Abstract
Administration of nitroglycerin (NTG) to rats induces a hyperalgesic condition and neuronal activation of central structures involved in migraine pain. In order to identify therapeutic strategies for migraine pain, we evaluated the anti-nociceptive activity of Andrographis Paniculata (AP), a herbaceous plant, in the hyperalgesia induced by NTG administration in the formalin test. We also analyzed mRNA expression of cytokines in specific brain areas after AP treatment. Male Sprague-Dawley rats were pre-treated with AP extract 30 minutes before NTG or vehicle injection. The data show that AP extract significantly reduced NTG-induced hyperalgesia in phase II of the test, 4 hours after NTG injection. In addition, AP extract reduced IL-6 mRNA expression in the medulla and mesencephalon and also mRNA levels of TNF-alpha in the mesencephalic region. These findings suggest that AP extract may be a potential therapeutic approach in the treatment of general pain, and possibly of migraine.
Keywords: cytokines, Andrographis Paniculata, formalin test, migraine, nitroglycerin
Introduction
Experimental research has led to considerable advances in understanding of migraine pain mechanisms, and several options for both acute and prophylactic treatment have emerged (Olesen and Ashina, 2011). However, alternative therapeutic approaches are still needed because of tolerability issues and/or high percentages of non-responders. The popularity of medicinal herbs has grown significantly over the past decade and several reports have focused on the therapeutic potential of various plant-derived compounds, particularly molecules derived from natural leads with anti-inflammatory activities. Bioactive chemical constituents from medicinal herbs, namely sesquiterpene lactones, monoterpenes/diterpenes and flavonoids, are known to elicit a variety of pharmacological activities. Some of these compounds act as enzyme inhibitors and antioxidants, and have been reported to have anti-inflammatory properties (Middleton et al., 2000; Havsteen, 2002). The inflammatory response is associated with increased production of nitric oxide (NO) and activation of proinflammatory mediators (Moilanen et al., 1999; Bogdan, 2001). Although the exact mechanisms remain elusive, certain flavonoids and diterpenoids down-regulate NO production in response to inflammatory stimuli (Díaz-Viciedo et al., 2008). Experimental data support the idea that compounds inhibiting expression or activity of NO synthase (NOS) and nuclear factor-kappa B (NF-κB) are potential anti-migraine agents (Reuter et al., 2001). NO is involved in pain transmission, hyperalgesia, chronic pain, inflammation and central sensitization, mostly in a cyclic guanosine monophosphate-dependent way (Neeb and Reuter, 2007). Systemic nitroglycerin (NTG), an NO donor, activates brain nuclei involved in pain transmission as well as in neuroendocrine and autonomic functions in rats. These changes are considered significant for migraine, because NTG consistently induces delayed migraine-like attacks only in migraineurs (Sances et al., 2004). Additionally, in rats, NTG injection in the formalin test (a model of inflammatory pain) induces a state of hyperalgesia that is detectable as an increase in nociceptive behavior (Greco et al., 2014, 2015). Various evidence points to the existence of a condition of trigeminal sensitization in migraineurs, which results in hyperalgesia, allodynia and cognitive dysfunction during and between episodes (Noseda and Burstein, 2013). Therefore, NTG-induced hyperalgesia in rodents is used as an experimental model for sensory hypersensitivity associated with migraine (Tassorelli et al., 1999; Greco et al., 2014, 2015). Andrographis Paniculata (AP), also known as “King of Bitters”, is a herbaceous plant belonging to the Acanthaceae family; AP has been widely used in traditional Asian medicines for centuries. The traditional uses and pharmacological aspects of AP have been well documented, and its major constituents are diterpenoids, flavonoids and polyphenols (Jarukamjorn and Nemoto, 2008). Phytochemical studies have identified a specific bioactive diterpene, known as andrographolide, as the main component of AP (Sareer et al., 2014). Andrographolide and its derivatives and synthetic analogs have been shown to have a broad range of pharmacological properties, including anti-inflammatory, immunostimulatory and anticancer activities (Akbar, 2011; Varma et al., 2011). In addition, the components of AP extract exhibit anti-inflammatory effects by interfering with the production of inflammatory mediators, in particular cyclooxygenase (COX) enzymes and pro-inflammatory cytokines (Parichatikanond et al., 2010). Notably, these effects are intimately associated with the modulation of the NF-κB signaling network and the down-regulation of pro-inflammatory responses (Lim et al., 2012). Moreover, another critical target for the inflammation suppressive activity regulated by andrographolide is the NO/inducible NOS (iNOS) pathway (Lim et al., 2012). The NO/iNOS system is involved in pain transmission, hyperalgesia, chronic pain, neuroinflammation and central sensitization (Barbanti et al., 2014). Despite the potent anti-inflammatory properties exhibited by AP, its anti-nociceptive and analgesic effects are still largely unexplored. However, recent studies reported an anti-nociceptive effect of AP in the acetic acid-induced writhing, hot-plate and formalin tests (Sulaiman et al., 2010; Adedapo et al., 2015).
The aim of the present study was to evaluate the anti-nociceptive activity of AP and its potential effect on the mRNA expression of specific cytokines in an animal model of hyperalgesia induced by NTG administration (Tassorelli et al., 2003; Greco et al., 2014). This experimental model has been tested over the years with different drugs and is considered a reliable animal model of migraine (Tassorelli and Joseph, 1995; Bergerot et al., 2006; Greco et al., 2011).
Materials and methods
Animals
Adult male Sprague-Dawley rats (weight 250–270 g) were evaluated in the present experiments. The principles of the Helsinki declaration and IASP’s guidelines for pain research in animals were rigorously applied (Zimmermann, 1983). The rats were housed two per cage, at 20–22 °C on a 12-h light-dark cycle, with food and water ad libitum at the Centralized Animal Facility of the University of Pavia. All procedures were approved by the local Animal Care Committee. All the rats were acclimatized to the test chamber before testing began.
Drugs
The ethanolic extract of aerial parts of AP (Product: L100235L, purified by high performance liquid chromatography; andrographolide average content 10%) was purchased from Truffini and Reggè Farmaceutici, Milan, Italy. The extract was dissolved in saline (used as vehicle) and administered to the animals as intraperitoneal (i.p.) suspensions. Suspensions were freshly prepared every day (Sulaiman et al., 2007, 2010). Nitroglycerin (Bioindustria L.I.M. Novi Ligure (AL), Italy) was prepared from a stock solution of 5.0 mg/1.5 ml dissolved in 27% alcohol and 73% propylene glycol (PG). For the i.p. injections, NTG was further diluted in saline to reach the final concentration of alcohol 6% and PG 16% and administered at a dose of 10 mg/kg. The vehicle control used in these experiments was 16% PG, 6% alcohol and 0.9% saline (NTG vehicle).
Formalin test: experimental design
The rats were randomly divided into groups of 6–8 animals, according to the following treatment schedule:
1) control group (n=8): saline (30′) + NTG vehicle, 12 ml/kg, i.p.
2) saline + NTG group (n=8): saline (30′) + NTG, 10 mg/kg, i.p.
3) AP 1 + NTG group (n=6): AP extract (30′), 50 mg/kg, i.p. + NTG;
4) AP 2 + NTG group (n=6): AP extract (30′), 25 mg/kg, i.p. + NTG.
In addition, to evaluate the possible interaction of AP extract with NTG vehicle we included another experimental group:
5) AP 1 + NTG vehicle group (n=6): AP extract (30′), 50 mg/kg, i.p. + NTG vehicle
AP or saline was administered 30 minutes (30′) before NTG or NTG vehicle injection. The formalin test was performed 4 hours after NTG or vehicle administration. The investigators responsible for the behavioral testing were blind to treatment group assignment. For the formalin test, a 100 μl volume of 1% formalin (formaldehyde diluted in 0.9% saline) was administered by intraplantar injection. Pain-related behavior was quantified for 1 h by counting spontaneous flinches and shakes of the injected paw: over 60-s periods for the first 5 min (min 1, 2, 3, 4 and 5) and thereafter following 4-min pauses, for 60-s periods up to the hour. Phase I was defined as the period from 1 to 5 min, phase II was defined as the period from 15 to 60 min inclusive. Phase I is considered the result of chemical activation of nociceptors, whereas phase II reflects the inflammatory reaction and central processing (Tjølsen et al., 1992).
Detection of mRNA expression of cytokines: experimental design
The rats were randomly divided into four groups of 5–6 animals, according to the following treatment schedule:
Control group (n=6): saline (30′) + NTG vehicle, 12 ml/kg, i.p.
saline + NTG group (n=6): saline (30′) + NTG, 10 mg/kg, i.p.
AP 1 + NTG group (n=5): AP extract (30′), 50 mg/kg, i.p. + NTG
AP 1 + NTG vehicle group (n=5): AP extract (30′), 50 mg/kg, i.p. + NTG vehicle
On the basis of the distribution of the nuclei that are known to be activated by NTG and involved in migraine pain, the following discrete brain areas were dissected out 4 hours after NTG or vehicle administration and used for analysis: the medulla (bregma, −13.30 to −14.60 mm), containing the nucleus trigeminalis caudalis (NTC), nucleus tractus solitarius and area postrema; the mesencephalon (bregma, −4.8 to −6.04 mm), containing the ventrolateral column of the periaqueductal gray and parabrachial nucleus.
Real-time polymerase chain reaction
Interleukin-6 (IL-6) (forward primer: TTCTCTCCGCAAGAGACTTC; reverse primer: GGTCTGTTGTGGGTGGTATC) and tumor necrosis factor alpha (TNF-alpha) (forward primer: CCTCACACTCAGATCATCTTCTC; reverse primer: CGCTTGGTGGTTTGCTAC) mRNA expression was analyzed by a real-time polymerase chain reaction (RT-PCR). Total RNA was isolated from the cerebral samples with Trizol reagent in accordance with the method of Chomczynski and Mackey (1995). RNA was quantified by measuring the absorbance at 260/280 nm. cDNA was generated using the iScript cDNA Synthesis kit (Bio-rad, Milan) following the supplier’s instructions. Gene expression was analyzed using the Fast Eva Green supermix (Bio-rad). As regards the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH; forward primer: AACCTGCCAAGTATGATGAC; reverse primer: GGAGTTGCTGTTGAAGTCA) was used. The expression of the housekeeping gene remained constant in all the experimental groups considered. The amplification was performed through two-step cycling (95–60°C) for 45 cycles in a light Cycler 480 Instrument RT-PCR Detection System (Roche, Milan) following the supplier’s instructions. All samples were assayed in triplicate. Gene expression was calculated using the ΔCt method.
Statistical analysis
Data are expressed as mean ± SEM. The total number of flinches/shakes evoked by formalin injection was counted separately for phase I and for phase II, as described above. An a priori power analysis was conducted to determine the minimal sample size needed to obtain a statistical power of 0.80 at an alpha level of 0.05. In our previous studies, evaluating the difference in nociceptive response in the second phase of the formalin test (total number flinches/shakes) between rats injected with NTG and rats injected with vehicle (Tassorelli et al., 2006), we calculated a standardized effect size of 2.38 for this variable. The analysis estimated a sample size of at least five rats per group. We used 5–8 rats per experimental group. The data were tested for normality using the KS normality test and considered normal. The differences between groups were analyzed by unpaired t test or one-way analysis of variance (ANOVA) followed by the Newman-Keuls Multiple Comparison Test when more than two groups were compared. The minimum level of statistical significance was set at p<0.05.
Results
Formalin test
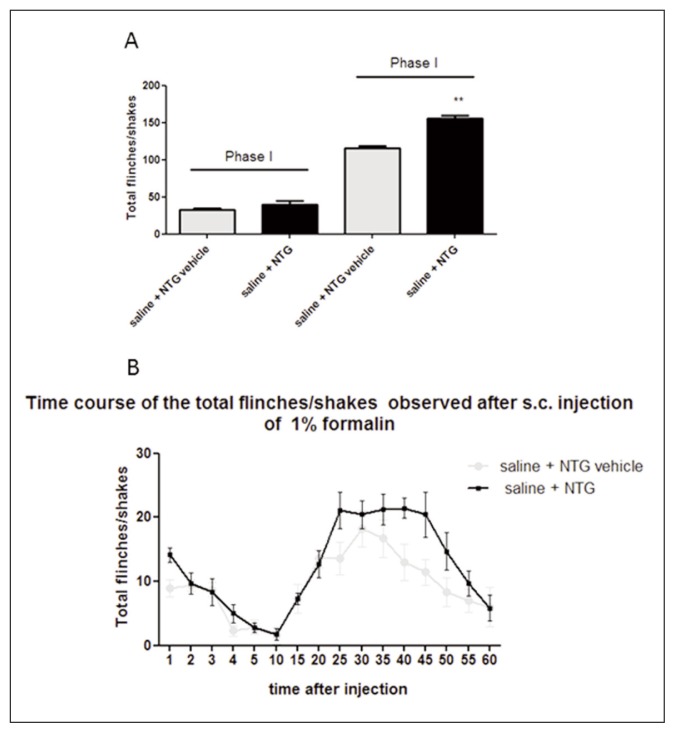
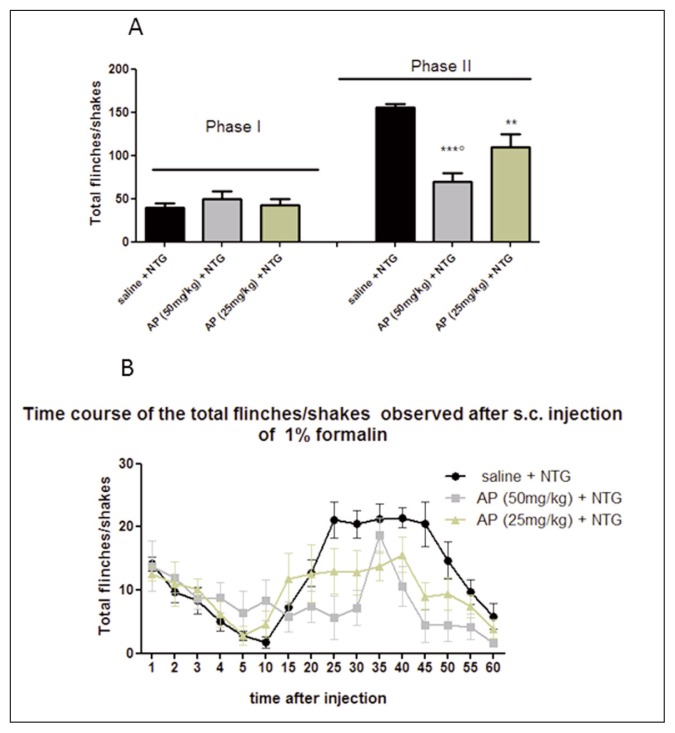
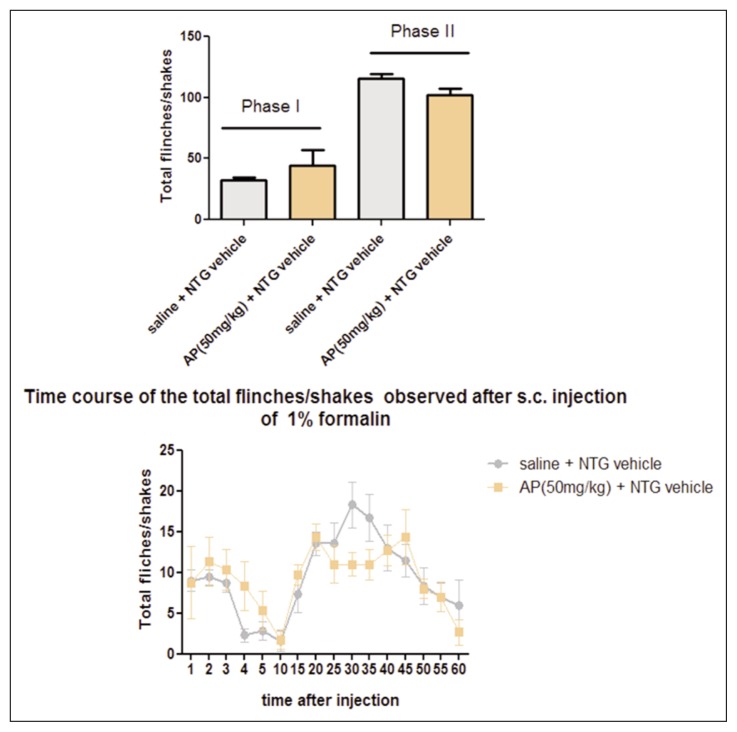
In the control group (saline + NTG vehicle), the formalin injection induced an initial acute phase of nociception within the first 5 min (phase I), followed by a prolonged tonic response from 15 to 60 min after formalin injection (phase II). As expected, NTG administration significantly increased the nocifensive behavior in phase II of the formalin test, compared with the control group (Fig. 1), confirming previous data (Tassorelli et al., 2006). AP ethanolic extract (25 mg/kg) significantly reduced the nocifensive behavior in phase II of the test after NTG administration. The effect was more evident when AP ethanolic extract was administered at a higher dose (50 mg/kg) before NTG administration (Fig. 2). No significant effect on nocifensive behavior was observed after AP extract (50 mg/kg) pre-treatment in the experimental group treated with NTG vehicle (Fig. 3).
Figure 1.
Nitroglycerin-induced hyperalgesia in the formalin test.
(A) NTG administration significantly increased the total number of flinches/shakes during phase II of the test, when compared with saline + NTG vehicle group (control group). No significant effect was reported in phase I. (B) Time course of the nocifensive behavior in the different groups. Data are expressed as mean ± SEM. Unpaired t test, **p<0.01 vs saline + NTG vehicle.
Figure 2.
Effects of Andrographis Paniculata (AP) on nitroglycerin-induced hyperalgesia in the formalin test.
(A) The histograms illustrate the total number of flinches and shakes per phase of the test. AP ethanolic extract (50 mg/kg or 25 mg/kg) is effective to counteract NTG-induced hype-ralgesia during phase II of the test, compared with saline + NTG group. (B) Time course of the nocifensive behavior in different groups. Data are expressed as mean ± SEM. ANOVA followed by Newman-Keuls Multiple Comparison Test; ***p<0.001 vs saline + NTG; **p< 0.01 vs saline + NTG; ∘p<0.05 vs AP (25 mg/kg).
Figure 3.
Andrographis Paniculata (AP) effects in baseline conditions (without NTG)
(A) The histograms illustrate the total number of flinches and shakes per phase of the test. After AP extract (50 mg/kg) pre-treatment no significant effect was observed on the nocifensive behavior in the experimental group treated with NTG vehicle.
(B) Time course of the nocifensive behavior in different groups. Data are expressed as mean ± SEM. Unpaired t test.
mRNA expression of cytokines
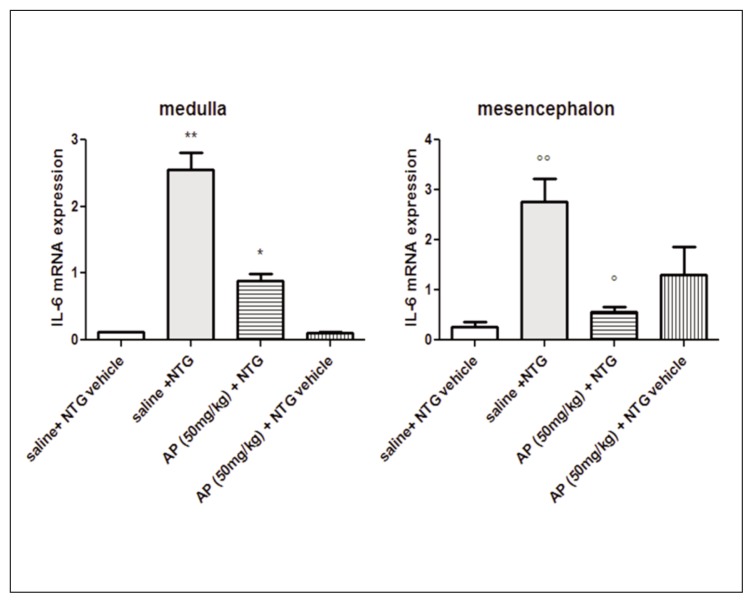
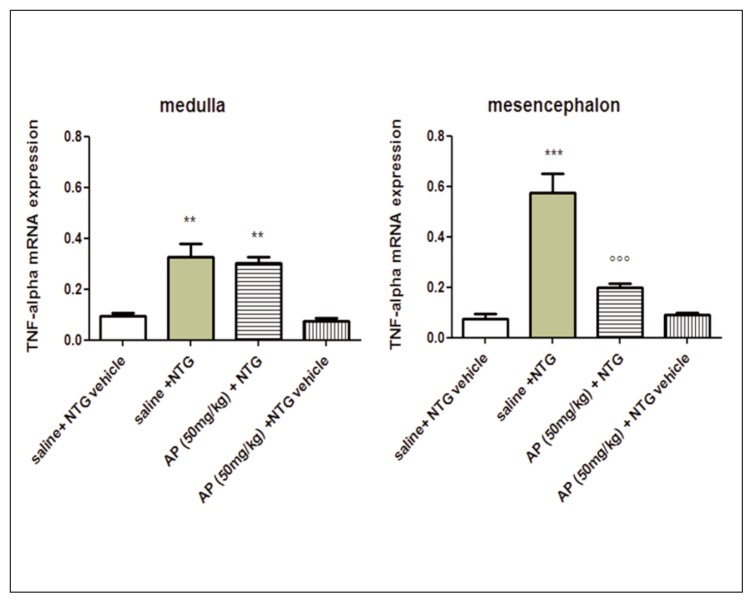
IL-6 and TNF-alpha mRNA expression was significantly increased in the medulla and in the mesencephalon of rats treated with NTG, compared with the control group (saline + NTG vehicle) (Figs. 4 and 5). AP ethanolic extract pre-treatment significantly reduced the expression of IL-6 mRNA in all cerebral areas. The level of TNF-alpha mRNA was reduced only in the mesencephalon (Figs. 4 and 5). No significant effect in cytokine mRNA expression was observed after AP extract pre-treatment in cerebral areas of rats treated with saline + NTG vehicle (Figs. 4 and 5).
Figure 4.
IL-6 mRNA expression in specific brain areas.
NTG administration significantly increased IL-6 mRNA expression in the medulla and mesencephalon compared with saline + NTG vehicle group. AP pre-treatment significantly reduced IL-6 mRNA expression in both brain areas, compared with saline + NTG group. Data reported as mean ± SEM, ANOVA followed by Newman-Keuls Multiple Comparison Test, **p<0.01 vs saline + NTG vehicle and AP (50 mg/kg) + NTG vehicle; * p<0.05 vs saline + NTG, saline + NTG vehicle and AP (50 mg/kg) + NTG vehicle; ∘∘p<0.01 vs saline + NTG vehicle; °p<0.05 vs saline + NTG.
Figure 5.
TNF-alpha mRNA expression in specific brain areas. NTG administration significantly increased TNF-alpha mRNA expression in the medulla and mesencephalon, compared with saline + NTG vehicle group. AP pre-treatment significantly reduced TNF-alpha mRNA level in the mesencephalon, compared with saline + NTG group. Data reported as mean ± SEM, ANOVA followed by Newman-Keuls Multiple Comparison Test. **p<0.01 vs saline + NTG vehicle and AP (50 mg/kg) + NTG vehicle; ***p<0.001 vs saline + NTG vehicle and AP (50 mg/kg) + NTG vehicle; ∘∘∘p<0.001 vs saline + NTG.
Discussion
Systemic NTG activates brain nuclei involved in nociceptive transmission as well as in neuroendocrine and autonomic functions in rats (Tassorelli et al., 1999). These changes are considered relevant for migraine because NTG consistently provokes spontaneous-like migraine attacks in migraineurs (Sances et al., 2004). NTG is able to induce a long-lasting hyperalgesic state in rats, detectable as an increase in the nociceptive behavior evoked by the formalin test (Tassorelli et al., 2006; Greco et al., 2015). This is probably due to complex mechanisms involving NO synthesis but also to sensitization of the trigeminovascular system at the meningeal and central level (Galeotti and Ghelardini, 2013). The formalin test was introduced as a model of tonic pain several years ago, and has since been used extensively in rats and mice (Tjølsen et al., 1992). In this study, we investigated the anti-hyperalgesic effect of AP ethanolic extract in baseline conditions and in NTG-induced hyperalgesia in the formalin test. In addition, in order to evaluate whether pretreatment with AP may modulate expression of pro-inflammatory cytokines, we also examined IL-6 and TNF-alpha mRNA expression in brain areas specific for migraine pain.
AP ethanolic extract proved capable of counteracting NTG-induced hyperalgesia in the second phase of the formalin test but did not alter phase I. This was also the case when a lower dose was used. Lack of phase I responses suggests that AP ethanolic extract does not alter basal pain but rather reduces pain transmission and alters nocifensive behavior in phase II compared with the control group (saline + NTG vehicle). Although several studies have reported a significant analgesic effect of AP in animal models of inflammatory pain, this discrepancy in the results may be due to differences in timing and route of administration (Sulaiman et al., 2007; Adedapo et al., 2015). It should also be pointed out that the possible mechanisms underlying NTG-induced hyperalgesia are currently elusive. However, it is becoming increasingly evident that NTG exerts its activity through a direct effect on the production of NO and pro-inflammatory mediators at central level (Tassorelli et al., 2006, 2007), or indirectly via inflammation mediated by NO-dependent mechanisms at the meningeal level as a consequence of sensitization of the trigeminovascular system (Reuter et al., 2001). The second phase of the formalin test reflects the activation of mediators of the inflammatory response and the activation of dorsal horn neurons (Tjølsen et al., 1992). Therefore, it is possible that the clear-cut anti-hyperalgesic effect observed following the AP administration reflects an interaction at the periphery as well as at central level. Cytokines play a key role in inflammatory responses, modulation of the pain threshold and also in trigeminal nerve fiber sensitization. These mediators are related to neurogenic inflammation in the pathogenesis of migraine. Moreover, it is known that increased expression of cytokines is implicated in the development and maintenance of sensitization of peripheral nociceptors and second order neurons (Miller et al., 2009). Migraine patients indeed show higher serum levels of IL-1beta and IL-6, and lower levels of IL-10 than healthy subjects (Uzar et al., 2011). By contrast, TNF-alpha levels increase after migraine pain onset and decrease progressively over time (Perini et al., 2005).
Our findings show that NTG significantly increased IL-6 and TNF-alpha expression in all cerebral areas examined. These findings demonstrate for the first time that NTG administration may induce changes in cerebral cytokine gene expression and not only at the dural level (Reuter et al., 2001). A recent study reported a significant increase of pro-inflammatory cytokines in the trigeminal ganglia and in spinal trigeminal nuclei following capsaicin injection in the eyebrow regions (Vause and Durham, 2012). In this study, pre-treatment with AP ethanolic extract significantly reduced IL-6 mRNA expression in all cerebral areas, while a significant reduction of TNF-alpha mRNA expression was found only in the mesencephalon. In accordance with these findings, Parichatikanond et al. (2010) demonstrated that components of AP extract induce a down-regulation of genes involved in inflammatory responses, including IL-6 and TNF-alpha. Andrographolide is one of the main ingredients of AP, and it was reported to possess anti-inflammatory activity (Akbar, 2011, Lim et al., 2012) and to inhibit NO production in lipopolysaccharide-stimulated murine macrophage (Liu et al., 2007). In a previous study, Hidalgo et al. (2005) reported the ability of andrographolide to inhibit NF-κB binding to DNA, which then leads to reduced expression of proinflammatory proteins, including COX-2 and cytokines. The capacity of AP to reduce inflammatory pathways by preventing binding of NF-κB to DNA thus represents a potential mechanism for its anti-hyperalgesic effect. It is noteworthy that NTG increases the transcriptional activity of NF-κB in the dura and medulla of rats (Reuter et al., 2001; Greco et al., 2005) and these changes may also be associated with increased COX-2 expression in the medulla (Tassorelli et al., 2007). The medulla is the area containing the NTC, one of the most important relay stations for pain transmission in the brain. In this area, we found increased IL-6 and TNF-alpha mRNA levels.
Taken together, these observations provide evidence that AP ethanolic extract has anti-hyperalgesic activity in an experimental animal model of sensory hypersensitivity associated with migraine. AP ethanolic extract seems to act through mechanisms that may be related to direct or indirect inhibition of pro-inflammatory responses in specific brain areas involved in migraine pain transmission.
Acknowledgments
This work was funded by FB Health S.p.A. (Ascoli Piceno – Italy)
References
- 1.Adedapo AA, Adeoye BO, Sofidiya MO, et al. Antioxidant, antinociceptive and anti-inflammatory properties of the aqueous and ethanolic leaf extracts of Androphaphis paniculata in some laboratory animals. J Basic Clin Physiol Pharmacol. 2015;26:327–334. doi: 10.1515/jbcpp-2014-0051. [DOI] [PubMed] [Google Scholar]
- 2.Akbar S. Andrographis paniculata: a review of pharmacological activities and clinical effects. Altern Med Rev. 2011;16:66–77. [PubMed] [Google Scholar]
- 3.Barbanti P, Egeo G, Aurilia C, et al. Drugs targeting nitric oxide synthase for migraine treatment. Expert Opin Investig Drugs. 2014;23:1141–1148. doi: 10.1517/13543784.2014.918953. [DOI] [PubMed] [Google Scholar]
- 4.Bergerot A, Holland PR, Akerman S. Animal models of migraine: looking at the component parts of a complex disorder. Eur J Neurosci. 2006;24:1517–1534. doi: 10.1111/j.1460-9568.2006.05036.x. [DOI] [PubMed] [Google Scholar]
- 5.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 6.Chomczynski P, Mackey K. Substitution of chloroform by bromo-chloropropane in the single-step method of RNA isolation. Anal Biochem. 1995;225:163–164. doi: 10.1006/abio.1995.1126. [DOI] [PubMed] [Google Scholar]
- 7.Díaz-Viciedo R, Hortelano S, Girón N, et al. Modulation of inflammatory responses by diterpene acids from Helianthus annuus L. Biochem Biophys Res Commun. 2008;369:761–766. doi: 10.1016/j.bbrc.2008.02.104. [DOI] [PubMed] [Google Scholar]
- 8.Galeotti N, Ghelardini C. St. John’s wort relieves pain in an animal model of migraine. Eur J Pain. 2013;17:369–381. doi: 10.1002/j.1532-2149.2012.00196.x. [DOI] [PubMed] [Google Scholar]
- 9.Greco R, Bandiera T, Mangione AS, et al. Effects of peripheral FAAH blockade on NTG-induced hyperalgesia-evaluation of URB937 in an animal model of migraine. Cephalalgia. 2015;35:1065–1076. doi: 10.1177/0333102414566862. [DOI] [PubMed] [Google Scholar]
- 10.Greco R, Mangione AS, Sandrini G, et al. Activation of CB2 receptors as a potential therapeutic target for migraine: evaluation in an animal model. J Headache Pain. 2014;15:14. doi: 10.1186/1129-2377-15-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greco R, Meazza C, Mangione AS, et al. Temporal profile of vascular changes induced by systemic nitroglycerin in the meningeal and cortical districts. Cephalalgia. 2011;31:190–198. doi: 10.1177/0333102410379887. [DOI] [PubMed] [Google Scholar]
- 12.Greco R, Tassorelli C, Cappelletti D, et al. Activation of the transcription factor NF-kappaB in the nucleus trigeminalis caudalis in an animal model of migraine. Neuro-toxicology. 2005;26:795–800. doi: 10.1016/j.neuro.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 2002;96:67–202. doi: 10.1016/s0163-7258(02)00298-x. [DOI] [PubMed] [Google Scholar]
- 14.Hidalgo MA, Romero A, Figueroa J, et al. Andrographolide interferes with binding of nuclear factor-kappaB to DNA in HL-60-derived neutrophilic cells. Br J Pharmacol. 2005;144:680–686. doi: 10.1038/sj.bjp.0706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarukamjorn L, Nemoto N. Pharmacological aspects of Andrographis paniculata on health and its major diterpenoid constituent andrographolide. Journal of Health Science. 2008;54:370–381. [Google Scholar]
- 16.Lim JC, Chan TK, Ng DS, et al. Andrographolide and its analogues: versatile bioactive molecules for combating inflammation and cancer. Clin Exp Pharmacol Physiol. 2012;39:300–310. doi: 10.1111/j.1440-1681.2011.05633.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Wang ZT, Ji LL, et al. Inhibitory effects of neoandrographolide on nitric oxide and prostaglandin E2 production in LPS-stimulated murine macrophage. Mol Cell Biochem. 2007;298:49–57. doi: 10.1007/s11010-006-9349-6. [DOI] [PubMed] [Google Scholar]
- 18.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 19.Moilanen E, Whittle BJR, Moncada S. Nitric oxide as a factor of inflammation. In: Gallin JI, Snyderman R, editors. Inflammation: Basic Principles and Clinical Correlates. Philadelphia, Pa, USA: Lippincott Williams & Wilkins; 1999. pp. 787–801. [Google Scholar]
- 20.Miller R, Jung H, Bhangoo S, et al. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol. 2009;(194):417–449. doi: 10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neeb L, Reuter U. Nitric oxide in migraine. CNS Neurol Disord Drug Targets. 2007;6:258–264. doi: 10.2174/187152707781387233. [DOI] [PubMed] [Google Scholar]
- 22.Noseda R, Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain. 2013;154(Suppl 1):S44–S53. doi: 10.1016/j.pain.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 23.Olesen J, Ashina M. Emerging migraine treatments and drug targets. Trends Pharmacol Sci. 2011;32:352–359. doi: 10.1016/j.tips.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Parichatikanond W, Suthisisang C, Dhepakson P, et al. Study of anti-inflammatory activities of the pure compounds from Andrographis paniculata (burm.f.) Nees and their effects on gene expression. Int Immunopharmacol. 2010;10:1361–1373. doi: 10.1016/j.intimp.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Perini F, D’Andrea G, Galloni E, et al. Plasma cytokine levels in migraineurs and controls. Headache. 2005;45:926–931. doi: 10.1111/j.1526-4610.2005.05135.x. [DOI] [PubMed] [Google Scholar]
- 26.Reuter U, Bolay H, Jansen-Olesen I, et al. Delayed inflammation in rat meninges: implications for migraine pathophysiology. Brain. 2001;124:2490–2502. doi: 10.1093/brain/124.12.2490. [DOI] [PubMed] [Google Scholar]
- 27.Sances G, Tassorelli C, Pucci E, et al. Reliability of the nitroglycerin provocative test in the diagnosis of neurovascular headaches. Cephalalgia. 2004;24:110–119. doi: 10.1111/j.1468-2982.2004.00639.x. [DOI] [PubMed] [Google Scholar]
- 28.Sareer O, Ahmad S, Umar S. Andrographis paniculata: a critical appraisal of extraction, isolation and quantification of andrographolide and other active constituents. Nat Prod Res. 2014;28:2081–2101. doi: 10.1080/14786419.2014.924004. [DOI] [PubMed] [Google Scholar]
- 29.Sulaiman MR, Zakaria ZA, Abdul Rahman A, et al. Antinociceptive and antiedematogenic activities of andrographolide isolated from Andrographis paniculata in animal models. Biol Res Nurs. 2010;11:293–301. doi: 10.1177/1099800409343311. [DOI] [PubMed] [Google Scholar]
- 30.Sulaiman MR, Sainan S, Zakaria ZA, et al. Anti-nociceptive profile of the ethanolic extract of andrographis paniculata in mice. Oriental Pharmacy and Experimental Medicine. 2007;7:390–394. [Google Scholar]
- 31.Tassorelli C, Greco R, Armentero MT, et al. A role for brain cyclooxygenase-2 and prostaglandin-E2 in migraine: effects of nitroglycerin. Int Rev Neurobiol. 2007;82:373–382. doi: 10.1016/S0074-7742(07)82020-4. [DOI] [PubMed] [Google Scholar]
- 32.Tassorelli C, Greco R, Wang D, et al. Prostaglandins, glutamate and nitric oxide synthase mediate nitroglycerin-induced hyperalgesia in the formalin test. Eur J Pharmacol. 2006;534:103–107. doi: 10.1016/j.ejphar.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 33.Tassorelli C, Greco R, Wang D, et al. Nitroglycerin induces hyperalgesia in rats—a time-course study. Eur J Pharmacol. 2003;464:159–162. doi: 10.1016/s0014-2999(03)01421-3. [DOI] [PubMed] [Google Scholar]
- 34.Tassorelli C, Joseph SA, Buzzi MG, et al. The effects on the central nervous system of nitroglycerin—putative mechanisms and mediators. Prog Neurobiol. 1999;57:607–624. doi: 10.1016/s0301-0082(98)00071-9. [DOI] [PubMed] [Google Scholar]
- 35.Tassorelli C, Joseph SA. Systemic nitroglycerin induces Fos immunoreactivity in brainstem and forebrain structures of the rat. Brain Res. 1995;682:167–181. doi: 10.1016/0006-8993(95)00348-t. [DOI] [PubMed] [Google Scholar]
- 36.Tjølsen A, Berge OG, Hunskaar S, et al. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- 37.Uzar E, Evliyaoglu O, Yucel Y, et al. Serum cytokine and pro-brain natriuretic peptide (BNP) levels in patients with migraine. Eur Rev Med Pharmacol Sci. 2011;15:1111–1116. [PubMed] [Google Scholar]
- 38.Varma A, Padh H, Shrivastava N. Andrographolide: a new plant-derived antineoplastic entity on horizon. Evid Based Complement Alternat Med. 20112011:815390. doi: 10.1093/ecam/nep135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vause CV, Durham PL. Identification of cytokines and signaling proteins differentially regulated by sumatriptan/naproxen. Headache. 2012;52:80–89. doi: 10.1111/j.1526-4610.2011.02048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]