Abstract
Background
Glycogen synthase kinase-3β (GSK-3β) inhibitor is a serine/threonine kinase with an inhibitory role in glycogen synthesis, which is essential in inflammatory and immunological diseases. The purpose of our study was to determine if TDZD-8 can alleviate collagen II-induced rheumatoid arthritis in rats.
Material/Methods
Twenty collagen II-induced rheumatoid arthritis rats were treated with selective GSK-3β inhibitor. The effects of GSK-3β inhibition on collagen II-induced rheumatoid arthritis in the rats were evaluated by paw edema, histological examination of arthritic synovium, radiographic examination of knee joint, and the level of inflammation mediators such as prostaglandin E2, 5-hydroxytryptamin, and histamine. The level of cytokines such as IL-6, IL-12, IL-10, and TNF-α, was examined by Elisa.
Results
GSK-3β inhibitor significantly reduced the development of rheumatoid arthritis in rats. The levels of inflammation mediators such as prostaglandin E2, 5-hydroxytryptamin, and histamine were decreased in the TDZD-8 group. Serum levels of IL-6, IL-12, and TNF-α were significantly reduced in the TDZD-8 group compared with the RA group.
Conclusions
Treatment with GSK-3β inhibitor suppressed inflammatory response in RA rats. These findings suggest that the inhibition of GSK-3β can be an effective treatment for RA.
MeSH Keywords: Arthritis, Experimental; Arthritis, Juvenile; Glycogen Synthase Kinase 3
Background
Rheumatoid arthritis (RA) is a chronic inflammatory disease in which the autoimmune reaction affects synovial joints [1]. Although the pathogenesis of rheumatoid arthritis is unknown, it is recognized that the autoimmunity of RA patients is active and inflammation chemokines are abnormally stimulated [2]. Therefore, RA treatment may focus on reducing immune and inflammatory responses [3].
Glycogen synthase kinase-3β (GSK-3β) inhibitor [4] is a serine/threonine kinase with an inhibitory role in glycogen synthesis, which is important in cell proliferation, apoptosis, differentiation, and many other cellular responses. GSK is a protein kinase involved in modulating inflammatory cytokines such as IL-6, IL-1β, and TNF-α [5]. GSK-3β inhibitors such as TDZD-8, SB216763, SB415286 can protect cells from inflammatory response [6]. The inflammation observed in RA is strongly associated with various pro-inflammatory mediators and transcription factors, which have been shown to be associated with GSK-3β.
The purpose of our study was to determine if TDZD-8 can alleviate the development of collagen II-induced rheumatoid arthritis in rats. We evaluated the following: (1) body weight, (2) radiographic examination of knee joint, (3) histological examination of arthritic synovium, (4) the level of inflammation mediators, (5) and serum level of cytokines.
Material and Methods
Animals
Male Wistar rats (150–200 g body weight) were used in this study. The animals were housed in a laboratory room with a 12 h/12 h light/dark cycle and provided with standard water and food. This study was approved by the local Animal Care Committee.
Experimental protocol
Rats were divided into 3 experimental groups:
RA group: 20 rats were randomly allocated to collagen-induced the rheumatoid arthritis group.
TDZD-8 group: 20 rats were subjected to collagen-induced rheumatoid arthritis and administrated 1 mg/kg TDZD-8 (i.p.) from day 12. TDZD-8 was administrated once daily for 9 consecutive days.
Control: 20 rats were randomly allocated to the control group.
Collagen-induced arthritis rats model
Bovine CII was dissolved in 0.05 mol/L acetic acid at a concentration of 2 mg/ml by stirring at room temperature. CII was diluted with an equal volume of Complete Freund’s adjuvant.
Radiographic examination
Rat were anesthetized and placed on a radiographic box. The radiographic examination was: Score 0, normal; Score 1, one joint swelling and edema; Score 2, two or more joints swelling and edema; Score 3, swelling of entire paw; Score 4, ankylosis or deformity [7].
Measurement of histamine, 5-HT, PGE2
The determination of histamine was evaluated as described by Yang et al. [8]. After the rats were sacrificed, the edema paws were cut and weighed immediately. After the skin of the edema paw was taken off, we cut 0.3 g of tissue into pieces and soaked them into 5 ml saline, after which it was mixed with 0.25 ml 5M NaOH, 1 g NaCl, and 5 ml n-butanol and then centrifuged at 3000 rpm for 10 min. We added 0.1M HCl into the n-butanol layer taken from the mixture. After 10-min centrifugation, 1 M NaOH and 0.2% o-phthalaldehyde were added into the HCl fraction and incubated in an ice bath for 40 min. After the addition of 2 M citric acid, samples were subjected to excitation wavelength of 355 nm and emission wavelength of 440 nm.
The determination of 5-HT was performed according to the method described by Sawynok et al. [9]. We mixed 1.5 g NaCl and 3.5 ml acetous n-butanol with the supernatants of edema. Heptane and HCl were added into the n-butanol fraction, and then 0.5% cysteine and 0.008% o-phthalaldehyde was incubated with aqueous fraction in a boiling water bath for 10 min. The absorbance was determined with excitation wavelength of 355 nm and emission wavelength of 475 nm.
PGE2 levels were determined by the method of Zhou et al. [10]. After KOH was mixed with the supernatants of edema, it is incubated in a 50°C water bath for 20 min, then methanol was added and the absorbance at 278 nm was recorded.
Histological examination
Arthritic synovium (1–2 per rat) were removed and fixed in 10% buffered formalin. Then we embedded synovium in paraffin, and sectioned them and stained them with hematoxylin and eosin for microscopic evaluation.
Measurement of cytokines
IL-6, IL-10, IL-12, and TNF-α were measured in the plasma from the 3 groups. The assay was carried out using a colorimetric commercial ELISA kit according to the manufacturer’s protocol.
Data analysis
All data are expressed as mean ±SEM, representing at least 3 independent experiments. In the experiments involving immunohistochemistry, the figures shown are representative of at least 3 experiments. Data were analyzed using SPSS219.0 with one-way ANOVA. Post hoc comparisons were made with Student-Newman-Keuls test. A p value less than 0.05 was deemed to indicate statistical significant.
Results
Effect of TDZD-8 on joint injury of collagen-induced arthritis rat
In both RA and TDZD-8 groups, we found onset of symptoms such as reduced activity, decreased appetite, and joint swelling compared with the control group on days 11–13. In the RA group, the more obvious symptoms tended to be emerged at 12–21 days. After 21 days, joint swelling and relevant symptoms were alleviated over time. In the RA group, the body weight of rats was significantly lower than in the control group and TDZD-8 group. We found that TDZD-8 treatment can significantly increase loss of body weight in collagen II-induced rheumatoid arthritis rats (Table 1).
Table 1.
The weight of rats in three group.
| Group | Day 0 (g) | Day 21 (g) | Growth rate (%) |
|---|---|---|---|
| Control group | 185.2±11.00** | 265.3±11.90* | 49.2±10.40 |
| RA group | 186.4±9.40** | 226.2±11.20 | 24.1±3.40 |
| TDZD-8 group | 184.2±11.30** | 246.4±10.40* | 45.4±5.20 |
Data were performed with means +SEM.
P<0.01 compared with RA group;
P>0.05 versus each group.
The paw volume was evaluated 21 days after administration of collagen II. The paw volume in the control group was 1.93±0.99 cm, an increase of 12.4%±1.89 compared with 21 days before. However, the paw volume in the RA group increased by 55.2%±0.12, which showed significant paw edema. In the TDZD-8 treatment group, the paw edema was significantly attenuated compared with the RA group (P<0.01) (Figure 1).
Figure 1.
Effect of TDZD-8 treatment on collagen II induced RA rats. (A1) and (A2) is control group. (B1) and (B2) is collagen II induced RA rats group. (C1) and (C2) is TDZD-8 treatment group, which showed a significant reduction on paw edema. (D) demonstrated the paw volume of three group, which TDZD-8 treatment group significantly decrease the paw volume of collagen II induced RA in rats.
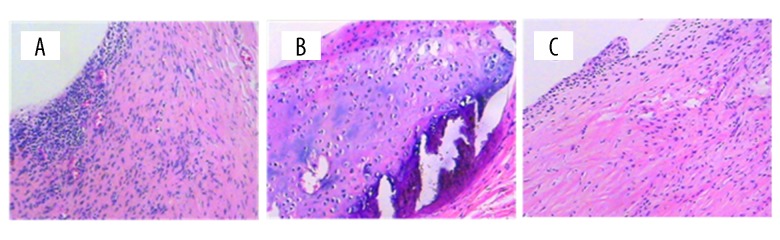
The histological examination of arthritic synovium in RA rats showed increased angiogenesis, bone erosion, cellular proliferation in synovium, and inflammation cell infiltration in the RA group, but there was no pathological change observed in the control group. In the TDZD-8 treatment group, inflammation, cell infiltration, and angiogenesis were significantly reduced. In addition, there was no bone erosion observed in the TDZD-8 group (Figure 2).
Figure 2.
The histological examination of arthritic synovium in three group. There is no evidence of histological change in control group (A). RA group (B) demonstrated that increased angiogenesis, bone erosion, cell proliferation in synovium and inflammation cell infiltration was observed. TDZD-8 group (C) showed reduced inflammation cell infiltration and angiogensis.
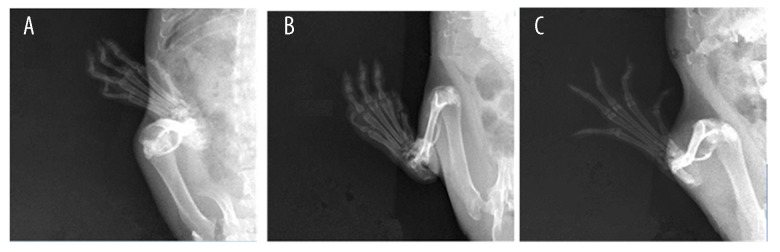
The radiographic examination of the knee joints in the RA group revealed bone erosion. The TDZD-8 group demonstrated less bone erosion compared with the RA group (Figure 2).
Effect of TDZD-8 on HA, 5-HT, PGE2
The results of the determination of prostaglandin E2 (PGE2), 5-hydroxytryptamin (5-HT), and histamine (HA) are shown in Figure 4. The treatment with TDZD-8 significantly decreased the production of PGE2, 5-HT in collagen II-induced rheumatoid arthritis rats (P<0.05) and also significantly reduced the production of HA in collagen II-induced rheumatoid arthritis rats (P<0.01).
Figure 4.
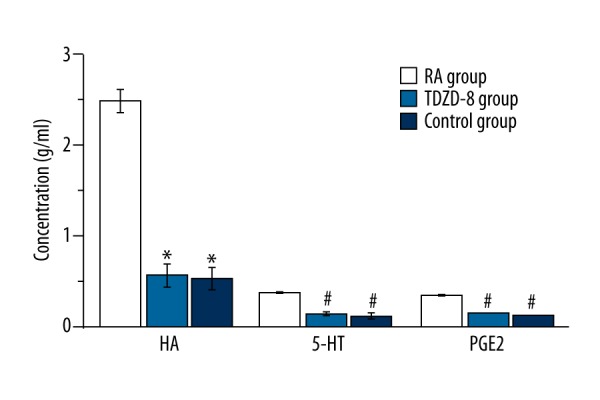
The determination of prostaglandin E2, 5-hydroxytryptamin and histamine in control group, RA group and TDZD-8 treatment group
Effect of TDZD-8 on cytokines
We performed this study to determine the effect of TDZD-8 on the expression of cytokines in serum. The expression of IL-6, IL-12, and TNF-α was significantly higher in the RA group than in the control group. TDZD-8 treatment can significantly reduce the expression of IL-6, IL-12, and TNF-α (p<0.01) and increase the expression of IL-10, but there was no significance between groups in the expression of IL-10 (Figure 5).
Figure 5.
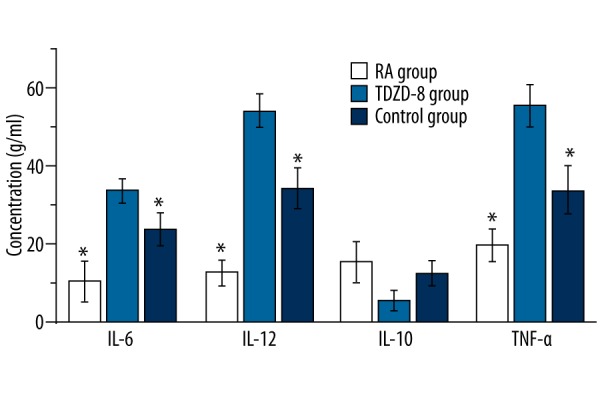
The expression level of cytokines such as IL-6, IL-10, IL-12 and TNF-α.
Discussion
Rheumatoid arthritis is an autoimmune dysfunction disease and can cause swollen and painful joints or even damage to bones [11]. There is currently no effective medicine to cure rheumatoid arthritis. The major goals of an effective medicines would be to relieve pain and discomfort [12]. Collagen II-induced rheumatoid arthritis is a useful model to predict the efficiency of GSK-3β inhibitor in rheumatoid arthritis patients [13,14], and has been widely used in mice [15], rats [16], and rabbits[17].
Here, we demonstrated that GSK-3β inhibitor can attenuate the development of rheumatoid arthritis in rats. This conclusion is supported by the following evidence. First, TDZD-8 treatment can significantly attenuate the paw edema of RA rats. Second, the histological examination of arthritic synovium in the TDZD-8 treatment group revealed reduced angiogenesis, bone erosion, and cellular proliferation compared with the RA group. Third, the radiographic examination of knee joints in the TDZD-8 treatment group demonstrated less bone erosion compared with the RA group.
The conclusion that inhibition of GSK-3β reduced the development of rheumatoid arthritis is also supported by a previous study performed in fibroblast-like synoviocytes, which revealed that GSK-3β inhibitor treatment suppressed inflammatory responses in fibroblast-like synoviocytes. The levels of pro-inflammatory mediators such as phosphorylated JNK, c-jun, ATF-2, and p-38 were decreased by GSK-3β inhibitor treatment [18].
If GSK-3β inhibitor is indeed critical for reducing the development of rheumatoid arthritis, several questions are raised by the results of our study. For example, does GSK-3β inhibitor reduce the expression of inflammation mediators? Our results suggest that GSK-3β inhibitor reduces the level of inflammation mediators such as prostaglandin E2, 5-hydroxytryptamin, and histamine. On the other hand, cytokines such as IL-6, IL-12, and TNF-α in the TDZD-8 treatment group were significantly decreased.
Conclusions
Our results demonstrate that the GSK-3β inhibitor TDZD-8 can alleviate the development of collagen II-induced rheumatoid arthritis in rats. (i) TDZD-8 treatment can attenuate paw edema and bone erosion in RA rats as shown by the histological examination of the knee joint. (ii) TDZD-8 treatment can alleviate the expression of inflammation mediators such as prostaglandin E2, 5-hydroxytryptamin, and histamine. (iii) TDZD-8 treatment can alleviate the expression of cytokines such as IL-6, IL-12, and TNF-α. These findings support that GSK-3β inhibitor may be useful in treating RA patients.
Figure 3.
The radiographic examination of the knee joint. There is no evidence of pathology in control group (A). RA group (B) demonstrated more bone erosion compared with TDZD-8 group (C).
Footnotes
Source of support: Departmental sources
References
- 1.Solomon DH, Bitton A, Katz JN, et al. Review: Treat to target in rheumatoid arthritis: Fact, fiction, or hypothesis? Arthritis Rheumatol. 2014;66:775–82. doi: 10.1002/art.38323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boissier M-C, Semerano L, Challal S, et al. Rheumatoid arthritis: From autoimmunity to synovitis and joint destruction. J Autoimmun. 2012;39:222–28. doi: 10.1016/j.jaut.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: Mechanisms, diagnosis and treatment. Nat Rev Rheumatol. 2012;8:656–64. doi: 10.1038/nrrheum.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou YX, Shi Z, Singh P, et al. Potential role of glycogen synthase kinase-3β in regulation of myocardin activity in human vascular smooth muscle cells. J Cell Physiol. 2016;231:393–402. doi: 10.1002/jcp.25084. [DOI] [PubMed] [Google Scholar]
- 5.Li B, Zhang C, He F, et al. GSK-3β inhibition attenuates LPS-induced death but aggravates radiation-induced death via down-regulation of IL-6. Cell Physiol Biochem. 2013;32:1720–28. doi: 10.1159/000356606. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Tong K. Glycogen synthase kinase-3β inhibitor ameliorates imbalance of connexin 43 in an acute kidney injury model. Toxicol Rep. 2015;2:1391–95. doi: 10.1016/j.toxrep.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yokoyama W, Kohsaka H, Kaneko K, et al. Abrogation of CC chemokine receptor 9 ameliorates collagen-induced arthritis of mice. Arthritis Res Ther. 2014;16:445. doi: 10.1186/s13075-014-0445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang D, He K, Cheng G. Effect of tremulacin on actions of SRS-A and histamine. Yao Xue Xue Bao. 1994;30:254–57. [in Chinese] [PubMed] [Google Scholar]
- 9.Sawynok J, Zarrindast M-R, Reid AR, Doak GJ. Adenosine A 3 receptor activation produces nociceptive behaviour and edema by release of histamine and 5-hydroxytryptamine. Eur J Pharmacol. 1997;333:1–7. doi: 10.1016/s0014-2999(97)01110-2. [DOI] [PubMed] [Google Scholar]
- 10.Zhou C, Gao H, Sun X, et al. Anti-inflammatory and anti-allergic action of aloperine. Zhongguo Yao Li Xue Bao. 1989;10:360–65. [PubMed] [Google Scholar]
- 11.van Schouwenburg PA, Rispens T, Wolbink GJ. Immunogenicity of anti-TNF biologic therapies for rheumatoid arthritis. Na Rev Rheumatol. 2013;9:164–72. doi: 10.1038/nrrheum.2013.4. [DOI] [PubMed] [Google Scholar]
- 12.Richards BL, Whittle SL, Buchbinder R. Neuromodulators for pain management in rheumatoid arthritis. Cochrane Database Syst Rev. 2012;1:CD008921. doi: 10.1002/14651858.CD008921.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muschter D, Göttl C, Vogel M, et al. Reactivity of rat bone marrow-derived macrophages to neurotransmitter stimulation in the context of collagen II-induced arthritis. Arthritis Res Ther. 2015;17:1–15. doi: 10.1186/s13075-015-0684-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonelli M, Savitskaya A, Steiner C, et al. Heme oxygenase-1 end products carbon monoxide and biliverdin ameliorate murine collagen-induced arthritis. Clin Exp Rheumatol. 2012;30(1):73–78. [PubMed] [Google Scholar]
- 15.Kwok SK, Cho ML, Park MK, et al. Interleukin-21 promotes osteoclastogenesis in humans with rheumatoid arthritis and in mice with collagen-induced arthritis. Arthritis Rheum. 2012;64:740–51. doi: 10.1002/art.33390. [DOI] [PubMed] [Google Scholar]
- 16.Peng C, Perera PK, Li Y-m, et al. Anti-inflammatory effects of Clematis chinensis Osbeck extract (AR-6) may be associated with NF-κB, TNF-α, and COX-2 in collagen-induced arthritis in rat. Rheumatol Int. 2012;32:3119–25. doi: 10.1007/s00296-011-2083-8. [DOI] [PubMed] [Google Scholar]
- 17.Hu Y, Cheng W, Cai W, et al. Advances in research on animal models of rheumatoid arthritis. Clin Rheumatol. 2013;32:161–65. doi: 10.1007/s10067-012-2041-1. [DOI] [PubMed] [Google Scholar]
- 18.Kwon Y-J, Yoon C-H, Lee S-W, et al. Inhibition of glycogen synthase kinase-3β suppresses inflammatory responses in rheumatoid arthritis fibroblast-like synoviocytes and collagen-induced arthritis. Joint Bone Spine. 2014;81:240–46. doi: 10.1016/j.jbspin.2013.09.006. [DOI] [PubMed] [Google Scholar]