Abstract
Purpose:
To determine the cross-linking effect of a riboflavin ultraviolet-A (UV-A) corneal cross-linking treatment that is both shorter and has lower energy than the Dresden protocol.
Methods:
In a first experiment, 12 human corneas were presoaked with riboflavin and then irradiated with UV-A at 3 mW/cm2 after clearing the surface of riboflavin, with no added riboflavin during irradiation. Percent UV-A transmission through the corneas was measured at intervals up to 30 minutes. A second experiment involved 24 porcine corneas. Eight were de-epithelialized, presoaked in riboflavin for 30 minutes, and irradiated at 1.5 mW/cm2 for 10 minutes. An additional 8 were riboflavin treated and similarly irradiated, but with epithelium intact and a final 8 corneas were not treated. Young modulus was measured in all 24 corneas at the end of the experiment.
Results:
The first experiment showed essentially complete riboflavin oxidation after only 10 minutes. Based on these results, a shortened UV-A exposure cross-linking experiment was designed using a reduced UV-A fluence of 1.5 mW/cm2, an endothelial exposure within safety limits in humans. With this protocol Young modulus was the same in the irradiated porcine corneas but with epithelium intact as in the untreated corneas. In contrast, Young modulus increased by a factor of 1.99 in the UV-A cross-linked corneas at 1.5 mW/cm2 for 10 minutes with the epithelium removed.
Conclusions:
A shorter, lower energy protocol than the Dresden protocol seems to provide a significant increase in Young modulus, similar to published results with higher energy, longer exposure protocols.
Key Words: corneal cross-linking, corneal UV-A absorption, low-energy treatment
Corneal collagen cross-linking (CXL) as introduced in the Dresden protocol1,2 has succeeded in reducing visual loss in an increasing number of people worldwide. The Bunsen–Roscoe Law suggests that the same cross-linking effect could be completed in a shorter time as long as the total ultraviolet (UV) irradiation energy is the same. Higher intensity, shorter duration treatments have been tried, but there have been safety and effectiveness questions with these variations. Kymionis et al3 recently demonstrated that the 30-minute, 3-mW/cm2 UV-A intensity Dresden protocol CXL yielded a deeper corneal effect than a shorter, more intense treatment of a 10-minute, 9-mW/cm2 CXL protocol, casting some doubt on the application of the Bunsen–Roscoe Law to cross-linking.
Studies on porcine eyes have shown toxicity to the endothelium at irradiation levels of 0.35 mW/cm2 for 30 minutes.4 Based on the Lambert–Beer law of exponential decline in UV transmission as a function of corneal depth, a recommendation was derived, and it is now standard, that cross-linking at 3 mW/cm2 should not be performed on corneas thinner than 400 μm. Furthermore, to prevent endothelial damage, there was a recommendation that a riboflavin layer be renewed on the cornea during UV-A irradiation, but variations in the thickness of the riboflavin covering layer over the treatment time and over different treatments is a potential cause of variation in the degree of cross-linking effect.
Two laboratory experiments, reported here, were conducted to determine if it was possible to produce a shorter duration, lower energy, time-variable UV intensity cross-linking treatment that would accomplish the goal of adequately increasing corneal stiffness while lowering the risk of endothelial cell damage.
MATERIALS AND METHODS
This research adhered to the tenets of the Declaration of Helsinki. Institutional Review Board/Ethics Committee, and Animal Care and Use committee approvals were obtained (authorization no. 1269).
Riboflavin Solution
The solution used contained 0.1% wt/vol riboflavin-5-phosphate and 15% (wt/vol) dextran T-500 (IROS, Naples, Italy), similar to those typically used in the standard CXL procedure described by the Dresden protocol.
Samples
Twelve human and 24 porcine corneas were used for the experimental protocol. Human corneas, not suitable for transplant because of biological contamination of the carrier solution (Optisol GS; Bausch & Lomb, Rochester, NY), were provided by the spin-off consortium Fast-linking Srl and by the Pellegrini Hospital Regional Eye Bank.
The porcine corneas, harvested from a local slaughterhouse, were preserved with the same type of carrier solution and were tested within 24 hours postmortem. All the samples showed intact epithelium, a normal density of endothelial cells, and absence of epithelial or stromal opacity. Before experimentation, each cornea was excised circularly 2 mm from the limbus, and the thickness (d) of the sample was measured with a 5-μm resolution ultrasound pocket pachymeter (Quantel Medical, Clermont, France).
Spectrophotometric Measurements
The UV-A source used in this study (CF–X Linker Modulated Megaride, IROS) uses light-emitting diodes with a 370-nm wavelength and an intensity (i) varying from 1 to 10 mW/cm2. The distance between this device and the corneal surface was set at 5 cm. The UV detector used was a Lasermate-Q laser (Coherent Inc, Santa Clara, CA), set orthogonal to the radiation wave front. The detector has been previously calibrated with a known intensity, so that standard error of the mean was always within ±0.01 mW/cm2. The UV-A intensity values (i0) were measured placing the microscope glass directly in contact with this device. The intensity of the beam emerging from the posterior surface of every microscope slide (i0) when exposed to UV-A (3 mW/cm2) was previously measured; only slides presenting clear and unscratched glass and i0 values of 2.527 ± 0.01 mW/cm2 were used in this study. The specific absorbance value ε of the riboflavin solution was then measured using a spectrophotometer (PerkinElmer Lambda 25 UV/Vis; PerkinElmer, Waltham, MA); the starting solution was diluted to 1:20, to obtain a molarity of 2.6 × 10−3 mol/L. The test was repeated with 10 different samples. The i0 values at the posterior surface of each corneal sample were initially measured, as described above. These values at time 0 (t0) were used as a comparative control for the subsequent readings. The cornea, with a 2-mm ring of sclera (approximate) was then mounted on a modified Franz-type diffusion cell (Ø 9 mm, 5-mL receptor volume; SES GmbH- Analysesysteme, Bechenheim, Germany), with the endothelium facing the receptor compartment. This compartment was constituted with isotonic phosphate buffer at pH 7.4 and was maintained at 37 ± 1°C. When stipulated, epithelium was removed using a surgical scraper and epithelial melting by ethanol as already described.5 Before each experiment, corneas were equilibrated with the use of 0.5-mL of balanced salt solution for 10 minutes, after which 1.0 mL of riboflavin solution was applied. After 30 minutes, cells were dismantled, the excess donor solution was washed away with balanced salt solution and the samples were set on the microscope slide to be exposed to UV-A radiation. Human corneas were divided into 2 groups. The first group (H1, 6 samples) was soaked with the riboflavin solution after the removal of the epithelium (Epi-off); the second group (H2, 6 samples) was soaked with intact epithelium (Epi-on). All the samples were then placed on glass slides and were irradiated for 30 minutes at 3 mW/cm2. The intensity of UV-A light emerging from the posterior surface of each cornea (i0) was measured every 5 minutes, with the last reading at 30 minutes.
Stress–Strain Measurements
Porcine corneas were divided into 3 groups of 8 samples each. The first group (P1) was not soaked with the riboflavin solution after epithelium removal and was not exposed to UV-A rays (baseline group). The second group (P2) was soaked with the riboflavin solution, in Epi-on conditions, and received a 10-minute UV-A exposure at 1.5 mW/cm2 (negative control group). The third group (P3) was soaked with the riboflavin solution, in Epi-off conditions, and received the same 10-minute UV-A exposure at 1.5 mW/cm2. After irradiation, 2 strips of each sample underwent static stress test measurements as described below. All procedures were carried out while shielding samples from external light sources. A previously described experimental model was used for stress-strain measurement.5 After CXL, 2 corneal strips of 5.0 ± 0.2 mm width, 11.0 ± 0.4 mm length (comprising 1 mm of sclera at both ends), and 890 ± 20 μm thickness were cut from each sample. The dimensions of the corneal strips were assessed using a digital caliper. Static stress-strain measurements were performed using an electronic dynamometer (Ametek Lloyd Instruments LRX plus, St. Clair Shores, MI). Young modulus was calculated at 2%, 4%, 6%, and 8% strain. The method used and the force parameters were the same as previously described.4
Statistical Analysis
Statistical analyses were conducted using Graph-Pad Prism (GraphPad Software Inc, San Diego, CA). Results are given as mean ± standard error of the mean. The significance of differences between groups was determined by one-way analyses of variance followed by Bonferroni post hoc tests for multiple comparisons. Differences with P < 0.05 were considered statistically significant in this study.
RESULTS
Transcorneal UV-A transmission data for the human cadaver eyes are shown in Table 1 for the cases of cross-linking with epithelium removed. After riboflavin presoaking for 30 minutes, those corneas with epithelium removed transmitted only 24% of the UV-A light through the cornea (second column), but after 5 minutes of UV-A irradiation fully 75% of the UV-A light was transmitted (third column); both these were statistically significantly different from baseline (P < 0.05). In the interval from 10 to 30 minutes after commencing irradiation, 100% UV-A transmission was observed, within the limits of statistical variation. There was no statistically significant difference between transmission from 10 to 30 minutes and baseline (P > 0.19 in all cases).
TABLE 1.
Time-related (Minutes) UV-A Intensity Readings (mW/cm2) (i0) in Group H1 (Epi-off, i = 3 mW/cm2)
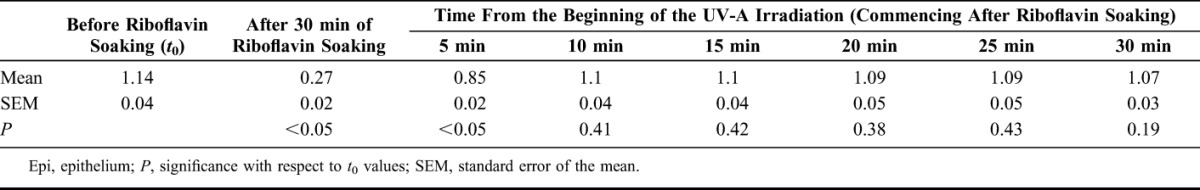
In the group where the epithelium was left intact, there was no statistically significant change in UV-A transmission after riboflavin application for 30 minutes or after UV-A exposure throughout the entire 30-minute period (P > 0.19 in all cases, data not shown).
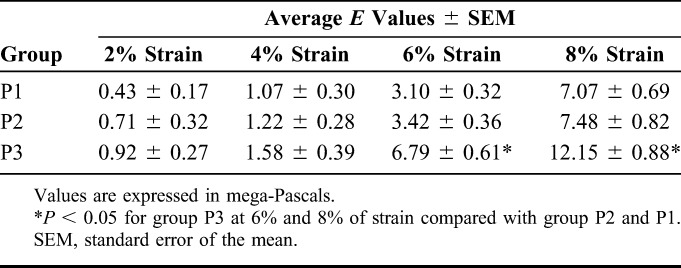
Table 2 shows the average values of Young modulus measured at various levels of strain for the 3 test groups. Group P3 demonstrated a statistically significantly higher Young modulus at 6% and 8% strain when compared with groups P1 and P2 (P < 0.05). Although the average Young modulus seemed slightly higher in group P2 than P1 at each level of strain, the differences were not statistically significant (P > 0.05).
TABLE 2.
Measured Young Modulus (E) at 2%, 4%, 6%, and 8% Strain in Porcine Corneas
DISCUSSION
Spectroscopic results obtained in this study show that the UV-A intensities emerging from the posterior surface of the cornea during standard CXL procedures are not constant over time, as suggested by the Lambert–Beer law. The average intensity of UV-A radiation emerging from the posterior corneal surface in the epithelium OFF group, calculated immediately after topical riboflavin application, was 0.27 ± 0.041 mW/cm2, a value considered safe as it is within the 0.35-mW/cm2 limit to avoid endothelial damage.4 However, by 10 minutes the apparent UV-A exposure exceeded this safety limit in all tested corneas. These results are consistent with those reported previously; several authors have noted that during cross-linking, the endothelial UV light dose would exceed safety limits.6,7 This is why the current standard CXL protocol mandates the presence of a riboflavin solution film on the cornea during the CXL procedure, to shield the endothelium from an excess of UV-A radiation. There is a negative consequence to this; by reducing the penetration of UV-A, the precorneal film allows only the cross-linking of the superficial stroma, thus producing the so-called “demarcation line” effect.8–10 Avoiding the use of the precorneal riboflavin film and lowering UV-A intensities should provide a deeper biomechanical effect while maintaining overall safety.
In addition, the results obtained in this study suggest that the riboflavin has been oxidized long before the standard UV-A irradiation period is complete. This implies that the shielding fades long before the standard 30-minute UV-A exposure ends. In the present study, the riboflavin shielding effect completely disappeared in 10 minutes. As such, a shorter irradiation time may be feasible, which would be likely to produce a similar effect to the Dresden protocol with lower potential UV-A exposure to the endothelium.
The differences between the groups in the second experiment were most notable at 6% and 8% strain, which is consistent with previous observations in the literature.5,11 Wollensak et al2 reported that the Young modulus of cross-linked porcine corneas increased by a factor of 1.8–2.0 with 6% and 8% of strain. Our results are very similar, with a corresponding increase of 1.7 and 2.2 times (Table 2) at those levels of strain. We encountered some variation among the porcine samples in measured values of stress at each strain level. We attribute these variations in Young modulus to the variations in porcine corneal thicknesses and to the fact that only the outer stromal layers were cross-linked.5 However, the SDs of our measurements were lower than those reported in previous studies.5,11
The Dresden protocol for cross-linking has been demonstrated to be effective in stabilizing keratoconus.1–5 It requires a precorneal riboflavin film and 30 minutes of UV-A exposure, and it hardens the outer portion of the cornea. Higher UV-A intensity and short-duration treatment produce an even more superficial effect and have the potential to exceed published safety limits to endothelial irradiation.3,6,12
Experimental cross-linking in the present study, using lower UV-A intensity and not adding riboflavin during UV irradiation, provided corneal stiffening similar to that achieved with the Dresden protocol. These effects were achieved with a 10-minute exposure time, stopping the treatment once the riboflavin has already oxidized. This suggests that a quicker, safer, and more reproducible cross-linking protocol may be viable.
The experimental data reported here, along with additional experimental results, are being used to develop a mathematical model describing the rate of photolysis of riboflavin by unit of volume (rate equation). It is hoped that such a model may allow for a UV-A energy optimization, further reducing unnecessary UV-A exposure. That material will be the subject of a separate article.
Associated clinical data related to the shorter protocol have been collected and recently published. Results in the clinical study demonstrate that stabilization of corneal and refractive parameters can be achieved with the shorter, lower energy protocol.13
The results of 2 laboratory experiments, reported here, indicate that the Lambert–Beer law must be modified in cross-linking to account for the reduction in UV filtration over time. They also suggest that it may be possible to produce a shorter duration, lower energy, time-variable UV-A intensity cross-linking treatment that will accomplish the goal of adequately increasing corneal stiffness while lowering the risk of endothelial cell damage. Such a treatment would not require a precorneal riboflavin film during UV-A exposure. A new CXL protocol based on these findings may yield a safer, equally effective, more reproducible, and more rapid cross-linking treatment. It must be kept in mind that the results presented here are based on in vitro experiments and cannot be generalized to in vivo experiments. Preliminary results have been obtained with the procedure in a clinical setting, but only cases of mild keratoconus were treated.13 Additional clinical research is required to determine if the procedure studied here can be generalized to broader clinical application.
ACKNOWLEDGMENTS
The authors thank the researchers from the spin-off consortium Fast Linking srl for their technical support.
Footnotes
The work described here was entirely funded by the spin-off consortium Fast Linking, approved and partially financed by the Italian Ministry of Education, Universities and Research (DM 593/2000, Prog. 04/11, Prot. 3693, April 4, 2011).
R. L. Epstein has received consultation fees from I.R.O.S. during 2014 in connection with a different topic. The other authors have no conflicts of interest to disclose.
REFERENCES
- 1.Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Exp Eye Res. 1998;66:97–103. [DOI] [PubMed] [Google Scholar]
- 2.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen cross-linking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–627. [DOI] [PubMed] [Google Scholar]
- 3.Kymionis GD, Tsoulnaras KI, Grentzelos MA, et al. Corneal stroma demarcation line after standard and high-intensity collagen cross-linking determined with anterior segment optical coherence tomography. J Cataract Refract Surg. 2014;40:736–740. [DOI] [PubMed] [Google Scholar]
- 4.Wollensak G, Spoerl E, Wilsch M, et al. Endothelial cell damage after riboflavin-ultraviolet-A treatment in the rabbit. J Cataract Refract Surg. 2003;29:1786–1790. [DOI] [PubMed] [Google Scholar]
- 5.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg. 2003;29:1780–1785. [DOI] [PubMed] [Google Scholar]
- 6.Wollensak G, Sporl E, Reber F, et al. Corneal endothelial cytotoxicity of riboflavin/UV-A treatment in vitro. Ophthalmic Res. 2003;35:324–328. [DOI] [PubMed] [Google Scholar]
- 7.Schumacher S, Mrochen M, Wernli J, et al. Optimization model for UV-riboflavin corneal cross-linking. Invest Ophthalmol Vis Sci. 2012;53:762–769. [DOI] [PubMed] [Google Scholar]
- 8.Lincoln VA, Ventura L, Faria e Sousa SJ. Ultraviolet analysis of donated corneas: a portable prototype. Appl Opt. 2010;49:4890–4897. [DOI] [PubMed] [Google Scholar]
- 9.Ringvold A, Davanger M, Olsen EG. Changes of the cornea endothelium after ultraviolet radiation. Acta Ophthalmol (Copenh). 1982;60:41–53. [DOI] [PubMed] [Google Scholar]
- 10.Caporossi A, Mazzotta C, Baiocchi S, et al. Transepithelial corneal collagen cross-linking for keratoconus: qualitative investigation by in vivo HRT II confocal analysis. Eur J Ophthalmol. 2012;22:S81–S88. [DOI] [PubMed] [Google Scholar]
- 11.Ostacolo C, Caruso C, Tronino D, et al. Enhancement of corneal permeation of riboflavin-5'-phosphate through vitamin E TPGS: a promising approach in corneal trans-epithelial cross linking treatment. Int J Pharm. 2013;440:148–153. [DOI] [PubMed] [Google Scholar]
- 12.Seiler T, Hafezi F. Corneal cross-linking induced stromal demarcation line. Cornea. 2006;25:1057–1059. [DOI] [PubMed] [Google Scholar]
- 13.Caruso C, Ostacolo C, Epstein RL, et al. Transepithelial corneal cross linking with vitamin E-enhanced riboflavin solution and abbreviated, low dose UV-A: 24-month clinical outcomes. Cornea. 2016;35:145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]


