Abstract
Lipocalin-2 (LCN2), also known as neutrophil gelatinase-associated lipocalin (NGAL), is a secreted glycoprotein that belongs to a group of transporters of small lipophilic molecules in circulation. LCN2 has been recently characterized as an adipose-derived cytokine. This adipokine is believed to bind small substances, such as steroids and lipopolysaccharides, and has been reported to have roles in the induction of apoptosis in hematopoietic cells, transport of fatty acids and iron, modulation of inflammation, and metabolic homeostasis. Recently, LCN2 has emerged as a useful biomarker and rheumatic diseases. This review provides an overview of LCN2 in inflammation, immunity, and metabolism.
Keywords: Adipokines, adipose tissue, immunity, kidney diseases, rheumatic diseases
Introduction
White adipose tissue (WAT) is now recognized to be a true endocrine organ, which is able to secrete a wide variety of adipose-derived factors that have been collectively termed “adipokines”. Adipokines, which are also synthesized in other tissues, include a variety of pro-inflammatory factors with most of them being increased in obesity and appearing to contribute to the so-called “low-grade inflammatory state” in obese subjects.
Inflammation in obesity is also closely related to a cluster of metabolic disorders including cardiovascular complications and autoimmune and inflammatory diseases (Gregor & Hotamisligil, 2011; Lago et al., 2007). For instance, adipokines dysregulation is a clear component of metabolic-triggered inflammation that appears to play a major role in osteoarthritis (OA) and rheumatoid arthritis (RA) (Hu et al., 2015; Malemud, 2015).
Among the members of the adipokines superfamily, lipocalin-2 (LCN2) has emerged as a pleiotropic molecule involve in a variety of physiological and pathophysiological processes, such as metabolic homeostasis, apoptosis, infection, immune response, or inflammation. In fact, this adipokine has been proposed as a biomarker of acute kidney injury (AKI), lupus nephritis (LN), cardiovascular diseases, or intestinal inflammation. Thus, the aim of the present review is to present the potential of LCN2 as biomarker in inflammatory and metabolic diseases.
LCN2 biology: structure and receptors
LCN2, also known as neutrophil gelatinase-associated lipocalin (NGAL), 24p3, p25, migration-stimulating factor inhibitor, human neutrophil lipocalin, α1-microglobulin-related protein, siderocalin, or uterocalin, is a 25 kDa secreted glycoprotein encoded by a gene located at the chromosome locus 9q34.11. The LCN2 gene produces at least five functional transcripts, the most common of which encodes for a 198 amino acid-secreted protein. The mouse homolog of LCN2 is called lipocalin 2 (Lcn2). It is denoted by lower case (Lcn2 or Ngal) to distinguish it from its human counterpart (LCN2 or NGAL) (Kjeldsen et al., 2000).
LCN2 was initially identified as a secreted protein from human neutrophils (Kjeldsen et al., 1994). Belonging to the same lipocalin superfamily members of fatty acid binding proteins and retinol binding proteins, LCN2 possesses a lipid binding domain, capable of binding small hydrophobic molecules (Chu et al., 1998). LCN2 also has a ligand binding cavity that can explain how LCN2 interacts with bacterial and mammalian proteins termed siderophores. Siderophores are low molecular weight proteins produced by microorganisms (including bacteria and fungi) that bind specifically to the ferric form of iron (Bao et al., 2010).
It is not yet clear if there is a definite receptor for LCN2. The observation that LCN2 is a secreted protein that is internalized through an endocytotic mechanism suggests the existence of a LCN2 cell-surface receptor. However, its identification remains still elusive. Two receptors have been proposed: the solute carrier family 22 member 17 (SLC22A17 or 24p3R) that binds to mouse Lcn2 (Devireddy et al., 2005) and the megalin/glycoprotein GP330, a low-density lipoprotein receptor that binds human LCN2 protein (Hvidberg et al., 2005).
LCN2 is expressed in multiple tissues including uterus (Huang et al., 1999), immune cells (Borregaard & Cowland, 2006), liver, spleen, kidney in mice (Aigner et al., 2007), bone marrow, and in the tissues that are exposed to microorganisms (Cowland & Borregaard, 1997), and it has been recently identified in chondrocytes (Owen et al., 2008), although WAT is thought to be the main source (Yan et al., 2007).
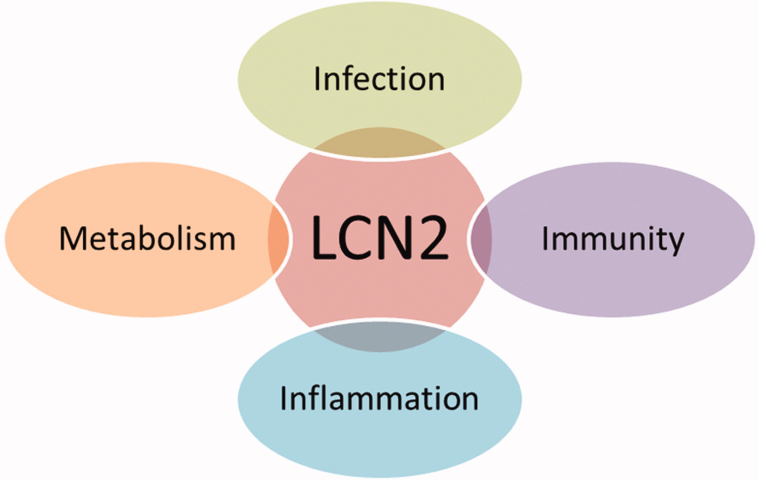
As outlined in the following subsections (Figure 1), LCN2 is involved in a series of processes such as apoptosis of hematopoietic cells (Devireddy et al., 2001), transport of fatty acids (Chu et al., 1998), and iron (Yang et al., 2002), modulation of inflammation (Cowland & Borregaard, 1997), and metabolic homeostasis (Yan et al., 2007). Moreover, LCN2 have been linked to the pathogenesis of metabolic disorders through its effects on inflammation (Gómez et al., 2011).
Figure 1.
Schematic representation of the main functions of lipocalin-2.
LCN2 in infection
LCN2 has been described as new component of the innate immune system and the acute phase response to infection. During infection, bacteria acquire much of their iron essential for grow from the host by synthesizing siderophores that scavenge iron and transport it into the pathogen. Enterobactin is a siderophore produced by gram-negative bacteria (such as Escherichia coli, Klebsiella, or Salmonella spp.). LCN2 binds to enterobactin, both in their iron-laden and iron-free state, and transports it through the LCN2 receptor (24p3R) into mammalian cells where the iron is stored. Bacteria require iron for their growth. Hence, by depleting iron stores, LCN2 inhibits bacterial growth (i.e. has a bacteriostatic effect) (Bao et al., 2010). This event is pivotal in the innate immune response to bacterial infection. Mice expressing LCN2 are more resistant to infections with such gram-negative bacteria, as compared to LCN2−/− littermates. Upon encountering invading bacteria the Toll-like receptors on immune cells stimulate the transcription, translation, and secretion of LCN2; secreted LCN2 then limits bacterial growth by sequestrating siderophores (Flo et al., 2004).
It is of interest, that LCN2 not only affects microbial iron delivery but also host iron homeostasis. This is most likely due to binding of 2,5-DHBA (2,5-dihydroxy benzoic acid), a recently identified mammalian siderophore, by LCN2 which then can shuttle iron across cellular membranes (Bao et al., 2010; Devireddy et al., 2010; Nairz et al., 2014). It has also been suggested that LCN2 stabilizes the labile iron/siderophore complex. LCN2-deficient mice (Lcn2KO) exhibit elevated intracellular labile iron. Srinivasan et al. (2012) reported that lipopolysaccharides (LPS)-induced systemic LCN2 by 150-fold in wild-type mice at 24 h. Further, cells from Lcn2KO mice were hyperresponsive to LPS ex vivo exhibiting elevated cytokine secretion. Desferroxamine, an iron chelator, significantly protects Lcn2KO mice from LPS-induced toxicity, including mortality, suggesting that LCN2 may act as an antioxidant in vivo by regulating iron homeostasis.
LCN2 not only protects against bacterial sepsis but also regulates host pro-inflammatory cytokine expression by limiting iron-mediated oxidative stress. Its small size and simple structure may make LCN2 as a deployable treatment for sepsis (Srinivasan et al., 2012). Furthermore, LCN2 exerts antiparasitic effects by maintaining host iron homeostasis and promoting innate and adaptive immune responses to blood-stage malaria infection (Zhao et al., 2012).
LCN2 in immunity
Recently, the work of La Manna et al. (2014) provided in vitro evidence that LCN2 is involved in cellular immunity. The potential role of LCN2 as an immunomodulatory molecule is based on its ability to induce immune tolerance by upregulating the expression of human leukocyte antigen G (HLA-G), a well-known tolerogenic molecule, on CD4 + T cells and expansion of T regulatory (Treg) cells in healthy donors. Deregulation of Treg cells may cause autoimmune diseases, including, type 1 diabetes mellitus, systemic lupus erythematosus (SLE), RA, and psoriasis (Dejaco et al., 2006). Thus, LCN2 may have an important protective function as an immune activator.
The complement system provides a link between the innate and adaptive immune systems. C3 components are important for recruiting inflammatory cells and have also been implicated in several acute and chronic inflammatory diseases (Hawlisch et al., 2004). Activated products of C3 are recognized by complement receptors CD21 and CD35. These receptors are predominantly expressed on B cells. Animals lacking the CD21/CD35 co-receptors are highly susceptible to infections. When gene expression in the spleen from CD21/CD35−/− mice was compared with that in the spleen from wild-type mice, a significant upregulation of LCN2 expression was observed. This upregulation of LCN2 could be decreased to levels close to that in the wild-type mice upon pretreatment with a pharmacological inhibitor of C3aR (receptor for C3a). This suggests a mechanism of CD21/CD35 regulated and C3-mediated regulation of LCN2 expression in the spleen resident immune cells of mice (Chakraborty et al., 2012; Jacobson et al., 2008).
The role of LCN2 in autoimmune diseases
During several acute and chronic inflammatory diseases, including immune complex (IC)-mediated inflammatory/autoimmune disorders, LCN2 is highly upregulated (Conde et al., 2011a). In IC-mediated autoimmune diseases, such as RA or SLE, autoantibodies bind to target host cells and initiate inflammation resulting in tissue injury (Nakamura & Takai, 2004). More recently, upregulation of LCN2, along with other inflammatory cytokines, has been reported in SLE patients (Li et al., 2014). However, the exact role of elevated LCN2 in autoimmune diseases is largely unknown. The study of Shashidharamurthy et al. (2013) was undertaken to investigate the role of LCN2 in IC-mediated diseases. Their results demonstrate that LCN2 may regulate immune cell recruitment to the site of inflammation, a process essential for the controlled initiation, perpetuation, and resolution of inflammatory processes. Thus, LCN2 may present a promising target in the treatment of IC-mediated inflammatory/autoimmune diseases.
LCN2 in inflammation
Although LCN2 is highly upregulated under a large number of inflammatory conditions, both pro- and anti-inflammatory properties of this adipokines have been reported.
In neutrophils, LCN2 secretion is highly regulated by the activation of inflammation and infection (Kjeldsen et al., 1994); LPS and TNFα are the two strong inducers of LCN2 production. Most intriguingly, LCN2 promoter possesses the binding sites of two key transcription factors, nuclear factor κB (NF-κB) and CCAAT/enhancer binding protein (C/EBP) (Shen et al., 2006), suggesting that transcriptional activation of this gene is associated with inflammation. Recently, Guo et al. suggest that LCN2 plays a role as an anti-inflammatory regulator of macrophage polarization and NF-κB/STAT3 pathway activation. They observed that in LCN2−/− bone marrow-derived macrophages (BMDMs) LPS stimulation elicited an increase in the activation of NF-κB, c-Jun, and STAT3 signaling pathways, as well as an upregulation of expression of NF-κB and STAT3 target genes IL-1β, IL-6, iNOS, and MCP-1 in LCN2−/− BMDMs compared with wild-type controls. Moreover, pretreatment of recombinant LCN2 attenuated LPS-stimulated degradation of IκBα and STAT3 phosphorylation as well as LPS-induced gene expression of IL-6 and iNOS in LCN2−/− BMDMs (Guo et al., 2014).
Furthermore, recent studies indicate that LCN2 expression and secretion by glial cells are induced by inflammatory stimuli in the central nervous system. After induction of experimental autoimmune encephalomyelitis (EAE), LCN2 expression was found to be strongly increased in spinal cord and secondary lymphoid tissues. In spleens, LCN2 and 24p3R were highly expressed in neutrophils and dendritic cells, respectively. Inflammatory infiltration and the expression of inflammatory mediators were significantly attenuated in LCN2-deficient mice as compared with wild-type animals. Recombinant LCN2 protein injection experiments suggested that LCN2 expression in spinal cord and peripheral immune organs contributes to EAE development. These results imply LCN2 as a critical pro-inflammatory mediator of autoimmune inflammation and disease development in EAE (Nam et al., 2014).
The role of LCN2 in musculoskeletal diseases
LCN2 is believed to have significant activity in inflammatory degenerative articular diseases, including OA and RA (Gómez et al., 2011). In addition, LCN2 has been proposed as a biomarker of cartilage degradation in arthritic diseases (Wilson et al., 2008).
LCN2 has been identified in chondrocytes (Owen et al., 2008). In these cells, LCN2 expression was modulated by IL-1β, leptin, adiponectin, LPS, and dexamethasone (Conde et al., 2011b). In addition, the synovial fluid from patients with knee OA was found to be enriched with MMP-9/LCN2 complexes that have been involved in matrix degradation (Gupta et al., 2007). Recently, the group of Katano confirmed that synovial fluid levels of LCN2 were significantly higher in patients with RA than in those with OA. Through a proteome analysis, they showed that granulocyte macrophage colony-stimulating factor (GM-CSF) can contribute to the pathogenesis of RA by upregulating LCN2 in neutrophils, followed by the induction of a series of enzymes, such as cathepsin D, transitional endoplasmic reticulum ATPase (TERA), and tranglutaminase 2 (tg2) in synoviocytes, which could contribute to the proliferation of synovial cells and infiltration of inflammatory cells inside the synovium (Katano et al., 2009). Very recently, the NEIRID group have showed that nitric oxide boosts TLR-4 mediated lipocalin expression in chondrocytes, suggesting the existence of a feedback loop regulating the expression of this adipokines (Gómez et al., 2013).
LCN2 in metabolism
LCN2 expression is altered in several pathologic conditions, such as adipose tissue hypoxia and obesity (Jang et al., 2012; Sommer et al., 2009). Nevertheless, whether LCN2 plays a role in the pathogenesis of obesity-related diseases has not been investigated so far.
Recent studies have reported the association between serum LCN2 concentrations and various metabolic parameters and inflammatory markers (Moreno-Navarrete et al., 2010; Wang et al., 2007; Yan et al., 2007). The study of Jang et al. provides the first clinical evidence demonstrating that serum concentrations of LCN2 are closely associated with obesity and its related chronic inflammation and metabolic complications. Patients with MetS showed higher levels of LCN2 than those without MetS. However, correlation between serum LCN2 concentration and the number of MetS components was not significant. Nonetheless, they suggest serum LCN2 as a useful biomarker for evaluating the outcomes in various clinical settings of obesity-related metabolic and cardiovascular disease (Jang et al., 2012).
LCN2 promoter possesses the binding sites of two key transcription factors, NF-κB and CCAAT/enhancer binding protein (C/EBP) (Shen et al., 2006), and glucocorticoid response element (Garay-Rojas et al., 1996), suggesting that transcriptional activation of this gene in adipose tissue may be associated with inflammation and obesity and may have a function in adipose tissue remodeling. LCN2 expression and secretion have been shown to be induced by two proinflammatory cytokines, IFNγ and TNFα, in cultured murine and human adipocytes. In the work of Zhao et al., they demonstrated that IFNγ and TNFα induced LCN2 expression and secretion in vivo. In knockdown experiments they showed that STAT1 is required for IFNγ-induced LCN2 expression in murine adipocytes. Similarly, knockdown of p65 in adipocytes demonstrated the necessity of the NF-κB signaling pathway for TNFα-mediated effects on LCN2. Activation of ERKs by IFNγ and TNFα also affected STAT1 and NF-κB signaling through modulation of serine phosphorylation. ERK activation-induced serine phosphorylation of both STAT1 and p65 mediated the additive effects of IFNγ and TNFα on LCN2 expression. They suggest that these same mechanisms occur in humans as they observed STAT1 and NF-κB binding to the human LCN2 promoter in chromatin immunoprecipitation assays performed in human fat cells (Zhao et al., 2014).
The molecular disruption of LCN2 increased body fat mass as well as exacerbated dyslipidemia, fatty liver, and insulin resistance upon high-fat diet (HFD) feeding compared with wild-type mice (Guo et al., 2010). However, in Law et al. work, LCN2 deficiency significantly alleviated HFD-induced insulin resistance, and the effect could be observed as early as 5 weeks after HFD feeding. Despite the enlarged fat mass they observed, inflammation and the accumulation of lipid peroxidation products are significantly attenuated by modulating 12-lipoxygenase and TNFα levels in the adipose tissues of LCN2-KO mice (Law et al., 2010).
In previous studies of the Xiaoli Chen group, they demonstrated that LCN2 is a critical regulator of energy metabolism, glucose and lipid homeostasis, and insulin resistance in LCN2-deficient mice (Guo et al., 2010,2012; Jin et al., 2011). Thiazolidinedione (TZD) treatment, which acts as a peroxisome proliferator-activated receptor-γ (PPARγ) agonist, was able to markedly improve HFD-induced insulin resistance and dyslipidemia in LCN2−/− mice (Jin et al., 2011). In a recent study, they investigated the regulation of LCN2 expression in adipose tissue in response to metabolic stress in mice, as well as the control of LCN2 expression and secretion by cytokines and nutrients in 3T3-L1 adipocytes. The mRNA expression of LCN2 was upregulated in white and brown adipose tissues as well as liver during fasting and cold stress in mice. Among pro-inflammatory cytokines, TNFα, IL-1β, and IL-6, IL-1β showed most profound effect on LCN2 expression and secretion in 3T3-L1 adipocytes. Insulin stimulated LCN2 expression and secretion in a dose-dependent manner; this insulin effect was significantly abolished in the presence of low concentration of glucose. Moreover, insulin-stimulated LCN2 expression and secretion was also attenuated by blocking NF-κB pathway activation (Zhang et al., 2014a). Furthermore, they reported LCN2 as a novel regulator of brown adipose tissue activation by modulating the adrenergic independent p38 MAPK-PGC-1α-UCP1 pathway (Zhang et al., 2014b).
LCN2 synthesis has also been described in bone (Bartsch & Tschesche, 1995). Costa and colleagues reported that LCN2 could modulate the bone marrow microenvironment by increasing the expression of one of the most important bone niche factors: stromal-derived factor 1, a chemokine involved in the recruitment of hemopoietic precursors (Costa et al., 2010). They recently found that LCN2 was expressed during osteoblast differentiation. By generating transgenic (Tg) mice over-expressing LCN2 in bone, they observed that Tg mice were smaller and presented thinner layer of cortical bone and a decreased trabecular number. In particular, Tg bones displayed a reduced osteoblast bone matrix deposition and osteoblast differentiation was slowed down. Differences were also observed in the growth plate of young Tg mice where chondrocyte displayed a more immature phenotype and a lower proliferation rate. Moreover, the expression of the conventional receptor activator of NF-κB ligand (RANKL) and of the IL-6 was enhanced in Tg mice. With this work, they found that LCN2 plays a role in bone development and turnover having both a negative effect on bone formation, by affecting growth plate development and interfering with osteoblast differentiation (Costa et al., 2013). Further studies are needed to clarify the function of LCN2 in modulating the bone microenvironment.
The potential of LCN2 as a biomarker
As an acute phase protein, LCN2 has become increasingly relevant in recent years as a potential clinical biomarker in inflammatory diseases. LCN2 levels in biological fluids are generally low, being upregulated in inflammatory state, which strongly indicates the potential of its use as a biomarker of disease onset and progression (Table 1).
Table 1. Potential value of LCN2 as a biomarker in several diseases.
| Biomarker | Disease | Reference |
|---|---|---|
| Plasma LCN2 | Arthritic diseases | Wilson et al. (2008) |
| Plasma LCN2 | Severe acute pancreatitis | Chakraborty et al. (2012) |
| Plasma LCN2 | Obesity-related metabolic | Jang et al. (2012) |
| Plasma LCN2 | Cardiovascular disease | Lindberg et al. (2014) |
| Urinary LCN2 | Acute kidney injury | Bolignano et al. (2008) |
| Urinary LCN2 | Lupus nephritis | Brunner et al. (2012) |
| Fecal LCN2 | Intestinal inflammation | Chassaing et al. (2012) |
| Plasma LCN2 | Multiple sclerosis | Berard et al. (2012) |
In a prospective study with 10-year outcome, plasma LCN2 associated strongly with all inflammatory markers. Even after adjustment for confounding risk factors by Cox regression analysis, LCN2 remained an independent biomarker of both all-cause mortality and major adverse cardiovascular event (Lindberg et al., 2014). In other study, assay of fecal Lcn2 by ELISA function as a noninvasive, sensitive, dynamic, stable, and cost-effective means to monitor intestinal inflammation in mice (Chassaing et al., 2012). In addition, as well it was pointed out before in this text, LCN2 has been proposed as a biomarker of cartilage degradation in arthritic diseases (Wilson et al., 2008).
Blood and urinary levels of LCN2 have been extensively studied as very promising biomarkers for an early diagnosis of AKI and for monitoring of chronic kidney disease severity, which may revolutionize our clinical practice in the near future.
LCN2 as biomarker in kidney inflammatory diseases
Plasma LCN2 is filtered by the glomerulus and almost totally reabsorbed in the proximal tubules (Filiopoulos et al., 2014). LCN2 is also released by renal tubular cells (Friedl et al., 1999). Urinary excretion of LCN2 is presumed when there is proximal renal tubular injury that avoids LCN2 reabsorption and/or increases de novo LCN2 synthesis. Thus, tubular cell damage leads to an increase in production and release of LCN2 in plasma and urine. It can readily be detected as it has low molecular weight and it is resistant against fragmentation.
Transcriptome and proteomic studies identified LCN2 to be one of the most upregulated genes and one of the most highly induced proteins in the kidney very early after AKI in animal models (Mishra et al., 2003; Yuen et al., 2006). The serendipitous detection of LCN2 in the urine and plasma in animal and human AKI has resulted in several translational studies to evaluate LCN2 as a noninvasive biomarker in human AKI. LCN2 is therefore one of the most promising next-generation biomarkers in the field of AKI (Bolignano et al., 2008). However, recent data suggest that LCN2 is not only an early predictor of AKI, but also seems to play an important role in chronic kidney disease (Nickolas et al., 2012).
Furthermore, the rapid determination of plasma LCN2 level provides valuable information quickly, concerning the distinction of acute pyelonephritis, for determining the clinical course of acute febrile urinary tract infection (Seo et al., 2014).
Nonetheless, there are some limitations in use LCN2 as a biomarker for the prediction of AKI, including lack of published studies that adhere to diagnostic study guidelines, heterogeneity in AKI definition, the lack of uniformly applicable cutoff values and variability in the performance of commercially available LCN2 assays (Haase-Fielitz et al., 2014).
Moreover, recent studies link together LCN2 and SLE. SLE is a multisystem inflammatory autoimmune disease in which renal involvement is common. It is a major cause of morbidity and mortality and predictor of poor prognosis. The diagnostic gold standard for LN is the renal biopsy, it is considered to give more information than laboratory tests used nowadays (creatinine levels and clearance, urine proteins amounts, and urine sediment or complement anti-double strand DNA antibodies). This is an invasive technique so biomarkers that ease the diagnosis, monitoring, and follow-up of this manifestation are being searched (Yang et al., 2012). Urinary LCN2 is a sensitive biomarker of renal injury (Elewa et al., 2015; Hammad et al., 2013). It is a predictor of LN in all SLE patients, but it also predicts LN flares in patients previously diagnosed with LN (Rubinstein et al., 2010). LCN2 has a role in LN activity, it is also a damage indicator, being a biomarker of LN chronicity (Brunner et al., 2012).
Conclusions
During the last two decades, there has been an increasing understanding of the relationships among metabolic syndrome, adipokines, and inflammatory diseases. In this regard, LCN2 can be considered one of the mediators responsible for the low-level systemic inflammation that is present in metabolic syndrome associated with obesity, emerging as a major player linking immunity and metabolism, acting at different levels as a regulator of multiple responses.
All the studies described above highlight the involvement of LCN2 in the modulation of both innate and adaptive immune response. Therefore, LCN2 could be considered a relevant potential therapeutic target in certain clinical situations, in which exists a dysfunctional immune system, as autoimmune diseases. Moreover, this adipokine has emerged as a potential biomarker for rheumatic and kidney diseases. However, more studies aimed at understanding the exact role of LCN2 in inflammatory processes and modulation of metabolism will be necessary.
Declaration of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Oreste Gualillo and Francisca Lago are Staff Personnel of Xunta de Galicia (SERGAS) through a research-staff stabilization contract (ISCIII/SERGAS). Oreste Gualillo is a member of RETICS Programme, RD12/0009/0008 (RIER: Red de Investigación en Inflamación y Enfermedades Reumáticas) via Instituto de Salud Carlos III (ISCIII) and FEDER. The Oreste Gualillo work was funded by Instituto de Salud Carlos III and FEDER (grants PI14/00016 and PIE13/00024). Ali Mobasheri is the co-ordinator of the D-BOARD Consortium funded by European Commission Framework 7 Programme (EU FP7; HEALTH.2012.2.4.5-2, project number 305815, Novel Diagnostics and Biomarkers for Early Identification of Chronic Inflammatory Joint Diseases). Ali Mobasheri is also a member of the Arthritis Research UK Centre for Sport, Exercise, and Osteoarthritis, funded by Arthritis Research UK (Grant Reference: 20194). Ali Mobasheri has received funding from the Deanship of Scientific Research (DSR), King AbdulAziz University (grant no. 1-141/1434 HiCi).Vanessa Abella is a recipient of a predoctoral fellowship from ESF (European Social Fund) grant from Xunta de Galicia through a contract signed with University of Coruña. Morena Scotece is recipient of the “FPU” Program of the Spanish Ministry of Education. Veronica Lopez is a recipient of a grant from Instituto de Salud Carlos III. Javier Conde is a recipient of a fellowship from the Foundation IDIS-Ramón Dominguez.
References
- Aigner F, Maier HT, Schwelberger HG, et al. Lipocalin-2 regulates the inflammatory response during ischemia and reperfusion of the transplanted heart. Am J Transplant. 2007;7:779–88. doi: 10.1111/j.1600-6143.2006.01723.x. [DOI] [PubMed] [Google Scholar]
- Bao G, Clifton M, Hoette TM, et al. Iron traffics in circulation bound to a siderocalin (Ngal)-catechol complex. Nat Chem Biol. 2010;6:602–9. doi: 10.1038/nchembio.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch S, Tschesche H. Cloning and expression of human neutrophil lipocalin cDNA derived from bone marrow and ovarian cancer cells. FEBS Lett. 1995;357:255–9. doi: 10.1016/0014-5793(94)01303-i. [DOI] [PubMed] [Google Scholar]
- Berard JL, Zarruk JG, Arbour N, et al. Lipocalin 2 is a novel immune mediator of experimental autoimmune encephalomyelitis pathogenesis and is modulated in multiple sclerosis. Glia. 2012;60:1145–59. doi: 10.1002/glia.22342. [DOI] [PubMed] [Google Scholar]
- Bolignano D, Donato V, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis. 2008;52:595–605. doi: 10.1053/j.ajkd.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Borregaard N, Cowland JB. Neutrophil gelatinase-associated lipocalin, a siderophore-binding eukaryotic protein. Biometals. 2006;19:211–15. doi: 10.1007/s10534-005-3251-7. [DOI] [PubMed] [Google Scholar]
- Brunner HI, Bennett MR, Mina R, et al. Association of noninvasively measured renal protein biomarkers with histologic features of lupus nephritis. Arthritis Rheum. 2012;64:2687–97. doi: 10.1002/art.34426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S, Kaur S, Guha S, et al. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta. 2012;1826:129–69. doi: 10.1016/j.bbcan.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing B, Srinivasan G, Delgado MA. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS One. 2012;9:e44328. doi: 10.1371/journal.pone.0044328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu ST, Lin HJ, Huang HL, Chen YH. The hydrophobic pocket of 24p3 protein from mouse uterine luminal fluid: fatty acid and retinol binding activity and predicted structural similarity to lipocalins. J Pept Res. 1998;52:390–7. doi: 10.1111/j.1399-3011.1998.tb00663.x. [DOI] [PubMed] [Google Scholar]
- Conde J, Gomez R, Bianco G, et al. Expanding the adipokine network in cartilage: identification and regulation of novel factors in human and murine chondrocytes. Ann Rheum Dis. 2011b;70:551–9. doi: 10.1136/ard.2010.132399. [DOI] [PubMed] [Google Scholar]
- Conde J, Scotece M, Gómez R, et al. Adipokines: biofactors from white adipose tissue. A complex hub among inflammation, metabolism, and immunity. BioFactors. 2011a;37:413–20. doi: 10.1002/biof.185. [DOI] [PubMed] [Google Scholar]
- Costa D, Biticchi R, Negrini S, et al. Lipocalin-2 controls the expression of SDF-1 and the number of responsive cells in bone. Cytokine. 2010;51:47–52. doi: 10.1016/j.cyto.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Costa D, Lazzarini E, Canciani B, et al. Altered bone development and turnover in transgenic mice over-expressing lipocalin-2 in bone. J Cell Physiol. 2013;228:2210–21. doi: 10.1002/jcp.24391. [DOI] [PubMed] [Google Scholar]
- Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics. 1997;45:17–23. doi: 10.1006/geno.1997.4896. [DOI] [PubMed] [Google Scholar]
- Dejaco C, Duftner C, Grubeck-Loebenstein B, et al. Imbalance of regulatory T cells in human autoimmune diseases. Immunology. 2006;117:289–300. doi: 10.1111/j.1365-2567.2005.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devireddy LR, Teodoro JG, Richard FA, Green MR. Induction of apoptosis by a secreted lipocalin that is transcriptionally regulated by IL-3 deprivation. Science. 2001;293:829–34. doi: 10.1126/science.1061075. [DOI] [PubMed] [Google Scholar]
- Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005;123:1293–305. doi: 10.1016/j.cell.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Devireddy LR, Hart DO, Goetz DH, Green MR. A mammalian siderophore synthesized by an enzyme with a bacterial homolog involved in enterobactin production. Cell. 2010;141:1006–17. doi: 10.1016/j.cell.2010.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elewa EA, El Tokhy MA, Fathy SE, et al. Predictive role of urinary neutrophil gelatinase-associated lipocalin in lupus nephritis. Lupus. 2015;24:138–46. doi: 10.1177/0961203314550225. [DOI] [PubMed] [Google Scholar]
- Filiopoulos V, Biblaki D, Vlassopoulos D. Neutrophil gelatinase-associated lipocalin (NGAL): a promising biomarker of contrast-induced nephropathy after computed tomography. Ren Fail. 2014;36:979–86. doi: 10.3109/0886022X.2014.900429. [DOI] [PubMed] [Google Scholar]
- Flo TH, Smith KD, Sato S, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–21. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- Friedl A, Stoesz SP, Buckley P, Gould MN. Neutrophil gelatinase-associated lipocalin in normal and neoplastic human tissues. Cell type-specific pattern of expression. Histochem J. 1999;31:433–41. doi: 10.1023/a:1003708808934. [DOI] [PubMed] [Google Scholar]
- Garay-Rojas E, Harper M, Hraba-Renevey S, Kress M. An apparent autocrine mechanism amplifies the dexamethasone- and retinoic acid-induced expression of mouse lipocalin-encoding gene 24p3. Gene. 1996;170:173–80. doi: 10.1016/0378-1119(95)00896-9. [DOI] [PubMed] [Google Scholar]
- Gómez R, Conde J, Scotece M, et al. What’s new in our understanding of the role of adipokines in rheumatic diseases? Nat Rev Rheumatol. 2011;7:528–36. doi: 10.1038/nrrheum.2011.107. [DOI] [PubMed] [Google Scholar]
- Gómez R, Scotece M, Conde J, et al. Nitric oxide boosts TLR-4 mediated lipocalin 2 expression in chondrocytes. J Orthop Res. 2013;31:1046–52. doi: 10.1002/jor.22331. [DOI] [PubMed] [Google Scholar]
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–45. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- Guo H, Jin D, Zhang Y, et al. Lipocalin-2 deficiency impairs thermogenesis and potentiates diet-induced insulin resistance in mice. Diabetes. 2010;59:1376–85. doi: 10.2337/db09-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Jin D, Chen X. Lipocalin 2 is a regulator of macrophage polarization and NF-κB/STAT3 pathway activation. Mol Endocrinol. 2014;28:1616–28. doi: 10.1210/me.2014-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Zhang Y, Brockman DA, et al. Lipocalin 2 deficiency alters estradiol production and estrogen receptor signaling in female mice. Endocrinology. 2012;153:1183–93. doi: 10.1210/en.2011-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K, Shukla M, Cowland JB, et al. Neutrophil gelatinase-associated lipocalin is expressed in osteoarthritis and forms a complex with matrix metalloproteinase 9. Arthritis Rheum. 2007;56:3326–35. doi: 10.1002/art.22879. [DOI] [PubMed] [Google Scholar]
- Haase-Fielitz A, Haase M, Devarajan P. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: a critical evaluation of current status. Ann Clin Biochem. 2014;51:335–51. doi: 10.1177/0004563214521795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad A, Mosaad Y, Elhanbly S, et al. Urinary neutrophil gelatinase-associated lipocalin as a marker of severe lupus nephritis in children. Lupus. 2013;22:486–91. doi: 10.1177/0961203313479419. [DOI] [PubMed] [Google Scholar]
- Hawlisch H, Wills-Karp M, Karp CL, Köhl J. The anaphylatoxins bridge innate and adaptive immune responses in allergic asthma. Mol Immunol. 2004;41:123–31. doi: 10.1016/j.molimm.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Hu X, Luo P, Peng X, et al. Molecular cloning, expression pattern analysis of porcine Rb1 gene and its regulatory roles during primary dedifferentiated fat cells adipogenic differentiation. Gen Comp Endocrinol. 2015;214:77–86. doi: 10.1016/j.ygcen.2015.01.016. [DOI] [PubMed] [Google Scholar]
- Huang HL, Chu ST, Chen YH. Ovarian steroids regulate 24p3 expression in mouse uterus during the natural estrous cycle and the preimplantation period. J Endocrinol. 1999;162:11–19. doi: 10.1677/joe.0.1620011. [DOI] [PubMed] [Google Scholar]
- Hvidberg V, Jacobsen C, Strong RK, et al. The endocytic receptor megalin binds the iron transporting neutrophil-gelatinase-associated lipocalin with high affinity and mediates its cellular uptake. FEBS Lett. 2005;579:773–7. doi: 10.1016/j.febslet.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Jacobson AC, Weis JJ, Weis JH. Complement receptors 1 and 2 influence the immune environment in a B cell receptor-independent manner. J Immunol. 2008;180:5057–66. doi: 10.4049/jimmunol.180.7.5057. [DOI] [PubMed] [Google Scholar]
- Jang Y, Lee JH, Wang Y, Sweeney G. Emerging clinical and experimental evidence for the role of lipocalin-2 in metabolic syndrome. Clin Exp Pharmacol Physiol. 2012;39:194–9. doi: 10.1111/j.1440-1681.2011.05557.x. [DOI] [PubMed] [Google Scholar]
- Jin D, Guo H, Bu SY, et al. Lipocalin 2 is a selective modulator of peroxisome proliferator-activated receptor-gamma activation and function in lipid homeostasis and energy expenditure. FASEB J. 2011;25:754–64. doi: 10.1096/fj.10-165175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katano M, et al. Implication of granulocyte-macrophage colony-stimulating factor induced neutrophil gelatinase-associated lipocalin in pathogenesis of rheumatoid arthritis revealed by proteome analysis. Arthritis Res Ther. 2009;11:R3. doi: 10.1186/ar2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjeldsen L, Cowland JB, Borregaard N. Human neutrophil gelatinase-associated lipocalin and homologous proteins in rat and mouse. Biochim Biophys Acta. 2000;1482:272–83. doi: 10.1016/s0167-4838(00)00152-7. [DOI] [PubMed] [Google Scholar]
- Kjeldsen L, Okamoto K, Arito M, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood. 1994;83:799–807. [PubMed] [Google Scholar]
- Lago F, Dieguez C, Gómez-Reino J, et al. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol. 2007;3:716–24. doi: 10.1038/ncprheum0674. [DOI] [PubMed] [Google Scholar]
- La Manna G, Ghinatti G, Tazzari PL, et al. Neutrophil gelatinase-associated lipocalin increases HLA-G(+)/FoxP3(+) T-regulatory cell population in an in vitro model of PBMC. PLoS One. 2014;9:e89497. doi: 10.1371/journal.pone.0089497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law IKM, Xu A, Lam KS, et al. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes. 2010;59:872–82. doi: 10.2337/db09-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hu FL, Dai YJ, et al. Serum anti-lipocalin 2 IgG is a novel biomarker in the diagnosis of systemic lupus erythematosus. Lupus. 2014;23:868–75. doi: 10.1177/0961203314530484. [DOI] [PubMed] [Google Scholar]
- Lindberg S, Jensen JS, Mogelvang R, et al. Plasma neutrophil gelatinase-associated lipocalinin in the general population: association with inflammation and prognosis. Arterioscler Thromb Vasc Biol. 2014;34:2135–42. doi: 10.1161/ATVBAHA.114.303950. [DOI] [PubMed] [Google Scholar]
- Malemud CJ. Biologic basis of osteoarthritis: state of the evidence. Curr Opin Rheumatol. 2015;27:289–94. doi: 10.1097/BOR.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–43. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- Moreno-Navarrete JM, Manco M, Ibáñez J, et al. Metabolic endotoxemia and saturated fat contribute to circulating NGAL concentrations in subjects with insulin resistance. Int J Obes (Lond) 2010;34:240–9. doi: 10.1038/ijo.2009.242. [DOI] [PubMed] [Google Scholar]
- Nairz M, Haschka D, Demetz E, et al. Iron at the interface of immunity and infection. Front Pharmacol 5: 2014:152. doi: 10.3389/fphar.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Takai T. A role of FcgammaRIIB in the development of collagen-induced arthritis. Biomed Pharmacother. 2004;58:292–8. doi: 10.1016/j.biopha.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Nam Y, Kim JH, Seo M, et al. Lipocalin-2 protein deficiency ameliorates experimental autoimmune encephalomyelitis: the pathogenic role of lipocalin-2 in the central nervous system and peripheral lymphoid tissues. J Biol Chem. 2014;289:16773–89. doi: 10.1074/jbc.M113.542282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickolas TL, Forster CS, Sise ME, et al. NGAL (Lcn2) monomer is associated with tubulointerstitial damage in chronic kidney disease. Kidney Int. 2012;82:718–22. doi: 10.1038/ki.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen HC, Roberts SJ, Ahmed SF, Farquharson C. Dexamethasone-induced expression of the glucocorticoid response gene lipocalin 2 in chondrocytes. Am J Physiol Endocrinol Metabol. 2008;294:1023–34. doi: 10.1152/ajpendo.00586.2007. [DOI] [PubMed] [Google Scholar]
- Rubinstein T, Pitashny M, Levine B, et al. Urinary neutrophil gelatinase-associated lipocalin as a novel biomarker for disease activity in lupus nephritis. Rheumatology (Oxford) 2010;49:960–71. doi: 10.1093/rheumatology/kep468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo WH, Nam SW, Lee EH, et al. A rapid plasma neutrophil gelatinase-associated lipocalin assay for diagnosis of acute pyelonephritis in infants with acute febrile urinary tract infections: a preliminary study. Eur J Pediatr. 2014;173:229–32. doi: 10.1007/s00431-013-2112-6. [DOI] [PubMed] [Google Scholar]
- Shashidharamurthy R, Machiah D, Aitken JD, et al. Differential role of lipocalin 2 during immune complex-mediated acute and chronic inflammation in mice. Arthritis Rheum. 2013;65:1064–73. doi: 10.1002/art.37840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F, Hu Z, Goswami J, Gaffen SL. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J Biol Chem. 2006;281:24138–48. doi: 10.1074/jbc.M604597200. [DOI] [PubMed] [Google Scholar]
- Sommer G, Weise S, Kralisch S, et al. Lipocalin-2 is induced by interleukin-1beta in murine adipocytes in vitro. J Cell Biochem. 2009;106:103–8. doi: 10.1002/jcb.21980. [DOI] [PubMed] [Google Scholar]
- Srinivasan G, Aitken JD, Zhang B, et al. Lipocalin 2 deficiency dysregulates iron homeostasis and exacerbates endotoxin-induced sepsis. J Immunol. 2012;189:1911–19. doi: 10.4049/jimmunol.1200892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lam KS, Kraegen EW, et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin Chem. 2007;53:34–41. doi: 10.1373/clinchem.2006.075614. [DOI] [PubMed] [Google Scholar]
- Wilson R, Belluoccio D, Little CB, et al. Proteomic characterization of mouse cartilage degradation in vitro. Arthritis Rheum. 2008;58:3120–31. doi: 10.1002/art.23789. [DOI] [PubMed] [Google Scholar]
- Yan QW, Yang Q, Mody N, et al. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes. 2007;56:2533–40. doi: 10.2337/db07-0007. [DOI] [PubMed] [Google Scholar]
- Yang CC, Hsieh SC, Li KJ, et al. Urinary neutrophil gelatinase-associated lipocalin is a potential biomarker for renal damage in patients with systemic lupus erythematosus. J Biomed Biotechnol. 2012;2012:759313. doi: 10.1155/2012/759313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Goetz D, Li JY, et al. An iron delivery pathway mediated by a lipocalin. Mol Cell. 2002;10:1045–56. doi: 10.1016/s1097-2765(02)00710-4. [DOI] [PubMed] [Google Scholar]
- Yuen PST, Jo SK, Holly MK, et al. Ischemic and nephrotoxic acute renal failure are distinguished by their broad transcriptomic responses. Physiol Genomics. 2006;25:375–86. doi: 10.1152/physiolgenomics.00223.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Foncea R, Deis JA, et al. Lipocalin 2 expression and secretion is highly regulated by metabolic stress, cytokines, and nutrients in adipocytes. PLoS One. 2014a;9:e96997. doi: 10.1371/journal.pone.0096997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Guo H, Deis JA, et al. Lipocalin 2 regulates brown fat activation via a nonadrenergic activation mechanism. J Biol Chem. 2014b;289:22063–77. doi: 10.1074/jbc.M114.559104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Konishi A, Fujita Y, et al. Lipocalin 2 bolsters innate and adaptive immune responses to blood-stage malaria infection by reinforcing host iron metabolism. Cell Host Microbe. 2012;12:705–16. doi: 10.1016/j.chom.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Zhao P, Elks CM, Stephens JM. The induction of lipocalin-2 protein expression in vivo and in vitro. J Biol Chem. 2014;289:5960–9. doi: 10.1074/jbc.M113.532234. [DOI] [PMC free article] [PubMed] [Google Scholar]