Summary
The aim of this pilot randomized controlled trial was to evaluate the effects of treadmill training on cognitive and motor performance in patients with Parkinson’s disease (PD). Seventeen persons with mild to moderate PD were enrolled. Nine patients were allocated to the Intervention group and received twelve 45-minute sessions of treadmill training: one session a day, three days a week, for four consecutive weeks. Eight patients were allocated to the Control group; these patients did not undergo physical training but were required to have regular social interactions, following a specific lifestyle program. All the patients were evaluated at baseline and one month later. The primary outcome measures were the Frontal Assessment Battery-Italian version (FAB-it) and the 6-minute walking test (6MWT). At the one-month evaluation significant differences were found between the groups in their performance on the FAB-it (p=0.005) and the 6MWT (p=0.018). Our findings support the hypothesis that treadmill training might effectively improve cognitive and motor features in patients with PD.
Keywords: basal ganglia, executive function, gait, movement disorders
Introduction
Parkinson’s disease (PD) is a common idiopathic neurodegenerative disorder characterized by a progressive loss of dopaminergic neurons in the substantia nigra pars compacta (Monchi et al., 2004). Patients with PD are typically debilitated, presenting with symptoms of muscular rigidity, impaired movement, loss of balance and tremor at rest (Pothakos et al., 2009). This results in abnormal gait patterns mainly characterized by reduced gait speed and shortened stride length (Picelli et al., 2010). As well as motor impairment, about 25% of newly diagnosed, non-demented people with PD show some cognitive deficits involving attention, memory and visuospatial and executive functions (Elgh et al., 2009; Mamikonyan et al., 2009).
To date, a wide range of physical therapy modalities has been proposed and employed to treat motor impairment in PD (Ceravolo et al., 2001; Picelli et al., 2012a,b; Carda et al., 2012; Picelli et al., 2013, 2015; Smania et al., 2013; Tomlinson et al., 2013). In particular, the implementation of physical activity programs for people with PD has resulted in improvements in daily activities, motor performance, ambulation and overall functional independence (Lau et al., 2011). Furthermore, in experimental animal models of PD, aerobic exercise has been shown to improve neurochemical and mitochondrial function, with a positive impact on cognitive and emotional aspects of behavior (Pothakos et al., 2009; Pietrelli et al., 2012; Tuon et al., 2012, 2014)
Cognitive-motor relationships have been explored in patients with PD (Domellöf et al., 2011; Poletti et al., 2012). Recently, a cross-sectional pilot study investigated, in depth, the relationship between cognitive deficits and motor dysfunctions in PD; balance and gait skills were found to show significant correlations with some cognitive features in parkinsonian patients (Varalta et al., 2015). These observations could have implications for rehabilitation, offering perspectives for clinical treatment protocols based on cognitive-motor relationships in patients with PD. In view of these considerations, the present study was conducted to evaluate the effects of treadmill training on cognitive and motor performance in patients with mild to moderate PD.
Materials and methods
This was a pilot, single-blind, single-center, randomized controlled trial. The inclusion criteria were a confirmed diagnosis of idiopathic PD according to the UK Brain Bank Criteria (Hughes et al., 1992); Hoehn and Yahr stage 3, determined in the “on” phase (Hoehn and Yahr, 1967); and a Mini-Mental State Examination score greater than 24 (Folstein et al., 1975). The exclusion criteria were: severe dyskinesias or “on-off” fluctuations; important modifications of PD medication during the study (i.e. drug changes); deficits of somatic sensation involving the lower limbs (as assessed by means of a physical and neurological examination); vestibular disorders or paroxysmal vertigo; other neurological or orthopedic conditions involving the lower limbs (musculoskeletal diseases, severe osteoarthritis, peripheral neuropathy, joint replacement); and cardiovascular comorbidity (recent myocardial infarction, heart failure, uncontrolled hypertension, orthostatic hypotension).
All the participants were outpatients and gave their informed written consent to participate in the current study, which was carried out in accordance with the Declaration of Helsinki and approved by the local ethics committee. Prior to testing, eligible participants were allocated, in a one-to-one ratio, to one of two arms according to a balanced (restricted) randomization scheme: a group that performed treadmill training without body-weight support and a group that received no physical treatment. The investigator (V.V.) who decided whether a subject was eligible for inclusion in the trial was unaware, when making this decision, of which group the subject would be allocated to (allocation was by sealed opaque envelopes). Another investigator (V.Z.) checked correct patient allocation according to the randomization list. After unmasking at the end of the study, we checked that no errors had been made in the allocation process. During the study, participants were instructed to take their PD medications regularly and were tested and trained during the on phase, 1 to 2.5 hours after taking their morning dose. The participants did not perform any type of rehabilitation in the three months leading up to the study, or undergo any form of physical therapy other than that scheduled in the study protocol.
Treatment procedures
Patients allocated to the Intervention group performed treadmill training without body-weight support using the Jog Now 500MD (Technogym, Cesena, Italy). The training program consisted of twelve 45-minute sessions (including rest periods): one session a day, three days a week (Monday, Wednesday, Friday), for four consecutive weeks (Picelli et al., 2013, 2015). Each training session comprised three parts with a 5-minute rest after each. First, patients were trained at a speed of 1.0 km/h for 10 minutes; then, at 1.5 km/h for 10 minutes; finally, at 2.0 km/h for 10 minutes (Picelli et al., 2013, 2015). Patients unable to maintain the established pace were excluded.
Patients allocated to the Control group did not perform physical training; however, they were instructed to have regular social interactions, according to a specific lifestyle program, during the study period in order to ensure that they had social interactions with the same frequency and of the same duration as the Intervention group attending the rehabilitation center.
Evaluation procedures and outcomes
The patients were evaluated at baseline (T0) and one month later (T1). To avoid facilitating the Intervention group, the T1 evaluation was not conducted at the training center. All the patients were evaluated by the same rater (C.M.), who was blinded to the group allocation. Asking the assessor to make an educated guess tested the success of the blinding.
Primary outcomes
The primary cognitive outcome measure was the Frontal Assessment Battery-Italian version (FAB-it) (Appollonio et al., 2005). The FAB-it assesses executive functions such as conceptualization, mental flexibility, programming, sensitivity to interference, inhibitory control and environmental autonomy. It consists of six tests (similarities, lexical fluency, Luria motor series, conflicting instructions, go no-go, prehension behavior), each rated on a scale from 0 to 3 points. The total score is the sum of all the items, and it ranges from 0 (worst performance) to 18 (best performance) (Appollonio et al., 2005).
The primary motor outcome was the 6-minute walking test (6MWT) (Enright, 2003), which was used to assess walking capacity. The subjects were asked to cover as much ground as possible in six minutes (walking continuously at their fastest possible speed without using walking aids) along a marked 40-meter circuit. The distance covered was recorded.
Secondary outcomes
Secondary cognitive outcomes were the Montreal Cognitive Assessment (MoCA), the trail making test (TMT) and a memory with interference (MI) test. The MoCA investigates a patient’s skills in seven domains: visuospatial/executive, naming, memory, attention, language, abstraction and orientation. The total score is the sum of all the items, with a maximum score of 30 (best performance) (Nasreddine et al., 2005). Attention capacity was evaluated with the TMT (parts A and B), specifically to assess selective attention, psychomotor speed and sequencing skills. Part B also investigates the ability to switch attention between two rules or tasks. The time taken to complete the trails is recorded (longer = worse performance) (Giovagnoli et al., 1996). Working memory was assessed with the MI test. In this test, subjects are asked to recall a consonant trigram after a ten-second interval during which they were required to count forward starting from a three-digit number randomly presented by the examiner immediately after the trigram. The maximum score is 9 (best performance) (Mondini et al., 2011).
The secondary motor outcome was the 10-meter walking test (10MWT), selected as a measure of gait speed (Bohannon et al., 1996). The subjects were asked to walk on a flat, hard floor at their fastest speed for 10 meters without assistance or the use of walking aids. A 10-m walkway was marked by two lines on the floor at 2 m and at 8 m. In order to minimize the effect of acceleration and deceleration, gait speed was measured in the 6 meters between the two marks (timing started when the toes of the leading foot crossed the 2-m mark and stopped when the toes of the leading foot crossed the 8-m mark) (Bohannon et al., 1996). The time taken was measured using a handheld stopwatch.
In addition to cognitive and motor skills, the patients were also administered the Beck Depression Inventory (BDI), to evaluate mood, and the Unified Parkinson’s Disease Rating Scale (UPDRS), to assess their disease course. The BDI, which focuses on psychological aspects of depression, consists of 21 items, each rated on a four-point scale of severity. The total score is the sum of all the items; the maximum score is 63 (worst mood) (Beck et al., 1961). The UPDRS has four subsections and its score ranges from 0 to 147 (higher scores = worse performance) (Song et al., 2009).
Statistical analysis
Statistical analysis was carried out using the Statistical Package for the Social Sciences (SPSS) software, version 20.0, for Macintosh (SPSS Inc., Chicago, Illinois). We assessed all the patients who were randomized (intention-to-treat principle). We used the Mann-Whitney U test to assess the homogeneity of the sample before the study and compare the effect of treatment between groups (to determine this, we computed the differences between T1-T0 performances for all outcomes). Within-group comparisons were performed using the Wilcoxon signed-ranks test. Descriptive analysis was used to evaluate the effect size measures between groups (Cohen’s d calculation) and the 95% confidence intervals (Benjamini and Hochberg, 1995). The alpha level for significance was set at p<0.05.
Results
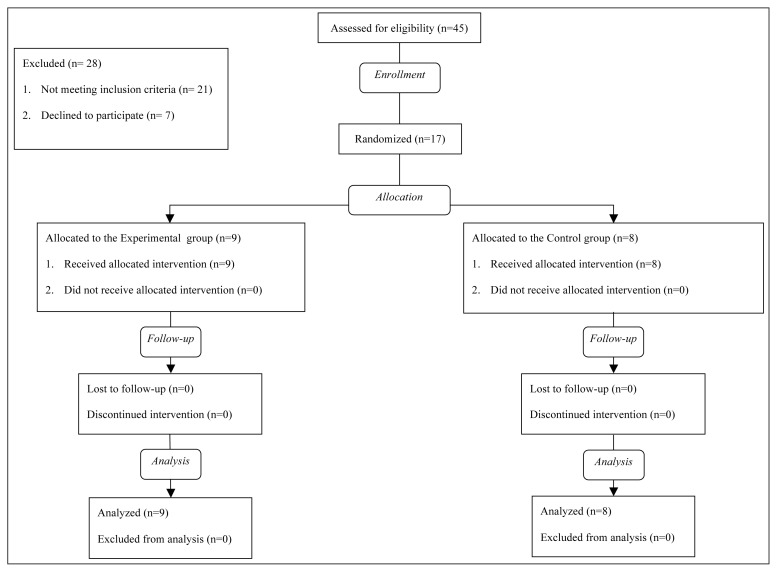
Seventeen persons (12 men and 5 women; mean age: 70.0 years) presenting with mild to moderate idiopathic PD (mean disease duration: 9.9 years) were recruited from among 45 outpatients referred to our research center. The enrollment period was from January 2014 to February 2015. Nine patients were allocated to the Intervention group and 8 patients were allocated to the Control group. No drop-out was observed and no adverse events occurred during the trial in either of the groups. The flow diagram of the study is shown in figure 1.
Figure 1.
Study flow.
Baseline
No significant difference was observed between the groups with regard to age (p=0.771), primary outcome measures (FAB-it: p=0.293; 6MWT: p=0.923) and secondary outcomes (MoCA: p=0.772; TMT-A: p=0.885; TMT-B: p=0.664; MI: p=0.286; 10MWT: p=0.178; BDI: p=0.961; UPDRS: p=0.962) at T0. The patients’ demographic and clinical features are detailed in table I.
Table I.
Demographic and clinical features of the patients.
| Intervention group (n=9) | Control group (n=8) | |
|---|---|---|
| Age mean (SD) | 71.2 (9.2) y | 71.6 (7.2) y |
| Gender, m/f | 5/4 | 4/4 |
| Disease duration, mean (SD) | 11.2 (5.6) y | 10.8 (4.1) y |
Abbreviations: SD=standard deviation; n=no. of patients; y=years
Primary outcomes
The FAB-it showed a significant difference between the groups at T1 (p=0.005; z=−2.791; effect size=0.63). Within-group comparisons showed a significant improvement at T1 versus T0 only in the Intervention group (p=0.011; z=−2.530). As for the 6MWT, a significant difference was found between groups at T1 (p=0.018; z=−2.360; effect size=0.53). Within-group comparisons showed a significant improvement at T1 versus T0 only in the Intervention group (p=0.008; z=−2.668). Group data and within-group comparisons are detailed in table II.
Table II.
Group data and within-group comparisons.
| Outcome | Group | Baseline | One month | Within-group comparisons |
|---|---|---|---|---|
|
| ||||
| One month vs baseline p value (95% CI) | ||||
| FAB-it (0–18) | Intervention | 14.00 (11.00; 15.00) | 16.00 (14.00; 17.00) | 0.011 (1.16; 3.95)* |
| median (IQR) | Control | 14.50 (14.00; 16.00) | 14.00 (14.00; 15.75) | 0.705 (−1.09; 1.09) |
| 6MWT (meters) | Intervention | 310.22 (83.28) | 346.67 (80.70) | 0.008 (18.99; 53.90)* |
| mean (SD) | Control | 298.75 (101.31) | 307.25 (89.47) | 0.362 (−9.92; 26.92) |
| MoCA (0–30) | Intervention | 24.00 (18.50; 27.00) | 25.00 (20.50; 28.00) | 0.017 (0.53; 2.58)* |
| median (IQR) | Control | 23.00 (20.25; 26.00) | 24.50 (22.00; 26.75) | 0.227 (−0.71; 1.96) |
| TMT-A (seconds) | Intervention | 141.00 (113.99) | 120.67 (104.59) | 0.018 (−37.32; −3.35)* |
| mean (SD) | Control | 123.50 (101.27) | 124.75 (108.55) | 0.735 (−10.14; 12.64) |
| TMT-B (seconds) | Intervention | 200.00 (80.19) | 149.56 (69.33) | 0.008 (−79.75; −21.14)* |
| mean (SD) | Control | 195.25 (93.92) | 181.13 (78.96) | 0.345 (−67.68; 39.43) |
| MI (0–9) | Intervention | 4.00 (2.00; 6.00) | 7.00 (5.00; 9.00) | 0.010 (1.53; 3.58)* |
| median (IQR) | Control | 6.50 (3.25; 8.75) | 5.50 (4.00; 8.75) | 1.000 (−1.84; 1.84) |
| 10MWT (seconds) | Intervention | 10.20 (1.52) | 7.61 (1.50) | 0.008 (−3.28; −1.90)* |
| mean (SD) | Control | 9.41 (3.02) | 9.30 (2.42) | 0.624 (−0.95; 0.72) |
| BDI (0–63) | Intervention | 11.00 (8.00; 25.00) | 6.00 (3.00; 16.50) | 0.012 (−8.24; −1.98)* |
| median (IQR) | Control | 13.00 (9.25; 20.25) | 13.00 (7.00; 19.75) | 0.914 (−2.18; 3.18) |
| UPDRS (0–147) | Intervention | 40.00 (33.50; 45.50) | 37.00 (30.50; 43.00) | 0.013 (−5.58; −1.46)* |
| median (IQR) | Control | 42.00 (33.75; 43.75) | 40.50 (34.25; 42.75) | 0.285 (−2.10; 0.85) |
Abbreviations: IQR=interquartile range; SD=standard deviation; CI=confidence interval; FAB-it=Frontal Assessment Battery-Italian version; 6MWT=6-minute walking test; MoCA=Montreal Cognitive Assessment; TMT=trail making test; MI=memory with interference test; 10MWT=10-meter walking test; BDI=Beck Depression Inventory; UPDRS=Unified Parkinson’s Disease Rating Scale.
= statistically significant (p<0.005).
Secondary outcomes
The MoCA showed no significant difference between the groups at T1 (p=0.365; z=−0.906; effect size=0.30). As regards the TMT, the assessment at T1 revealed a significant difference between the groups in both TMT-A (p=0.027; z=−2.213; effect size=−0.51) and TMT-B (p=0.009; z=−2.600; effect size=−0.33) scores. Significant between-group differences at T1 were also found in the MI (p=0.014; z=−2.459; effect size=0.57), 10MWT (p=0.001; z=−3.418; effect size= −0.80), BDI (p=0.009; z=−2.616; effect size=−0.61), and UPDRS (p=0.022; z=−2.294; effect size=−0.54) scores. Group data and within-group comparisons are reported in table II.
Discussion
This pilot, randomized, controlled trial was conducted to evaluate the effects of treadmill training on cognitive and motor performance in mild to moderate PD. We found significant improvements in cognitive performance (as measured by the FAB-it, the TMT and the M test) and motor performance (as measured by the 6MWT and the 10MWT) in patients with mild to moderate PD who underwent a training program consisting of four weeks of treadmill training without bodyweight support. Furthermore, the PD patients who underwent treadmill training also showed significant mood and disease course improvements (as measured by the BDI and the UPDRS).
Conventionally, exercise is thought to produce an overall benefit in terms of physical fitness and mental stimulation, to slow down the aging process, and to help prevent the onset of chronic disease (Lau et al., 2011; van Praag et al., 2005; Pereira et al., 2007). Furthermore, the impact of exercise in promoting brain angiogenesis and neurogenesis has been well established, supporting the notion that exercise can act to slow decline of cognitive and memory function during the course of normal aging (van Praag et al., 2005; Pereira et al., 2007). In PD, physical activity has been found to potentially reduce the risk of further neurological impairment (Lau et al., 2011; Tuon et al., 2012). In particular, long-term treadmill exercise training has been shown to protect against neurotoxin-induced protein oxidation (by reducing the level of striatal carbonylated proteins), impaired mitochondrial function (by restoring mitochondrial respiration, adenosine triphosphate and superoxide dismutase levels in the striatum), and loss of dopaminergic neurons and transmission (by increasing striatal dopamine receptors); furthermore, it has been found to elevate nigrostriatal neurotrophic factors in chronic experimental models of PD (Lau et al., 2011; Tuon et al., 2012). The internal generation of movements depends on a decision-making process (i.e. the selection of an action, among several alternatives, for the performance of a task) (Nagano-Saito et al., 2014). The basal ganglia, whose activity is mostly modulated by dopaminergic projections, seems to play an important role in mediating cognitive and motor modules, and thus in allowing the selection and generation of an appropriate action for the task in hand (Nagano-Saito et al., 2014). Patients with PD, in whom the dopaminergic projections to the striatum are significantly reduced, show some difficulties in performing internally generated movements as well as cognitive deficits that are often manifested as impaired executive functions (Nagano-Saito et al., 2014; Varalta et al., 2015). On these bases, it has been suggested that dopamine, first processed in cognitive brain networks, is involved in the transfer of information toward motor-related networks, and thus in task performance (Nagano-Saito et al., 2014). In short, the dopaminergic projections of the basal ganglia may be involved in the formation of an ideal network, combining the cognitive and motor networks in the brain, for the conducting of a series of tasks (Nagano-Saito et al., 2014). Our findings are in line with these concepts. Indeed, our observation of significant improvements in motor and cognitive performances after four weeks of treadmill training in the Intervention group provides further confirmation of the close relationship between impaired cognitive performance and motor dysfunction in patients with PD (Varalta et al., 2015; Kelly et al., 2015). These findings have relevance to rehabilitation, considering that PD-associated cognitive deficits are important features of the disease, contributing to reduced quality of life and an increased risk of disability and mortality (Kelly et al., 2015). In our view, these findings not only highlight the possibility of obtaining improvements in cognitive performance through motor training in PD, but also suggest a role for new rehabilitation approaches integrating both cognitive and motor training. In particular, it would be interesting to use physical aerobic exercise (i.e. treadmill training) in order to prime cognitive rehabilitation in people with PD, in line with what is already proposed for patients with other neurological disorders such as stroke (Mang et al., 2013).
Investigation of neuropsychiatric symptoms has revealed the presence of comorbid depression in a high percentage of patients with PD (Elgh et al., 2009; Mamikonyan et al., 2009; Tuon et al., 2014). This may be explained by the fact that mood symptoms are related to alterations in serotoninergic pathways, which are known to interact with the dopaminergic degeneration associated with PD (Jellinger, 2015). Physical exercise was recently found to prevent depressive symptoms in PD by increasing the levels of brain-derived neurotrophic factor and preventing neurodegeneration (Tuon et al., 2014). Accordingly, in the present pilot study we observed some positive effects on depression in patients who performed treadmill training. As regards walking, gait in PD is characterized by reduced speed, a shortened stride length and a longer double support phase, leading to mobility problems, instability and falls, and thus a reduction in quality of life and mental well-being. Treadmill training without body-weight support has been shown to effectively improve walking ability in patients with PD (Mehrholz, 2010; Carda et al., 2012; Picelli et al., 2013, 2015). Our results confirm previous findings about the usefulness of treadmill training for promoting mobility (in particular walking capacity and gait speed) in PD through restoration of stride length, gait rhythmicity and a more stable walking pattern.
This study has several limitations. First, the sample size was small. We estimated that a total of 66 patients (33 per group) would provide a power of 80% to detect a between-groups difference of 1.26 points (standard deviation 1.81 points) on the FAB-it (Lima et al., 2008). Second, the Control group did not perform any specific training during the study period. Thus, we cannot exclude that changes observed in the Intervention group might be consequent to a placebo effect. Third, no long-term follow-up was considered. Fourth, we did not test participants “off” medication and thus cannot draw conclusions on the unmedicated state. Fifth, even though we did not include patients with severe fluctuation, since possible levodopa effects were not controlled we cannot exclude some degree of fluctuation in our patients.
In conclusion, our preliminary findings support the hypothesis that aerobic physical exercise consisting of treadmill training without body-weight support may improve some cognitive and motor features in non-demented patients with mild to moderate PD. Properly-sized randomized controlled trials are needed to further validate these findings.
References
- 1.Appollonio I, Leone M, Isella V, et al. The Frontal Assessment Battery (FAB): normative values in an Italian population sample. Neurol Sci. 2005;26:108–116. doi: 10.1007/s10072-005-0443-4. [DOI] [PubMed] [Google Scholar]
- 2.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 3.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B. 1995;57:289–300. [Google Scholar]
- 4.Bohannon RW, Andrews AW, Thomas MW. Walking speed: reference values and correlates for older adults. J Orthop Sports Phys Ther. 1996;24:86–90. doi: 10.2519/jospt.1996.24.2.86. [DOI] [PubMed] [Google Scholar]
- 5.Carda S, Invernizzi M, Baricich A, et al. Robotic gait training is not superior to conventional treadmill training in parkinson disease: a single-blind randomized controlled trial. Neurorehabil Neural Repair. 2012;26:1027–1034. doi: 10.1177/1545968312446753. [DOI] [PubMed] [Google Scholar]
- 6.Ceravolo MG, Paoloni L, Provinciali L. Rehabilitation of parkinsonian patients. Funct Neurol. 2001;16:157–162. [PubMed] [Google Scholar]
- 7.Domellöf ME, Elgh E, Forsgren L. The relation between cognition and motor dysfunction in drug-naive newly diagnosed patients with Parkinson’s disease. Mov Disord. 2011;26:2183–2189. doi: 10.1002/mds.23814. [DOI] [PubMed] [Google Scholar]
- 8.Elgh E, Domellöf M, Linder J, et al. Cognitive function in early Parkinson’s disease: a population-based study. Eur J Neurol. 2009;16:1278–1284. doi: 10.1111/j.1468-1331.2009.02707.x. [DOI] [PubMed] [Google Scholar]
- 9.Enright PL. The six-minute walk test. Respir Care. 2003;48:783–785. [PubMed] [Google Scholar]
- 10.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician”. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 11.Giovagnoli AR, Del Pesce M, Mascheroni S, et al. Trail making test: normative values from 287 normal adult controls. Ital J Neurol Sci. 1996;17:305–309. doi: 10.1007/BF01997792. [DOI] [PubMed] [Google Scholar]
- 12.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 13.Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinicopathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jellinger KA. Neuropathobiology of non-motor symptoms in Parkinson disease. J Neural Transm (Vienna) 2015;122:1429–1440. doi: 10.1007/s00702-015-1405-5. [DOI] [PubMed] [Google Scholar]
- 15.Kelly VE, Johnson CO, McGough EL, et al. Association of cognitive domains with postural instability/gait disturbance in Parkinson’s disease. Parkinsonism Relat Disord. 2015;21:692–697. doi: 10.1016/j.parkreldis.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau YS, Patki G, Das-Panja K, et al. Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson’s disease with moderate neurodegeneration. Eur J Neurosci. 2011;33:1264–1274. doi: 10.1111/j.1460-9568.2011.07626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lima CF, Meireles LP, Fonseca R, et al. The Frontal Assessment Battery (FAB) in Parkinson’s disease and correlations with formal measures of executive functioning. J Neurol. 2008;255:1756–1761. doi: 10.1007/s00415-008-0024-6. [DOI] [PubMed] [Google Scholar]
- 18.Mamikonyan E, Moberg PJ, Siderowf A, et al. Mild cognitive impairment is common in Parkinson’s disease patients with normal Mini-Mental State Examination (MMSE) scores. Parkinsonism Relat Disord. 2009;15:226–231. doi: 10.1016/j.parkreldis.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mang CS, Campbell KL, Ross CJ, et al. Promoting neuroplasticity for motor rehabilitation after stroke: considering the effects of aerobic exercise and genetic variation on brain-derived neurotrophic factor. Phys Ther. 2013;93:1707–1719. doi: 10.2522/ptj.20130053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehrholz J, Friis R, Kugler J, et al. Treadmill training for patients with Parkinson’s disease. Cochrane Database Syst Rev. 2010;(1):CD007830. doi: 10.1002/14651858.CD007830.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Monchi O, Petrides M, Doyon J, et al. Neural bases of set-shifting deficits in Parkinson’s disease. J Neurosci. 2004;24:702–710. doi: 10.1523/JNEUROSCI.4860-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mondini S, Mapelli D, Vestri A, et al. Esame Neuropsicologico Breve 2. Milan: Cortina Editions; 2011. [Google Scholar]
- 23.Nagano-Saito A, Martinu K, Monchi O. Function of basal ganglia in bridging cognitive and motor modules to perform an action. Front Neurosci. 2014;8:187. doi: 10.3389/fnins.2014.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 25.Pereira AC, Huddleston DE, Brickman AM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Picelli A, Melotti C, Origano F, et al. Robot-assisted gait training is not superior to balance training for improving postural instability in patients with mild to moderate Parkinson’s disease: a single-blind randomized controlled trial. Clin Rehabil. 2015;29:339–347. doi: 10.1177/0269215514544041. [DOI] [PubMed] [Google Scholar]
- 27.Picelli A, Melotti C, Origano F, et al. Robot-assisted gait training versus equal intensity treadmill training in patients with mild to moderate Parkinson’s disease: a randomized controlled trial. Parkinsonism Relat Disord. 2013;19:605–610. doi: 10.1016/j.parkreldis.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Picelli A, Melotti C, Origano F, et al. Robot-assisted gait training in patients with Parkinson disease: a randomized controlled trial. Neurorehabil Neural Repair. 2012a;26:353–361. doi: 10.1177/1545968311424417. [DOI] [PubMed] [Google Scholar]
- 29.Picelli A, Melotti C, Origano F, et al. Does robotic gait training improve balance in Parkinson’s disease? A randomized controlled trial. Parkinsonism Relat Disord. 2012b;18:990–993. doi: 10.1016/j.parkreldis.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Picelli A, Camin M, Tinazzi M, et al. Three-dimensional motion analysis of the effects of auditory cueing on gait pattern in patients with Parkinson’s disease: a preliminary investigation. Neurol Sci. 2010;31:423–430. doi: 10.1007/s10072-010-0228-2. [DOI] [PubMed] [Google Scholar]
- 31.Pietrelli A, Lopez-Costa J, Goñi R, et al. Aerobic exercise prevents age-dependent cognitive decline and reduces anxiety-related behaviors in middle-aged and old rats. Neuroscience. 2012;202:252–266. doi: 10.1016/j.neuroscience.2011.11.054. [DOI] [PubMed] [Google Scholar]
- 32.Poletti M, Frosini D, Pagni C, et al. Mild cognitive impairment and cognitive-motor relationships in newly diagnosed drug-naive patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2012;83:601–606. doi: 10.1136/jnnp-2011-301874. [DOI] [PubMed] [Google Scholar]
- 33.Pothakos K, Kurz MJ, Lau YS. Restorative effect of endurance exercise on behavioral deficits in the chronic mouse model of Parkinson’s disease with severe neurodegeneration. BMC Neurosci. 2009;10:6. doi: 10.1186/1471-2202-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smania N, Picelli A, Geroin C, et al. Robot-assisted gait training in patients with Parkinson’s disease. Neurodegenerative Disease Management. 2013;3:321–330. [Google Scholar]
- 35.Song J, Fisher BE, Petzinger G, et al. The relationships between the unified Parkinson’s disease rating scale and lower extremity functional performance in persons with early-stage Parkinson’s disease. Neurorehabil Neural Repair. 2009;23:657–661. doi: 10.1177/1545968309332878. [DOI] [PubMed] [Google Scholar]
- 36.Tomlinson CL, Patel S, Meek C, et al. Physiotherapy versus placebo or no intervention in Parkinson’s disease. Cochrane Database Syst Rev. 2013;9:CD002817. doi: 10.1002/14651858.CD002817.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tuon T, Valvassori SS, Dal Pont GC, et al. Physical training prevents depressive symptoms and a decrease in brain-derived neurotrophic factor in Parkinson’s disease. Brain Res Bull. 2014;108:106–112. doi: 10.1016/j.brainresbull.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Tuon T, Valvassori SS, Lopes-Borges J, et al. Physical training exerts neuroprotective effects in the regulation of neurochemical factors in an animal model of Parkinson’s disease. Neuroscience. 2012;227:305–312. doi: 10.1016/j.neuroscience.2012.09.063. [DOI] [PubMed] [Google Scholar]
- 39.van Praag H, Shubert T, Zhao C, et al. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varalta V, Picelli A, Fonte C, et al. Relationship between cognitive performance and motor dysfunction in patients with Parkinson’s disease: a pilot cross-sectional study. Biomed Res Int. 2015;2015;2015:365959. doi: 10.1155/2015/365959. [DOI] [PMC free article] [PubMed] [Google Scholar]