Abstract
Objectives
This study aimed to characterise feline audiogenic reflex seizures (FARS).
Methods
An online questionnaire was developed to capture information from owners with cats suffering from FARS. This was collated with the medical records from the primary veterinarian. Ninety-six cats were included.
Results
Myoclonic seizures were one of the cardinal signs of this syndrome (90/96), frequently occurring prior to generalised tonic–clonic seizures (GTCSs) in this population. Other features include a late onset (median 15 years) and absence seizures (6/96), with most seizures triggered by high-frequency sounds amid occasional spontaneous seizures (up to 20%). Half the population (48/96) had hearing impairment or were deaf. One-third of cats (35/96) had concurrent diseases, most likely reflecting the age distribution. Birmans were strongly represented (30/96). Levetiracetam gave good seizure control. The course of the epilepsy was non-progressive in the majority (68/96), with an improvement over time in some (23/96). Only 33/96 and 11/90 owners, respectively, felt the GTCSs and myoclonic seizures affected their cat’s quality of life (QoL). Despite this, many owners (50/96) reported a slow decline in their cat’s health, becoming less responsive (43/50), not jumping (41/50), becoming uncoordinated or weak in the pelvic limbs (24/50) and exhibiting dramatic weight loss (39/50). These signs were exclusively reported in cats experiencing seizures for >2 years, with 42/50 owners stating these signs affected their cat’s QoL.
Conclusions and relevance
In gathering data on audiogenic seizures in cats, we have identified a new epilepsy syndrome named FARS with a geriatric onset. Further studies are warranted to investigate potential genetic predispositions to this condition.
Introduction
A reflex seizure is defined as a seizure that is objectively and consistently precipitated by environmental or internal stimuli. It is differentiated from spontaneous epileptic seizures, in which precipitating factors cannot be identified. 1 Reflex epilepsy syndrome is therefore one in which all seizures are precipitated by sensory stimuli. However, some authors suggest that this definition is too restrictive and only the majority of seizures need to be precipitated by sensory stimuli to constitute a reflex epilepsy syndrome. 2 Audiogenic reflex seizures describe those predominantly induced by sounds.
Reflex seizures may occur in humans with idiopathic, symptomatic or probably symptomatic epilepsies. 2 Reflex seizures are reported to be either generalised, such as absences (non-convulsive), myoclonic jerks or tonic–clonic seizures, or focal. 1 A specific stimulus may result in isolated absences, myoclonic jerks or generalised tonic–clonic seizures (GTCSs), or may cause combinations of all three. Patients may exhibit reflex and spontaneous seizures. 2 Myoclonic jerks are by far the most common type of reflex seizures and may manifest initially in the limbs and body, or focally involving just the face or a single limb. Reflex absence seizures are common in people, with reflex focal seizures being much less common.
In summary, reflex seizures can occur with any epilepsy type and the stimulus may be very specific. Numerous epileptic seizure syndromes involving reflex seizures are described, although a robust classification scheme for reflex epilepsies remains elusive.
We have become increasingly aware of audiogenic reflex seizures in cats. A questionnaire-based method was used to gather data to better define and characterise the phenotype of feline audiogenic reflex seizures (FARS). The purpose of this article is to provide a description of this syndrome.
Materials and methods
Case recruitment
Cases were recruited via the veterinary media (The Veterinary Times and Veterinary Record), the internet (solicitation on cat forums, Facebook and the International Cat Care website) and the international press (radio and newspaper driven) asking primary veterinarians and owners to contact us regarding suspected cases of audiogenic seizures in cats. Pedigrees, medical history information, litter information and cheek swab samples for DNA isolation were collected where possible. A detailed invitation-only questionnaire was prepared and was preceded by owner interview via telephone conversation or email in which an open description of the seizure types was given by the owner. On obtaining a description considered compatible with a seizure (as determined by one of the authors [ML]) the questionnaire invitation was given. Where available, video recordings of episodes were reviewed to characterise that the episodes truly represented an epileptic seizure. In the cases recruited via primary veterinarians, a full history was also requested and reviewed. If the owner had contacted us directly and was included in the study, consent was obtained to request and review the history from their primary veterinarian. This history supplemented the information gained from the questionnaire. Data were acquired from September 2013 to March 2014.
Questionnaire design
The questions used in this questionnaire are detailed in the Supplementary material. The questionnaire was divided into four sections, with each section having its own purpose. The questionnaire contained numerous open-ended questions, to which the answer was recorded verbatim, followed by specific leading questions. The majority of questions were closed questions with multiple choice answers. In order to ensure they were correctly excluded, questions were also included to help recognise those participants that did not fulfil the inclusion criteria. Questions directed towards the types and characteristics of the episodes also included an opportunity to provide a description verbatim of the episodes. Where more than one type of episode was observed, participants were asked to characterise each individually. The questions were therefore divided across the sections into those that screened cats for audiogenic reflex seizures and those that provided detailed phenotypic information in terms of the signalment of animals, possible precipitating factors, general health, therapeutic trials, where relevant, and the characteristics of the episodes. Questions were randomised within each section to prevent owners from drawing conclusions about the expected answer. The project questionnaire was presented online utilising the online survey software tool SurveyMonkey (http://www.surveymonkey.com).
Inclusion criteria
Inclusion criteria were in place to exclude other disease processes that may be misinterpreted as an epileptic seizure or paroxysmal behaviour. It was important that all cats had to have suffered three or more GTCSs that had been precipitated by the same sound stimulus. A GTCS was considered only if strict criteria were met, notably the presence of tonic–clonic contractions of all limbs, and the presence of an altered consciousness with autonomic signs (specifically urination and/or salivation). A GTCS was also excluded if the duration was reported to be >5 mins. If other types of episodes were present in addition to a confirmed GTCS, the features of these episodes were recorded individually in the questionnaire. A myoclonic seizure or jerk was considered when there was a sudden, brief involuntary contraction of a muscle or muscle group. Absence seizures were considered as the occurrence of an abrupt, transient apparent loss of consciousness with no motor activity. All cats had to have at least a 1 year history of seizures.
Results
There were 128 respondents to the questionnaire. Thirty-two cats were excluded because they did not meet the inclusion criteria. This included 16 cats that had suffered fewer than three GTCSs, 10 cats that had a history of GTCSs for <1 year, four cats that had no reported autonomic signs during the episodes and two cats that had seizures reported to be >5 mins in duration.
Signalment
Details on 96 cats were therefore collected; these comprised 45 domestic shorthairs, 30 Birmans, six Burmeses five domestic longhairs, four Bengals, two Maine Coons, and one of each of British Shorthair, European Shorthair, Norwegian Forest and Birman cross. Of the Birman cats, 12 (40%) were blue point and 18 (60%) were seal point.
The mean age of the cats at seizure onset was 15 years (median 15 years; range 10–19 years). Forty-seven cats were female (64% were neutered; 30/47) and 49 were male (71% were neutered; 35/49).
Sound stimulus
All seizures, as per the inclusion criteria, occurred following a noise stimulus, but in some cats spontaneous myoclonic jerks (18/90; 20%) and GTCSs (8/96; 8%) were observed on rare occasions without an obvious noise stimulus. All sounds triggering these seizures were high pitched. The sounds were capable of triggering different seizure types with no distinct sound predicting or producing a distinct seizure pattern. In some cases, a repeated sound initiated a myoclonic seizure that, when prolonged, could progress to a GTCS. This phenomenon of audiogenic kindling was reported in 86 cats. These sounds had not always caused cats to seizure and could occur without causing seizures on occasion in 32/96 cats (33%). Avoiding these noises eliminated seizures in 72/96 cats (75%), although many owners remarked that the nature of the sounds made it very difficult to eradicate the seizures completely. All owners felt they could reliably induce a seizure by making one of the described trigger noises. The loudness of the sound (amplitude) also seemed to increase the severity of seizures, regardless of the type of seizure. This was reported by 23% (22/96) of owners.
Sound stimuli identified to evoke seizures in cats included the crinkling of tin foil (n = 82); a metal spoon dropping into a ceramic feeding bowl (n = 79); the chinking or tapping of glass (n = 72); paper or plastic bags crinkling (n = 71); computer keyboard tapping or mouse clicking (n = 61); the clinking of coins or keys (n = 59); the hammering of a nail (n = 38); the clicking of an owner’s tongue (n = 24); the sound of breaking the tin foil from treatment or tablet packaging (n = 12); texting (n = 8); a digital alarm (n = 6); the sound of Velcro (n = 6); the clicking of a piezo lighter for a gas stove or the sound made by igniting the gas hob (n = 4); a mobile phone ring (n = 4); running water (n = 2); the sound created by a dog scratching its neck and jangling its collar (n = 2); a computer printer (n = 2); firewood spitting (n = 1); wooden building blocks being knocked together (n = 1); walking on a wooden floor with bare feet or squeaky shoes (n = 1); and the short, sharp scream of a young child (n = 1). There were no other reported precipitating factors that could induce a seizure or make one more likely to occur.
Clinical features
Apart from GTCSs, cats developed other seizure types (see Figure 1): myoclonic jerks (90/96; 94%) and clinical absences or presumed absence seizures (6/96; 6%). The majority of cats (84/96; 88%), had myoclonic jerks and GTCSs; with six (6/96; 6%) cats having the triad of absence seizures, myoclonic jerks and GTCSs. Six cats (6/96; 6%) had only GTCSs. Absence seizures antedated myoclonic jerks in all six cats (6/96) with this seizure type. The first seizure observed by the owner was a GTCS in 17% (16/96) of cats, a myoclonic seizure in 70% (67/96) of cats and an absence seizure in 6% (6/96) of cats. Seven (7/96; 7%) owners were uncertain which seizure type was observed first. All cats were reported to be normal between the seizures.
Figure 1.
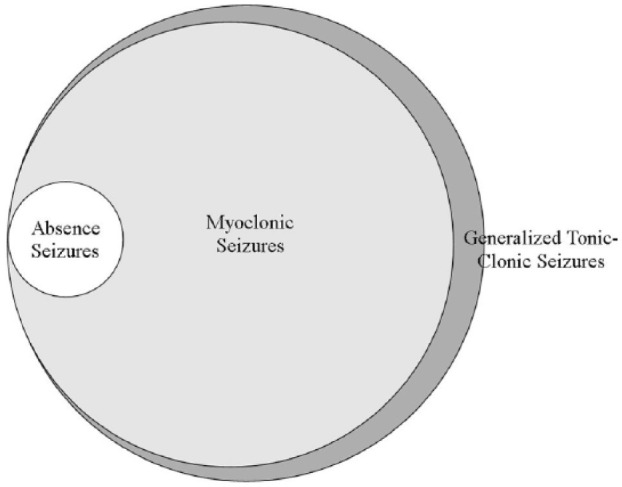
Venn diagram demonstrating the seizure types associated with feline audiogenic reflex seizures and their frequency in this population
GTCSs
GTCSs were present in all cats, as per the inclusion criteria. Therefore, all had an altered consciousness with episodes lasting <5 mins. Urination was recorded in 74/96 (77%) and salivation in 83/96 (86%) cats. Videos were provided in three cases, which confirmed their nature as GTCSs. The seizures never occurred more frequently than one per 24 h period (ie, no clusters). GTCSs were often (86/96; 90%) preceded by generalised myoclonic jerks, most commonly of the head (82/96; 85%). GTCSs indicated their coming just minutes in advance, with a series of rapid myoclonic jerks of increasing intensity. GTCSs most commonly occurred at a frequency of one every 3–6 months, although, overall, this was highly variable, with periods as long as 18 months between seizures.
Fifty-eight cats (82/96; 85%) were ataxic following a GTCS, with hunger (69/96; 72%), restlessness (42/96; 44%), seeking to be near their owners (21/96; 22%) and thirst (6/96; 6%) also being reported. These postictal signs abated within 24 h in all cats. However, many cats improved within hours (31/96; 32%) or (30/96; 31%) minutes of a GTCS.
Myoclonic seizures
Myoclonic jerks or seizures without apparent loss of consciousness were frequently reported (90/96; 94%). The jerks were described as brief (always <30 s), commonly bilateral, rhythmic contractions that mainly started in the head and neck (79/90; 88%) or occasionally in the back/abdomen (11/90; 12%). However, all owners reported that the jerking could spread to involve the shoulders and forelimbs (86/90; 96%), and/or hips and pelvic limbs (83/90; 92%). Some jerks occurred unilaterally (12/90; 13%) but none were reported to involve only a single part of the body. Videos were provided in 23/96 cats, which confirmed the nature of these myoclonic seizures (see video in Supplementary material). All owners reported that consciousness was unaffected during these jerks. However, video footage suggested there was some degree of impairment, albeit brief. This discrepancy is understandable given the short duration of the seizures and so impairment of consciousness should be considered likely in these cats.
Myoclonic jerks were more likely to occur in clusters (88/90; 98%) than as single episodes. The frequency and intensity of myoclonic jerks varied from up to 20 in a day to one every 3–6 months. Owners reported that more violent jerks could result in cats falling to the floor. Rapid successive jerks would frequently evolve into a GTCS (86/90; 96%).
The majority of the cats were normal immediately after a myoclonic episode (88/90; 98%), although two cats were reported to be more sleepy than normal within the first few hours only. No other postictal signs were noted for this type of seizure.
The 16 cats that were excluded because they suffered fewer than three GTCSs included 14/16 that reported regular myoclonic jerks in response to specific sounds. Some of these cats (12/16) had no reported episodes of GTCSs. However, these cats have not been included in the study population.
Absence seizures
Periods of absence were rarely reported within this population. Absences were considered when the owners reported periods of staring with no motor activity (6/96; 6%). These episodes would almost always precede myoclonic jerks. Episodes lasted between 30 s and 1 min in all six cats. A few seconds after each episode, the cats were reported to be quieter than usual by the owners and slower to respond. Postictal signs were varied, with all owners describing sleepiness and a perceived feeling of their cat experiencing disorientation. These signs could last up to a few hours (4/6; 67%) or even up to 1 day (2/6; 33%).
Investigations
Diagnostic investigations were pursued in 85/96 cats (89%). Haematology and biochemistry were performed in 82/85 cats. Urinalysis (including specific gravity and dipstick) was performed in 72/85 cats. Blood pressure recording was performed in 52/85 cats. Magnetic resonance imaging (MRI) of the brain had been performed in 32/85 cats, and computed tomography (CT) in 3/85 cats. The results of cross-sectional imaging in these 35 cats were normal. Cerebrospinal fluid investigation was performed in 18 cats and the results were unremarkable. Toxoplasma and Neospora serology was performed in 48/96 and 12/96 cats, respectively, and revealed no evidence of active infection. Feline leukaemia virus and feline immunodeficiency virus testing was performed in 12/85 cats and was negative in all.
Concurrent diseases
Concurrent diseases reported by the owners and corroborated by primary veterinary surgeon notes were diagnosed in 35 cats (35/85; 40%). These included chronic renal disease (21/85), hyperthyroidism (6/85), hypertrophic cardiomyopathy (3/85), hypertension (3/85) and diabetes mellitus (2/85). The cats with concurrent diseases did not have a progressive seizure course, with seizures remaining static in 21/35 and improving in 14/35 cats.
The mean weight of the cats was 4.2 kg (median 4 kg; range 2–10 kg). Sixty percent of the cats (58/96) were described as ‘eating all meals’, with 25% (24/96) described as picky eaters and 6% (6/96) having a poor appetite. Eight percent of the cats (8/96) were described as hungry all the time. Episodes of diarrhoea or vomiting were described as frequent (6%; 6/96), occasional (42%; 40/96) or infrequent (20%; 19/96), with 31/96 cats having no reported episodes (32%). Twenty-seven percent of the cats (26/96) were reported to have itchy skin, excessive licking or feet/ear problems, although no known allergies were reported by the owners. No owners reported coughing, collapsing or breathing difficulties. Fifty percent of the cats (48/96) were described as having hearing impairment, including five cats where the owners considered they were completely deaf. Sixteen of these cats (16/48) had cross-sectional imaging of the head (4/16 had CT and 12/16 had MRI), revealing no obvious cause for this apparent hearing impairment. Further, 21% (20/96) of cats were described as having visual impairment, including three cats where the owners thought they were completely blind.
Treatment
Treatment was pursued in 44/96 cats. Fifteen cats had received phenobarbital and 29 cats had received levetiracetam. Among the 15 cats taking phenobarbital, 4/15 (27%) experienced adequate GTCS control, although only one owner felt it reduced the frequency of the myoclonic seizures (7%). The remainder were reported by the owners to have no change in seizure frequency. All cats had received this drug for a minimum of 6 weeks before discontinuing the medication. Serum concentrations were only available in 3/15 cats and were within published reference intervals but dosages were available in all 15 cats and were considered acceptable. Levetiracetam gave good control of both the GTCSs and myoclonic seizures in 20/29 (69%) and 27/29 (93%) cats, respectively. The remaining cats were reported by the owners to have no change in seizure frequency. Dosages were available in 27/29 cats and were considered adequate, and all cats had received the medication for at least 2 months. Additionally, those cats receiving no medication for seizure control did not appear to progress or deteriorate during the course of their seizures.
Outcome
Twenty-two cats were dead at the time of writing, all having been euthanased. All 22 cats had suffered seizures up until the point of euthanasia; therefore, the average duration of seizures in these cats was 24 months (range 13–38 months); median duration was 23 months. The reason for euthanasia was seizure-related in just one cat. The remaining 21 cats were euthanased owing to concurrent diseases or a decline in condition.
The onset of epilepsy was highly variable, with single seizures or clusters being observed. The course of the epilepsy was non-progressive in the majority of cats (68/96; 71%), with some owners reporting an improvement with time (23/96; 25%). Thirty-three of 96 (33%) owners stated that the GTCSs affected their cat’s quality of life (QoL); however, a much lower proportion (12%; 11/90) felt the myoclonic seizures affected their cat’s QoL.
Although the seizures were not a concern to the majority, many owners (50/96; 52%) reported a slow decline in their cat’s health in that they became incoordinated or weak in the pelvic limbs (24/50), less responsive (43/50), stopped jumping (41/50), experienced dramatic (>1 kg) weight loss (39/50), toileted inappropriately in the house (12/50) and got stuck in corners (8/50) since seizure onset. However, these signs were only reported in cats that had experienced seizures for >2 years. Only nine cats that had experienced seizures for >2 years were reported to be normal between seizures. Of these 50 cats with a decline in general health, only eight had been diagnosed with a concurrent disease. Eighty-four percent (42/50) of owners felt that these non-seizure signs affected their cat’s QoL.
Discussion
FARS are characterised by GTCSs, myoclonic seizures and absence seizures. In gathering these data the consistency of agreement between owners’ responses has identified a degenerative syndrome of FARS in older cats. There appears to be no sex bias, although cats exclusively suffer from FARS later in life within their second decade. The sounds responsible are high-pitched sounds, often relatively quiet sounds, with increasing loudness and persistence of a sound only serving to enhance the severity of epileptic seizures. Myoclonic jerks or seizures with or without impairment of consciousness appear as one of the cardinal signs of FARS, frequently occurring prior to a GTCS in this population. One-third of the population was diagnosed with concurrent diseases; however, this most likely reflected the age of the population rather than a causal relationship. This rationale is made on the basis of the static or improving nature of the epilepsy in the cats with concurrent medical conditions. However, 50% of the population were reported to have hearing impairment or were deaf. Therapeutic trials with levetiracetam suggest this may be more suitable than phenobarbital to control myoclonic seizures and GTCSs associated with this condition. Although seizures remained relatively non-progressive, other signs developed that were slowly progressive exclusively in cats suffering this epilepsy syndrome for >2 years. However, it cannot be determined whether this decline was due to concurrent disease and entirely coincidental to the association of FARS, or part of the same syndrome.
We found a high number of Birman cats in the study cohort (31% of cats), strongly suggesting a breed predisposition to the condition and thus a hereditary tendency. The ancestry of the Birman is such that the seal and blue points are the original breed colours and cross-mating with other breeds such as Persians and Siamese cats have led to the development of other colours, which appear unaffected by this syndrome. To date, all known Birmans with this condition are of the seal and blue point variety. A recessive or dominant mode of inheritance seems unlikely based on the limited pedigree data obtained. It is also important to state that Birmans had the same clinical features of FARS as cats of other breeds, so although the Birman may represent a different genotype or aetiology, the phenotype was comparable.
Animal models that recapitulate human epilepsies are a useful and convenient tool for studying epilepsy. Audiogenic epilepsy in rodents has become one such influential model, with sound-induced seizures initiated and driven by a brainstem network independent of the forebrain – so-called ‘brainstem seizures’. 3 The caudal colliculus is critical for the initiation of audiogenic seizures. 4 Seizure discharges then spread to other brainstem nuclei such as the rostral colliculus, pontine reticular formation and periaqueductal gray. 5 Repetitive acoustic stimulation transforms these midbrain electrical stimulations into limbic ones. The spread of these seizure discharges to these forebrain structures after such repetitive stimulation results in the clinical manifestation of an epileptic seizure. In the context of the cats reported in this study, the term audiogenic kindling refers to myoclonic seizures and/or GTCSs, which develop after numerous daily sound exposures. This process of epileptic activation of the forebrain by repeated sound-induced brainstem seizures has been referred to as audiogenic kindling.6,7
Levetiracetam has been shown to reduce the frequency and progression of audiogenic seizures.8,9 The antiepileptic effect of levetiracetam against audiogenic seizures has been found to correlate with the affinity for the synaptic vesicle glycoprotein 2A. 10 Mapping of levetiracetam-selective binding sites in the rat brain identified high concentrations in structures such as the rostral colliculus and periaqueductal gray; that is, the structures involved in propagation of brainstem seizures. 11 In contrast to other antiepileptic drugs, levetiracetam appears to exert a more profound antiepileptic effect in kindled audiogenic seizures than in non-kindled epileptic animals, supporting the hypothesis put forward by Klitgaard et al, 8 proposing that levetiracetam has increased efficacy in the kindled epileptic brain.
The results of our study suggest that levetiracetam may aid in reducing the frequency of audiogenic seizures in cats, as well as their progression (preventing audiogenic kindling), providing an exciting opportunity to trial levetiracetam therapy for FARS. However, the small number of cats receiving treatment and the potential for variability in administration means this aspect would require further investigation before any claims are made about levetiracetam being a suitable choice for epileptic myoclonus and FARS. It is interesting to note that human studies also show that myoclonic seizures are highly responsive to treatment with levetiracetam.12–14
The reason for cats being so sensitive to these seemingly benign high-pitch sounds may have an origin in the ultrasonic hearing range of the species. Mice and rats communicate in the ultrasonic frequency range (around 40 kHz). 15 It is believed that cats developed a secondary ultrasonic sensitive hearing range at these frequencies, presumably as an evolutionary advantage in catching rats and mice; their natural prey. 15 Common domestic noises with a high component of ultrasonic frequencies – such as tearing paper, opening cans, jangling keys and hitting solid surfaces – may sound innocuous to us but actually sound more startling to cats that are sensitive to these frequencies. Deafness or hearing impairment was reported in half the cats in this study, and no apparent cause was found in those in which cross-sectional imaging of the head was performed. We therefore speculate from our data that sensorineural deafness may be associated with audiogenic seizures in cats, although these signs, especially given the age of the population, may have had no relationship at all to the FARS. Audiogenic seizures in cats that are deaf seem paradoxical. It is speculated that cats become susceptible to cochlear damage as a consequence of age and exposure to loud noises. The frequency of the noise determines the position on the cochlea that the irreversible damage to the outer hair cells occurs. 16 Work by Miller et al showed that the main area of damage was in the middle and lower portions of the second turn of the cochlea (corresponding to 7600Hz – everyday ‘loud’ noises to us) but that the area of the cochlea associated with detecting higher-frequency sounds was not affected. 16 Therefore, these cats will appear deaf to us, although complete hearing loss is not present. Further investigations will be required in this area before final conclusions are drawn as hearing impairment was perceived and reported by owners and was not confirmed clinically, making it impossible to confirm this clinical feature or its pathogenesis.
An interesting feature of audiogenic seizures in rodents is that one particular strain displays concurrent sensorineural deafness. Mutations in GIPC3 have been found to cause progressive sensorineural hearing loss and audiogenic seizures (Jams1) in mice and autosomal recessive deafness in humans. 17 Another important set of proteins in epilepsy are those characterised by the presence of tandem epilepsy associated repeats, or epitempin as it is also known. 18 This family consists of several proteins, 18 including the four members of the LGI (LGI-1, LG1-2, LG1-3, LG1-4) subfamily and VLGR1. 19 Leucine-rich glioma inactivated protein, a protein-truncating mutation in LGI2 in the Lagotto Romagnolo dog, results in benign familial juvenile epilepsy. 20 Mutations in Vlgr1 have been found to be responsible for a monogenic form of auditory seizures in certain strains of mice,21–23 and are associated with hearing impairment in these same mice. 24 This makes them suitable candidate genes for genetic analysis in cases of FARS. FARS also share similarities with the progressive myoclonic epilepsies (PMEs). PMEs are widely reported in humans and are characterised by myoclonic seizures, tonic–clonic seizures and progressive neurological deterioration, typically with cerebellar signs and dementia. 24 In different disease entities various types of seizures and neurological signs predominate. Myoclonus in PME is typically fragmentary and multifocal, and is often triggered by an environmental or internal stimulus. 25 The age of onset, presenting signs, predominance of signs such as seizures, or myoclonus over cerebellar signs and dementia vary substantially across the different disorders. In humans, there are five main causes of PME that have been more accurately defined with recent advances in genetic studies: Lafora disease, neuronal ceroid lipofuscinosis (NCLs), myoclonic epilepsy with ragged red fibres, Unverricht–Lundborg disease (UCL) and sialidoses. 25 Only Lafora disease, NCLs and UCL have been associated with reflex seizures. However, few are reported in veterinary medicine, with only Lafora disease and several different subtypes of the NCLs having been shown to have a clear genetic cause.26–34
Lafora disease has been reported in dogs,26,35–45 and has some clinical correlation with FARS in that reflex myoclonic seizures are a feature. The disease has a late onset (median 7 years of age) with a slowly progressive course,26,35–37 similar to the cats reported herein. The myoclonic seizures may occur in response to auditory and/or visual stimuli.35–37,46–48 Lafora disease in Miniature Wirehaired Dachshunds has been shown to be caused by the recessive inheritance of a biallelic expansion of a dodecamer repeat in the malin gene (EPM2B or NHLRC1). 26 Mutations for the human disease have also been identified in the laforin (EPM2A) and the malin (EPM2B or NHLRC1) genes,49–51 making these candidates for FARS.
The NCLs are a group of inherited PMEs resulting from lysosomal storage disorders. They typically cause myoclonic seizures, often in the terminal phase, alongside other degenerative neurological signs. Eight genes have now been described in canine NCLs: PPT1, 27 TPP1 or CLN2, 28 CLN5, 29 CLN6, 30 CLN8, 31 CTSD, 32 ATP13A2 33 and ARSG. 34 In general, the descriptions of cats with NCLs report progressive visual dysfunction and profound neurological decline in animals exclusively younger than 2 years of age.52–56 Myoclonic seizures are only reported in end-stage disease. No gene defects have as yet been identified in feline NCLs.55,56
The nature of this study meant that set criteria were necessary for the phenotype and for inclusion of cats in the study – this may be helpful for performing a genetic study, which will form the second part of our investigations. Initial recognition of this disorder requires a select collection of cases using defined criteria. However, it is important to note that other seizure types, such as absence and myoclonic seizures, are likely to occur as the sole seizure type in this syndrome. As an example, it is interesting to note that 12/16 excluded cats had myoclonic jerks alone with no GTCSs. Conversely, it is possible that some cats presented here may have suffered absence seizures that the owners had not previously recognised. Furthermore, it is known that the frequency and intensity of myoclonic jerks varies. For instance, in humans they may be perceived only internally, as an electric shock-like sensation. For this reason it is possible that owners underestimated the number of episodes that their cat was suffering. Therefore, the reliance upon clinical historical evidence is likely to overstate the prevalence of GTCS when compared with absences and myoclonic seizures. The spectrum of this syndrome can be further defined should a genetic marker become apparent for this condition.
It is accepted that this study has a number of limitations; not least the fact that we were relying on owner accounts, albeit with clinical correlation to the medical history provided by primary veterinarians and video footage (in 24% cats). For example, clinical confirmation of hearing impairment would require brainstem auditory-evoked response or some other form of auditory testing, which was not performed here. Furthermore, histopathological examination has not been performed, making it difficult to know if one disease process is more likely than another. However, the consistent observations of this syndrome, combined with the large number of cats and stringent inclusion criteria, suggest that this has not adversely affected our results. It is accepted that the inclusion of cats with absence seizures is debatable as this seizure type has never been reported in this species. However, these individuals had the features that allowed inclusion in our cohort as audiogenic seizures and, given the consistent descriptions by their owners of absence seizures, it is worthy of mention in this report. However, electroencephalography would be required to confirm or refute this finding, and to further characterise the myoclonic jerks and GTCSs.
Conclusions
This study has defined a previously unreported syndrome by using a carefully screened questionnaire and medical records. In doing this it has allowed a large cohort to be examined with the purposes of genotyping this syndrome. The geriatric nature of this condition is such that it may be overlooked in older cats that may potentially suffer from other concurrent conditions, and so this study serves a purpose in informing veterinary practitioners of this syndrome. The phenotype is likely to be broader than that described but specific criteria were applied to ensure a homogeneous population. Work is ongoing to identify the genetic basis of this disorder.
Supplemental Material
A copy of the questionnaire given to owners of cats with suspected feline audiogenic reflex seizures
Acknowledgments
We thank all owners and primary veterinarians for participating in this study. Special thanks are given to Rory Burke, Jasper Copping, Melva Eccles, Dr Malcolm Gamble and Dr Kim Kendall for their work with this study.
Footnotes
Supplementary material: The following files are available: A copy of the questionnaire given to owners of cats with suspected feline audiogenic reflex seizures. Three short video clips: (1) a cat exhibiting characteristic myoclonic jerks in response to a noise stimulus; (2) a different cat suffering myoclonic seizures triggered by sound progressing into a generalised tonic–clonic seizure; (3) an audiogenic generalised tonic–clonic seizure in an elderly Birman cat.
The authors do not have any potential conflicts of interest to declare.
Funding: This research received no grant from any of the public, commercial or not-for-profit funding agencies.
Accepted: 23 March 2015
References
- 1. Engel J, Jr. A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia 2001; 42: 796–803. [DOI] [PubMed] [Google Scholar]
- 2. Panayiotopoulos CP. Reflex seizures and reflex epilepsies. In: The epilepsies: seizures, syndromes and management. Oxford: Bladon Medical Publishing, 2005, pp 449–496. [PubMed] [Google Scholar]
- 3. Browning RA, Wang C, Nelson DK, et al. Effect of precollicular transection on audiogenic seizures in genetically epilepsy-prone rats. Exp Neurol 1999; 155: 295–301. [DOI] [PubMed] [Google Scholar]
- 4. Kesner RP. Subcortical mechanisms of audiogenic seizures. Exp Neurol 1966; 15: 192–205. [DOI] [PubMed] [Google Scholar]
- 5. Garcia-Cairasco N. A critical review on the participation of inferior colliculus in acoustic-motor and acoustic-limbic networks involved in the expression of acute and kindled audiogenic seizures. Hear Res 2002; 168: 208–222. [DOI] [PubMed] [Google Scholar]
- 6. Vergnes M, Kiesmann M, Marescaux C, et al. Kindling of audiogenic seizures in the rat. Int J Neurosci 1987; 36: 167–176. [DOI] [PubMed] [Google Scholar]
- 7. Kiesmann M, Marescaux C, Vergnes M, et al. Audiogenic seizures in Wistar rats before and after repeated auditory stimuli: clinical, pharmacological, and electroencephalographic studies. J Neural Transm 1988; 72: 235–244. [DOI] [PubMed] [Google Scholar]
- 8. Klitgaard H, Matagne A, Gobert K, et al. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur J Pharmacol 1998; 353: 191–206. [DOI] [PubMed] [Google Scholar]
- 9. Ji-qun C, Ishihara K, Nagayama T, et al. Long-lasting antiepileptic effects of levetiracetam against epileptic seizures in the spontaneously epileptic rat (SER): differentiation of levetiracetam from conventional antiepileptic drugs. Epilepsia 2005; 46: 1362–1370. [DOI] [PubMed] [Google Scholar]
- 10. Noyer M, Gillard M, Matagne A, et al. The novel antiepileptic drug levetiracetam (ucb L059) appears to act via a specific binding site in CNS membranes. Eur J Pharmacol 1995; 286: 137–146. [DOI] [PubMed] [Google Scholar]
- 11. Fuks B, Gillard M, Michel P, et al. Localization and photoaffinity labelling of the levetiracetam binding site in rat brain and certain cell lines. Eur J Pharmacol 2003; 478: 11–19. [DOI] [PubMed] [Google Scholar]
- 12. Krauss GL, Bergin A, Kramer RE, et al. Suppression of post-hypoxic and post encephalitic myoclonus with levetiracetam. Neurology 200; 56: 411–412. [DOI] [PubMed] [Google Scholar]
- 13. Noachtar S, Andermann E, Meyvisch P, et al. Levetiracetam for the treatment of idiopathic generalized epilepsy with myoclonic seizures. Neurology 2008; 70: 607–616. [DOI] [PubMed] [Google Scholar]
- 14. Rosenfeld WE, Benbadis S, Edrich P, et al. Levetiracetam as add-on therapy for idiopathic generalized epilepsy syndromes with onset during adolescence: analysis of two randomized, double-blind, placebo-controlled studies. Epilepsy Res 2009; 85: 72–80. [DOI] [PubMed] [Google Scholar]
- 15. Gamble MR. Sound and its significance for laboratory animals. Biol Rev Camb Philos Soc 1982; 57: 395–421. [DOI] [PubMed] [Google Scholar]
- 16. Miller JD, Watson CS, Covell WP. Deafening effects of noise on the cat. Acta Otolaryngol Suppl 1963; 176: 3–91. [Google Scholar]
- 17. Charizopoulou N, Lelli A, Schraders M, et al. Gipc3 mutations associated with audiogenic seizures and sensorineural hearing loss in mouse and human. Nat Commun 2011; 2: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kegel L, Aunin E, Meijer D, et al. LGI proteins in the nervous system. ASN Neuro 2013; 5: 167–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Delmaghani S, Aghaie A, Michalski N. Defect in the gene encoding the EAR/EPTP domain-containing protein TSPEAR cases DFNB98 profound deafness. Hum Mol Genet 2012; 21: 3835–3844. [DOI] [PubMed] [Google Scholar]
- 20. Seppälä EH, Jokinen TS, Fukata, et al. LGI2 truncation causes a remitting focal epilepsy in dogs. PLoS Genet 2011; 7: e1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Skradski SL, Clark AM, Jiang H, et al. A novel gene causing a mendelian audiogenic mouse epilepsy. Neuron 2001; 31: 537–544. [DOI] [PubMed] [Google Scholar]
- 22. Yagi H, Takamura Y, Yoneda T, et al. Vlgr1 knockout mice show audiogenic seizure susceptibility. J Neurochem 2005; 92: 191–202. [DOI] [PubMed] [Google Scholar]
- 23. McMillan DR, White PC. Loss of the transmembrane and cytoplasmic domains of the very large G-protein-coupled receptor-1 (VLGR1 or Mass1) causes audiogenic seizures in mice. Mol Cell Neurosci 2004; 26: 322–329. [DOI] [PubMed] [Google Scholar]
- 24. Johnson KR, Zheng QY, Weston MD, et al. The Mass1 frings mutation underlies early onset hearing impairment in BUB/BnJ mice, a model for the auditory pathology of Usher syndrome IIC. Genomics 2005; 85: 582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shahwan A, Farrell M, Delanty N. Progressive myoclonic epilepsies: a review of genetic and therapeutic aspects. Lancet Neurol 2005; 4: 239–248. [DOI] [PubMed] [Google Scholar]
- 26. Lohi H, Young EJ, Fitzmaurice SN, et al. Expanded repeat in canine epilepsy. Science 2005; 307: 81. [DOI] [PubMed] [Google Scholar]
- 27. Sanders DN, Farias FH, Johnson GS, et al. A mutation in canine PPT1 causes early onset neuronal ceroid lipofuscinosis in a Dachshund. Mol Genet Metab 2010; 100: 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Awano T, Katz ML, O’Brien DP, et al. A frame shift mutation in canine TPP1 (the ortholog of human CLN2) in a juvenile Dachshund with neuronal ceroid lipofuscinosis. Mol Genet Metab 2006; 89: 254–260. [DOI] [PubMed] [Google Scholar]
- 29. Melville SA, Wilson CL, Chiang CS, et al. A mutation in canine CLN5 causes neuronal ceroid lipofuscinosis in Border collie dogs. Genomics 2005; 86: 287–294. [DOI] [PubMed] [Google Scholar]
- 30. Katz ML, Farias FH, Sanders DN, et al. A missense mutation in canine CLN6 in an Australian shepherd with neuronal ceroid lipofuscinosis. J Biomed Biotechnol 2011; 2011: 198042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Katz ML, Khan S, Awano T, et al. A mutation in the CLN8 gene in English Setter dogs with neuronal ceroid-lipofuscinosis. Biochem Biophys Res Commun 2005; 327: 541–547. [DOI] [PubMed] [Google Scholar]
- 32. Awano T, Katz ML, O’Brien DP, et al. A mutation in the cathepsin D gene (CTSD) in American Bulldogs with neuronal ceroid lipofuscinosis. Mol Genet Metab 2006; 87: 341–348. [DOI] [PubMed] [Google Scholar]
- 33. Farias FH, Zeng R, Johnson GS, et al. A truncating mutation in ATP13A2 is responsible for adult-onset neuronal ceroid lipofuscinosis in Tibetan terriers. Neurobiol Dis 2011; 42: 468–474. [DOI] [PubMed] [Google Scholar]
- 34. Abitbol M, Thibaud JL, Olby NJ, et al. A canine Arylsulfatase G (ARSG) mutation leading to a sulfatase deficiency is associated with neuronal ceroid lipofuscinosis. Proc Natl Acad Sci U S A 2010; 107: 14775–14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fitzmaurice SN, Powell HC, Shelton GD. Familial myoclonic epilepsy with skeletal muscle polyglucosan bodies in the miniature wirehaired dachshund. J Vet Intern Med 1999; 13: 239. [Google Scholar]
- 36. Fitzmaurice SN, Rusbridge C, Shelton GD, et al. Familial myoclonic epilepsy in the miniature wirehaired dachshund. J Vet Intern Med 2001; 15: 72–73. [Google Scholar]
- 37. Rusbridge C, Fitzmaurice SN, Lohi H, et al. Treatment of Lafora disease (inherited myoclonic epilepsy) in dogs. J Vet Intern Med 2005; 19: 289. [Google Scholar]
- 38. Kaiser E, Krauser K, Schwartz-Porsche D. Lafora’s disease (progressive myoclonus epilepsy) in the Basset Hound – early diagnosis by muscle biopsy. Tierärztl Prax 1991; 19: 290–295. [PubMed] [Google Scholar]
- 39. Holland JM, Davis WC, Prieur DJ, et al. Lafora’s disease in the dog. A comparative study. Am J Pathol 1970; 58: 509–530. [PMC free article] [PubMed] [Google Scholar]
- 40. Jian Z, Alley MR, Cayzer J, et al. Lafora’s disease in an epileptic Basset hound. N Z Vet J 1990; 38: 75–79. [DOI] [PubMed] [Google Scholar]
- 41. Tomchick TL. Familial Lafora’s disease in the beagle dog. Fed Proc 1973; 32: 821. [Google Scholar]
- 42. Hegreberg GA, Padgett GA. Inherited progressive epilepsy of the dog with comparisons to Lafora’s disease of man. Fed Proc 1976; 35: 1202–1205. [PubMed] [Google Scholar]
- 43. Davis KE, Finnie JW, Hooper PT. Lafora’s disease in a dog. Aust Vet J 1990; 67: 192–193. [DOI] [PubMed] [Google Scholar]
- 44. Whitenack DL. Neuronal glycoproteinosis (Lafora’s disease) in the dog. American Association of Veterinary Laboratory Diagnosticians, 21st Annual Proceedings; 1978, Madison, WI. 1978, pp 493–496. [Google Scholar]
- 45. Cusick PK, Cameron AM, Parker AJ. Canine neuronal glycoproteinosis – Lafora’s disease in the dog. J Am Anim Hosp Assoc 1976; 12: 518–521. [Google Scholar]
- 46. Schoeman T, Williams J, van Wilpe E. Polyglucosan storage disease in a dog resembling Lafora’s disease. J Vet Intern Med 2002; 16: 201–207. [DOI] [PubMed] [Google Scholar]
- 47. Gredal H, Berendt M, Leifsson PS. Progressive myoclonus epilepsy in a beagle. J Small Anim Pract 2003; 44: 511–514. [DOI] [PubMed] [Google Scholar]
- 48. Webb AA, McMillan C, Cullen CL, et al. Lafora disease as a cause of visually exacerbated myoclonic attacks in a dog. Can Vet J 2009; 50: 963–967. [PMC free article] [PubMed] [Google Scholar]
- 49. Minassian BA, Lee JR, Herbrick JA, et al. Mutations in a gene encoding a novel protein tyrosine phosphatase cause progressive myoclonus epilepsy. Nat Genet 1998; 20: 171–174. [DOI] [PubMed] [Google Scholar]
- 50. Chan EM, Bulman DE, Paterson AD, et al. Genetic mapping of a new Lafora progressive myoclonus epilepsy locus (EPM2B) on 6p22. J Med Genet 2003; 40: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gómez-Abad C, Gómez-Garre P, Gutiérrez-Delicado E, et al. Lafora disease due to EPM2B mutations: a clinical and genetic study. Neurology 2005; 64: 982–986. [DOI] [PubMed] [Google Scholar]
- 52. Green PD, Little PB. Neuronal ceroid-lipofuscin storage in Siamese cats. Can J Comp Med 1974; 38: 207–212. [PMC free article] [PubMed] [Google Scholar]
- 53. Bildfell R, Matwichuk C, Mitchell S, et al. Neuronal ceroid-lipofuscinosis in a cat. Vet Pathol 1995; 32: 485–488. [DOI] [PubMed] [Google Scholar]
- 54. Kuwamura M, Nakagawa M, Nabe M, et al. Neuronal ceroid-lipofuscinosis in a Japanese domestic shorthair cat. J Vet Med Sci 2009; 71: 665–667. [DOI] [PubMed] [Google Scholar]
- 55. Furusawa Y, Mizukami K, Yabuki A, et al. Mutational analysis of the feline CLN3 gene and an ultrastructural evaluation of lysosomal storage materials in a cat with neuronal ceroid lipofuscinosis: an investigation into the molecular basis of the disease. Vet J 2012; 194: 425–428. [DOI] [PubMed] [Google Scholar]
- 56. Chalkley MD, Armien AG, Gilliam DH. Characterization of neuronal ceroid-lipofuscinosis in 3 cats. Vet Pathol 2014; 51: 796–804. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A copy of the questionnaire given to owners of cats with suspected feline audiogenic reflex seizures


