Abstract
Purpose of review
Globally, the number of deaths associated with tuberculosis (TB) and HIV coinfection remains unacceptably high. We review the evidence around the impact of strengthening the HIV treatment cascade in TB patients and explore recent findings about how best to deliver integrated TB/HIV services.
Recent findings
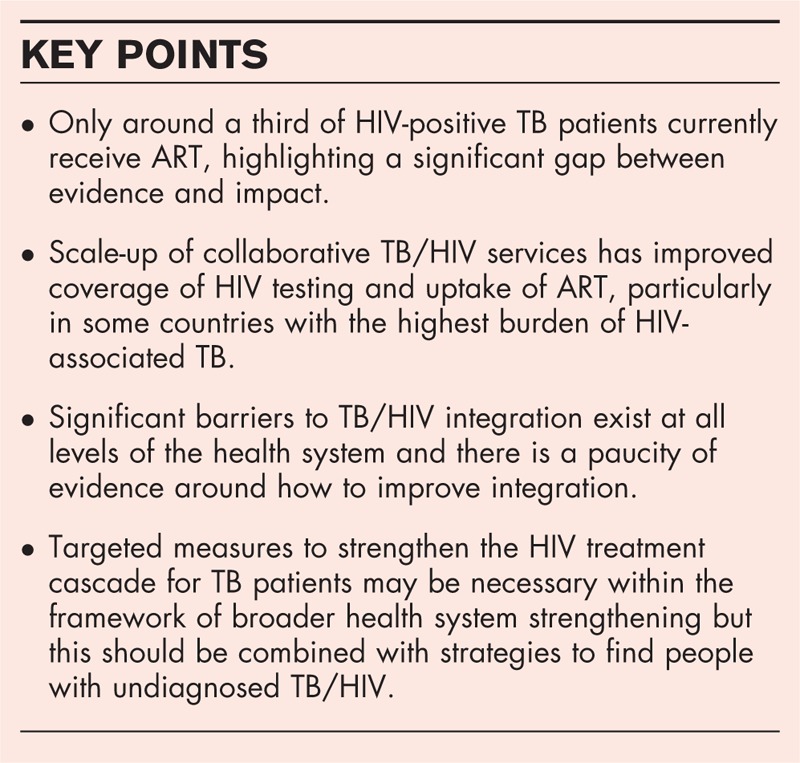
There is clear evidence that the timely provision of antiretroviral therapy (ART) reduces mortality in TB/HIV coinfected adults. Despite this, globally in 2013, only around a third of known HIV-positive TB cases were treated with ART. Although there is some recent evidence exploring the barriers to achieve high coverage of HIV testing and ART initiation in TB patients, our understanding of which factors are most important and how best to address these within different health systems remains incomplete. There are some examples of good practice in the delivery of integrated TB/HIV services to improve the HIV treatment cascade. However, evidence of the impact of such strategies is of relatively low quality for informing integrated TB/HIV programming more broadly. In most settings, there remain barriers to higher-level organizational and functional integration.
Summary
There remains a need for commitment to patient-centred integrated TB/HIV care in countries affected by the dual epidemic. There is a need for better quality evidence around how best to deliver integrated services to strengthen the HIV treatment cascade in TB patients, both at primary healthcare level and within community settings.
Keywords: antiretroviral therapy, HIV, HIV testing, integrated care, tuberculosis
INTRODUCTION
In 2013, there were an estimated 1.1 million cases of tuberculosis (TB) disease in people living with HIV and 360 000 deaths attributable to HIV-associated TB [1]. Africa is home to around four in every five cases of HIV-associated TB disease [1]. Although there is evidence of decreasing mortality from HIV-associated TB (reduction by one-third in the last decade), the rate of mortality decline is slower than for TB in individuals who are HIV negative [1,2].
The main steps in the HIV treatment cascade for TB patients involve diagnosis of HIV infection, linkage to care, initiation of cotrimoxazole prophylaxis and antiretroviral therapy (ART), and achieving and maintaining viral load suppression (Fig. 1) [3,4]. Delivery of these services is guided by the World Health Organization (WHO) policy on collaborative TB/HIV activities [5]. Most countries with a high burden of TB/HIV now have specific policies promoting HIV counselling and testing for those with presumptive or confirmed TB, and most now recommend ART for all TB cases regardless of CD4+ cell count [6]. Routine TB programme reports indicate that despite scale-up of collaborative TB/HIV services, there is still significant attrition along the HIV cascade for TB patients. In 2013, only 48% of TB cases notified globally had a documented HIV test result, and of those known to be HIV positive, only 70% were started on ART (Fig. 2) [1]. This suggests that overall only around a third of HIV-positive TB cases were treated with ART. Even these figures mask the fact that 3 million TB cases are estimated to be undiagnosed each year and do not enter the cascade, many of whom are likely to have HIV-associated TB [1,7].
FIGURE 1.
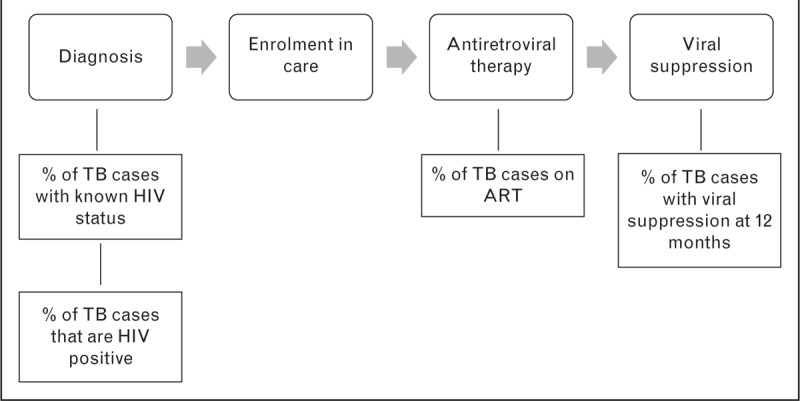
HIV treatment cascade in TB patients and indicators used to evaluate the cascade.
FIGURE 2.
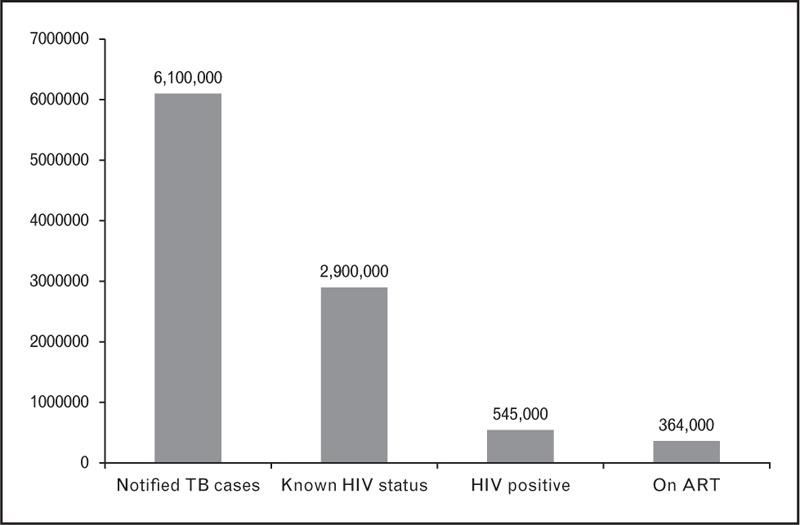
Cascade graph of diagnosis and treatment of HIV in TB cases, 2013 (global data) [1].
Strengthening the HIV treatment cascade is important to reduce the number of deaths from HIV-associated TB. There is quite significant heterogeneity between countries in the key measures of HIV testing and ART initiation for TB patients (Tables 1 and 2). These differences highlight that a one-size-fits-all approach to strengthen the cascade may not be appropriate. There do continue to be issues about the quality of routine programme data, which are emphasized in the context of TB/HIV wherein there may be discrepancies in reporting the same indicator by TB and HIV programmes [1]. Caution is therefore required when interpreting routine aggregated national data alongside data collected in research settings or well defined implementation projects.
Table 1.
Proportion of notified tuberculosis cases with known HIV status in high-burden TB/HIV countries, 2013 [1]
| Country | Proportion | |
| ≥90% | Rwanda | 98 |
| Mali | 97 | |
| Togo | 97 | |
| Burkina Faso | 96 | |
| Kenya | 94 | |
| Malawi | 92 | |
| Namibia | 92 | |
| Zimbabwe | 92 | |
| Swaziland | 91 | |
| Botswana | 91 | |
| Mozambique | 91 | |
| Lesotho | 91 | |
| Uganda | 91 | |
| Sierra Leone | 91 | |
| Zambia | 90 | |
| South Africa | 90 | |
| 70–89% | Côte d’Ivoire | 89 |
| Nigeria | 88 | |
| Ukraine | 88 | |
| Burundi | 87 | |
| Haiti | 86 | |
| United Republic of Tanzania | 83 | |
| Thailand | 83 | |
| Cambodia | 82 | |
| Ghana | 73 | |
| Ethiopia | 71 | |
| Viet Nam | 70 | |
| 50–69% | Brazil | 65 |
| India | 63 | |
| Djibouti | 51 | |
| 30–49% | Central African Republic | 45 |
| Democratic Republic of Congo | 44 | |
| Cameroon | 41 | |
| Angola | 40 | |
| Chad | 40 | |
| China | 39 | |
| Congo | 30 | |
| <30% | Sudan | 27 |
| Myanmar | 12 | |
| Indonesia | 2 | |
No data for Russian Federation.
Table 2.
Proportion of notified HIV-positive tuberculosis cases started on antiretroviral therapy in high-burden TB/HIV countries, 2013 [1]
| Country | Proportion | |
| ≥90% | Mali | 100 |
| Congo | 100 | |
| Burkina Faso | 98 | |
| 70–89% | Cambodia | 89 |
| Malawi | 88 | |
| India | 88 | |
| Kenya | 84 | |
| Namibia | 80 | |
| Swaziland | 80 | |
| Rwanda | 79 | |
| Zimbabwe | 77 | |
| Myanmar | 74 | |
| United Republic of Tanzania | 73 | |
| Togo | 72 | |
| Botswana | 72 | |
| Mozambique | 72 | |
| Lesotho | 70 | |
| 50–69% | Ethiopia | 68 |
| Zambia | 67 | |
| Nigeria | 67 | |
| China | 67 | |
| South Africa | 67 | |
| Uganda | 65 | |
| Sierra Leone | 64 | |
| Burundi | 64 | |
| Cameroon | 64 | |
| Viet Nam | 61 | |
| Thailand | 59 | |
| Haiti | 57 | |
| Côte d’Ivoire | 55 | |
| 30–49% | Ukraine | 48 |
| Democratic Republic of Congo | 48 | |
| Ghana | 42 | |
| Sudan | 39 | |
| Djibouti | 30 | |
| <30% | Indonesia | 21 |
No data for Russian Federation, Brazil, Central African Republic, Angola, Chad.
This review will summarize recent data that provide insight into the cascade in different settings, with a particular focus on evidence around interventions to strengthen the cascade and more broadly to support the delivery of integrated TB/HIV services.
Box 1.
no caption available
HIV TESTING FOR TUBERCULOSIS PATIENTS
There was quite substantial variation globally in the proportion of TB cases with known HIV status in 2013 – highest in the WHO African region at 76% and below 50% in south-east Asia, Western Pacific, and Eastern Mediterranean regions [1]. Even within these regions there is considerable heterogeneity in performance between countries (Table 1) [8].
There are several recent examples of good performance in different high-burden TB/HIV countries. In South Africa, individual reports have confirmed the high national-level uptake of HIV testing and have shown that high uptake (>90%) can be achieved in primary healthcare facilities regardless of the level of integration of TB and HIV services [9], and in drug-resistant TB services regardless of whether centralized or decentralized [10]. Although the overall proportion of TB patients tested for HIV in India is low, one study described new referral pathways for TB patients in Delhi, allowing direct referral to ART centres rather than first sending patients to separate HIV counselling and testing sites. Over the 9-month period following implementation, 92% of TB patients received HIV counselling and testing, and of those that tested positive, 93% engaged in care [11▪]. More broadly in India, there have been concerted efforts to scale-up an intensified package of collaborative TB/HIV activities facilitated by a joint national TB/HIV policy [12▪]. Colocation of TB and HIV testing facilities in India has been shown to increase HIV testing coverage and is now a priority intervention within the national strategic plan [12▪].
Despite calls to move testing upstream and ensure that all people investigated for TB are offered HIV testing [5,13], there is a scarcity of data to show to what extent programmes are achieving this. There are also few data describing HIV testing for children undergoing investigation and treatment for TB disease. National-level data in South Africa showed some evidence of recent programmatic improvement, with the proportion of child TB cases (under 15 years) reported to have unknown HIV status declining from 77 to 25% between 2008 and 2012 [14].
In order to optimize the uptake of HIV testing, there is a need to better understand the factors behind TB patients not being tested for HIV. In terms of patient-level factors, it would be interesting to know whether or not there are issues particular to TB patients that could potentially be addressed with improved counselling or other specific interventions. One study from Ethiopia, with a high uptake of HIV testing among TB patients (92%), suggested that patient-level barriers were not issues specific to TB/HIV but rather general factors, such as lack of formal education and lower levels of knowledge about HIV [15▪].
In general, however, other than colocation of services, there is a lack of high-quality evidence around interventions to maximize uptake of HIV testing in TB patients. Although there are several reports of high uptake of HIV testing in various settings, in themselves, these may have limited impact as there is often no clear evidence of what precisely has contributed to high uptake.
UPTAKE AND TIMING OF ANTIRETROVIRAL THERAPY FOR TUBERCULOSIS PATIENTS
Since 2010, WHO guidelines have recommended that all HIV-positive TB patients should be commenced on ART, regardless of CD4+ cell count [16,17]. Despite this, coverage of ART in TB patients remains suboptimal – globally in 2013 only 70% of known HIV-positive TB patients were commenced on ART prior to or during TB treatment [1].
There is high-quality evidence from randomized controlled trials (RCTs) that the initiation of ART during TB treatment improves survival in HIV-positive TB patients [18–21]. A recent meta-analysis of 12 observational studies supported this result, finding that the use of ART led to a reduction of between 44 and 72% in mortality during TB treatment [22▪]. There is also recent evidence that confirms that ART substantially improves survival for patients with multidrug-resistant and extensively drug-resistant TB [23▪,24▪].
Observational data from Cape Town has confirmed earlier findings from the RCTs that the impact of ART is most pronounced amongst patients with low CD4+ cell counts (<350 cells/μl) [25▪▪]. In view of this, some continue to question the benefits of ART during TB treatment in patients with higher CD4+ cell counts. A recent multicentre RCT in Africa reported that for HIV-positive TB patients with CD4+ cell count of at least 220 cells/μl, there was no difference in the composite endpoint of TB treatment failure, TB recurrence, and death between those randomized to early ART (after 2 weeks of TB treatment) or delayed ART (at the end of 6 months of TB treatment) [26▪▪]. However, the trial was not powered to detect a difference between the arms in mortality alone and overall mortality was low. Also the proportion of those allocated to the delayed ART arm that nonetheless initiated ART during TB treatment was not reported. There was no evidence of any adverse effect of early ART and therefore this evidence does not support any change in treatment guidelines.
In terms of the timing of ART initiation, a meta-analysis of six RCTs demonstrated that early ART initiation (within 4 weeks of starting TB treatment) was associated with a reduction in mortality of 25% compared with later initiation of ART (8–12 weeks after starting TB treatment) [27]. A post-hoc analysis of the Cambodian Early versus Late Introduction of Antiretrovirals (CAMELIA) RCT in Cambodia showed that, compared with early ART (after 2 weeks of TB treatment), late ART (after 8 weeks of TB treatment) was associated with more than double the risk of late mortality (after week 50), suggesting a long-lasting benefit of early ART among patients with advanced immunodeficiency [28▪]. A recent RCT from Ethiopia explored the impact of even earlier initiation of ART (1 week after starting TB treatment, compared with 4 or 8 weeks) [29]. There was no evidence that starting ART after 1 week of TB treatment reduced mortality for all patients, or specifically for those with most advanced immunosuppression (CD4+ cell count <50 cells/μl).
Interventions to improve uptake of ART might include integration and decentralization of TB and HIV services, and task shifting with nurse-initiated ART. A systematic review explored whether providing ART at the TB health facility improves uptake of ART, compared with systems in which patients were referred to a separate facility [30]. This review of 12 studies, all but one from Africa, suggested that integration improved ART coverage [relative risk (RR) 1.83, 95% confidence interval (CI) 1.48–2.25]. There was also weak evidence that integrated services led to a reduction in mortality amongst HIV/TB patients (RR 0.55, 95% CI 0.29–1.05) [30].
The Integrating Tuberculosis and Anti-Retroviral Treatment (ITART) study was a prospective cohort study in five primary healthcare clinics in the Democratic Republic of Congo. This study explored outcomes with nurse-delivered decentralized and integrated TB/HIV care and compared this to a historical cohort wherein TB patients were referred to a centralized facility for ART initiation [31▪▪]. ART uptake was 69% in the integrated TB/HIV model, compared with 17% in the historical cohort. Mortality during TB treatment was also lower with the integrated TB/HIV model (10 vs. 20% in the historical cohort) [31▪▪]. Further analyses of the ITART study uncovered delays in initiating ART with the integrated TB/HIV care model [32▪]. For this analysis, delayed ART for TB patients was defined quite tightly as more than 5 days beyond the 1, 2, or 6-month threshold for ART (depending on CD4+ cell count). Overall, almost half of all patients experienced delayed ART initiation or did not initiate ART at all – delay was particularly high (59%) for those who should have started ART within 1 month (CD4+ cell count <100 cells/μl or WHO clinical stage 4). In this analysis, delayed ART was associated with having a contraindication to at least one antiretroviral drug, intolerance to TB drugs, nondisclosure of HIV status, and lower CD4+ cell count [32▪]. Modelling of the same data suggested that around a third of the observed mortality during the study could have been prevented with perfect fidelity to the recommended timing of ART initiation [33▪▪].
Elsewhere, there are other examples wherein ART coverage has improved with models of integrated service delivery. In a before–after study in a regional TB hospital in rural Guatemala, ART uptake increased from 22 and 72% and mortality within 50 weeks decreased from an extremely high level of 78 to 21% with decentralized integrated TB/HIV care [34]. In a before–after study in 17 health facilities in western Kenya, integration of TB and HIV services led to an improvement in ART uptake during TB treatment from 39 to 61% and a reduction in the median time to ART initiation from 42 to 34 days [35]. In Malawi, a before–after study compared ART uptake in an integrated TB/HIV clinic before and after the implementation of new guidelines recommending ART within 2 weeks for all TB patients. Overall, ART uptake improved from 70 to 78% and the proportion starting ART within 2 weeks increased from 30 to 46% [36▪].
Although studies consistently demonstrate that a significant number of TB patients do not start ART, there is relatively limited understanding about the factors that contribute to this. One qualitative study in the Kingdom of Swaziland involved in-depth interviews with HIV-positive TB patients that had not initiated ART [37▪]. This highlighted factors operating at an individual level, such as concern about medication side-effects and pill burden as well as deeper issues such as lack of readiness for lifelong treatment. However, the interviews also uncovered important health system barriers, such as poor relationships with clinic staff, failure to receive adequate counselling, and limited availability of diagnostic tests [37▪]. In Namibia, healthcare workers also identified significant structural barriers to ART initiation for TB patients, most notably human resource shortages, lack of training, and lack of physical space in which to provide care [38]. Even in an inpatient setting in Tanzania, significant health system barriers to TB/HIV integration were identified by healthcare workers [39].
Data on uptake and timing of ART initiation for children with TB disease are particularly scarce. In the ITART study, 30 ART-naïve children aged 3–18 years were included. Overall, 73% initiated ART either during TB treatment or on completion of TB treatment, yet in some cases, similarly to the adults in the same study, there were significant delays in starting ART [40].
VIRAL SUPPRESSION FOR TUBERCULOSIS PATIENTS ON ANTIRETROVIRAL THERAPY
With the potential for drug–drug interactions, drug toxicity, and challenges to adherence with combined TB treatment and ART, it is important to understand whether virologic outcomes are compromised by concurrent TB treatment. A systematic review of 17 studies found no evidence of a difference in virologic suppression between those on TB treatment and those not on TB treatment at the time of ART initiation [41▪].Overall, the random-effects RR for virologic suppression at 11–12 months was 0.99 (95% CI 0.94–1.05). In a recent single study in five Ethiopian health centres, the overall proportion with virologic suppression (<50 copies/ml) at 6 months was similar for participants with and without concurrent TB at the time of ART initiation (71 vs. 72%, P = 0.74) [42]. In patients unable to tolerate efavirenz in first-line ART, nevirapine remains an option in the absence of alternative drugs, although efavirenz-based ART is associated with superior rates of virological suppression when coadministered with TB treatment [43].
There are specific issues with combined TB treatment and ART in children, particularly young children using protease inhibitors. In a large hospital programme in South Africa, 92 of 199 children aged 0–8 years were on TB treatment at the time of ART initiation. Overall, TB treatment did not affect the likelihood of achieving virological suppression (proportion with viral load <50 copies/ml at 12 months 63% for TB patients vs. 62% for those without TB) or of experiencing viral rebound over 24 months of follow-up [44▪].
MODELS OF TUBERCULOSIS AND HIV SERVICE INTEGRATION
Integration of TB/HIV services is aimed at improving the efficiency and effectiveness of the health system and reducing opportunities for attrition in the cascade of care [45▪▪]. It is not clear how integration should be measured, although attempts have been made to develop instruments capable of determining the extent of clinical and organizational integration [45▪▪]. This tool was used to evaluate the impact of integration on clinical outcomes in 33 clinics within Cape Town. There was some evidence that, after adjusting for individual-level and clinic-level factors, two of the derived domains of TB/HIV integration (integrated service delivery and care provided by the same clinician) were associated with lower mortality in TB/HIV coinfected patients [46▪].
There is a paucity of evidence around how different models of TB/HIV integration might influence retention in care and viral suppression. Encouragingly, two ongoing clinical trials are exploring models of integrated TB/HIV care and the impact on retention in care [47,48]. In the MERGE cluster randomized trial in South Africa, the intervention being studied is a set of clinic-specific activities to optimize integration of TB and HIV services [47]. The Start TB Patients on ART and Retain on Treatment study in Lesotho is testing a multifaceted intervention package incorporated into integrated HIV/TB care – nurse training and clinical mentoring as well as support for patients (health education, adherence support, and financial support) [48].
Integration of TB and HIV services can encompass different models and the organization and delivery of services needs to be context specific and focused on the needs of patients, families, and communities. Colocation of services within health facilities in itself does not necessarily meet the needs of patients if service delivery remains fragmented [45▪▪]. As the delivery of HIV treatment and care is increasingly decentralized and moved out of health facilities into the community, there is a need for research into community-based integrated models of TB/HIV care [49,50▪,51]. Most of the published evidence about integration focuses on clinical integration, yet there remain significant barriers to higher-level organizational or functional integration of programmes within the broader health system [45▪▪,52▪▪]. In most settings, HIV and TB programmes remain vertical programmes with separate organizational structures, separate policies and guidelines, separate budgeting, and separate monitoring and evaluation systems. There are often historical and cultural differences between TB and HIV programmes that provide challenges to integration [53▪,54]. In many countries, HIV and TB programmes are also dependent on funding from international donors and may be somewhat restricted in how services can be developed. Encouragingly, the Global Fund now stipulates that the 41 countries with a high burden of TB–HIV coinfection should submit applications that encompass integrated and joint programming for the two diseases [55].
CONCLUSION
For TB patients globally, considerable progress will be needed to achieve the Joint United Nations Programme on HIV/AIDS (UNAIDS) 90–90–90 targets by 2020. Strengthening of the HIV treatment cascade for TB patients needs to be coordinated with initiatives to find undiagnosed TB/HIV cases within the health system and more broadly in the community. There is a need for higher quality evidence, combined with economic analysis, to inform the scale-up of efficient TB/HIV services in different settings.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.World Health Organization. Global tuberculosis report 2014. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 2.World Health Organization. Gear up to end TB: introducing the end TB strategy. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 3.World Health Organization. Global update on HIV treatment 2013: results, impact and opportunities: WHO report in partnership with UNICEF and UNAIDS. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 4.World Health Organization. Metrics for monitoring the cascade of HIV testing, care and treatment services in Asia and the Pacific. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 5.World Health Organization. WHO policy on collaborative TB/HIV activities: guidelines for national programmes and other stakeholders. Geneva, Switzerland: World Health Organization; 2012. [PubMed] [Google Scholar]
- 6.Gupta S, Granich R, Date A, et al. Review of policy and status of implementation of collaborative HIV-TB activities in 23 high-burden countries. Int J Tuberc Lung Dis 2014; 18:1149–1158. [DOI] [PubMed] [Google Scholar]
- 7.Herbert N, George A, Baroness Masham of Ilton, et al. World TB Day 2014: finding the missing 3 million. Lancet 2014; 383:1016–1018. [DOI] [PubMed] [Google Scholar]
- 8.Trinh QM, Nguyen HL, Nguyen VN, et al. Tuberculosis and HIV co-infection-focus on the Asia-Pacific region. Int J Infect Dis 2015; 32:170–178. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan R, Caldwell J, Bekker LG, et al. Integration of TB and ART services fails to improve TB treatment outcomes: comparison of ART/TB primary healthcare services in Cape Town, South Africa. S Afr Med J 2014; 104:204–209. [DOI] [PubMed] [Google Scholar]
- 10.Loveday M, Wallengren K, Brust J, et al. Community-based care vs. centralised hospitalisation for MDR-TB patients, KwaZulu-Natal, South Africa. Int J Tuberc Lung Dis 2015; 19:163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11▪.Gupta AK, Singh GP, Goel S, et al. Efficacy of a new model for delivering integrated TB and HIV services for people living with HIV/AIDS in Delhi: case for a paradigm shift in national HIV/TB cross-referral strategy. AIDS Care 2014; 26:137–141. [DOI] [PubMed] [Google Scholar]; This study from Delhi highlights the importance of colocation of services to limit fragmentation of care.
- 12▪.World Health Organization. Scaling up of collaborative TB/HIV activities in concentrated HIV epidemic settings: a case study from India. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]; An excellent and comprehensive report detailing successes and challenges of scaling up collaborative TB/HIV activities in India.
- 13.Kumar AM, Gupta D, Gupta RS, et al. HIV testing in people with presumptive tuberculosis: time for implementation. Lancet Respir Med 2013; 1:7–9. [DOI] [PubMed] [Google Scholar]
- 14.Smith J, Moyo S, Day C. A review of TB in children and adolescents in South Africa 2008–2012. In South African Health Review 2013/14. Padarath A, English R, eds. Durban: Health Systems Trust; 2014. [Google Scholar]
- 15▪.Kebede W, Keno F, Ewunetu T, Mamo G. Acceptance of provider initiated HIV testing and counseling among tuberculosis patients in East Wollega Administrative Zone, Oromia Regional State, Western Ethiopia. Tuberc Res Treat 2014; 2014:935713. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated high uptake of HIV testing and suggested that uptake was associated with general knowledge of HIV and individual perception of HIV risk.
- 16.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach – 2010 revision. Geneva, Switzerland: WHO; 2010. [PubMed] [Google Scholar]
- 17.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach June 2013. Geneva, Switzerland: WHO; 2013. [PubMed] [Google Scholar]
- 18.Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med 2010; 362:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdool Karim SS, Naidoo K, Grobler A, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med 2011; 365:1492–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanc FX, Sok T, Laureillard D, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med 2011; 365:1471–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Havlir DV, Kendall MA, Ive P, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med 2011; 365:1482–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22▪.Odone A, Amadasi S, White RG, et al. The impact of antiretroviral therapy on mortality in HIV positive people during tuberculosis treatment: a systematic review and meta-analysis. PLoS One 2014; 9:e112017. [DOI] [PMC free article] [PubMed] [Google Scholar]; A meta-analysis of predominantly observational studies that highlights the impact of early ART initiation on mortality.
- 23▪.Padayatchi N, Abdool Karim SS, Naidoo K, et al. Improved survival in multidrug-resistant tuberculosis patients receiving integrated tuberculosis and antiretroviral treatment in the SAPiT Trial. Int J Tuberc Lung Dis 2014; 18:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study from South Africa highlights the importance of early ART for survival in multidrug-resistant TB.
- 24▪.Pietersen E, Ignatius E, Streicher EM, et al. Long-term outcomes of patients with extensively drug-resistant tuberculosis in South Africa: a cohort study. Lancet 2014; 383:1230–1239. [DOI] [PubMed] [Google Scholar]; This study also from South Africa demonstrates that ART is associated with reduced mortality in extensively drug-resistant TB.
- 25▪▪.Kaplan R, Caldwell J, Middelkoop K, et al. Impact of ART on TB case fatality stratified by CD4 count for HIV-positive TB patients in Cape Town, South Africa (2009-2011). J Acquir Immune Defic Syndr 2014; 66:487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates increasing ART uptake in Cape Town over a 3-year period and a modest reduction in mortality over the same period. The findings also highlight that the mortality is largely driven by those with lower CD4+ cell counts and that gaps still exist in getting these cases onto ART.
- 26▪▪.Mfinanga SG, Kirenga BJ, Chanda DM, et al. Early versus delayed initiation of highly active antiretroviral therapy for HIV-positive adults with newly diagnosed pulmonary tuberculosis (TB-HAART): a prospective, international, randomised, placebo-controlled trial. Lancet Infect Dis 2014; 14:563–571. [DOI] [PubMed] [Google Scholar]; A multicentre RCT in Africa suggesting that there is no clear benefit from early ART initiation in those with less-advanced immunodeficiency. Although the evidence may not change policy, it does highlight that people with low CD4+ cell counts remain the priority for early initiation of ART.
- 27.Yan S, Chen L, Wu W, et al. Early versus delayed antiretroviral therapy for HIV and tuberculosis co-infected patients: a systematic review and meta-analysis of randomized controlled trials. PLoS One 2015; 10:e0127645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28▪.Marcy O, Laureillard D, Madec Y, et al. Causes and determinants of mortality in HIV-infected adults with tuberculosis: an analysis from the CAMELIA ANRS 1295-CIPRA KH001 randomized trial. Clin Infect Dis 2014; 59:435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]; A post-hoc analysis from one of the RCTs exploring early ART initiation during TB treatment. The findings highlight the long-term protective effect of ART started early during TB treatment.
- 29.Amogne W, Aderaye G, Habtewold A, et al. Efficacy and safety of antiretroviral therapy initiated one week after tuberculosis therapy in patients with CD4 counts < 200 cells/μL: TB-HAART study, a randomized clinical trial. PLoS One 2015; 10:e0122587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suthar AB, Rutherford GW, Horvath T, et al. Improving antiretroviral therapy scale-up and effectiveness through service integration and decentralization. AIDS 2014; 28 Suppl 2:S175–S185. [DOI] [PubMed] [Google Scholar]
- 31▪▪.Van Rie A, Patel MR, Nana M, et al. Integration and task shifting for TB/HIV care and treatment in highly resource-scarce settings: one size may not fit all. J Acquir Immune Defic Syndr 2014; 65:e110–e117. [DOI] [PubMed] [Google Scholar]; Main findings from the ITART study in the Democratic Republic of Congo demonstrating that integrated, nurse-delivered TB/HIV care improved uptake of ART and reduced mortality in comparison to a historical cohort.
- 32▪.Patel MR, Nana M, Yotebieng M, et al. Delayed antiretroviral therapy despite integrated treatment for tuberculosis and HIV infection. Int J Tuberc Lung Dis 2014; 18:694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]; Analysis of data from the ITART study, highlighting persistent delays in initiation of ART and some of the factors contributing to that delay.
- 33▪▪.Patel MR, Westreich D, Yotebieng M, et al. The impact of implementation fidelity on mortality under a CD4+ stratified timing strategy for antiretroviral therapy in patients with tuberculosis. Am J Epidemiol 2015; 181:714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]; Analysis of data from the ITART study, highlighting the potential to save lives by more timely initiation of ART.
- 34.Ikeda JM, Tellez CA, Hudes ES, et al. Impact of integrating HIV and TB care and treatment in a regional tuberculosis hospital in rural Guatemala. AIDS Behav 2014; 18 Suppl 1:S96–S103. [DOI] [PubMed] [Google Scholar]
- 35.Owiti P, Zachariah R, Bissell K, et al. Integrating tuberculosis and HIV services in rural Kenya: uptake and outcomes. Public Health Action 2015; 5:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36▪.Tweya H, Ben-Smith A, Kalulu M, et al. Timing of antiretroviral therapy and regimen for HIV-infected patients with tuberculosis: the effect of revised HIV guidelines in Malawi. BMC Public Health 2014; 14:183. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that implementing guideline changes can lead to improvements in ART uptake but that these improvements may be modest and so achieving high levels of uptake may require more targeted intervention.
- 37▪.Kebogile M, Elias P. Why do patients refuse antiretroviral therapy before they complete tuberculosis treatment?. A qualitative enquiry. J AIDS HIV Res 2014; 6:33–38. [Google Scholar]; This study explores why some people choose to defer ART and highlights areas that could be addressed in education and counselling.
- 38.Seeling S, Mavhunga F, Thomas A, et al. Barriers to access to antiretroviral treatment for HIV-positive tuberculosis patients in Windhoek, Namibia. Int J Mycobacteriol 2014; 3:268–275. [DOI] [PubMed] [Google Scholar]
- 39.Wajanga BM, Peck RN, Kalluvya S, et al. Healthcare worker perceived barriers to early initiation of antiretroviral and tuberculosis therapy among Tanzanian inpatients. PLoS One 2014; 9:e87584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel MR, Yotebieng M, Behets F, et al. Outcomes of integrated treatment for tuberculosis and HIV in children at the primary healthcare level. Int J Tuberc Lung Dis 2013; 17:1206–1211. [DOI] [PubMed] [Google Scholar]
- 41▪.Soeters HM, Napravnik S, Patel MR, et al. The effect of tuberculosis treatment on virologic and CD4+ cell count response to combination antiretroviral therapy: a systematic review. AIDS 2014; 28:245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]; This systematic review suggests that virological response to first-line ART is not affected by TB treatment.
- 42.Reepalu A, Balcha TT, Skogmar S, et al. High rates of virological suppression in a cohort of human immunodeficiency virus-positive adults receiving antiretroviral therapy in Ethiopian health centers irrespective of concomitant tuberculosis. Open Forum Infect Dis 2014; 1:ofu039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang HY, Zhang MN, Chen HJ, et al. Nevirapine versus efavirenz for patients co-infected with HIV and tuberculosis: a systematic review and meta-analysis. Int J Infect Dis 2014; 25:130–135. [DOI] [PubMed] [Google Scholar]
- 44▪.Soeters HM, Sawry S, Moultrie H, Rie AV. The effect of tuberculosis treatment on virologic and immunologic response to combination antiretroviral therapy among South African children. J Acquir Immune Defic Syndr 2014; 67:136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the very few articles focused on paediatric TB/HIV, providing reassurance that even in children virological outcomes do not seem to be adversely affected by TB treatment.
- 45▪▪.Uyei J, Coetzee D, Macinko J, et al. Measuring the degree of integrated tuberculosis and HIV service delivery in Cape Town, South Africa. Health Policy Plan 2014; 29:42–55. [DOI] [PubMed] [Google Scholar]; This article describes an attempt to measure integration, taking into account various aspects of healthcare delivery and organization.
- 46▪.Uyei J, Coetzee D, Macinko J, et al. The influence of integrated tuberculosis and human immunodeficiency virus service delivery on patient outcomes. Int J Tuberc Lung Dis 2014; 18:315–321. [DOI] [PubMed] [Google Scholar]; This follows on from the previous article in applying the tool measuring integration to an analysis of individual patient outcomes. The results suggest that higher levels of integration do impact on patient outcomes, although the results were not consistent across all domains of integration.
- 47.Kufa T, Hippner P, Charalambous S, et al. A cluster randomised trial to evaluate the effect of optimising TB/HIV integration on patient level outcomes: the “merge” trial protocol. Contemp Clin Trials 2014; 39:280–287. [DOI] [PubMed] [Google Scholar]
- 48.Columbia University. Start TB patients on ART and retain on treatment (START study), vol. 2015. Bethesda, MD: National Library of Medicine (US). [Google Scholar]
- 49.Frasca K, Cohn J. Integration of HIV and tuberculosis in the community. J Int Assoc Provid AIDS Care 2014; 13:534–538. [DOI] [PubMed] [Google Scholar]
- 50▪.Gilbert JA, Long EF, Brooks RP, et al. Integrating community-based interventions to reverse the convergent TB/HIV epidemics in rural South Africa. PLoS One 2015; 10:e0126267. [DOI] [PMC free article] [PubMed] [Google Scholar]; Results from mathematical modelling which emphasize the potential impact of moving TB and HIV interventions into the community in a particularly high-burden district of South Africa.
- 51.Arshad A, Salam RA, Lassi ZS, et al. Community based interventions for the prevention and control of tuberculosis. Infect Dis Poverty 2014; 3:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52▪▪.Department of Health RoSA. Joint review of HIV, TB and PMTCT programmes in South Africa. Pretoria, South Africa: Department of Health RoSA; 2014. [Google Scholar]; A comprehensive independent evaluation of the national HIV and TB programmes in South Africa, which provides rich detail and context, particularly around challenges to HIV/TB integration.
- 53▪.Amo-Adjei J, Kumi-Kyereme A, Fosuah Amo H, Awusabo-Asare K. The politics of tuberculosis and HIV service integration in Ghana. Soc Sci Med 2014; 117:42–49. [DOI] [PubMed] [Google Scholar]; Another interesting discursive article, pointing out some of the structural and functional barriers toTB/HIV integration.
- 54.Daftary A, Calzavara L, Padayatchi N. The contrasting cultures of HIV and tuberculosis care. AIDS 2015; 29:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Global Fund Interagency Working Group on TB/HIV. Joint tuberculosis and HIV programming information note. Geneva, Switzerland: Global Fund Interagency Working Group on TB/HIV; 2014. [Google Scholar]


