Abstract
The β-galactosidases from Lactobacillus reuteri L103 (Lreuβgal), Lactobacillus delbrueckii subsp. bulgaricus DSM 20081 (Lbulβgal), and Bifidobacterium breve DSM 20281 (Bbreβgal-I and Bbreβgal-II) were investigated in detail with respect to their propensity to transfer galactosyl moieties onto lactose, its hydrolysis products d-glucose and d-galactose, and certain sugar acceptors such as N-acetyl-d-glucosamine (GlcNAc), N-acetyl-d-galactosamine (GalNAc), and l-fucose (Fuc) under defined, initial velocity conditions. The rate constants or partitioning ratios (kNu/kwater) determined for these different acceptors (termed nucleophiles, Nu) were used as a measure for the ability of a certain substance to act as a galactosyl acceptor of these β-galactosidases. When using Lbulβgal or Bbreβgal-II, the galactosyl transfer to GlcNAc was 6 and 10 times higher than that to lactose, respectively. With lactose and GlcNAc used in equimolar substrate concentrations, Lbulβgal and Bbreβgal-II catalyzed the formation of N-acetyl-allolactosamine with the highest yields of 41 and 24%, respectively, as calculated from the initial GlcNAc concentration.
Keywords: β-galactosidases, transgalactosylation, galactosyl acceptors, galacto-oligosaccharides, hetero-oligosaccharides
Introduction
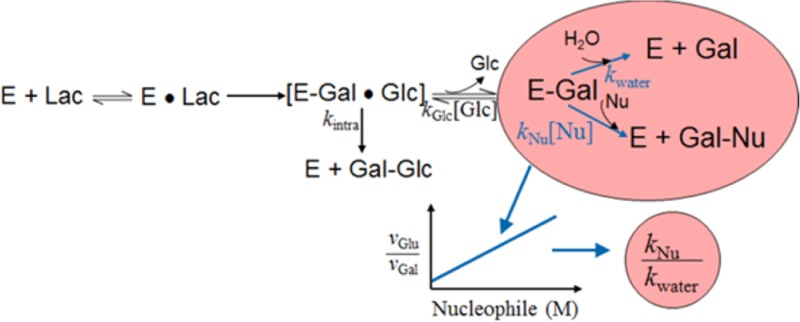
β-Galactosidases (β-d-galactoside galactohydrolase, EC 3.2.1.23; βgal) have long been known to catalyze the hydrolysis of lactose into glucose and galactose, as well as the transfer of a galactosyl moiety to suitable acceptors. If lactose is present in excess, βgal will use lactose, or its hydrolysis products, glucose and galactose, as alternative galactosyl acceptors to form galacto-oligosaccharides (GOS) (Scheme 1). The source of βgal, lactose concentration, and working temperature influence the GOS type, GOS yield, and specific linkages formed, thus creating a wide array of GOS.1
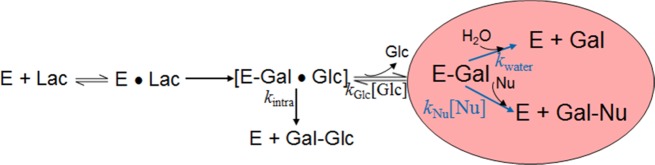
Scheme 1. Hydrolysis and Galactosyl Transfer Reactions during the β-Galactosidase-Catalyzed Conversion of Lactose.
E, enzyme; Lac, lactose; Gal, galactose; Glc, glucose; Nu, nucleophile.
β-Galactosidases have also been used to form hetero-oligosaccharides (HeOS) or other galactosylated compounds, with mannose, fructose, N-acetylneuraminic acid, glucuronic acid, and a number of aromatic compounds used as galactosyl acceptor.2−7 Using this approach, Sulfolobus solfataricus and Kluyveromyces lactis β-galactosidases were used to produce lactulose, a commercial prebiotic disaccharide, and galactosylated aromatic primary alcohols.5,8 Part of the human milk oligosaccharide (HMO) core structure or structurally related compounds can also be accessed, albeit not in pure form, through an approach based on β-galactosidase-catalyzed transglycosylation with lactose as donor (thus transferring galactose onto suitable acceptors) and N-acetylglucosamine (GlcNAc) as acceptor. Thereby, lacto-N-biose (LNB; Gal-β-1,3-GlcNAc) and N-acetyl-lactosamine (LacNAc; Gal-β-1,4-GlcNAc) together with their regioisomers can be obtained. β-Galactosidases from Bacillus circulans, K. lactis, and Lactobacillus bulgaricus were proved to be suitable biocatalysts for the formation of N-acetyl-oligosaccharides using lactose and GlcNAc as substrates.9−11 Although the feasibility of this transferase reaction has been shown, no study has dealt with a more detailed biochemical characterization of this reaction.
The intermolecular transfer of galactose to acceptors other than water typically presents the major pathway for the formation of GOS during lactose hydrolysis by retaining β-galactosidase (Scheme 1). As the sugar species in the mixture that can act as a nucleophile and hence as a galactosyl acceptor change constantly during the reaction, an exact prediction of product formation and degradation cannot be made. However, the partitioning of the galactosylated enzyme (which is formed as an intermediate in the enzymatic reaction; E-Gal in Scheme 1) between the reaction with water and, hence, hydrolysis and the reaction with a galactosyl acceptor can be studied under defined initial velocity conditions. When complete hydrolysis of the disaccharide lactose occurs, equimolar amounts of d-glucose and d-galactose are formed, and therefore the initial velocities at which the two sugars are released are identical and the ratio vGlu/vGal is 1.0. In the presence of an appropriate Nu (e.g., a sugar acceptor for galactose) vGlu/vGal will increase because some of the galactosyl moieties will not be released but transferred onto the acceptor. Richard et al.12 derived eq 1 from Scheme 1, where the rate constant ratio kNu/kwater is obtained as the slope from the linear correlation of vGlu/vGal with increasing concentrations of Nu:
| 1 |
With regard to the process for the formation of HeOS that mimics HMO, the main candidates for galactosyl acceptors are N-acetylglucosamine, N-acetylgalactosamine, and l-fucose (which will be added to the reaction mixture), the substrate lactose, and its hydrolysis products d-glucose and d-galactose. The rate constant ratios determined for the different acceptors can therefore be used as a measure of the ability of a certain substance to act as a galactosyl acceptor (i.e., nucleophile), which in turn allows an estimation of the transgalactosylation products obtained for a known reaction mixture or the suitability of a certain enzyme for the efficient synthesis of HeOS.
In this paper, the propensity of the β-galactosidases from Lactobacillus delbrueckii subsp. bulgaricus DSM 20081 (L. bulgaricus, Lbulβgal), L. reuteri L103 (Lreuβgal), and Bifidobacterium breve DSM 20281 βgal I (Bbreβgal-I) and βgal-II (Bbreβgal-II) to transfer galactosyl moiety to different acceptors such as lactose (Lac), glucose (d-Glc), galactose (d-Gal), l-fucose (Fuc), N-acetyl-d-glucosamine (GlcNAc), and N-acetyl-d-galactosamine (GalNAc) was determined to deepen the understanding of the relative extent of galactosyl transfer of a specific β-galactosidase to different sugar acceptors. The four β-galactosidases belong to glycoside hydrolase family 2 (GH 2) and are of the hetero-oligomeric LacLM type (Lreuβgal) or the homo-oligomeric LacZ type (Lbulβgal, Bbreβgal-I, Bbreβgal-II). The enzymatic synthesis of N-acetyl oligosaccharides using the two enzymes Lbulβgal and Bbreβgal-II will be also presented.
Materials and Methods
Chemicals
All chemicals and enzymes were purchased from Sigma (St. Louis, MO, USA), unless stated otherwise, and were of the highest quality available. The test kit for the determination of d-glucose was obtained from Megazyme (Wicklow, Ireland). Galacto-oligosaccharide standards of β-d-Galp-(1→3)-d-Glc, β-d-Galp-(1→6)-d-Glc, β-d-Galp-(1→3)-d-Gal, β-d-Galp-(1→4)-d-Gal, β-d-Galp-(1→6)-d-Gal, β-d-Galp-(1→3)-β-d-Galp-(1→4)-d-Glc, β-d-Galp-(1→4)-β-d-Galp-(1→4)-d-Glc, and β-d-Galp-(1→6)-β-d-Galp-(1→4)-d-Glc were purchased from Carbosynth (Berkshire, UK), whereas β-d-Galp-(1→3)-d-GlcNAc (lacto-N-biose I, LNB I) and β-d-Galp-(1→4)-d-GlcNAc (N-acetyl-d-lactosamine, LacNAc) were purchased from Dextra Laboratories (Reading, UK).
Enzyme Preparation
β-Galactosidase from L. reuteri L103 (Lreuβgal) was recombinantly produced in Escherichia coli and purified as reported previously,13 whereas the lacZ gene encoding βgal from L. bulgaricus DSM 20081 was expressed in L. plantarum WCFS1 and the corresponding protein was purified as described before.14B. breve DSM 20231 βgal-I and βgal-II were prepared according to the method of Arreola et al.15
β-Galactosidase Assays
The measurement of β-galactosidase activity using o-nitrophenyl β-d-galactopyranoside (oNPG) or lactose as substrate was carried out as described previously.16 Briefly, these assays were performed in 50 mM sodium phosphate buffer of pH 6.5 at 30 °C, and the final substrate concentrations in the 10 min assay were 22 mM for oNPG and 600 mM for lactose. Protein concentrations were determined using the method of Bradford with bovine serum albumin (BSA) as a standard.
GOS Synthesis
The ability of the four recombinant β-galactosidases to synthesize GOS was compared by carrying out discontinuous conversion reactions in a 2 mL scale. The activities (ULac/mL) of the recombinant βgals used were as follows: L. reuteri, 0.8; L. bulgaricus, 1.5; B. breve βgal-I 1.0; B. breve βgal-II, 2.5. Reaction conditions were 600 mM initial lactose concentration in sodium phosphate buffer (50 mM, pH 6.5) containing 1 mM Mg2+ with the incubation temperature set at 30 °C and continuous agitation at 300 rpm. At certain time intervals, samples were withdrawn and the reaction was stopped by heating at 95 °C for 5 min. The composition of the GOS mixture was analyzed by HPAEC-PAD following the method described previously.15d-Glc, d-Gal, lactose, and GOS components were identified and quantified using the external standard technique.
Intermolecular Galactosyl Transfer under Defined Initial Velocity Conditions
Initial velocities of d-Glc or d-Gal release were determined using 50 mM sodium phosphate buffer (pH 6.5) at 30 °C using either 10 mM oNPG or 100 mM lactose as substrate. These substrate concentrations were a compromise between the practical requirement to measure the initial velocity of d-Gal (and/or d-Glc) formation and to maximize the transfer of d-Gal to the external added nucleophile but not to the substrate. The final enzyme concentration used was ≤1.0 U/mL. The relationship between [oNP] (or [d-Glc]) and [d-Gal] was found to be linear up to 30 min. Thus, the standard reaction time of 20 min was used. voNP, vGlc, and vGal were obtained from measuring the molar concentrations of oNP, d-Glc, and d-Gal, respectively. The ratios of voNP and vGal were measured in the absence and presence of d-glucose with its concentration varied between 2.5 and 20 mM.
The intermolecular transgalactosylation to lactose was performed with various initial lactose concentrations (9–600 mM), whereas galactosyl transfer to either GlcNAc, GalNAc, or l-fucose was determined using 100 mM lactose with acceptor concentrations varying from 12.5 to 200 mM. After incubation of the reaction mixture for 20 min at 30 °C, the reaction was stopped by heating for 5 min at 95 °C. The rate of formation of oNP (voNP) was measured using the standard β-galactosidase assay, whereas d-galactose (vGal) or d-glucose (vGlc) measurement was carried out by HPLC (Dionex, Chelmsford, MA, USA) using an Aminex HPX-87K column (300 × 7.8 mm; Bio-Rad, Hercules, CA, USA) equipped with a refractive index detector. Water was used as mobile phase at a flow rate of 0.80 mL min–1, and the column temperature was 80 °C.
N-Acetyl Oligosaccharide Formation
N-Acetyl-oligosaccharide synthesis was carried out using lactose and GlcNAc (or GalNAc) as substrate with either Lbulβgal or Bbreβgal-II. The effects of temperature (30 and 50 °C), substrate concentration (0.6 and 1 M), molar ratio of donor to acceptor (1:2, 1:1 and 2:1), and enzyme concentration (2.5 and 5.0 U/mL) on the synthesis were also investigated. Substrates were dissolved in 50 mM sodium phosphate buffer (pH 6.5) containing 1 mM Mg2+. The enzyme was added, and the incubation was done at the required temperature at 300 rpm with a thermomixer (Eppendorf, Hamburg, Germany). Aliquots of samples were withdrawn at certain time intervals to determine the residual activities and carbohydrate content using either HPAEC-PAD as described by Splechtna et al.17 or an HPLC system consisting of a UV detector and the Hypercarb column (0.32 × 150 mm, inner diameter = 5 μm; Thermo Scientific). Ammonium formate buffer (0.3% formic acid, pH 9.0) was used as buffer A, and a gradient was performed from 0 to 35% acetonitrile within 35 min using a Dionex Ultimate 3000 pump (cap flow, 1 mL/min). The GlcNAc transgalactosylation yield was determined on the basis of the starting GlcNAc concentration and was calculated using eq 2.
| 2 |
Purification of N-Acetyl-oligosaccharides
For purification and identification of GlcNAc transfer products, a 10 mL discontinuous batch reaction using Bbreβgal-II (5 ULac/mL) was carried out at 30 °C using initial equimolar concentrations of lactose and GlcNAc (600 mM each) dissolved in 50 mM sodium phosphate buffer (pH 6.5) with 1 mM Mg2+. Agitation was at 300 rpm on a rotary shaker. After 4 h, the reaction was stopped by heating at 95 °C. GlcNAc-containing oligosaccharides were prepared also with Lbulβgal in a similar way, and here the reaction conditions were 2.5 ULac/mL with 1 M lactose and 1 M GlcNAc at 50 °C. Due to the complex course of transgalactosylation reactions, the reaction mixture was partially purified by gel permeation chromatography on Bio-Gel P2 (2.0 × 100 cm) equilibrated in water containing 5% (v/v) ethanol and 0.0015% (w/v) NaCl. The elution was followed by UV reading at 210 nm to detect the presence of GlcNAc and transgalactosylation products. The fractions containing the desired transgalactosylation products were pooled, freeze-dried, and redissolved in acetonitrile. The complete purification of the transgalactosylation products was obtained by using an HPLC system and the Hypercarb column as described above.
NMR Measurements
NMR spectra were recorded at 27 °C in 99.9% D2O on a Bruker Avance III 600 spectrometer (1H at 600.13 MHz and 13C at 150 MHz) equipped with a BBFO broad-band inverse probe head and z-gradients using standard Bruker NMR software. COSY experiments were recorded using the program cosygpqf with 2048 × 256 data points, respectively, per t1 increment. Multiplicity-edited HSQC spectra were recorded using hsqcedetgp with 1024 × 128 data points and 16 scans, respectively, per t1 increment. 1H NMR spectra were referenced to internal DSS (δ = 0); 13C NMR spectra were referenced to external 1,4-dioxane (δ = 67.4).
Results and Discussion
We studied in detail different β-galactosidases from probiotic strains of lactic acid bacteria (Lreuβgal and Lbulβgal) and bifidobacteria (Bbreβgal-I and Bbreβgal-II) with respect to their propensity to transfer galactosyl moieties onto certain sugar acceptors. Although the biochemical properties of these enzymes have been investigated in earlier works,14−17 detailed kinetic analyses of the formation of oligosaccharides based on the transfer constants (kNu/kwater) (Scheme 1) have not been reported.
Hydrolysis versus Transgalactosylation Using Lactose as Substrate
The measurement of the d-Glc/d-Gal ratio as a function of the reaction time provides a good estimate as to what extent transgalactosylation (onto lactose, d-Glc, or d-Gal as acceptors) competes with hydrolysis during lactose conversion. This ratio, however, does not accurately reflect the true extent of lactose conversion because a transfer of the galactosyl moiety can occur via either intramolecular or intermolecular reaction.
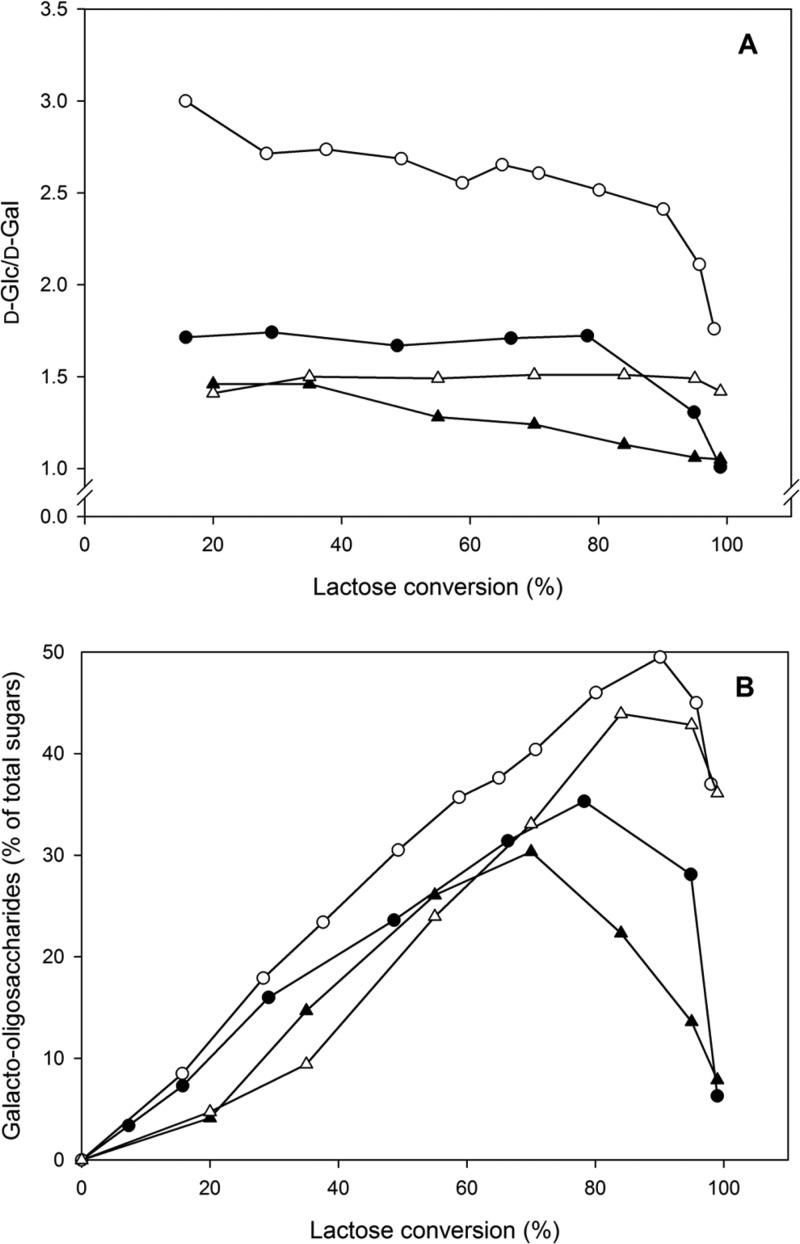
The formation of d-Glc and d-Gal was monitored over the entire course of the conversion using an initial lactose concentration of 600 mM (Figure 1A). At all times, the ratio of d-Glc/d-Gal was higher for Lbulβgal when compared with that of Lreuβgal, Bbreβgal-I, and Bbreβgal-II, indicating that this enzyme shows a more pronounced transferase activity. The maximum value of the d-Glc/d-Gal ratio for Lbulβgal was 3.0 at 16% lactose conversion, where trisaccharides form predominantly. This had also been confirmed in our previous work showing that β-d-Galp-(1→6)-β-d-Galp-(1→4)-d-Glc and β-d-Galp-(1→3)-β-d-Galp-(1→4)-d-Glc are the main transgalactosylation products at the beginning of the reaction when using this enzyme. This ratio decreased to 2.71 at ∼30% lactose conversion, then remained constant until lactose conversion was ∼70%, and decreased dramatically with >90% lactose conversion. The same trend was observed for Bbreβgal-I, where the highest d-Glc/d-Gal ratio was observed at the initial stage of the reaction and further decreased as the reaction progressed. For Lreuβgal and Bbreβgal-II, maximum values of the d-Glc/d-Gal ratio were found over a rather broad range of lactose conversion (20–80%). At about 98–99% lactose conversion, the d-Glc/d-Gal ratio was close to 1.0 for Lreuβgal and Bbreβgal-I, suggesting that lactose and all GOS formed were extensively hydrolyzed. In contrast, the d-Glc/d-Gal ratio with Lbulβgal and Bbreβgal-II was still 1.76 and 1.42, respectively, at almost complete lactose conversion, implying that a significant amount of GOS resisted hydrolysis even when lactose conversion was almost 100%.
Figure 1.
d-Glucose/d-galactose ratio (A) and formation and degradation of galacto-oligosaccharide (B) during lactose conversion by β-galactosidases from L. reuteri (●), L. bulgaricus (○), B. breve βgal-I (▲), and B. breve βgal-II (△). The reactions were performed at 30 °C at an initial lactose concentration of 200 g L–1 in sodium phosphate buffer (pH 6.5) and 1 mM MgCl2.
The ratio of d-Glc/d-Gal measured can be related directly to the level of GOS formation when these enzymes are used for the reaction with lactose. Lbulβgal, which exhibited the highest d-Glc/d-Gal ratio, showed the highest GOS yield at all lactose conversion levels compared to that of the other three βgals (Figure 1B). The yields as well as the type of GOS formed differ significantly among the βgals studied. Overall, Lreuβgal and Lbulβgal yielded the same mixture of GOS, which is different from those of Bbreβgal-I and Bbreβgal-II. Typical HPLC chromatograms of GOS formed by Lbulβgal and Bbreβgal-II are depicted in Figure S1.
Partitioning Analysis
The transfer constant kNu/kwater, which is obtained from the velocity ratios of vGlc/vGal or voNP/vGal, provides a useful tool to measure the ability of a certain substance to act as a galactosyl acceptor (i.e., a nucleophile, Nu), which in turn allows an estimation of the level of transgalactosylation products obtained from a known reaction mixture. Initial velocities were measured at 30 °C in 50 mM sodium phosphate buffer (pH 6.5). Plots of (voNP/vGal) or (vGlc/vGal) against [Nu] were linear for a specific range of the acceptor concentration. Deviation from linearity, which occurred mainly at low and high concentrations of the nucleophile, may be due to competition for binding to the nucleophile-binding site of the galactosyl-enzyme intermediate [E-Gal].18 The F test at 95% probability level confirmed the validity of the linear fit for the range of [Nu] as shown in Figure S2. Moreover, the fit of the lines as represented by r2 was usually >0.98. When kGlc/kwater was determined, Bbreβgal-II showed the lowest partitioning ratio (3.91 ± 0.44 M–1), whereas Lbulβgal gave the highest (9.36 ± 0.56 M–1) as shown in Table 1. Likewise, when lactose alone was used as the substrate, where the only possible galactosyl acceptors are lactose and its hydrolysis products, d-Gal and d-Glc, Bbreβgal-II showed the lowest kLac/kwater ratio (0.53 M–1), whereas that of Lbulβgal was the highest (2.79 M–1), again confirming the high transferase activity of this latter enzyme.
Table 1. Partitioning Ratios (kNu/kwater, M–1) for the Reaction of β-Galactosidases with Exogenous Nucleophiles and with Water.
| nucleophile |
|||||
|---|---|---|---|---|---|
| β-galactosidase source | d-Glca | lactose | GlcNAc | GalNAc | l-fucose |
| B. breve | |||||
| βgal-I | 6.73 ± 0.62 | 1.61 ± 0.05 | 1.01 ± 0.03 | 0.36 ± 0.03 | 1.27 ± 0.12 |
| βgal-II | 3.91 ± 0.44 | 0.53 ± 0.02 | 5.42 ± 0.05 | 0.39 ± 0.02 | 1.16 ± 0.05 |
| L. bulgaricus | 9.36 ± 0.56 | 2.79 ± 0.15 | 16.8 ± 0.7 | 3.21 ± 0.26 | 0.54 ± 0.05 |
| L. reuteri | 6.7 ± 0.3b | 1.91 ± 0.12b | 0.27 ± 0.01 | 1.07 ± 0.09 | 0.67 ± 0.06 |
Measured with 10 mM oNPGal as substrate and calculated from νoNP/νGal
Reference (17).
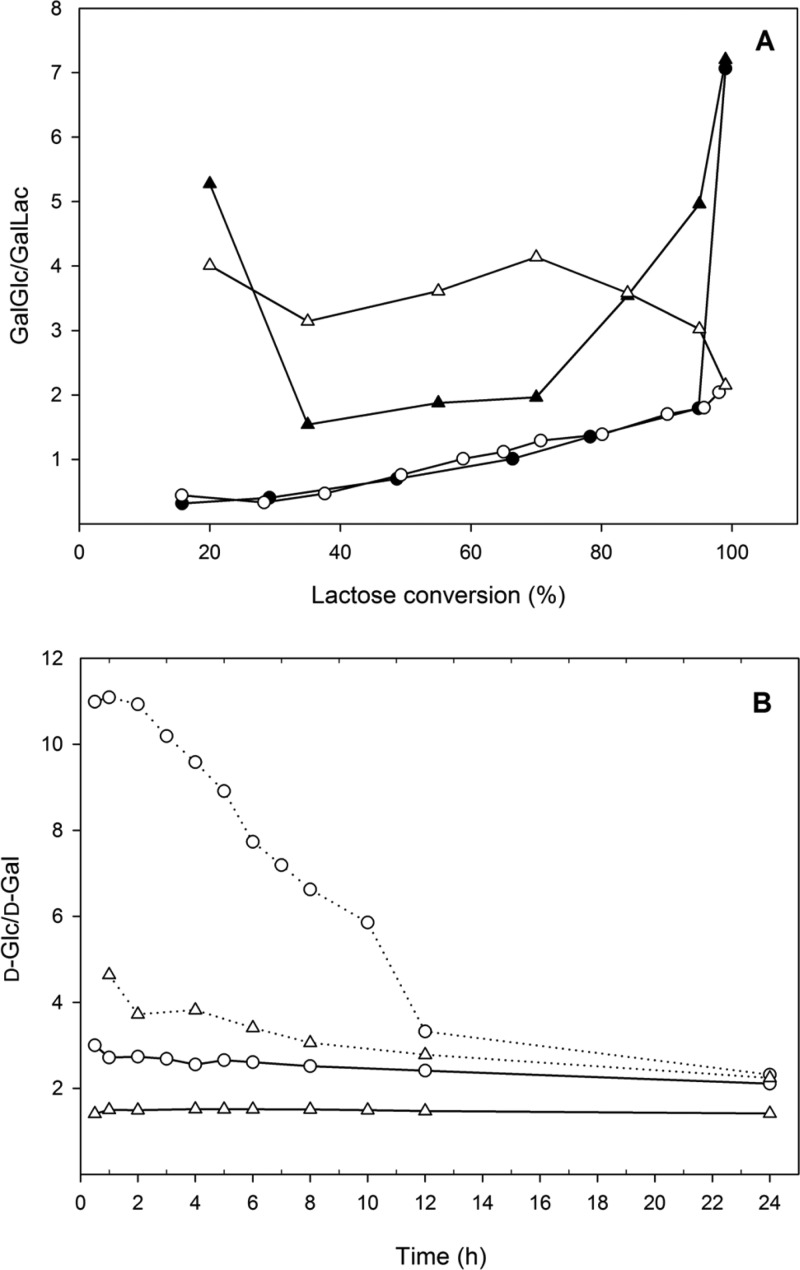
When kGlc/kLac was determined (obtained from the ratio of kGlc/kwater to kLac/kwater), Bbreβgal-II showed the highest ratio of 7.4 and Bbreβgal-I, a ratio of 4.2, whereas Lreuβgal and Lbulβgal showed lower ratios of 3.4 and 3.3, respectively. These values suggest that d-Glc is a far better galactosyl acceptor for the two bifidobacterial β-galactosidases than lactose when compared to the two lactobacillal β-galactosidases. Hence, disaccharides other than lactose will make up a large proportion of GOS mixtures formed with the two bifidobacterial enzymes. Figure 2A confirms that d-glucose is in fact a better galactosyl acceptor than d-lactose when looking at the ratio of total GalGlc disaccharides to Galβ-d-Galp-(1→4)-d-Glc trisaccharides formed. This was especially pronounced at the beginning of the reaction, where this ratio was ∼5 for Bbreβgal-I or ∼4 for Bbreβgal-II at 20% lactose conversion, whereas it was 0.44 for both Lbulβgal and Lreuβgal. We reported in our recent study that the predominant oligosaccharide product of both bifidobacterial enzymes was β-d-Galp-(1→6)-d-Glc (allolactose), accounting for approximately 45 and 50% of the GOS formed by transgalactosylation by Bbreβgal-I and Bbreβgal-II, respectively, at maximum total GOS yield,15 confirming the results predicted by the partionining coefficients.
Figure 2.
GalGlc/Galβ-d-Galp-(1→4)-d-Glc (or GalGlc/GalLac) ratio during lactose conversion (A) and d-Glc/d-Gal ratio during galacto-oligosaccharides (solid line) and N-acetyl-oligosaccharides (dashed line) formation (B). The reactions for galacto-oligosaccharide production were performed at 30 °C at an initial lactose concentration of 200 g L–1 in sodium phosphate buffer (pH 6.5) and 1 mM MgCl2. The reactions for N-acetyl-oligosaccharide production were at 30 °C with equimolar lactose and GlcNAc (600 mM each) in sodium phosphate buffer (pH 6.5) and 1 mM MgCl2. The enzymes are β-galactosidases from L. reuteri (●), L. bulgaricus (○), B. breve βgal-I (▲), and B. breve βgal-II (△).
Furthermore, the propensity of the four β-galactosidases to transfer the galactose moiety to either GlcNAc, GalNAc, or Fuc was determined using a fixed lactose concentration (100 mM) as galactosyl donor and the respective nucleophile in various concentrations as galactosyl acceptor. In the presence of 100 mM lactose and the absence of any external galactosyl acceptor, vGlc/vGal was found to be ∼1.3 for Lbulβgal, suggesting GOS formation even at this low lactose concentration, whereas vGlc/vGal of the other three βgals was nearly 1.0, indicating that hydrolysis of lactose is the main reaction.
Both Lbulβgal and Bbreβgal-II effectively transferred the galactosyl moiety to GlcNAc rather than to water as indicated by the rate constant ratios kGlcNAc/kwater of 16.8 and 5.42 M–1 (Table 1), respectively. Bbreβgal-II and Lbulβgal also showed high preference to transfer galactosyl moiety to GlcNAc rather than to lactose, and GlcNAc is a ∼10- and 6-fold better galactosyl acceptor than lactose for these two enzymes. The kGlcNAc/kGlc ratios of Lbulβgal and Bbreβgal-II (1.8 and 1.4, respectively) indicate furthermore that GlcNAc is also the preferred galactosyl acceptor over glucose. This altogether suggests that GlcNAc is an excellent acceptor for Lbulβgal and Bbreβgal-II and that the disaccharide Gal-GlcNAc (positional isomers thereof) will be the main products rather than tri-GOS or other GOS when using a mixture of GlcNAc and lactose as substrate.
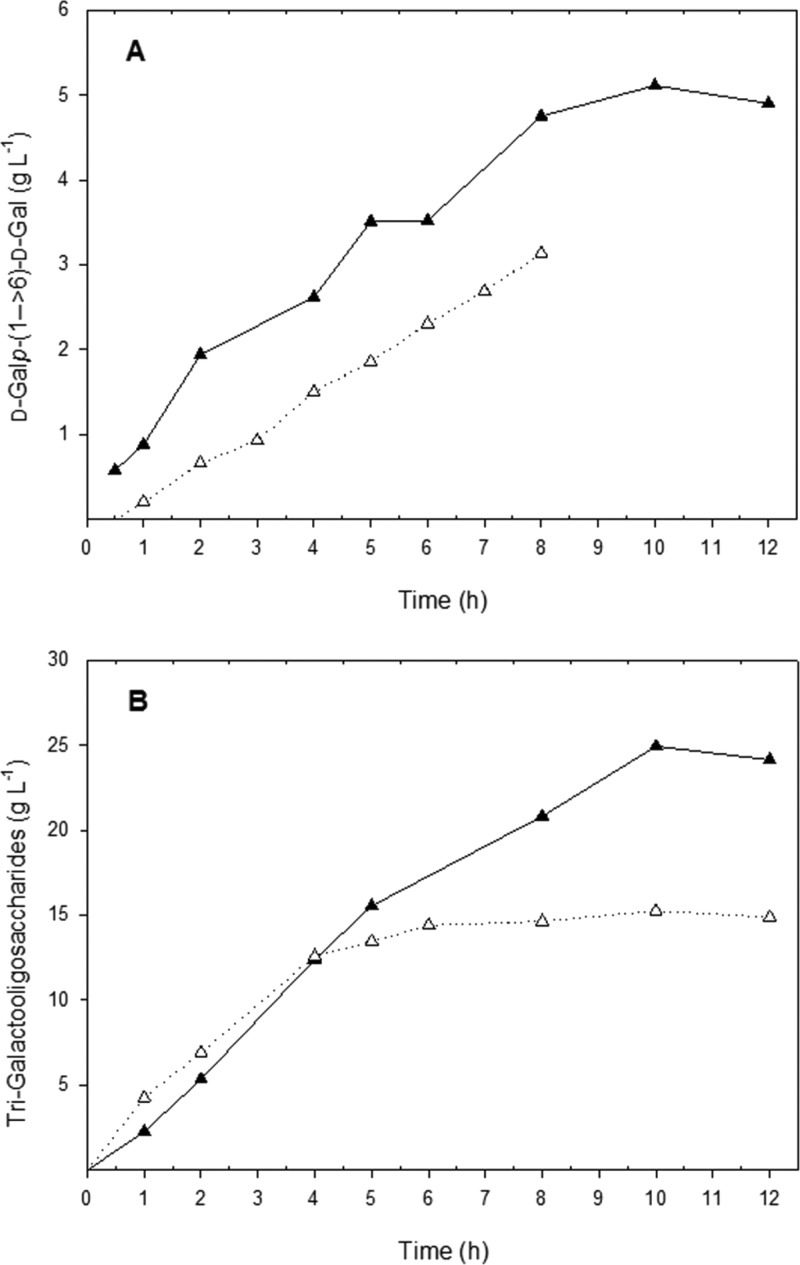
To confirm this assumption, discontinuous conversion reactions using either Bbreβgal-II or Lbulβgal (2.5ULac/mL) were carried out with 600 mM lactose in the absence and presence of 600 mM GlcNAc. The formation of d-Glc, d-Gal, and oligosaccharides was determined at different time intervals. Figure 2B shows that when using lactose alone as the substrate, the maximum d-Glc/d-Gal ratios with Lbulβgal and Bbreβgal-II were 3.0 and 1.5, respectively, whereas the presence of GlcNAc with lactose as substrates resulted in significantly higher d-Glc/d-Gal ratios of 11.0 and 4.6, respectively. The 3-fold increase in the d-Glc/d-Gal ratio suggests that the transferase activity of the enzymes was significantly increased over hydrolysis and that the galactosyl moiety was transferred preferentially to GlcNAc rather than onto lactose or glucose. For example, the presence of GlcNAc resulted in a decrease of GalGal formation by Bbreβgal-II, particularly of 6′-galactobiose (β-d-Galp-(1→6)-d-Gal), as is shown in Figure 3A. Moreover, the synthesis of GOS trisaccharides by Bbreβgal-II decreased significantly from 24 to 13 g/L when GlcNAc was present together with lactose as substrates (Figure 3B). In addition, HPLC analysis showed the presence of a prominent, novel oligosaccharide peak when GlcNAc was added to the reaction mixture (see below). Overall, this indicates that GlcNAc can be an excellent galactosyl acceptor for certain β-galactosidases such as Lbulβgal and Bbreβgal-II.
Figure 3.
Formation of 6′-galactobiose (β-d-Galp-(1→6)-d-Gal) (A) and trigalacto-oligosaccharides (B) during lactose conversion catalyzed by B. breve βgal-II in the absence (solid line) and presence (broken line) of N-acetyl-d-glucosamine. The reactions were performed at 30 °C at an initial lactose concentration of 600 mM in sodium phosphate buffer (pH 6.5) and 1 mM MgCl2. Initial GlcNAc concentration used was 600 mM.
N-Acetyl-d-galactosamine can also serve as galactosyl acceptor when using Lbulβgal as judged by the measured kGalNAc/kwater ratio (3.21 M–1). Bbreβgal-I, Bbreβgal-II, and Lreuβgal showed kGalNAc/kwater ≤ 1.0 M–1, suggesting that hydrolysis is the preferred reaction in the presence of GalNAc. l-Fucose, on the other hand, was shown to be a weak nucleophilic acceptor for all four enzymes based on the kFuc/kwater ratio (≤1.27 M–1).
The ratio of kGal/kwater would also be an essential kinetic parameter to measure the propensity to transfer the galactosyl moiety to another galactose unit. Unfortunately, kGal/kwater could not be determined because the amount of galactose released cannot be measured accurately in the presence of an excess of free galactose.
Formation of GalNAc-Containing Transgalactosylation Products
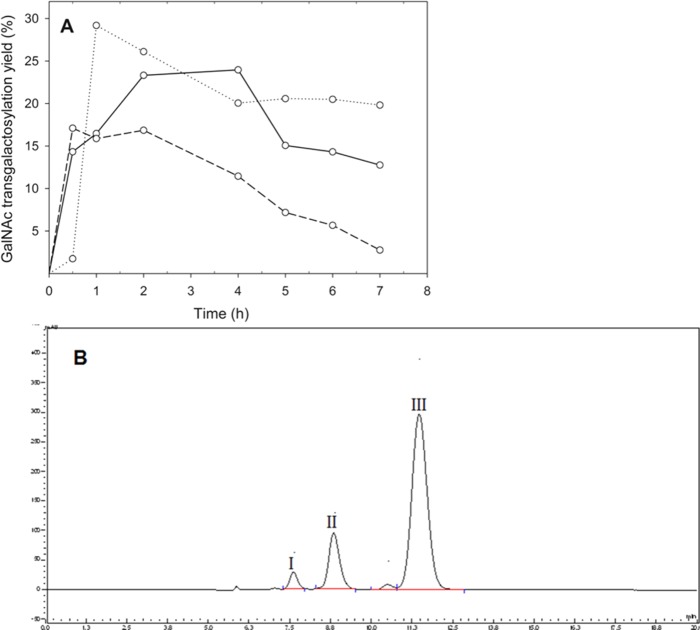
It was shown earlier that GalNAc can also serve as galactosyl acceptor when using Lbulβgal, based on the measured kGalNAc/kwater ratio (3.21 M–1). The formation of N-acetyl-oligosaccharides with lactose and N-acetyl-d-galactosamine (GalNAc) using Lbulβgal as biocatalyst was hence investigated. The maximum GalNAc transgalactosylation yield (29.2%) was obtained after 1 h with a donor/acceptor molar ratio of 2:1 and initial concentrations of 600 mM lactose and 300 mM GalNAc (Figure 4A). The HPLC profile showed that both di- and trisaccharides containing GalNAc were formed; however, the individual components were not structurally identified or quantified (Figure 4B).
Figure 4.
(A) GalNAc transgalactosylation yield at different initial lactose and GlcNAc molar ratios (solid line, 600 mM lactose and 600 mM GalNAc; dotted line, 600 mM lactose and 300 mM GalNAc; short dash line, 300 mM lactose and 600 mM GalNAc) and (B) HPLC-UV profile of GalNAc-containing oligosaccharides catalyzed by β-galactosidase from L. bulgaricus. The reaction was done in 50 mM sodium phosphate buffer (pH 6.5) containing 1 mM Mg2+ incubated at 50 °C. Peaks I and II are the tri- and di-GalNAc-containing oligosaccharides, respectively, and peak III is the free GalNAc.
Structural Characterization of GlcNAc Transgalactosylation Products
The effects of enzyme concentrations and the molar ratios of the donor (lactose) to the acceptor (GlcNAc) were studied to obtain the maximum yield of GlcNAc-containing transgalactosylation products. The molar ratio of the donor (lactose) to the acceptor (GlcNAc) of 1:1 (0.6 M lactose and 0.6 M GlcNAc) was found to be optimal for both enzymes, βgal from L. bulgaricus and βgal-II from B. breve (data not shown). Hence, the optimal conditions for the transgalactosylation reactions for βgal from L. bulgaricus (2.5 ULac/mL with 1 M lactose and 1 M GlcNAc at 50 °C) and for βgal-II from B. breve (5.0 ULac/mL with 0.6 M lactose and 0.6 M GlcNAc at 30 °C) were employed for the formation of N-acetyl-oligosaccharides. The reaction at 50 °C was carried out only with Lbulβgal because Bbreβgal-II is not stable at higher temperature.
The products formed in these reactions were then separated using a Hypercarb column. This chromatographic column can also separate the anomeric forms of reducing sugars, and thus each oligosaccharide is represented by two peaks constituting the anomeric isomers.19 The chromatographic patterns and the compounds synthesized by Lbulβgal and Bbreβgal-II were found to be similar as judged by HPLC analysis (Figure S3).
The major product was purified as described under Materials and Methods. This major disaccharide product was found to be β-d-Galp-(1→6)-d-GlcNAc (N-acetyl-allolactosamine) as identified by the NMR data. Despite the presence of the anomeric forms of the reducing glucosamine unit (α/β ratio ∼ 1.4:1), which led to two sets of spin-coupled systems, a full assignment could be achieved based on COSY and edited HSQC spectra (Figure S4; Table S1) and showed a low-field shift of carbon 6 of the reducing GlcNAc to 69.4 ppm. The data, when corrected for different referencing of chemical shifts, were in full agreement with published 13C NMR data of N-acetyl-allolactosamine.20−22 Peaks 2, 4, 6, and 7 (Figure S3) were not identified; however, on the basis of the elution rate, peaks 6 and 7 might be assigned to the trisaccharides GalGalGlcNAc, but the exact identities of these compounds have not been determined.
Under the optimal conditions, the maximum yields of N-acetyl-allolactosamine, calculated as the percentage of initial GlcNAc, were 41 and 24% with Lbulβgal and Bbreβgal-II, respectively (data not shown). The formation of disaccharides as a product of transgalactosylation of GlcNAc using β-galactosidases from different organisms has been reported, and the linkage preference of the transgalactosylation products varies for different enzymes. βgals from K. lactis, L. bulgaricus, and L. plantarum synthesized N-acetyl-allolactosamine as the major product and N-acetyl-lactosamine (LacNAc) as a minor product.9,11 βgals from Bifidobacterium bifidum and Bacillus circulans favored the formation of LacNAc over N-acetyl-allolactosamine,22−24 whereas N-acetyl-allolactosamine was exclusively synthesized with βgals from Penicillum multicolor, Aspergillus oryzae, and Bifidobacterium longum.22 The presence of higher DP N-acetyl-oligosaccharides in the reaction mixtures of transgalactosylation by using βgals from Sulfolobus solfataricus, A. oryzae, or E. coli was also observed, but they were not identified.25
In conclusion, kinetic analyses of β-galactosidases from L. reuteri, L. bulgaricus, B. breve (βgal-I and βgal-II) with various sugars as nucleophile provided an insight into the specificities of the given enzymes for transgalactosylation and formation of hetero-oligosaccharides. The transgalactosylation reaction with GlcNAc using Lbulβgal and Bbreβgal-II showed high yields of N-acetyl-oligosaccharides, of which N-acetyl-allolactosamine β-d-Galp-(1→6)-d-GlcNAc is dominant. Although the major product formed is not similar to the core structures of human milk oligosaccharides (HMO), which are lacto-N-biose (LNB, β-d-Galp-(1→3)-d-GlcNA, type I) or N-acetyl-lactosamine (LacNAc, β-d-Galp-(1→4)-d-GlcNAc, type II), this hetero-oligosaccharide might be of interest because of its potentially extended functionality in addition to galacto-oligosaccharides.
Acknowledgments
We thank Dr. Andreas Hofinger (Department of Chemistry, BOKU) for recording the NMR spectra.
Glossary
Abbreviations Used
- βgal
β-galactosidase
- Lreuβgal
β-galactosidase from L. reuteri L103
- Lbulβgal
β-galactosidase from L. delbrueckii subsp. bulgaricus DSM 20081
- Bbreβgal-I
β-galactosidase from B. breve DSM 20281 (βgal-I)
- Bbreβgal-II
β-galactosidase from B. breve DSM 20281 (βgal-II)
- Lac
lactose
- Glc
glucose
- Gal
galactose
- Fuc
l-fucose
- GlcNAc
N-acetyl-d-glucosamine
- GalNAc
N-acetyl-d-galactosamine
- LacNAc
N-acetyl-lactosamine
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jafc.5b06009.
Figure S1. Separation and quantification by HPAEC-PAD of individual GOS produced during lactose conversion. Figure S2. Ratio of initial velocities vGlu/vGal in the presence of different exogenous nucleophiles. Figure S3. HPLC-UV chromatogram of N-acetyl oligosaccharides produced by β-galactosidases from L. bulgaricus and B. breve βgal-II. Figure S4. Multiplicity edited HSQC spectrum of β-d-Galp-(1→6)-d-GlcNAc. Table S1. NMR assignments for N-acetyl-allolactosamine. (PDF)
This work was supported by the Austrian Science Fund FWF (Grant P24868-B22 to T.-H.N.). S.L.A. and M.I. are thankful for a Technology Grant Southeast Asia, and P.W. acknowledges receipt of an Ernst Mach Grant, both jointly supported by the ASEAN–European Academic University Network (ASEA-UNINET), the Austrian Federal Ministry of Science, Research and Economy, and the Austrian Agency for International Cooperation in Education and Research (OeAD-GmbH).
The authors declare no competing financial interest.
Supplementary Material
References
- Boon M. A.; Janssen A. E. M.; van’t Riet K. Effect of temperature and enzyme origin on the enzymatic synthesis of oligosaccharides. Enzyme Microb. Technol. 2000, 26, 271–281. 10.1016/S0141-0229(99)00167-2. [DOI] [PubMed] [Google Scholar]
- Yanahira S.; Yabe Y.; Nakakoshi M.; Miura S.; Matsubara N.; Ishikawa H. Structures of novel acidic galactooligosaccharides synthesized by Bacillus circulans β-galactosidase. Biosci., Biotechnol., Biochem. 1998, 62, 1791–1794. 10.1271/bbb.62.1791. [DOI] [PubMed] [Google Scholar]
- Lee Y. J.; Kim C. S.; Oh D. K. Lactulose production by β-galactosidase in permeabilized cells of Kluyveromyces lactis. Appl. Microbiol. Biotechnol. 2004, 64, 787–793. 10.1007/s00253-003-1506-1. [DOI] [PubMed] [Google Scholar]
- Miyasato M.; Ajisaka K. Regioselectivity in β-galactosidase-catalyzed transglycosylation for the enzymatic assembly of d-galactosyl-d-mannose. Biosci., Biotechnol., Biochem. 2004, 68, 2086–2090. 10.1271/bbb.68.2086. [DOI] [PubMed] [Google Scholar]
- Bridiau N.; Taboubi S.; Marzouki N.; Legoy M. D.; Maugard T. β-Galactosidase catalyzed selective galactosylation of aromatic compounds. Biotechnol. Prog. 2006, 22, 326–330. 10.1021/bp050230n. [DOI] [PubMed] [Google Scholar]
- Gänzle M. G.; Haase G.; Jelen P. Lactose: crystallization, hydrolysis and value-added derivatives. Int. Dairy J. 2008, 18, 685–694. 10.1016/j.idairyj.2008.03.003. [DOI] [Google Scholar]
- Gänzle M. G. Enzymatic synthesis of galacto-oligosaccharides and other lactose derivatives (hetero-oligosaccharides) from lactose. Int. Dairy J. 2012, 22, 116–122. 10.1016/j.idairyj.2011.06.010. [DOI] [Google Scholar]
- Kim Y. S.; Park C. S.; Oh D. K. Lactulose production from lactose and fructose by a thermostable β-galactosidase from Sulfolobus solfataricus. Enzyme Microb. Technol. 2006, 39, 903–908. 10.1016/j.enzmictec.2006.01.023. [DOI] [Google Scholar]
- Black B. A.; Lee V. S.; Zhao Y. Y.; Hu Y.; Curtis J. M.; Gänzle M. G. Structural identification of novel oligosaccharides produced by Lactobacillus bulgaricus and Lactobacillus plantarum. J. Agric. Food Chem. 2012, 60, 4886–4894. 10.1021/jf300917m. [DOI] [PubMed] [Google Scholar]
- Li W.; Sun Y.; Ye H.; Zeng X. Synthesis of oligosaccharides with lactose and N-acetylglucosamine as substrates by using β-d-galactosidase from Bacillus circulans. Eur. Food Res. Technol. 2010, 231, 55–63. 10.1007/s00217-010-1254-2. [DOI] [Google Scholar]
- Bridiau N.; Maugard T. A comparative study of the regioselectivity of the beta-galactosidases from Kluyveromyces lactis and Bacillus circulans in the enzymatic synthesis of N-acetyl-lactosamine in aqueous media. Biotechnol. Prog. 2011, 27, 386–394. 10.1002/btpr.542. [DOI] [PubMed] [Google Scholar]
- Richard J. P.; Westerfeld J. G.; Lin S.; Beard J. Structure-reactivity relationships for β-galactosidase (Escherichia coli, lac Z). 2. Reactions of the galactosyl-enzyme intermediate with alcohols and azide ion. Biochemistry 1995, 34, 11713–11724. 10.1021/bi00037a008. [DOI] [PubMed] [Google Scholar]
- Nguyen T.-H.; Splechtna B.; Yamabhai M.; Haltrich D.; Peterbauer C. Cloning and expression of the β-galactosidase genes from Lactobacillus reuteri in Escherichia coli. J. Biotechnol. 2007, 129, 581–591. 10.1016/j.jbiotec.2007.01.034. [DOI] [PubMed] [Google Scholar]
- Nguyen T. T.; Nguyen H. A.; Arreola S. L.; Mlynek G.; Djinovic-Carugo K.; Mathiesen G.; Nguyen T.-H.; Haltrich D. Homodimeric β-galactosidase from Lactobacillus delbrueckii subsp. bulgaricus DSM 20081: expression in Lactobacillus plantarum and biochemical characterization. J. Agric. Food Chem. 2012, 60, 1713–1721. 10.1021/jf203909e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arreola S. L.; Intanon M.; Suljic J.; Kittl R.; Pham N. H.; Kosma P.; Haltrich D.; Nguyen T.-H. Two β-galactosidases from the human isolate Bifidobacterium breve DSM 20213: molecular cloning and expression, biochemical characterization and synthesis of galacto-oligosaccharides. PLoS One 2014, 9, 0104056. 10.1371/journal.pone.0104056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.-H.; Splechtna B.; Steinböck M.; Kneifel W.; Lettner H. P.; Kulbe K. D.; Haltrich D. Purification and characterization of two novel beta-galactosidases from Lactobacillus reuteri. J. Agric. Food Chem. 2006, 54, 4989–4998. 10.1021/jf053126u. [DOI] [PubMed] [Google Scholar]
- Splechtna B.; Nguyen T.-H.; Steinböck M.; Kulbe K. D.; Lorenz W.; Haltrich D. Production of prebiotic galacto-oligosaccharides from lactose using β-galactosidases from Lactobacillus reuteri. J. Agric. Food Chem. 2006, 54, 4999–5006. 10.1021/jf053127m. [DOI] [PubMed] [Google Scholar]
- Petzelbauer I.; Splechtna B.; Nidetzky B. Galactosyl transfer catalyzed by thermostable β-glycosidases from Sulfolobus solfataricus and Pyrococcus furiosus: kinetic studies of the reactions of galactosylated enzyme intermediates with a range of nucleophiles. J. Biochem. 2001, 130, 341–349. 10.1093/oxfordjournals.jbchem.a002992. [DOI] [PubMed] [Google Scholar]
- Bao Y.; Chen C.; Newburg D. S. Quantification of neutral human milk oligosaccharides by graphitic carbon high-performance liquid chromatography with tandem mass spectrometry. Anal. Biochem. 2013, 433, 28–35. 10.1016/j.ab.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch P.; Yoon J. H. The effects of organic solvents on the synthesis of galactose disaccharides using β-galactosidases. Carbohydr. Res. 1997, 303, 339–345. 10.1016/S0008-6215(97)00182-1. [DOI] [Google Scholar]
- Sakai K.; Katsumi R.; Ohi H.; Usui T.; Ishido Y. Enzymatic syntheses of N-acetyllactosamine and N-acetylallolactosamine by the use of β-d-galactosidases. J. Carbohydr. Chem. 1992, 11, 553–565. 10.1080/07328309208016148. [DOI] [Google Scholar]
- Yoon J. H.; Rhee J. S. The efficient enzymatic synthesis of N-acetyllactosamine in an organic co-solvent. Carbohydr. Res. 2000, 327, 377–383. 10.1016/S0008-6215(00)00063-X. [DOI] [PubMed] [Google Scholar]
- Vetere A.; Paoletti S. High-yield synthesis of N-acetyllactosamine by regioselective transglycosylation. Biochem. Biophys. Res. Commun. 1996, 219, 6–13. 10.1006/bbrc.1996.0172. [DOI] [PubMed] [Google Scholar]
- Kaftzik N.; Wasserscheid P.; Kragl U. Use of ionic liquids to increase the yield and enzyme stability in the β-galactosidase catalysed synthesis of N-acetyllactosamine. Org. Process Res. Dev. 2002, 6, 553–557. 10.1021/op0255231. [DOI] [Google Scholar]
- Reuter S.; Nygaard A. R.; Zimmermann W. β-Galactooligosaccharide synthesis with β-galactosidases from Sulfolobus solfataricus, Aspergillus oryzae, and Escherichia coli. Enzyme Microb. Technol. 1999, 25, 509–516. 10.1016/S0141-0229(99)00074-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.