ABSTRACT
Acute mesenteric ischemia (AMI) is caused by considerable intestinal injury, which is associated with intestinal ischemia followed by reperfusion. To elucidate the mechanisms of ischemia/reperfusion injuries, a C5a inhibitory peptide termed AcPepA was used to examine the role of C5a anaphylatoxin, induction of inflammatory cells, and cell proliferation of the intestinal epithelial cells in an experimental AMI model. In this rat model, the superior mesenteric artery was occluded and subsequently reperfused (Induce‐I/R). Other groups were treated with AcPepA before ischemia or reperfusion. Induce‐I/R induced injuries in the intestine and AcPepA significantly decreased the proportion of severely injured villi. Induce‐I/R induced secondary receptor for C5a‐positive polymorphonuclear leukocytes in the vessels and CD204‐positive macrophages near the injured site; this was correlated with hypoxia‐induced factor 1‐alpha‐positive cells. Induction of these inflammatory cells was attenuated by AcPepA. In addition, AcPepA increased proliferation of epithelial cells in the villi, possibly preventing further damage. Therefore, Induce‐I/R activates C5a followed by the accumulation of polymorphonuclear leukocyte and hypoxia‐induced factor 1‐alpha‐producing macrophages, leading to villus injury. AcPepA, a C5a inhibitory peptide, blocks the deleterious effects of C5a, indicating it has a therapeutic effect on the inflammatory consequences of experimental AMI.
Keywords: complement C5a, hypoxia‐induced factor 1‐alpha, ischemia, macrophage
List of Abbreviations
- AMI
acute mesenteric ischemia
- C5L2
secondary receptor for C5a
- CD68+MAC
CD68‐positive macrophage
- CD204+MAC
CD204‐positive macrophage
- HIF‐1α
hypoxia‐induced factor 1‐alpha
- IHC
immunohistochemical staining
- I/R
Intestinal ischemia followed by reperfusion
- Induce‐I/R
induction of intestinal ischemia
- Induce‐I/R
induction of intestinal ischemia followed by reperfusion
- PCNA
proliferating cell nuclear antigen
- PMN
polymorphonuclear leukocyte
Acute mesenteric ischemia, a critical circulatory condition, is caused by an arterial or venous thrombosis or embolism 1, 2. The overall mortality rate of AMI has remained at 60% to 80% over the last 25 years and the incidence of this disease is increasing 3, 4. AMI comprises a group of pathologic processes that have a common end point—intestinal necrosis 4. The intestinal epithelium is probably one of the most sensitive tissues to I/R injury in the body 5; intestinal ischemia rapidly progresses to severe metabolic derangements, infiltration of inflammatory cells, loss of villi and epithelial cells, and mucosal destruction, culminating in irreversible bowel necrosis 3.
Reoxygenation is crucial for cell survival, however, it has been well established that I/R causes much more severe tissue injury than that induced by ischemia alone 6. Investigation of the development of I/R damage has revealed significant regeneration of the mucosa, which parallels necrosis and apoptosis of the epithelial cells of the villi 7, 8. Published studies have suggested that I/R injury involves multiple processes, including activation of inflammatory cells with cytokine production followed by decay or regeneration of the injured epithelial cells. A variety of endogenous compounds and effector cells have been identified as mediators of I/R injury, including platelet‐activating factor 9, TNF‐α 10, IL‐6 11 and oxygen radicals 12. It is also well‐known that I/R induces the so‐called antigen‐independent inflammatory pathway via which cellular and molecular participants of the immune system can be activated 13.
The complement system has been implicated as a major candidate in I/R injury, several studies having suggested that complement activation is involved in I/R injury in the gut 14, 15, 16. Complement activation results in production of C5a, which has been shown to be fundamental in exacerbation of I/R injuries 17. Complement activation occurs in the early stages of inflammation. In the case of gut I/R, activated complement induces activation of inflammatory cells, such as PMNs and macrophages, which have been demonstrated to play central roles in the development of I/R injury 18, 19, 20. In addition, C5a has been demonstrated to enhance the release of a number of pro‐inflammatory cytokines from activated PMNs and macrophages 21, 22, 23. Furthermore, inhibition of C5a by a complementary peptide to C5a (AcPepA) reportedly suppresses the release of high mobility group box 1 resulting in rescue of monkeys injected with lethal doses of LPS 24.
C5a is considered to be a major factor in complement‐mediated I/R tissue injury. Thus, the development of I/R injury involves multiple processes, such as C5a generation, induction of inflammatory cells and cytokine production, all of which lead to apoptosis and regeneration of the injured intestinal epithelial cells. These processes would involve cellular and molecular cross‐talk among inflammatory cells, which has not yet been investigated in detail. C5a is believed to be a major factor in complement‐mediated I/R tissue injury. Accordingly, the present study aimed to shed more light on the possible cellular and molecular pathways involved in the proliferative consequences of I/R events.
To this end we used AcPepA, which we have generated as an inhibitory complementary peptide (C‐peps) of C5a 25, 26, 27 and examined its modulating effects on certain inflammatory responses, such as induction of C5L2‐positive cells, induction of activated macrophages and involvement of HIF1‐α in I/R injury, in a clinically relevant animal model of AMI.
MATERIALS AND METHODS
Animals and surgical preparation
The experiments were performed in full accordance with the National Institutes of Health guidelines on the handling and care of experimental animals and the study was approved by the Animal Welfare Committee of the University of Szeged.
A total of 35 male Sprague–Dawley rats (250–350 g body weight) were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneally) and placed in a supine position on a heating pad. Tracheostomy was performed to facilitate spontaneous breathing, after which the right jugular vein was cannulated with PE50 tubing for administration of Ringer's lactate infusion (10 mL/kg/hr) and to facilitate maintenance of anesthesia with sodium pentobarbital throughout the experiment.
The right common carotid artery was cannulated to measure the mean arterial pressure, which was measured at 30‐min intervals and monitored throughout the investigation. The i.v. administration of AcPepA did not influence the mean arterial pressure of any of the treated animals (data not shown).
Experimental protocol
After confirming cardiovascular stabilization during the 30‐minute recovery from anesthesia, a median laparotomy was performed to carry out the following experimental protocol. The animals were divided into five groups. Rats in group A served as a sham‐operated group (n = 5). Rats in groups B (n = 8) and C (n = 8), were exposed to ischemic insult, in which the superior mesenteric artery was occluded for 45 min with an atraumatic vascular clamp (Induce‐I). Rats in groups D (n = 7) and E (n = 7), were subjected to ischemia followed by reperfusion for 30 min (Induce‐I/R). Rats in groups C and E were given AcPepA (courtesy of Alan Okada, Research Institute for Protein Science, Nagoya, Japan) (4 mg/kg iv. in Ringer's lactate solution) 30 min after initiation of ischemia. The tissue samples were divided into two portions. One was used for histological and immunohistochemical analyses, whereas the other served as materials for proteomic investigation and was stored at −70°C. For histology, samples were fixed with 4% paraformaldehyde and then embedded and processed for further analysis. The fixed tissue was attached to hard backing with staples to ensure the optimal longitudinal orientation of the section.
Evaluation of the degree of injury to the villi
To evaluate the effects of ischemia, I/R and AcPepA treatment on small intestinal villi, the percentage of injured villi was calculated for each animal. The total number of villi in each hematoxylin and eosin‐stained section was counted (20–25 fields at 400× magnification). Each villus was assigned to one of four categories (uninjured, slight, moderate or severe), depending on the degree of damage (length of the villus, infiltration of inflammatory cells, presence of surface erosion and amount of necrotic epithelium in the lumen).
In vivo detection of structural damage
The extent of microvascular and epithelial damage in the terminal ileum was evaluated by fluorescence real‐time laser scanning confocal endomicroscopy (Five1, Ex. 488 nm, Em. 505–585 nm; Optiscan, Melbourne, Victoria, Australia) 30 min after the beginning of reperfusion. The mucosal surface of the terminal ileum was surgically exposed 5 cm proximal to the cecum and laid flat for examination. The microvascular structure was recorded after i.v. administration of 0.3 mL of fluorescein isothiocyanate‐dextran (150 kDa, Sigma‐Aldrich, St. Louis, Missouri, USA, 20 mg mL−1 solution dissolved in physiological saline). Confocal imaging was performed 5 min after dye administration (one scan/image, 1024 × 512 pixels and 475 × 475 µm per image). The villous architecture was examined following topical application of the fluorescent dye acridine orange (Sigma‐Aldrich), surplus dye being flushed away from the mucosal surface of the ileum with physiological saline 2 min before imaging.
Immunohistochemical analysis
CD68 receptor, PCNA, C5L2 and CD204 receptors and HIF‐1α expression were evaluated by IHC of sections of the small intestine. For this IHC study, the following diluted primary antibodies were prepared: PCNA (Clone PC10, 1:500; Dako Japan., Tokyo, Japan), C5L2 (1:100; kindly provided by Masaki Imai, Department of Immunology, Nagoya City University, Nagoya, Japan), CD68 primary antibody (1:100; BMA Biomedicals, Augst, Switzerland), CD204 (1:100; Trans Genic, Kumamoto, Japan), and HIF1‐α (1:100; Thermo Fisher Scientific, Cheshire, UK). The entire IHC investigation was carried out using an automatic IHC machine, Leica Bond‐max (Leica Microsystems, Tokyo, Japan) according to the manufacturer's instructions. For quantitative analysis, immunostained sections were examined under a light microscope, and the numbers of nuclei and cells positive for PCNA, C5L2, CD68, CD204 and HIF1‐α enumerated at a magnification of ×400 for each region of the normal and injured villi, respectively.
Statistical analysis
Statistical analysis of the in vivo data was performed using Kruskal–Wallis and Bonferroni/Dunn multiple comparison tests. Data are presented as means ± SD. Values of P < 0.05 were deemed significant.
RESULTS
Effect of AcPepA on the degree of small intestinal injury
Induce‐I and Induce‐I/R induced various degrees of injury to the small intestine. The villi were sorted into four categories: uninjured (Fig. 1a), slightly (Fig. 1b), moderately (Fig. 1c) and severely injured (Fig. 1d), based on various histological criteria. About 96% of the intestinal villi in the control group were classified as uninjured (Table 1). The degrees of injury to the villi induced by Induce‐I and Induce‐I/R are shown in Table 1. The proportion of severely injured villi in the Induce‐I/R group (76%) was significantly higher than that in the Induce‐I group (6%) and was significantly reduced by AcPepA administration (to 24%; Table 1, Figure 1e).
Figure 1.
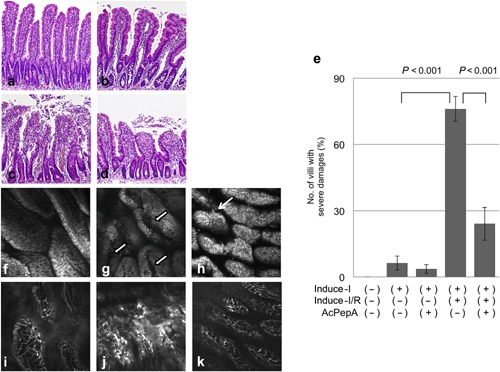
Effect of AcPepA on the degree of small intestinal injury. Small intestinal injury (×600) was classified as (a) normal, (b) slight, (c) moderate and (d) severe. (e) The percentages of severely injured villi in the variously treated groups. In vivo histology images of the mucosal surface of distal rat ileum recorded under fluorescence confocal endomicroscopy (f, g, h) after i.v. administration of FITC‐dextran and (i, j, k) topical administration of acridine orange. (f) Normal epithelium on the surface of the villi of the control group. (g) Longitudinal fissures on the surface of villi (white arrows) are apparent in the Induce‐I/R group. (h) A few fissures on the surface of villi (thin white arrow) were observed in the Induce‐I/R + AcPepA group. (i) Mucosal vasculature was normal in the control group. (j) Severe dye leakage from vessel lumina was observed 30 min after reperfusion in the Induce‐I/R group. (k) Little dye leakage was observed in the Induce‐I/R + AcPepA group.
Table 1.
Degree of damage observed in intestinal villi
| No. of damaged villi (%) | |||||
|---|---|---|---|---|---|
| Intervention | No. of villi examined | Uninjured | Slightly | Moderately | Severely |
| Control | 266 | 96 | 4 | 0 | 0 |
| Induce‐I | 413 | 3 | 25 | 66 | 6 |
| Induce‐I + AcPep | 436 | 2 | 47 | 47 | 4 |
| Induce‐I/R | 289 | 0 | 2 | 22 | 76† |
| Induce‐I/R + AcPep | 417 | 0 | 14 | 62 | 24‡ |
†, P < 0.001 versus Induce‐I/R
‡, P < 0.001 versus Induce‐I
Because Induce‐I/R caused more severe injury to the small intestinal epithelium (76%) than did Induce‐I (6%), the surface and micro‐vessels of the small intestinal villi were further examined by confocal laser scanning endomicroscopy. Acridine orange staining revealed that the surfaces of the villi in the control group were smooth (Fig. 1f). Although longitudinal fissures and epithelial gaps filled with tissue debris were observed in the Induce‐I/R group (Fig. 1g), only a few shed cells and epithelial gaps were observed in the AcPepA administration following Induce‐I/R group (Figure 1h). Similarly, microvessel structures observed in the control group (Fig. 1i) were disorganized and fluorescent dye leakage was recorded in several areas of small intestinal villi (Fig. 1j). The extent of leakage was diminished by AcPepA administration following Induce‐I/R (Fig. 1k). Thus, AcPepA treatment significantly reduced the degree of microvascular damage and preserved the epithelial morphology.
Taken together, these results suggest that Induce‐I/R causes more severe injury to the small intestinal epithelium than does Treat‐I alone, indicating that C5a activation may be involved in the increased damage that can be suppressed by the C5a‐inhibitory peptide AcPepA.
Effects of AcPepA administration on proliferative changes in the epithelium with Induce‐I/R
Because injuries to the small intestine change the resulted in a distributed balance between proliferation and apoptosis in the epithelium, the extent of proliferation of epithelial cells in the villi (except in strongly proliferative lesions) was examined to enable detection of small differences in the proliferation index (Fig. 2a). In normal villi, the PCNA index of the epithelium was 0.6–0.8% regardless of the treatment (Fig. 2b, c). In the injured villi, PCNA indices in the Induce‐I group were similar to those observed in the Induce‐I + AcPepA as well as in the Induce‐I/R group (Fig. 2c). Administering AcPepA following Induce‐I/R significantly increased the PCNA index compared with Induce‐I/R without AcPepA administration (Fig. 2d, f). These results indicate that C5a inhibition by AcPepA alleviates I/R injury and increases cell proliferation in the epithelium.
Figure 2.
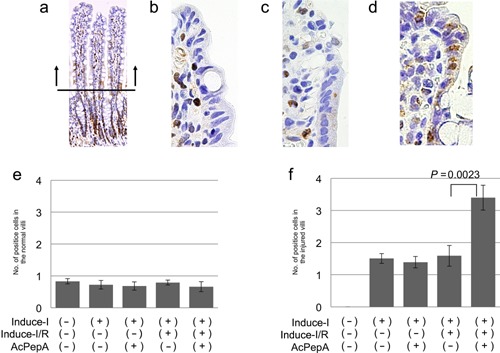
Effect of AcPepA on proliferation of small intestinal epithelium. Recovery of the epithelium was evaluated by cell proliferation by counting PCNA‐positive cells (PCNA index). (a) Positive epithelial cells were counted in the villi to avoid the strong proliferative region (below the horizontal bar in [a]) to facilitate detection of small differences in the cell proliferation index. Epithelial cells of injured small intestinal villi were visualized by PCNA staining of tissue from rats in the (b) Induce‐I alone, (c) Induce‐I/R and (d) Induce‐I/R + AcPepA groups. (e) PCNA indices (number of positive cells in each villus) of the epithelium of normal and (f) injured villi are summarized.
Induction of C5L2‐positive PMNs in the villi
The localization of C5L2, a C5a receptor, was analyzed to identify cells in which C5a/C5L2 signaling is possibly transduced. Circulating inflammatory cells were often observed in dilated vessels located in the centers of villi, this phenomenon being associated with an inflammatory response (square in Fig. 3a). C5L2‐positive cells were observed among PMNs in the vessels (Fig. 3b). A few positive cells were observed outside the vessels such as in the erosion front of the injured villi (data not shown). C5L2+PMNs were also observed in the dilated vessels of moderately or severely injured villi (Fig. 3c, d). The average number of C5L2+PMNs was less than one in both the control and uninjured villi groups (Fig. 3E), the number of C5L2+PMNs in the injured villi of the Induce‐I/R group was significantly higher than in the Induce‐I group. Additionally, C5L2+PMNs were remarkably reduced by AcPepA administration in the Induce‐I/R group (Fig. 3f). Because of our observation of a drastic increase in the number of C5L2‐positive cells in vessels of the villi of the Induce‐I/R group, C5a concentrations in the sera of the control, Induce‐I/R and Induce‐I/R + AcPepA groups were examined next (Fig. 3g). The serum concentration of C5a was 12 ng/mL in the control group, whereas in the Induce‐I and Induce‐I/R groups it was undetectable (Fig. 3g). An additional treatment with AcPepA restored the C5a concentration to >12 ng/mL, which is equivalent to that of the control group (Fig. 3g). These results suggest that C5a stimulates C5L2+PMNs, causing a release of cytokines which exacerbate inflammation; thus, C5L2+PMNs may contribute indirectly to I/R injury. Although Induce‐I alone completely exhausted C5a in serum, Induce‐I/R increased the C5a concentration up to 5 ng/mL, this possibly being attributable to further generation of C5a by reperfusion (R) following ischemia (I). The increased concentration of C5a (over 12 ng/mL) in rats treated with AcPepA following Induce‐I/R may indicate protection of C5 by AcPepA from catabolism by an inhibitor of C5a, namely carboxypeptidase R 28, 29, which is also known as thrombin activatable fibrinolysis inhibitor.
Figure 3.
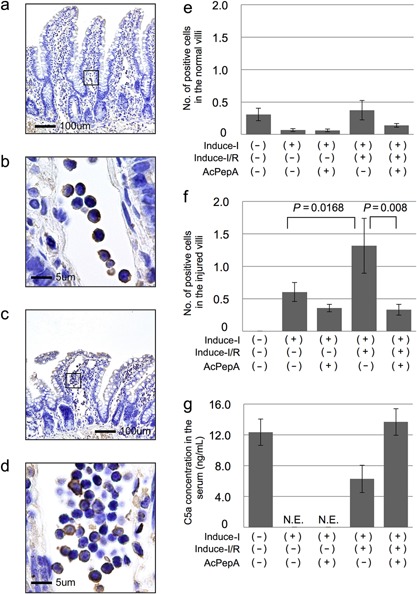
Induction of C5L2‐positive cells in villi. (a) Circulating inflammatory cells are often visible in dilated vessels located in the centers of the villi, this being associated with an inflammatory response (Square in 3a). (b) C5L2‐positive cells among inflammatory cells in vessels. (c, d), C5L2‐positive cells are also present in dilated vessels in severely injured villi. (e, f) The number of C5L2‐positive cells in (e) normal and in (f) injured villi. (g) C5a serum concentrations were significantly lower in the Induce‐I/R group than in the Induce‐I/R + AcPepA group.
Induction of CD68‐positive macrophages in the villi
Next, evidence of macrophage induction in the control and injured villi was examined. In contrast with C5L2+PMNs, most CD68+MACs were observed in stromal lesions outside the vessels in slightly injured villi (arrowheads in Figure 4a). CD68+MACs were observed in the erosion fronts of the injured villi (arrowheads in Fig. 4b). Although many macrophages were observed with Induce‐I/R (Fig. 4b), fewer were observed with Induce‐I/R + AcPepA (Figure 4c). Regardless of the form of treatment, about one macrophage was observed in each normal villus (Fig. 4d). In the injured villi, Induce‐I/R significantly increased the number of macrophages, this effect being significantly suppressed by subsequent AcPepA administration (Fig. 4e). These results indicate that CD68+MACs are induced in the small intestine in association with I/R and play a direct role in I/R injury in a manner that is dependent on C5a activation.
Figure 4.
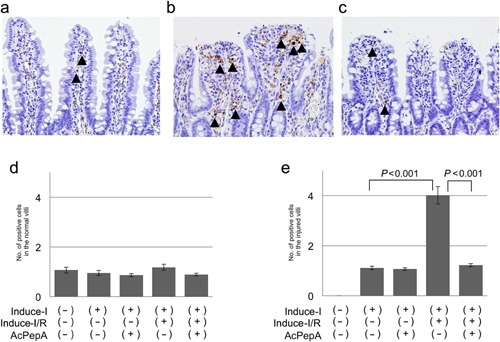
Induction of macrophages in villi. (a) CD68‐positive macrophages are present in the stromal region outside vessels in the villi (arrowheads). (b) CD68‐positive macrophages are present in the erosion fronts of injured villi of rats in which I/R was induced (arrowheads). (c) Fewer macrophages are present in the Induce‐I/R + AcPepA group. Numbers of the macrophages in (d) normal villi and (e) injured villi.
Induction of CD204‐positive macrophages in villi
CD204‐positive macrophages are known to modulate inflammation by producing various cytokines; accordingly, their induction in villi was investigated. These cells were observed not only in stromal lesions outside vessels in slightly injured villi (arrowheads in Fig. 5a), but also infiltrating the erosion fronts of severely injured villi (Fig. 5b). Fewer CD204+MACs were observed in the Induce‐I/R + AcPepA than in the Induce‐I/R group (Fig. 5c), indicating that inhibition of C5a by AcPepA suppresses activation of macrophages. A mean of approximately one CD204+MAC was present in each control and uninjured villus (Fig. 5d). The average number of CD204+MACs was significantly larger in the uninjured villi of the Induce‐I/R group than in those of the controls; additionally, AcPepA significantly decreased the number of CD204+MACs (Fig. 5d). In the injured villi, Induce‐I/R induced significantly more numerous CD204+MACs than in the Induce‐I alone group (Fig. 5e). There were significantly fewer positive cells in the AcPepA with Induce‐I/R than in the Induce‐I/R group alone; thus, again AcPepA with Induce‐I tended to decrease their numbers (Fig. 5e). These results indicate that I/R induces significant CD204+MAC activation in injured villi and suggest that M2‐type macrophages may contribute to I/R damage.
Figure 5.
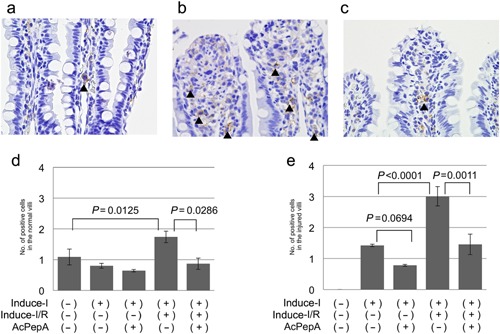
Induction of CD204‐positive macrophages in villi. (a) CD204‐positive macrophages are present in the stromal region outside vessels in slightly injured villi (arrowheads). (b) CD204‐positive macrophages are present in the erosion fronts of severely injured villi. (c) Fewer CD204‐positive macrophages are present in the Induce‐I/R + AcPepA group than in the Induce‐I/R group. The number of CD204‐positive macrophages in (d) normal and (e) injured villi.
Induction of HIF‐1α‐positive cells
Hypoxia‐induced factor 1‐alpha is up‐regulated in association with ischemic conditions. Therefore, whether HIF1‐α‐positive cells are involved in I/R‐induced changes in the villi was investigated. HIF1‐α‐positive cells were detected by IHC both in stromal lesions outside vessels in slightly injured villi (Fig. 6a) and in moderately injured villi (Fig. 6b). Many HIF1‐α‐positive cells were observed in the erosion fronts of severely injured villi (Fig. 6c). The average number of HIF1‐α‐positive cells was similar to that of CD204+MACs in control, uninjured (Fig. 6d) and injured villi (Fig. 6e), suggesting that CD204+MACs produce HIF1‐α.
Figure 6.
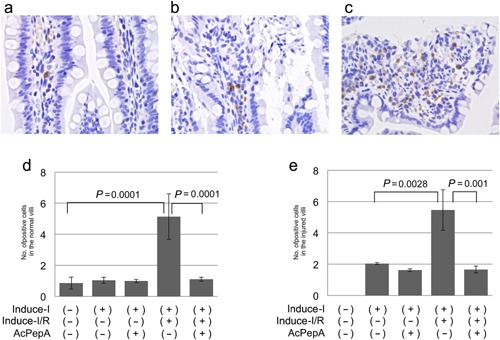
Induction of HIF1‐positive cells. (a, b) HIF1‐α‐positive cells are present in the stromal regions outside vessels in both (a) slightly injured and (b) moderately injured villi. (c) Many HIF1‐α‐positive cells are present in the erosion fronts of severely injured villi. (d, e) Changes in numbers of HIF1‐α‐positive cells in (d) normal and (e) injured villi.
DISCUSSION
Acute mesenteric ischemia is a serious multifactorial condition that develops from an occlusion of a main artery or vein. Interruption to the intestinal blood flow can lead to macro‐ and micro‐circulatory failure of abrupt onset, frequently resulting in bowel necrosis 4. In this study, we designed an experimental setup for inducing severe injuries based on an AMI model that we had used previously 30, 31, 32. Similarly to in ileal specimens from AMI patients 33, 34, in our rat model we observed patchy evidence of damage to the villous mucosa, including detachment of cells, particularly in the villi's apical regions, giving rise to degraded mature epithelial cells in their lumens. We therefore evaluated the effects of I/R and the consequences of using a C5a antagonist to treat epithelial injury and examined regeneration using a clinically relevant experimental AMI protocol.
Acute mesenteric ischemia induces not only structural damage and circulatory deficiencies, but also leads to a great abundance of inflammatory mediators that can result in multi‐organ failure 35. The complement system has been demonstrated to be a crucial mediator of I/R injury 36, 37. In the present study, we applied a newly synthesized C5a antagonist peptide, AcPepA 25, 27 and found that it significantly decreased the degree of I/R injury in our AMI model. Thus, we confirmed that C5a is involved in I/R injury in this model.
Because the C5a antagonist peptide AcPepA significantly decreased the degree of I/R damage in this study, we postulated that both structural damage to the villi and repair of their intestinal epithelial cells would be induced by the inflammatory cytokines released from cells stimulated with C5a generated following complement activation. We therefore examined the cell proliferation activity of intestinal epithelial cells in normal and injured villi by counting the number of proliferating cells visualized by PCNA staining and found that AcPepA significantly increased proliferation of intestinal epithelial cells in injured villi. These results indicate that C5a restriction by AcPepA suppresses cytokine production by inflammatory cells, resulting in suppression of inflammation; any remaining C5a may have directly stimulated the growth of epithelial cells.
We have previously determined the localization of the C5a receptor C5L2 38 to investigate how C5a/C5L2‐mediated signaling modulates the inflammatory responses that lead to I/R injury 39, 40. It has been demonstrated in vitro that C5L2 is expressed in neutrophils, macrophages and fibroblasts 41. Additionally, C5a has been shown to exert a chemotactic effect on neutrophils 42, releasing superoxide anions from them. Thus, C5a is believed to be largely responsible for exacerbating PMN‐mediated I/R tissue injuries. In the present study, C5a serum concentrations were decreased although C5L2+ PMNs were significantly more numerous in the Induce‐I/R group, indicating that serum C5a is consumed because of greater binding to C5L2. It is also possible that the generated C5a is inactivated by carboxypeptidase R 28, 29, also known as thrombin activatable fibrinolysis inhibitor, which removes the carboxy‐terminal arginine of C5a causing inactivation of the molecule, resulting in C5a‐desArg. However, C5L2 + PMNs were observed mainly in vessels of the villi, a location somewhat distant from the site of epithelial injury. Thus, these results suggest that C5a/C5L signaling has an indirect influence on I/R damage. On the other hand, C5a has been shown to enhance release of pro‐inflammatory cytokines from activated macrophages and monocytes 22, 43. Previous studies have suggested that alveolar macrophage activation is a key initiation signal for acute lung I/R injury 44, 45, whereas studies of complement inhibition in mice have suggested that intestinal I/R injury is unaffected by neutrophil depletion 36, 46. We found that CD68+MACs were significantly more numerous after I/R and decreased in number after subsequent AcPepA treatment. In addition, we mainly observed CD68+MACs near the site of injury. Taken together, even though there is some controversy about the direct contribution of neutrophils, C5a has an effect on PMNs and the subsequent activation of macrophages plays an important role in small intestinal I/R injury.
Several studies have demonstrated that M2 type macrophages produce cytokines such as TNF‐α, IL‐6 and IL‐12 in response to inflammatory stimuli 47, 48, 49; M2 macrophages are considered to be important effectors of fatal cellular mechanisms during cancer‐related inflammation. CD204, a class A scavenger receptor, is a multifunctional molecule that is expressed in M2 macrophages. We postulated that CD204+MACs, which produce HIF1‐α, are involved in I/R injury. In the present study, I/R treatment significantly increased the number of CD204+MACs in both injured and normal villi. Again, this increase was suppressed by AcPepA treatment, which suggests that CD204+MACs are indeed activated in response to I/R.
Hypoxia‐induced factor 1‐alpha is a heterodimeric transcription factor composed of a constitutively expressed alpha‐subunit 50 and is important for promoting a variety of cellular responses to hypoxia 51. Induce‐I/R would be expected to activate HIF1‐α; however, its role in I/R injury is controversial. The protective role of HIF1‐α in I/R injury has been demonstrated in proximal tubule cells in the kidney 52, 53, 54 as well as in astrocytes 55; additionally, HIF1‐ α expression is known to be essential for the development of I/R injury in the gut, especially with prolonged ischemia 56, 57. In the present study, I/R significantly increased the number of HIF1‐α‐positive cells in both injured and uninjured villi. Induction of CD204+MACs correlated closely with the number of HIF1‐α‐positive cells and both types of cells were suppressed by AcPepA treatment. These results suggest that, on activation with treatment I/R, C5a directly or indirectly induces activation of CD204+MACs in the intestinal villi; these cells then secrete HIF1‐α.
The mechanisms for I/R injury involve cellular/molecular processes that begin with hypoxia and hypoxia‐induced C5a formation; concerted communication between C5a, MACs and PMNs is necessary for the mediation of I/R damage in the villi. We propose that activated leukocytes flowing into the villus microcirculation spread signals further towards resident macrophages in the lamina propria and that this leads to stimulation of macrophage‐derived HIF‐1α production at the injury site. According to the outlined scenario, the greatest structural destruction would be expected to occur when C5a, activated PMNs and MACs are all present in the reperfused villi. Furthermore, this cellular casting might also explain the patchy pattern of mucosal damage; the damage is less severe if one or more of the players is not present or inactive.
Several studies have used blocking antibodies or inhibitors to target C5 or C5a. In I/R models of rat intestine, both C5 and C5a blockade result in protection from I/R injury 16, 58, 59. In myocardial infarcts in rats, antibodies to C5 significantly inhibit necrosis, cell apoptosis and neutrophil infiltration 60. In pigs, use of antibodies directed against C5a results in reduced myocardial injury and reduced coronary endothelial dysfunction after I/R 61, 62. AcPepA, which we generated as an inhibitory C5a peptide, is effective in reducing the incidence of lethal shock in rats 25 and mice 27, as well as sepsis induced by lethal doses of bacterial LPS in cynomolgus monkeys 26. In the present study, we demonstrated that AcPepA suppressed I/R tissue injury in our AMI rat model. Combined together, targeting C5/C5a may represent the best strategy for inhibiting complement‐mediated I/R‐induced tissue injury and may therefore prove useful for therapeutic interventions in clinical settings. These experimental studies should lead to clinical trials in patients with AMI, lethal shock and sepsis.
In summary, we examined the mechanisms of C5a‐induced I/R injury using our clinically relevant rat AMI model. The proposed mechanism includes the effect of C5a on PMNs and the subsequent induction of CD204+MACs, which secrete HIF1‐α, in the intestinal villi. Overall, targeting of C5/C5a is a potential strategy for inhibiting PMNs and reducing macrophage‐ mediated I/R injury and may therefore be a good option for future therapeutic interventions.
DISCLOSURE
The authors have no conflict of interest to declare.
ACKNOWLEDGEMENTS
The authors are grateful to Nikolett Beretka, Csilla Mester, Anita Németh, Károly Tóth and Kálmán Vas for their skillful assistance. The study was supported by Hungarian Science Research Fund (OTKA) grants K104656 and Social Renewal Operational Programme (TÁMOP)‐4.2.2/A‐11/1/KONV‐2012‐0035 and a grant in aid for JST Research Grant A‐STEP (AS2316910G) and Scientific Support Programs for Cancer Research Grant‐in‐Aid for Scientific Research on Innovative Areas Ministry of Education, Culture, Sports, Science and Technology in Japan.
REFERENCES
- 1. Wilson C., Gupta R., Gilmour, D.G. , Imrie, C.W. ( 1987) Acute superior mesenteric ischaemia. Br J Surg 74: 279–81. [DOI] [PubMed] [Google Scholar]
- 2. Oldenburg W.A., Lau L.L., Rodenberg T.J., Edmonds H.J., Burger, C.D. ( 2004) Acute mesenteric ischemia: A clinical review. Arch Intern Med 164: 1054–62. [DOI] [PubMed] [Google Scholar]
- 3. Benjamin E., Oropello, J.M. , Iberti, T.J. ( 1993) Acute mesenteric ischemia: pathophysiology, diagnosis, and treatment. Dis Mon 39: 131–210. [DOI] [PubMed] [Google Scholar]
- 4. Berland T., Oldenburg W.A. ( 2008) Acute mesenteric ischemia. Curr Treat Options Gastroenterol 11: 3–10. [DOI] [PubMed] [Google Scholar]
- 5. Rijke R.P., Van Der Meer‐Fieggen W., Galjaard H. ( 1974) Effect of villus length on cell proliferation and migration in small intestinal epithelium. Cell Tissue Kinet 7: 577–86. [DOI] [PubMed] [Google Scholar]
- 6. Yasue N., Guth P.H. ( 1988) Role of exogenous acid and retransfusion in hemorrhagic shock‐induced gastric lesions in the rat. Gastroenterology 94: 1135–43. [DOI] [PubMed] [Google Scholar]
- 7. Noda T., Iwakiri R., Fujimoto K., Matsuo S., Aw, T.Y. ( 1998) Programmed cell death induced by ischemia‐reperfusion in rat intestinal mucosa. Am J Physiol 274: G270–6. [DOI] [PubMed] [Google Scholar]
- 8. Ikeda H., Suzuki Y., Suzuki M., Koike M., Tamura J., Tong J., Nomura M., Itoh G. ( 1998) Apoptosis is a major mode of cell death caused by ischaemia and ischaemia/reperfusion injury to the rat intestinal epithelium. Gut 42: 530–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carter M.B., Wilson M.A., Wead W.B., Garrison R.N. ( 1996) Platelet‐activating factor mediates pulmonary macromolecular leak following intestinal ischemia‐reperfusion. J Surg Res 60: 403–8. [DOI] [PubMed] [Google Scholar]
- 10. Yao Y.M., Sheng Z.Y., Yu Y., Tian H.M., Wang Y.P., Lu L.R., Xu S.H. ( 1995) The potential etiologic role of tumor necrosis factor in mediating multiple organ dysfunction in rats following intestinal ischemia‐reperfusion injury. Resuscitation 29: 157–68. [DOI] [PubMed] [Google Scholar]
- 11. Tamion F., Richard V., Lyoumi S., Daveau M., Bonmarchand G., Leroy J., Thuillez C., Lebreton J.P. ( 1997) Gut ischemia and mesenteric synthesis of inflammatory cytokines after hemorrhagic or endotoxic shock. Am J Physiol 273: G314–21. [DOI] [PubMed] [Google Scholar]
- 12. Parks D.A., Bulkley G.B., Granger D.N., Hamilton S.R., McCord J.M. ( 1982) Ischemic injury in the cat small intestine: Role of superoxide radicals. Gastroenterology 82: 9–15. [PubMed] [Google Scholar]
- 13. Riedemann N.C., Ward P.A. ( 2003) Complement in ischemia reperfusion injury. Am J Pathol 162: 363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heller T., Hennecke M., Baumann U., Gessner J.E., Zu Vilsendorf A.M., Baensch M., Boulay F., Kola A., Klos A., Bautsch W., Kohl J. ( 1999) Selection of a C5a receptor antagonist from phage libraries attenuating the inflammatory response in immune complex disease and ischemia/reperfusion injury. J Immunol 163: 985–94. [PubMed] [Google Scholar]
- 15. Kimura T., Andoh A., Fujiyama Y., Saotome T., Bamba T. ( 1998) A blockade of complement activation prevents rapid intestinal ischaemia‐reperfusion injury by modulating mucosal mast cell degranulation in rats. Clin Exp Immunol 111: 484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wada K., Montalto M.C., Stahl G.L. ( 2001) Inhibition of complement C5 reduces local and remote organ injury after intestinal ischemia/reperfusion in the rat. Gastroenterology 120: 126–33. [DOI] [PubMed] [Google Scholar]
- 17. Van Beek J., Bernaudin M., Petit E., Gasque P., Nouvelot, A. , Mackenzie E.T., Fontaine M. ( 2000) Expression of receptors for complement anaphylatoxins C3a and C5a following permanent focal cerebral ischemia in the mouse. Exp Neurol 161: 373–82. [DOI] [PubMed] [Google Scholar]
- 18. Bremer C., Bradford B.U., Hunt K.J., Knecht K.T., Connor H.D., Mason R.P., Thurman R.G. ( 1994) Role of Kupffer cells in the pathogenesis of hepatic reperfusion injury. Am J Physiol 267: G630–6. [DOI] [PubMed] [Google Scholar]
- 19. Henrion J. ( 2000) Ischemia/reperfusion injury of the liver: pathophysiologic hypotheses and potential relevance to human hypoxic hepatitis. Acta Gastroenterol Belg 63: 336–47. [PubMed] [Google Scholar]
- 20. Wanner G.A., Ertel W., Muller P., Hofer Y., Leiderer R., Menger M.D., Messmer K. ( 1996) Liver ischemia and reperfusion induces a systemic inflammatory response through Kupffer cell activation. Shock 5: 34–40. [DOI] [PubMed] [Google Scholar]
- 21. Cavaillon J.M., Fitting C., Haeffner‐Cavaillon N. ( 1990) Recombinant C5a enhances interleukin 1 and tumor necrosis factor release by lipopolysaccharide‐stimulated monocytes and macrophages. Eur J Immunol 20: 253–7. [DOI] [PubMed] [Google Scholar]
- 22. Haynes D.R., Harkin D.G., Bignold L.P., Hutchens M.J., Taylor S.M., Fairlie D.P. ( 2000) Inhibition of C5a‐induced neutrophil chemotaxis and macrophage cytokine production in vitro by a new C5a receptor antagonist. Biochem Pharmacol 60: 729–33. [DOI] [PubMed] [Google Scholar]
- 23. Tyagi S., Klickstein L.B., Nicholson‐Weller A. ( 2000) C5a‐stimulated human neutrophils use a subset of beta2 integrins to support the adhesion‐dependent phase of superoxide production. J Leukoc Biol 68: 679–86. [PubMed] [Google Scholar]
- 24. Okada N., Imai M., Okada A., Ono F., Okada H. ( 2011) HMGB1 release by C5a anaphylatoxin is an effective target for sepsis treatment. natureprecedings hdl:10101/npre.2011.5727.1. [Google Scholar]
- 25. Fujita E., Farkas I., Campbell W., Baranyi L., Okada H., Okada N. ( 2004) Inactivation of C5a anaphylatoxin by a peptide that is complementary to a region of C5a. J Immunol 172: 6382–7. [DOI] [PubMed] [Google Scholar]
- 26. Okada H., Imai M., Ono F., Okada A., Tada T., Mizue Y., Terao K., Okada N. ( 2011) Novel complementary peptides to target molecules. Anticancer Res 31: 2511–6. [PubMed] [Google Scholar]
- 27. Okada N., Asai, S. , Hotta A., Miura N., Ohno N., Farkas I., Hau, L. , Okada H. ( 2007) Increased inhibitory capacity of an anti‐C5a complementary peptide following acetylation of N‐terminal alanine. Microbiol Immunol 51: 439–43. [DOI] [PubMed] [Google Scholar]
- 28. Campbell W., Okada H. ( 1989) An arginine specific carboxypeptidase generated in blood during coagulation or inflammation which is unrelated to carboxypeptidase N or its subunits. Biochem Biophys Res Commun 162: 933–9. [DOI] [PubMed] [Google Scholar]
- 29. Campbell W.D., Lazoura E., Okada N., Okada H. ( 2002) Inactivation of C3a and C5a octapeptides by carboxypeptidase R and carboxypeptidase N . Microbiol Immunol 46: 131–4. [DOI] [PubMed] [Google Scholar]
- 30. Szabo A., Vollmar B., Boros M., Menger, M.D. ( 2008) In vivo fluorescence microscopic imaging for dynamic quantitative assessment of intestinal mucosa permeability in mice. J Surg Res 145: 179–85. [DOI] [PubMed] [Google Scholar]
- 31. Boros M., Ghyczy M., Erces D., Varga, G. , Tokes T., Kupai, K. , Torday C., Kaszaki J. ( 2012) The anti‐inflammatory effects of methane. Crit Care Med 40: 1269–78. [DOI] [PubMed] [Google Scholar]
- 32. Adamicza A., Kaszaki J., Boros M., Hantos Z. ( 2012) Pulmonary mechanical responses to intestinal ischaemia‐reperfusion and endotoxin preconditioning. Acta Physiol Hung 99: 289–301. [DOI] [PubMed] [Google Scholar]
- 33. Chiu C.J., Mcardle A.H., Brown R., Scott H.J., Gurd F.N. ( 1970) Intestinal mucosal lesion in low‐flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg 101: 478–83. [DOI] [PubMed] [Google Scholar]
- 34. Thuijls G., Van Wijck K., Grootjans J., Derikx J.P., Van Bijnen A.A., Heineman E., Dejong C.H., Buurman W.A., Poeze M. ( 2011) Early diagnosis of intestinal ischemia using urinary and plasma fatty acid binding proteins. Ann Surg 253: 303–8. [DOI] [PubMed] [Google Scholar]
- 35. Roumen R.M., Redl H., Schlag G., Zilow G., Sandtner W., Koller W., Hendriks T., Goris R.J. ( 1995) Inflammatory mediators in relation to the development of multiple organ failure in patients after severe blunt trauma. Crit Care Med 23: 474–80. [DOI] [PubMed] [Google Scholar]
- 36. Austen W.G., Jr. , Kyriakides C., Favuzza J., Wang Y., Kobzik L., Moore F.D., Jr. , Hechtman H.B. ( 1999) Intestinal ischemia‐reperfusion injury is mediated by the membrane attack complex. Surgery 126: 343–8. [PubMed] [Google Scholar]
- 37. De Vries B., Kohl J., Leclercq W.K., Wolfs T.G., Van Bijnen A.A., Heeringa P., Buurman W.A. ( 2003) Complement factor C5a mediates renal ischemia‐reperfusion injury independent from neutrophils. J Immunol 170: 3883–9. [DOI] [PubMed] [Google Scholar]
- 38. Bamberg C.E., Mackay C.R., Lee H., Zahra D., Jackson J., Lim Y.S., Whitfeld P.L., Craig S., Corsini E., Lu B., Gerard C., Gerard N.P. ( 2010) The C5a receptor (C5aR) C5L2 is a modulator of C5aR‐mediated signal transduction. J Biol Chem 285: 7633–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hawlisch H., Belkaid Y., Baelder R., Hildeman D., Gerard C., Kohl J. ( 2005) C5a negatively regulates toll‐like receptor 4‐induced immune responses. Immunity 22: 415–26. [DOI] [PubMed] [Google Scholar]
- 40. Hopken U.E., Lu B., Gerard N.P., Gerard C. ( 1996) The C5a chemoattractant receptor mediates mucosal defence to infection. Nature 383: 86–9. [DOI] [PubMed] [Google Scholar]
- 41. Chen N.J., Mirtsos C., Suh D., Lu Y.C., Lin W.J., Mckerlie C., Lee T., Baribault H., Tian H., Yeh W.C. ( 2007) C5L2 is critical for the biological activities of the anaphylatoxins C5a and C3a. Nature 446: 203–7. [DOI] [PubMed] [Google Scholar]
- 42. Shin H.S., Snyderman R., Friedman E., Mellors A., Mayer M.M. ( 1968) Chemotactic and anaphylatoxic fragment cleaved from the fifth component of guinea pig complement. Science 162: 361–3. [DOI] [PubMed] [Google Scholar]
- 43. Okusawa S., Yancey K.B., Van Der Meer J.W., Endres S., Lonnemann G., Hefter K., Frank M.M., Burke J.F., Dinarello C.A., Gelfand J.A. ( 1988) C5a stimulates secretion of tumor necrosis factor from human mononuclear cells in vitro. Comparison with secretion of interleukin 1 beta and interleukin 1 alpha. J Exp Med 168: 443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Amaral F.A., Fagundes C.T., Guabiraba R., Vieira A.T., Souza A.L., Russo R.C., Soares M.P., Teixeira M.M., Souza D.G. ( 2007) The role of macrophage migration inhibitory factor in the cascade of events leading to reperfusion‐induced inflammatory injury and lethality. Am J Pathol 171: 1887–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhao M., Fernandez L.G., Doctor A., Sharma A.K., Zarbock A., Tribble C.G., Kron I.L., Laubach V.E. ( 2006) Alveolar macrophage activation is a key initiation signal for acute lung ischemia‐reperfusion injury. Am J Physiol Lung Cell Mol Physiol 291: L1018–26. [DOI] [PubMed] [Google Scholar]
- 46. Rehrig S., Fleming S.D., Anderson J., Guthridge J.M., Rakstang J., Mcqueen C.E., Holers V.M., Tsokos G.C., Shea‐Donohue T. ( 2001) Complement inhibitor, complement receptor 1‐related gene/protein y‐Ig attenuates intestinal damage after the onset of mesenteric ischemia/reperfusion injury in mice. J Immunol 167: 5921–7. [DOI] [PubMed] [Google Scholar]
- 47. Haworth R., Platt N., Keshav S., Hughes D., Darley E., Suzuki H., Kurihara Y., Kodama T., Gordon S. ( 1997) The macrophage scavenger receptor type A is expressed by activated macrophages and protects the host against lethal endotoxic shock. J Exp Med 186: 1431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Beamer C.A., Holian A. ( 2005) Scavenger receptor class A type I/II (CD204) null mice fail to develop fibrosis following silica exposure. Am J Physiol Lung Cell Mol Physiol 289: L186–95. [DOI] [PubMed] [Google Scholar]
- 49. Coller S.P., Paulnock D.M. ( 2001) Signaling pathways initiated in macrophages after engagement of type A scavenger receptors. J Leukoc Biol 70: 142–8. [PubMed] [Google Scholar]
- 50. Semenza G.L. ( 2001) HIF‐1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol 13: 167–71. [DOI] [PubMed] [Google Scholar]
- 51. Weidemann A., Johnson R.S. ( 2008) Biology of HIF‐1alpha. Cell Death Differ 15: 621–7. [DOI] [PubMed] [Google Scholar]
- 52. Bernhardt W.M., Campean V., Kany S., Jurgensen J.S., Weidemann A., Warnecke C., Arend M., Klaus S., Gunzler V., Amann K., Willam C., Wiesener M.S., Eckardt K.U. ( 2006) Preconditional activation of hypoxia‐inducible factors ameliorates ischemic acute renal failure. J Am Soc Nephrol 17: 1970–8. [DOI] [PubMed] [Google Scholar]
- 53. Hill P., Shukla D., Tran M.G., Aragones J., Cook H.T., Carmeliet P., Maxwell P.H. ( 2008) Inhibition of hypoxia inducible factor hydroxylases protects against renal ischemia‐reperfusion injury. J Am Soc Nephrol 19: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Manotham K., Tanaka T., Ohse T., Kojima I., Miyata T., Inagi R., Tanaka H., Sassa R., Fujita T., Nangaku M. ( 2005) A biologic role of HIF‐1 in the renal medulla. Kidney Int 67: 1428–39. [DOI] [PubMed] [Google Scholar]
- 55. Du F., Zhu L., Qian Z.M., Wu X.M., Yung W.H., Ke Y. ( 2010) Hyperthermic preconditioning protects astrocytes from ischemia/reperfusion injury by up‐regulation of HIF‐1 alpha expression and binding activity. Biochim Biophys Acta 1802: 1048–53. [DOI] [PubMed] [Google Scholar]
- 56. Sutton T.A., Wilkinson J., Mang H.E., Knipe N.L., Plotkin Z., Hosein M., Zak K., Wittenborn J., Dagher P.C. ( 2008) p53 regulates renal expression of HIF‐1{alpha} and pVHL under physiological conditions and after ischemia‐reperfusion injury. Am J Physiol Renal Physiol 295: F1666–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Feinman R., Deitch E.A., Watkins A.C., Abungu B., Colorado I., Kannan K.B., Sheth S.U., Caputo F.J., Lu Q., Ramanathan M., Attan S., Badami C.D., Doucet D., Barlos D., Bosch‐Marce M., Semenza G.L., Xu D.Z. ( 2010) HIF‐1 mediates pathogenic inflammatory responses to intestinal ischemia‐reperfusion injury. Am J Physiol Gastrointest Liver Physiol 299: G833–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Arumugam T.V., Shiels I.A., Woodruff T.M., Reid R.C., Fairlie D.P., Taylor S.M. ( 2002) Protective effect of a new C5a receptor antagonist against ischemia‐reperfusion injury in the rat small intestine. J Surg Res 103: 260–7. [DOI] [PubMed] [Google Scholar]
- 59. Hill J., Lindsay T.F., Ortiz F., Yeh C.G., Hechtman H.B., Moore F.D., Jr. ( 1992) Soluble complement receptor type 1 ameliorates the local and remote organ injury after intestinal ischemia‐reperfusion in the rat. J Immunol 149: 1723–8. [PubMed] [Google Scholar]
- 60. Vakeva A.P., Agah A., Rollins S.A., Matis L.A., Li L., Stahl G.L. ( 1998) Myocardial infarction and apoptosis after myocardial ischemia and reperfusion: Role of the terminal complement components and inhibition by anti‐C5 therapy. Circulation 97: 2259–67. [DOI] [PubMed] [Google Scholar]
- 61. Tofukuji M., Stahl G.L., Agah A., Metais C., Simons M., Sellke F.W. ( 1998) Anti‐C5a monoclonal antibody reduces cardiopulmonary bypass and cardioplegia‐induced coronary endothelial dysfunction. J Thorac Cardiovasc Surg 116: 1060–8. [DOI] [PubMed] [Google Scholar]
- 62. Amsterdam E.A., Stahl G.L., Pan H.L., Rendig S.V., Fletcher M.P., Longhurst J.C. ( 1995) Limitation of reperfusion injury by a monoclonal antibody to C5a during myocardial infarction in pigs. Am J Physiol 268: H448–57. [DOI] [PubMed] [Google Scholar]


