Summary
Objectives
The aim of this study was to assess treatment patterns of lipid‐lowering therapy (LLT) in patients with hyperlipidaemia or prior cardiovascular (CV) events who experience new CV events.
Methods
A retrospective population‐based cohort study was conducted using Swedish medical records and registers. Patients were included in the study based on a prescription of LLT or CV event history and followed up for up to 7 years for identification of new CV events and assessment of LLT treatment patterns. Patients were stratified into three cohorts based on CV risk level. All outcomes were assessed during the year following index (the date of first new CV event). Adherence was defined as medication possession ratio (MPR) > 0.80. Persistence was defined as no gaps > 60 days in supply of drug used at index.
Results
Of patients with major cardiovascular disease (CVD) history (n = 6881), 49% were not on LLT at index. Corresponding data for CV risk equivalent and low/unknown CV risk patients were 37% (n = 3226) and 38% (n = 2497) respectively. MPR for patients on LLT at index was similar across cohorts (0.74–0.75). The proportions of adherent (60–63%) and persistent patients (56–57%) were also similar across cohorts. Dose escalation from dose at index was seen within all cohorts and 2–3% of patients switched to a different LLT after index while 5–6% of patients augmented treatment by adding another LLT.
Conclusions
Almost 50% of patients with major CVD history were not on any LLT, indicating a potential therapeutic gap. Medication adherence and persistence among patients on LLT were suboptimal.
What's known?
It is well established that drug adherence, especially in chronic conditions, is far from optimal and that medication non‐adherence is a serious problem in healthcare. Several studies have linked treatment non‐adherence of lipid‐lowering therapies and other drug types to increased risk of cardiovascular disease and mortality. However, there are only limited data on the contemporary treatment of hyperlipidaemia in clinical practice, especially among high‐risk patients.
What's new?
Of 6881 patients with prior diagnosis of myocardial infarction, unstable angina pectoris, ischaemic stroke or revascularization procedures, 49% were not on lipid‐lowering therapy at the time of a new cardiovascular event. Of the patients who were on treatment at that time, 39% were not adherent during the first year after the event. Greater efforts to review and modify lipid‐lowering treatment for hyperlipidaemia patients, especially at high risk, are warranted.
Introduction
Cardiovascular disease (CVD), with the usual underlying pathology of atherosclerosis, is the leading cause of death and disability in the world 1. Consequently, CVD contributes extensively to the escalating costs of healthcare 2. The most common manifestation of CVD is coronary heart disease (CHD). CHD has been estimated to be the leading cause of disability in Europe, accounting for approximately 10% of total disability‐adjusted life years 3.
Hyperlipidaemia is one of the most important factors for developing atherosclerosis 4. The importance of pharmacological treatment in the prevention of cardiovascular disease has been demonstrated in a large number of clinical trials where the use of lipid‐lowering therapy (LLT) has been found to decrease rates of cardiovascular events and early death 5, 6. There is strong evidence that secondary prevention for CVD lowers the rates of CV events and mortality 7, 8, 9, 10, 11, 12, and primary prevention for patients with diabetes has been found to lower the risk for CVD 13.
The serious nature of CVD and the major economic impact of the disease calls for optimal treatment in real world settings. Despite this, there is a wealth of clinical trials demonstrating that several subgroups of statin‐treated patients would benefit from a more intense LLT regimen 14. In addition, for obvious reasons, ‘drugs do not work if patients do not take them’ 15. It is well established that drug adherence, especially in chronic conditions, is far from optimal and that medication non‐adherence is a serious problem in healthcare 13. Several studies have linked treatment non‐adherence for LLT and other drug types to increased risk of cardiovascular disease and mortality 16, 17, 18, 19, 20.
There are only limited data on the contemporary treatment of hyperlipidaemia, especially among high‐risk patients 21, 22, 23. The objective of the study was therefore to assess treatment patterns of lipid‐lowering drugs in Swedish patients with hyperlipidaemia or prior cardiovascular (CV) events (myocardial infarction, unstable angina, revascularization, ischaemic stroke, transient ischaemic attack or heart failure) who experience new CV events. The study also investigated adherence aspects of LLT in a real‐world setting.
Methods
Study design and population
This was a retrospective register cohort study conducted using patient‐level data. The primary data sources for the study were electronic medical records in primary care and three national compulsory health registers which are governed by the Swedish National Board of Health and Welfare. By merging data from the medical records with data from the National Patient Register, the Cause of Death Register, and the Swedish Prescribed Drug Register, information on a patient‐level basis was available for the following resource and cost domains:
Pharmaceuticals: The Prescribed Drug Register captures data on prescriptions filled at pharmacies, including drug type, prescription date, dispensing date, dose and pack size.
Inpatient and outpatient care: All hospitalizations, surgical procedures and outpatient specialist visits were collected from the National Patient Register for the complete observable period for each patient.
Primary care: All physical contacts and contacts by phone with nurses, general practitioners and other healthcare personnel in the primary care centres were collected from the electronic medical records.
Death: The Cause of Death Register provided confirmed dates of death, allowing for censoring of patients.
Unique patient ID numbers were available in all data sources which allowed for linkage of individual patients between datasets. The linkage of de‐identified patients was performed by the National Board of Health and Welfare. The study was approved by the Swedish Ethics Review Board.
Patients were included in the study population based on having hyperlipidaemia, defined as treatment with LLT as this is the most accurate way of identifying hyperlipidaemia patients in Sweden. Patients were included on the date of the first prescription of LLT during 2006 if a second filled prescription followed within 6 months. The study inclusion date was defined as the date of the first of the filled prescriptions for LLT. In addition, patients without LLT during 2006 but with a prior history of CV events (within the past 5 years) were also included in the study to capture patients with CVD at baseline. For patients that did not receive LLT but who had a history of CV events, the study inclusion date was defined as 1 January 2006.
Patients were stratified into three separate cohorts based on CVD risk ascertained from 10 years prior to and up until study inclusion according to the National Cholesterol Education Program Adult Treatment Panel III guidelines 24 and the European Society of Cardiology guidelines 25. The stratification was done according to the following definitions:
History of major CVD cohort: prior diagnosis of myocardial infarction, unstable angina pectoris, ischaemic stroke or revascularization
CHD risk equivalent cohort: patients not included in the history of major CVD cohort and with prior diagnosis of diabetes, peripheral artery disease, abdominal aortic aneurysm, transient ischaemic attack or stable angina pectoris
Low/unknown risk cohort: patients not included in the history of major CVD cohort or CHD risk equivalent cohort
Patients were observed from study inclusion until 31 December 2009 for identification of new CV events (exposure time). The date of the first new CV event during this period was defined as the index date. Patients were followed up for 1 year (up until 31 December 2012) after the index date (or until death, whichever occurred first) for assessment of treatment patterns on an individual patient level.
Outcome measures
The index drug was defined by the filled prescription of one LLT closest to but prior to the index date, with the supply covering the index date. It was assumed that patients were prescribed one pill per day to calculate length of each prescription. Switching was defined as discontinued the index drug and filling a prescription for another lipid‐lowering medication. Treatment augmentation was defined as adding another lipid‐lowering drug within a year after index date and dosage escalation as increasing the starting dose after a new CV event. A patient was defined as adherent if the medication possession ratio (MPR), the number of days of index drug supplied during the first 12 months after the new CV event divided by 365 days (or the number of days between index date and death), was greater than 0.8. Persistence was defined as no gaps of more than 60 days in the supply of index drug during the first 12 months after the new CV event. All outcomes were assessed during the year following index date.
Statistical analysis
This was a descriptive study and summary statistics were reported for each cohort. Tests for statistical comparisons across cohorts were not done because of known differences by CV risk level. All data management and statistical analysis was performed using MySQL and Stata 12.
Results
Patient attrition and characteristics
A total of 96,256 patients were identified for inclusion in the study (Figure 1).
Figure 1.
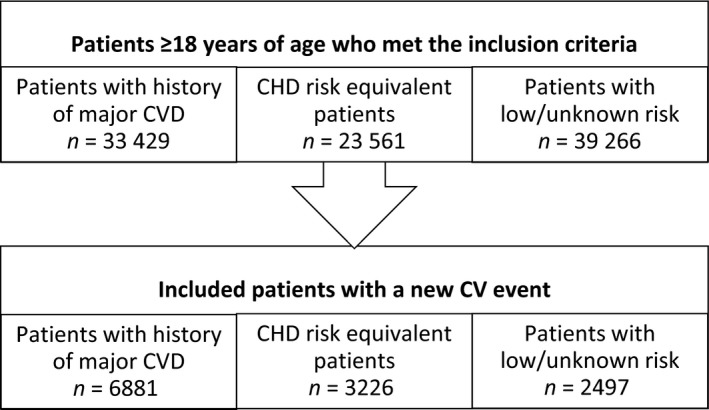
Patient attrition
Of these, 12,604 (9.1%) experienced a CV event during exposure time and were included in the study. Among the included patients, over half of them had a history of major CVD (55%), with somewhat more patients belonging to the CHD risk equivalent cohort (26%) than to the low/unknown risk cohort (20%). The characteristics of the different cohorts at index date are presented in Table 1.
Table 1.
Patient baseline characteristics at time of new CV event
| History of major CVD cohort | CHD risk equivalent cohort | Low/unknown risk cohort | |
|---|---|---|---|
| Number of patients | 6881 | 3226 | 2497 |
| Mean age (SD) | 75.26 (11.03) | 74.47 (10.29) | 73.09 (11.00) |
| Gender (% females) | 39.2 | 43.6 | 46.3 |
| Mean Charlson comorbidity index (SD) | 2.81 (2.11) | 2.59 (2.12) | 1.20 (1.55) |
| Myocardial infarction (%) | 43 | – | – |
| Peripheral vascular disease (%) | 15 | 18 | 4 |
| Cerebrovascular disease (%) | 42 | 20 | 9 |
| Diabetes mellitus (%) | 25 | 41 | – |
| Antihypertensive medication (%) | 90 | 92 | 85 |
| Anti‐diabetic medication (%) | 25 | 45 | – |
Patients with a history of major CVD were older and had higher comorbidity compared with the other cohorts. The low/unknown risk cohort had a substantially lower Charlson comorbidity index score compared with the higher risk cohorts. The low/unknown risk cohort had the highest proportion of females. Between 85% and 92% of the patients in the different risk groups had a filled prescription of an antihypertensive medication at time of study inclusion.
Hyperlipidaemia treatment and index drug
The proportion of patients with a filled prescription of LLT at the date of the new CV event and type of prescribed LLT are shown in Figure 2.
Figure 2.
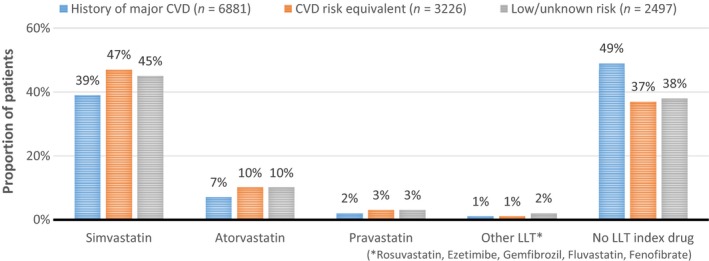
Type of lipid‐lowering therapy at time of new CV event
Almost half of the patients in the history of major CVD cohort did not have a filled prescription of LLT covering the index date. A slightly higher share of patients in the other two cohorts had a prescription of LLT, but still 37–38% of the patients lacked LLT at the time of their new CV event. Statin treatment was by far the most common choice of LLT and only 1–2% of the patients held a filled prescription for any other type of LLT at index.
Treatment patterns
The results on treatment patterns in a post‐event period included analyses on dose escalation, augmentation and switching of drug (Figure 3).
Figure 3.
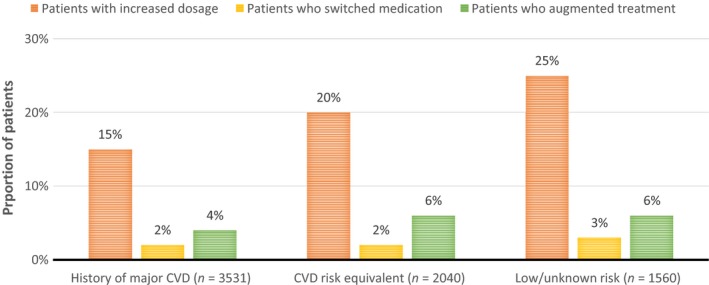
Treatment patterns for lipid‐lowering therapy during 1 year after a new cardiovascular event compared with treatment at index
Since all treatment patterns are described in relation to the prescribed LLT at index date, only those patients with a filled prescription at index date are included in these analyses (corresponding to 57% of the study population). Switching of therapy and drug augmentation were similar across cohorts, with 2–3% of patients switching and 4–6% augmenting LLT. There was some variation in terms of dosage escalation, however, with increases in dosage being more common for patients in the lower risk cohorts (25% in the low/unknown risk cohort, 20% in the CHD risk equivalent cohort, and 15% in the history of major CVD). In all three cohorts, statins were the only treatment type for which dosage escalation occurred.
Adherence and persistence
The study findings on how patients adhere and persist to LLT treatment after a new CV event (Figure 4) are only for the patients who had a filled prescription of LLT at index date (corresponding to 57% of the total study population).
Figure 4.
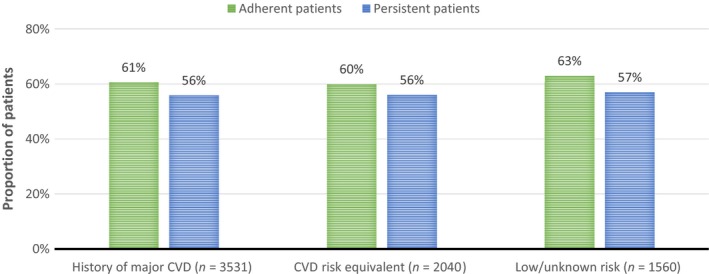
Adherence and persistence aspects for lipid‐lowering therapy during 1 year after a new cardiovascular event
As MPR was similar across cohorts (0.74–0.75), therapy adherence after a new CV event was similar in the three cohorts. Approximately two of five patients did not adhere to LLT treatment 1 year after a new CV events. Persistence rates were also similar across the cohorts and fewer patients were found persistent than adherent to LLT.
Discussion
As hyperlipidaemia is one of the most prominent risk factors for CHD, a key approach to reducing the risk of CVD in both primary and secondary prevention is to decrease LDL‐C 26, 27. Several clinical trials have additionally indicated that there are subgroups of statin‐treated patients who could benefit from a more intense LLT regimen 14. Reduction in CV event rates by improved treatment strategies could also mean an opportunity for lower healthcare costs – a recent Swedish registry study established the substantial healthcare resource use and associated costs that CV events result in, especially for patients with high CVD risk 28.
The current guidelines for treatment with LLT in Sweden are developed by the Medical Products Agency 29 and the National Board of Health and Welfare 30. They state that patients with a clinical history of CVD should primarily be offered statins unless the drug is unsuitable because of contraindications or intolerance. In primary prevention, a risk calculator designed to estimate a person's 10‐year CV event risk is used as an aid to making clinical decisions about how intensively to intervene with lifestyle measures and drug treatments 29. If the risk is estimated to be above 5%, the patient is initially advised to lifestyle changes (such as exercise, diet, lowering of stress). If these lifestyles changes do not prove sufficient, treatment with statins should be considered.
Despite these recommendations by the Medical Products Agency 29 and the National Board of Health and Welfare 30, this study showed that 38–49% of the patients did not have a filled prescription for LLT at the time of their new CV event. Changes in LLT treatment after a new event could be seen considering dosage increases, particularly for the low/unknown CV risk cohort, but fewer patients switched medication or added another LLT drug to the treatment regimen. For the secondary prevention patients, a subsequent event leads to fewer alterations of treatment than for the group of patients without a history of CVD. Since the patients in the history of major CVD cohort are at the highest risk of having new CV events, the finding that few of these high‐risk patients who experience a subsequent event alter their LLT treatment may indicate a gap in care.
The adherence to LLT in CV prevention has been evaluated in a number of studies internationally. In a large review of published literature on adherence rates, the 12‐month adherence rate, measured by MPR, was 74% for LLT 31. The proportion of patients who were persistent after 1 year was 66% for LLT 31. This study also used MPR as a measure of adherence and the results showed that 61% of the patients with a history of major CVD had a MPR ≥ 0.8 the first year after a new CV event. Corresponding figures for CHD risk equivalent patients and patients with low/unknown risk were 60% and 63% respectively. This was lower than the international review, but in accordance with a previous study in Swedish setting with statins where 59% of the patients had a compliance measure over 0.8 32. The proportion of persistent patients was also lower than in the international review. A major difference between the Swedish studies, however, is that this study observes MPR directly after a new CV event, while the previous study measured MPR for patients that had not filled a prescription for statins during the previous year. It was anticipated that more patients would be adherent to LLT in a period directly ensuing a new CV event.
The negative consequences of suboptimal LLT use was demonstrated in a study on real‐world data conducted in the Netherlands, a country with a similar healthcare system to Sweden. The authors assessed the impact of statin discontinuation on myocardial infarction outcomes in low‐risk (no prior CVD) and high‐risk (prior CVD) populations and when compared with non‐continuous users, 2 years continuous statin users had a lower risk of myocardial infarction in both the primary and secondary prevention groups 33. These findings highlights that the lack of treatment adherence shown by this study is important for clinicians to address.
This study is based on retrospective data from three national registers and does therefore not suffer from sampling bias or run the risk of bias because of changing participant behaviour. The high representativeness, large samples, long follow‐up and data quality that underlie the analyses are important strengths of this study. Sweden is well‐known for having excellent registers with a high degree of validity and completeness that allow for generating real‐world evidence of high quality. The study can as such be deemed a high degree of external validity, particularly in relevance to the studied healthcare system.
The study also had some limitations. The analyses did not incorporate intensities of background LLT or differentiate treatment patterns by type of LLT. Another potential study limitation concerns patients who receive a prescription for a lipid‐lowering therapy but never filled the prescription. This feature of non‐adherence could not be included in this study since the analysis was based on pharmacy records of prescriptions that were filled, and not for all prescriptions issued by healthcare personnel. Persistence measures for all patient groups could therefore have been overestimated, but the degree of the bias cannot be measured. It is however reasonable to assume that patients would not continue to refill a prescription without the intention to adhere to treatment 34.
This study contributes to the current evidence on treatment patterns in real‐world setting. The notion that there is a gap between evidence‐based medicine and real‐world clinical practice has been established in prior studies 35, 36, 37. Studies based on real‐world evidence are therefore important to establish the actual treatment of patients in different clinical practices. The results from this study demonstrate that even after a new CV event, approximately two out of five of these now secondary prevention patients were neither adherent nor persistent to LLT during the first 12 months after the event. An increase in dose compared with the index dose was seen for 15–25% of the patients after the new event, which could indicate suboptimal treatment pre‐event. A potential reason for this could be found in a previous study which examined physicians' perception of patients' CVD risk and found that physicians often underestimate this risk 38. In order for patients to reap the established benefits of LLT found in clinical trials, efforts need to be made to ensure that the current discrepancy between evidence‐based medicine and real‐world clinical practice is reduced.
Conclusion
Almost 50% of secondary prevention patients in this study were not on any hyperlipidaemia treatment, indicating a potential therapeutic gap leaving patients at unnecessary risk for CV events which are costly to both patients and payers. Furthermore, medication adherence and persistence among patients on hyperlipidaemia treatment were suboptimal, with 37–40% of all patients deemed as non‐adherent and 43–44% of all patients as non‐persistent. In most patients, a new CV event did not appear to result in major modifications in therapy.
Greater efforts to review and modify LLT for hyperlipidaemia patients may help in preventing future CV events. Further analyses into the reasons for non‐persistence or non‐adherence for LLT are warranted.
Author contributions
KMF, GJ, LÅL, PS and SRG designed the study. SH performed the data analysis. SH, JB and JM interpreted the results. SH drafted the manuscript, which was critically reviewed by JB, KMF, JM, GJ, LÅL, PS and SRG. All authors read and approved the final version of the manuscript.
Supporting information
Appendix S1. Codes for diagnoses and medication.
Acknowledgements
This study was supported by Amgen, Inc. The authors thank Carly J. Paoli for her help in developing the manuscript.
Disclosures
S. Hallberg, J. Banefelt, KM. Fox, J. Mesterton, G. Johansson, L.‐Å. Levin and P. Sobocki have received industry sponsored grants from Amgen, Inc. Author SR. Gandra is employed by Amgen, Inc. and owns stock and/or options in Amgen, Inc.
References
- 1. Mendis S, Puska P, Norrving B. Global Atlas on Cardiovascular Disease Prevention and Control. Geneva: World Health Organization, 2011. [Google Scholar]
- 2. Graham I, Atar D, Borch‐Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice: full text. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Eur J Cardiovasc Prev Rehabil 2007; 14(Suppl. 2): S1–113. [DOI] [PubMed] [Google Scholar]
- 3. National Institute for Health and Clinical Excellence . Statins for the prevention of cardiovascular events in patients at increased risk of developing cardiovascular disease or those with established cardiovascular disease. Technology appraisals, TA94 2006.
- 4. Wilson PW, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation 1998; 97: 1837–47. [DOI] [PubMed] [Google Scholar]
- 5. Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366: 1267–78. [DOI] [PubMed] [Google Scholar]
- 6. Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta‐analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009; 338: b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heart Protection Study Collaborative G . MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high‐risk individuals: a randomised placebo‐controlled trial. Lancet 2002; 360: 7–22. [DOI] [PubMed] [Google Scholar]
- 8. Sacks FM, Alaupovic P, Moye LA, et al. VLDL, apolipoproteins B, CIII, and E, and risk of recurrent coronary events in the Cholesterol and Recurrent Events (CARE) trial. Circulation 2000; 102: 1886–92. [DOI] [PubMed] [Google Scholar]
- 9. Scandinavian Simvastatin Survival Study Group . Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344: 1383–9. [PubMed] [Google Scholar]
- 10. The Long‐Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group . Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long‐Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med 1998; 339: 1349–57. [DOI] [PubMed] [Google Scholar]
- 11. Ray KK, Cannon CP, McCabe CH, et al. Early and late benefits of high‐dose atorvastatin in patients with acute coronary syndromes: results from the PROVE IT‐TIMI 22 trial. J Am Coll Cardiol 2005; 46: 1405–10. [DOI] [PubMed] [Google Scholar]
- 12. LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352: 1425–35. [DOI] [PubMed] [Google Scholar]
- 13. Cholesterol Treatment Trialists Collaborators , Kearney PM, Blackwell L et al. Efficacy of cholesterol‐lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta‐analysis. Lancet 2008; 371: 117–25. [DOI] [PubMed] [Google Scholar]
- 14. de Lemos J, Braunwald E, Blazing M, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐‐analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010; 376: 1670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005; 353: 487–97. [DOI] [PubMed] [Google Scholar]
- 16. Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Persistent use of evidence‐based pharmacotherapy in heart failure is associated with improved outcomes. Circulation 2007; 116: 737–44. [DOI] [PubMed] [Google Scholar]
- 17. Ho PM, Magid DJ, Shetterly SM, et al. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J 2008; 155: 772–9. [DOI] [PubMed] [Google Scholar]
- 18. Perreault S, Dragomir A, Blais L, et al. Impact of better adherence to statin agents in the primary prevention of coronary artery disease. Eur J Clin Pharmacol 2009; 65: 1013–24. [DOI] [PubMed] [Google Scholar]
- 19. Perreault S, Ellia L, Dragomir A, et al. Effect of statin adherence on cerebrovascular disease in primary prevention. Am J Med 2009; 122: 647–55. [DOI] [PubMed] [Google Scholar]
- 20. Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence‐based pharmacotherapy and long‐term mortality after acute myocardial infarction. J Am Med Assoc 2007; 297: 177–86. [DOI] [PubMed] [Google Scholar]
- 21. Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co‐sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Circulation 2006; 113: e409–49. [PubMed] [Google Scholar]
- 22. Antonopoulos S. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 2002; 106: 3421. [PubMed] [Google Scholar]
- 23. Fodor JG, Frohlich JJ, Genest JJ, McPherson PR. Recommendations for the management and treatment of dyslipidemia Report of the Working Group on Hypercholesterolemia and Other Dyslipidemias. Can Med Assoc J 2000; 162: 1441–7. [PMC free article] [PubMed] [Google Scholar]
- 24. Grundy SM, Cleeman JI, Merz CNB, et al. Implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines. J Am Coll Cardiol 2004; 44: 720–32. [DOI] [PubMed] [Google Scholar]
- 25. Perk J, De Backer G, Gohlke H et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). Eur Heart J 2012; 33(13): ehs092. [DOI] [PubMed] [Google Scholar]
- 26. Yusuf S, Hawken S, Ôunpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case‐control study. Lancet 2004; 364: 937–52. [DOI] [PubMed] [Google Scholar]
- 27. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults . Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on Detection, Evaluation, and Treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA: The Journal of the American Medical Association 2001; 285: 2486. [DOI] [PubMed] [Google Scholar]
- 28. Hallberg S, Gandra SR, Fox KM et al. Healthcare costs associated with cardiovascular events in patients with hyperlipidemia or prior cardiovascular events: estimates from Swedish population‐based register data. Eur J Health Econ 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. (Läkemedelsverket) MPA . Prevention av aterosklerotisk hjärt‐kärlsjukdom med lipidreglerande medel. Information Från Läkemedelsverket 2005; 16: 9–11. [Google Scholar]
- 30. Socialstyrelsen . Nationella riktlinjer för hjärtsjukvård 2008. Beslutsstöd för prioriteringar, Socialstyrelsen, Stockholm, 2008.
- 31. Cramer JA, Benedict A, Muszbek N, et al. The significance of compliance and persistence in the treatment of diabetes, hypertension and dyslipidaemia: a review. Int J Clin Pract 2008; 62: 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berglind IA, Kieler H, Linder M et al. Värdet av statiner‐ användningsmönster och följsamhet vid behandling. SNS, Stockholm, 2013. [Google Scholar]
- 33. Penning‐van Beest FJ, Termorshuizen F, Goettsch WG, et al. Adherence to evidence‐based statin guidelines reduces the risk of hospitalizations for acute myocardial infarction by 40%: a cohort study. Eur Heart J 2007; 28: 154–9. [DOI] [PubMed] [Google Scholar]
- 34. Dezii CM. Persistence with drug therapy: a practical approach using administrative claims data. Manag Care 2001; 10: 42–5. [PubMed] [Google Scholar]
- 35. Pearson TA, Laurora I, Chu H, Kafonek S. The lipid treatment assessment project (L‐TAP): a multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid‐lowering therapy and achieving low‐density lipoprotein cholesterol goals. Arch Intern Med 2000; 160: 459–67. [DOI] [PubMed] [Google Scholar]
- 36. Wood DA. Clinical reality of coronary prevention guidelines: a comparison of EUROASPIRE I and II in nine countries. The Lancet 2001; 357: 995–1001. [DOI] [PubMed] [Google Scholar]
- 37. Ovbiagele B, Saver J, Bang H, et al. Statin treatment and adherence to national cholesterol guidelines after ischemic stroke. Neurology 2006; 66: 1164–70. [DOI] [PubMed] [Google Scholar]
- 38. Sager HB, Linsel‐Nitschke P, Mayer B, et al. Physicians' perception of guideline‐recommended low‐density lipoprotein target values: characteristics of misclassified patients. Eur Heart J 2010; 31: 1266–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Codes for diagnoses and medication.


