Int J Cardiol 2015;183:47–53. 10.1016/j.ijcard.2015.01.046 [DOI] [PubMed] [Google Scholar]
- 78.Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: underlying mechanisms and clinical application. Atherosclerosis 2009;204:334–41. 10.1016/j.atherosclerosis.2008.10.029 [DOI] [PubMed] [Google Scholar]
- 79.Er F, Nia AM, Dopp H, et al. Ischemic preconditioning for prevention of contrast medium-induced nephropathy: randomized pilot renpro trial (renal protection trial). Circulation 2012;126:296–303. 10.1161/CIRCULATIONAHA.112.096370 [DOI] [PubMed] [Google Scholar]
- 80.Whittaker P, Przyklenk K. Remote-conditioning ischemia provides a potential approach to mitigate contrast medium-induced reduction in kidney function: a retrospective observational cohort study. Cardiology 2011;119:145–50. 10.1159/000330930 [DOI] [PubMed] [Google Scholar]
- 81.Deftereos S, Giannopoulos G, Tzalamouras V, et al. Renoprotective effect of remote ischemic post-conditioning by intermittent balloon inflations in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol 2013;61:1949–55. 10.1016/j.jacc.2013.02.023 [DOI] [PubMed] [Google Scholar]
- 82.Brar SS, Aharonian V, Mansukhani P, et al. Haemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney injury: The poseidon randomised controlled trial. Lancet 2014;383:1814–23. [DOI] [PubMed] [Google Scholar]
- 83.Briguori C, Visconti G, Focaccio A, et al. Renal insufficiency after contrast media administration trial ii (REMEDIAL ii): RenalGuard system in high-risk patients for contrast-induced acute kidney injury. Circulation 2011;124:1260–9. 10.1161/CIRCULATIONAHA.111.030759 [DOI] [PubMed] [Google Scholar]
- 84.Minsinger KD, Kassis HM, Block CA, et al. Meta-analysis of the effect of automated contrast injection devices versus manual injection and contrast volume on risk of contrast-induced nephropathy. Am J Cardiol 2014;113:49–53. 10.1016/j.amjcard.2013.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morcos SK, Thomsen HS, Webb JA. Contrast Media Safety Committee of the European Society of Urogenital R. Dialysis and contrast media. Eur Radiol 2002;12:3026–30. 10.1007/s00330-002-1629-2 [DOI] [PubMed] [Google Scholar]
- 86.Marenzi G, Lauri G, Campodonico J, et al. Comparison of two hemofiltration protocols for prevention of contrast-induced nephropathy in high-risk patients. Am J Med 2006;119:155–62. 10.1016/j.amjmed.2005.08.002 [DOI] [PubMed] [Google Scholar]
- 87.Choi MJ, Yoon JW, Han SJ, et al. The prevention of contrast-induced nephropathy by simultaneous hemofiltration during coronary angiographic procedures: a comparison with periprocedural hemofiltration. Int J Cardiol 2014;176:941–5. 10.1016/j.ijcard.2014.08.095 [DOI] [PubMed] [Google Scholar]
- 88.Klarenbach SW, Pannu N, Tonelli MA, et al. Cost-effectiveness of hemofiltration to prevent contrast nephropathy in patients with chronic kidney disease. Crit Care Med 2006;34:1044–51. 10.1097/01.CCM.0000206287.22318.C3 [DOI] [PubMed] [Google Scholar]
- 89.Duffy SJ, Ruygrok P, Juergens CP, et al. Removal of contrast media from the coronary sinus attenuates renal injury after coronary angiography and intervention. J Am Coll Cardiol 2010;56:525–6. 10.1016/j.jacc.2010.01.065 [DOI] [PubMed] [Google Scholar]
- 90.Seeliger E, Sendeski M, Rihal CS, et al. Contrast-induced kidney injury: Mechanisms, risk factors, and prevention. Eur Heart J 2012;33:2007–15. 10.1093/eurheartj/ehr494 [DOI] [PubMed] [Google Scholar]
- 91.Ungprasert P, Cheungpasitporn W, Crowson CS, et al. Individual non-steroidal anti-inflammatory drugs and risk of acute kidney injury: a systematic review and meta-analysis of observational studies. Eur J Intern Med 2015;26:285–91. 10.1016/j.ejim.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 92.Levin ML. Aminoglycoside nephrotoxicity: keys to prevention. J Crit Illn 1994;9:911–12, 915. [PubMed] [Google Scholar]
- 93.Bates DW, Su L, Yu DT, et al. Correlates of acute renal failure in patients receiving parenteral amphotericin b. Kidney Int 2001;60:1452–9. 10.1046/j.1523-1755.2001.00948.x [DOI] [PubMed] [Google Scholar]
- 94.Izzedine H, Launay-Vacher V, Deray G. Antiviral drug-induced nephrotoxicity. Am J Kidney Dis 2005;45:804–17. 10.1053/j.ajkd.2005.02.010 [DOI] [PubMed] [Google Scholar]
- 95.McNally PG, Feehally J. Pathophysiology of cyclosporin a nephrotoxicity: Experimental and clinical observations. Nephrol Dial Transplant 1992;7:791–804. [PubMed] [Google Scholar]
- 96.Ries F, Klastersky J. Nephrotoxicity induced by cancer chemotherapy with special emphasis on cisplatin toxicity. Am J Kidney Dis 1986;8:368–79. 10.1016/S0272-6386(86)80112-3 [DOI] [PubMed] [Google Scholar]
- 97.Spargias K, Alexopoulos E, Kyrzopoulos S, et al. Ascorbic acid prevents contrast-mediated nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. Circulation 2004;110:2837–42. 10.1161/01.CIR.0000146396.19081.73 [DOI] [PubMed] [Google Scholar]
- 98.Ix JH, McCulloch CE, Chertow GM. Theophylline for the prevention of radiocontrast nephropathy: a meta-analysis. Nephrol Dial Transplant 2004;19:2747–53. 10.1093/ndt/gfh468 [DOI] [PubMed] [Google Scholar]
- 99.Spargias K, Adreanides E, Demerouti E, et al. Iloprost prevents contrast-induced nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. Circulation 2009;120:1793–9. 10.1161/CIRCULATIONAHA.109.863159 [DOI] [PubMed] [Google Scholar]
- 100.Li WH, Li DY, Qian WH, et al. Prevention of contrast-induced nephropathy with prostaglandin e1 in high-risk patients undergoing percutaneous coronary intervention. Int Urol Nephrol 2014;46:781–6. 10.1007/s11255-014-0674-5 [DOI] [PubMed] [Google Scholar]
- 101.Shehata M. Impact of trimetazidine on incidence of myocardial injury and contrast-induced nephropathy in diabetic patients with renal dysfunction undergoing elective percutaneous coronary intervention. Am J Cardiol 2014;114:389–94. 10.1016/j.amjcard.2014.04.052 [DOI] [PubMed] [Google Scholar]
- 102.Morikawa S, Sone T, Tsuboi H, et al. Renal protective effects and the prevention of contrast-induced nephropathy by atrial natriuretic peptide. J Am Coll Cardiol 2009;53:1040–6. 10.1016/j.jacc.2008.10.061 [DOI] [PubMed] [Google Scholar]
- 103.Naeem M, McEnteggart GE, Murphy TP, et al. Fenoldopam for the prevention of contrast-induced nephropathy (CIN)-do we need more trials? A meta-analysis. Clin Imaging 2015;39:759–64. 10.1016/j.clinimag.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 104.Gare M, Haviv YS, Ben-Yehuda A, et al. The renal effect of low-dose dopamine in high-risk patients undergoing coronary angiography. J Am Coll Cardiol 1999; 34:1682–8. 10.1016/S0735-1097(99)00422-2 [DOI] [PubMed] [Google Scholar]
- 105.Beyazal H, Caliskan Z, Utaç C. Comparison of effects of isotonic sodium chloride with diltiazem in prevention of contrast-induced nephropathy. Ren Fail 2014;36:351–5. 10.3109/0886022X.2013.866016 [DOI] [PubMed] [Google Scholar]
- 106.Miller HI, Dascalu A, Rassin TA, et al. Effects of an acute dose of l-arginine during coronary angiography in patients with chronic renal failure: a randomized, parallel, double-blind clinical trial. Am J Nephrol 2003;23:91–5. doi:68036 [DOI] [PubMed] [Google Scholar]
- 107.Gu G, Zhang Y, Lu R, et al. Additional furosemide treatment beyond saline hydration for the prevention of contrast-induced nephropathy: a meta-analysis of randomized controlled trials. Int J Clin Exp Med 2015;8:387–94. [PMC free article] [PubMed] [Google Scholar]
- 108.Solomon R, Werner C, Mann D, et al. Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. N Engl J Med 1994;331:1416–20. 10.1056/NEJM199411243312104 [DOI] [PubMed] [Google Scholar]
- 109.Wang A, Holcslaw T, Bashore TM, et al. Exacerbation of radiocontrast nephrotoxicity by endothelin receptor antagonism. Kidney Int 2000;57:1675–80. 10.1046/j.1523-1755.2000.00012.x [DOI] [PubMed] [Google Scholar]