Abstract
Objectives New dual antiplatelet therapies (DAPTs) have been introduced in clinical practice for patients with acute coronary syndrome (ACS). This nationwide study investigated DAPT patterns over time and patient characteristics associated with the various treatments in a population with ACS. Design This observational cohort study linked morbidity, mortality and medication data from Swedish national registries. Results Overall, 91% (104 012 patients) of all patients admitted to the hospital with an ACS (2009–2013) were alive after discharge and included in this study. Compared with 2009, in 2013 patients investigated with angiography increased by 10%, patients revascularized with percutaneous coronary intervention (PCI) increased by 11% and patients prescribed DAPT increased by 8%. Mean DAPT duration increased from 225 to 298 days in patients investigated with angiography, and from 155 to 208 days in patients who were not investigated with angiography. Furthermore, in patients undergoing angiography a treatment switch from clopidogrel to ticagrelor was observed. DAPT with prasugrel was used to a low extent. Approximately 10% of patients initiated on prasugrel or ticagrelor switched to clopidogrel during the first year of treatment. Conclusion During the study more patients underwent angiography and PCI. There was an increase in the proportion of ACS patients receiving DAPT, as well as longer duration of DAPT in line with ESC guidelines. Among DAPT-treated patients, ticagrelor has emerged as the preferred P2Y12 antagonist in patients undergoing angiography, whereas clopidogrel tended to be prescribed to patients treated non-invasively.
Keywords: Acute coronary syndrome, dual antiplatelet treatment, nationwide registry data
Introduction
Acute coronary syndrome (ACS) remains as a leading cause of mortality worldwide, despite improved cardiovascular disease management.[1,2] Antiplatelet therapy is a key target in the treatment of ACS.[3–5] European Society of Cardiology (ESC) guidelines recommend dual antiplatelet therapy (DAPT) with low-dose acetylsalicylic acid (ASA) and a P2Y12 antagonist (clopidogrel, prasugrel or ticagrelor) to reduce the risk of acute ischaemic complications and recurrent atherothrombotic events for up to 12 months in patients with ACS, regardless of revascularization with percutaneous coronary intervention (PCI) or not.[6,7]
The time period following ACS onset represents a critical stage of coronary heart disease, with a high risk of recurrent unstable coronary disease.[8] In previous randomized trials, DAPT has been shown to reduce major adverse cardiac events (MACEs) in patients with ACS with treatment durations up to 12 months.[9–13]
To our knowledge, there are scarce published data describing the adherence to DAPT treatment and clinical characteristics associated with reduced adherence in large unselected patient populations. Previously, a nationwide Danish study examining initiation and persistence patterns of DAPT with clopidogrel and ASA treatment in an unselected cohort of post-myocardial infarction patients showed high treatment persistence among patients receiving PCI compared with those not receiving PCI.[14] The choice of drug, time of initiation and duration of treatment with P2Y12 antagonist depend on the clinical setting and patient-related factors, such as the ischemic risk, bleeding risk and other baseline clinical characteristics.[15]
The uptake of the new DAPT alternatives has been rapid and nationally extensive in Sweden.[16] However, to our knowledge, there is a lack of published data describing the changes over time in overall DAPTs in patients with ACS after the introduction of new treatment options.
The aim of this nationwide study, including all patients with an ACS, hospitalized and discharged alive between 2009 and 2013, was to compare the overall DAPT patterns over time and present descriptive data of patient selection for the various DAPT treatments.
Methods
This observational, retrospective cohort study analyzed data from mandatory Swedish national registries: the National Inpatient Register (IPR) (inpatient admission and discharge dates and diagnoses according to International Classification of Diseases, 10th revision, Clinical Modification [ICD-10 CM codes]), the Swedish Prescribed Drug Register (all drugs dispensed from pharmacies in Sweden, from 1 July 2005) and the Cause of Death Register (nationwide coverage of date and cause[s] of death). The National IPR covers more than 99% of all somatic and psychiatric hospital discharges. A validation of the IPR, in which diagnoses of myocardial infarction (MI) recorded in patient journals were compared with IPR data, revealed that >95% of all MI diagnoses in the IPR are valid.[17] All drugs were classified according to the Anatomical Therapeutic Chemical (ATC) classification system. Individual patient-level data from these registries were linked via the unique personal identification number, which was then replaced by a study identification number prior to further data processing. The study protocol was reviewed and approved by the regional ethics committee at Karolinska Institutet in Stockholm, Sweden. The linkage of data was approved and performed by the Swedish National Board of Health and Welfare. The linked database was managed at Statisticon AB, Stockholm, Sweden.
Study population
Patients had to have a primary diagnosis, i.e., main reason for hospitalization of ACS (MI [ICD-10:I21] or unstable angina pectoris [ICD-10:I20.0] from 1 January 2009 through 30 November 2013 to be eligible for the study. Patients were sub-classified into ST segment elevation myocardial infarction (STEMI) [ICD-10:I21.0-3] and non-segment ST elevation myocardial infarction (NSTEMI) [ICD-10:I21.4 + I20.0] and MI diagnosis with no sub-classification. Patients were included in the study at the qualifying event, which was the admission date of first registered admission for an ACS episode within the time period. A sensitivity analysis was performed with inclusion of patients with only first (lifetime) time ACS episode in order to study the potential impact of the prolonged event-free period for patients included in the end of the study period. Patients had to be alive within 30 days after the start of the qualifying event to be classified into the treatment groups. The DAPT-treated population included patients dispensed a P2Y12 antagonist in combination with ASA within 30 days after the start of the qualifying event. The date of dispatch of the P2Y12 antagonist was defined as the start of the DAPT treatment. The non-DAPT-treated population included patients who were not dispensed P2Y12 antagonist in combination with ASA within 30 days after the start of the qualifying event.
Statistical analyses
Patient characteristics at baseline included hospitalization data from 1987 and until the day before the qualifying event. Data for invasive procedures included procedures performed within 30 days after the qualifying event. Baseline drug treatment data were based on dispensed drugs 1 year within and until 30 days after the qualifying event. Follow-up data on DAPT persistence were collected from time of first dispensing of P2Y12 antagonist until 18 months after dispensing or time of death.
In order to identify patients targeted for invasive treatment or not, the DAPT- and non-DAPT-treated populations were stratified based on whether or not they underwent angiography for the qualifying event.
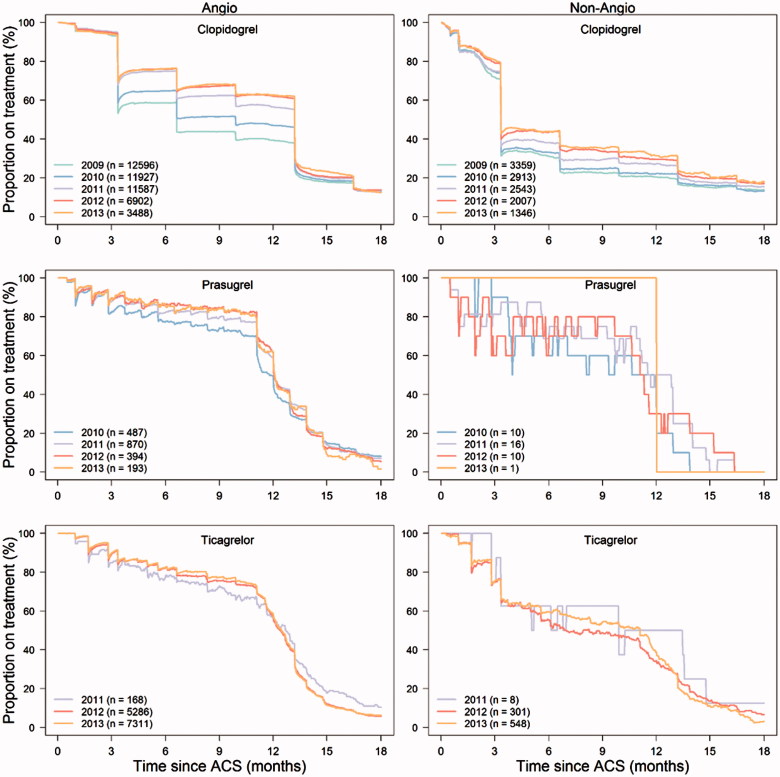
To describe persistence with treatment, the proportion of all subjects over time still alive who used the same P2Y12 antagonist as they did following discharge was estimated.[18] A subject was considered to be a P2Y12 antagonist user corresponding to the number of days based on the number of tablets dispensed from pharmacy for clopidogrel and prasugrel (used once daily) or half the number of tablets for ticagrelor (used twice daily). If a patient had a calculated treatment gap of more than 30 days, the patient is classified as non-user (drop out) in the proportion covered figure from last calculated day with available P2Y12 antagonist (Figure 3). Furthermore, in the figures, if a patient with treatment gap re-collected the same P2Y12 antagonist from the pharmacy during follow-up, the patient was classified as a user from that actual day. If a patient was switched to another P2Y12 antagonist during the first 18 months after the ACS episode, the patient is classified as a non-user from the day the new P2Y12 antagonist was dispensed.
Figure 3.
Persistence with different dual antiplatelet therapy in patients undergoing or not undergoing angiography from 2009 to 2013. Each point represents the number of patients with available medication divided by the number of patients alive at that time.
The average number of days on DAPT treatment was calculated as total days on treatment divided by the number of patients for all patients with at least 365 days’ observation time for actual P2Y12 antagonist.
The DAPTs treatment switch pattern was evaluated by assessing patients alive 1 year after the qualifying event and the proportion that were dispensed another P2Y12 antagonist other than the initial one within 12 months after DAPT initiation.
Results
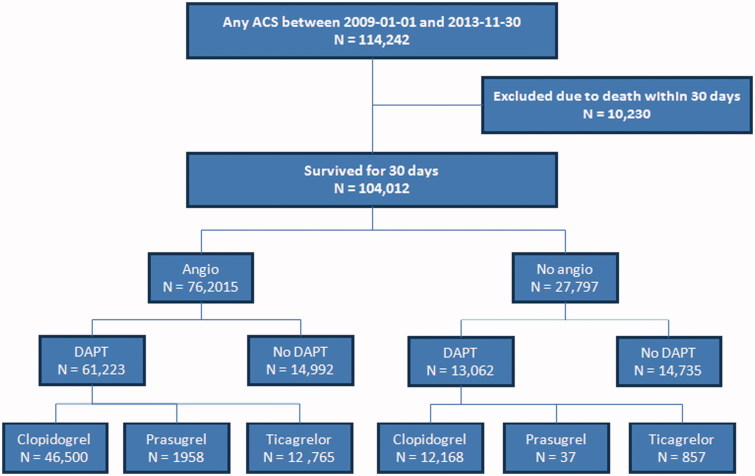
From 1 January 2009 to 30 November 2013, a total of 114 242 patients were admitted with ACS in Sweden, of whom 104 012 (91.0%) survived for 30 days and were included in the study (Figure 1) (see Supplementary data, Table 1, for overall baseline characteristics).
Figure 1.
Flow chart.
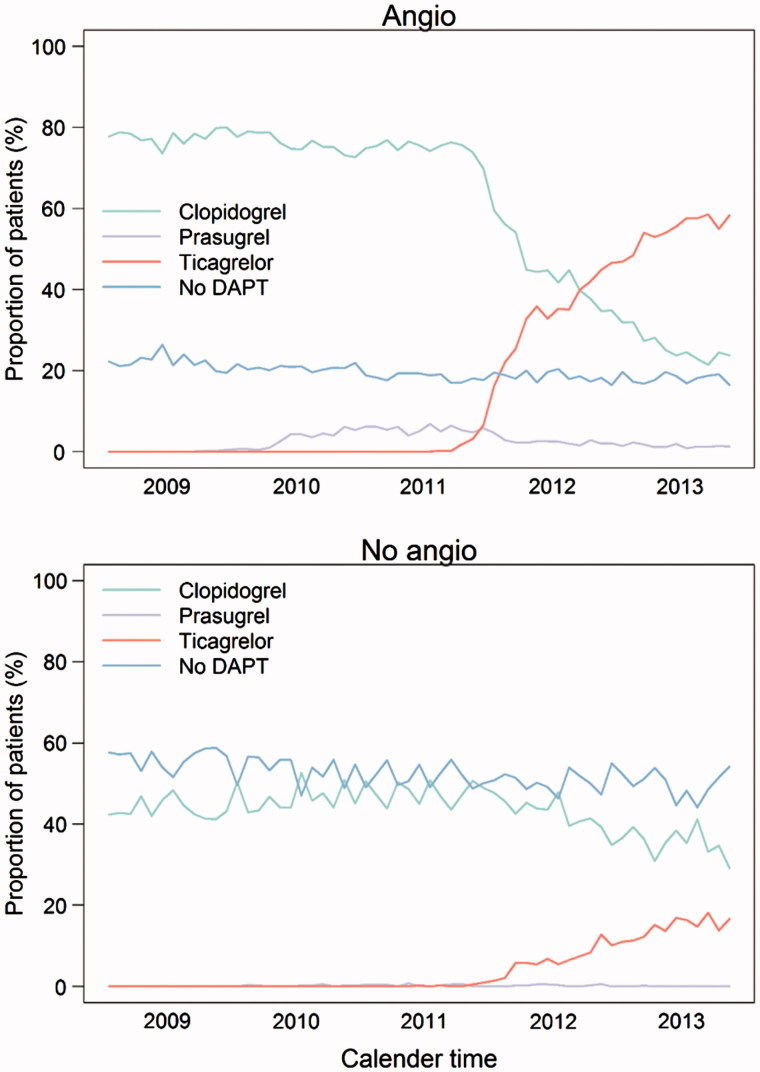
During the study period the proportion of patients receiving DAPT increased from 67% (2009) to 75% (2013) and the proportion of patients investigated with coronary angiography increased from 68% (2009) to 78% (2013) (Figure 2 and Tables 1 and 2). Among patients undergoing coronary angiography revascularization with PCI increased from 67% (2009) to 73% (2013) and with CABG decreased from 12% (2009) to 11% (2013) (Tables 1 and 2). Patients revascularized with PCI were prescribed DAPT in 94% of the cases with little variance during the study period (94% in 2009 versus 93% in 2013), whereas post-operative DAPT prescription increased from 12% (2009) to 27% (2013) in patients revascularized with CABG (Tables 1 and 2). Among patients not undergoing angiography, the proportion of patients prescribed DAPT increased from 44% in 2009 to 50% in 2013.
Figure 2.
Proportion of patients discharged alive undergoing angiography and prescribed different types of dual antiplatelet treatment from 2009 to 2013.
Table 1.
Baseline demographic and clinical characteristics for the 2009 acute coronary syndrome population.
| Angiography performed (n = 16 204, 68%) |
No angiography performed (n = 7703, 32%) |
|||||
|---|---|---|---|---|---|---|
| Clopidogrel (n = 12 596, 78%) | Prasugrel (n = 14, <1%) | No DAPT (n = 3594, 22%) | Clopidogrel (n = 3359, 43%) | Prasugrel (n = 0) | No DAPT (n = 4344, 56%) | |
| Age (years) | ||||||
| Mean (SD) | 67.1 (11.3) | 63.9 (11.5) | 68.0 (10.3) | 80.5 (9.5) | – | 82.9 (9.5) |
| Median | 67 | 64 | 69 | 83 | – | 85 |
| Age groups (%) | ||||||
| 18–49 years | 915 (7.3) | 1 (7.1) | 194 (5.4) | 29 (0.9) | – | 36 (0.8) |
| 50–64 years | 4212 (33.4) | 7 (50.0) | 1055 (29.4) | 236 (7.0) | – | 216 (5.0) |
| 65–69 years | 1963 (15.6) | 2 (14.3) | 638 (17.8) | 174 (5.2) | – | 149 (3.4) |
| 70–74 years | 1914 (15.2) | 2 (14.3) | 647 (18.0) | 272 (8.1) | – | 249 (5.7) |
| 75–79 years | 1713 (13.6) | 1 (7.1) | 598 (16.6) | 426 (12.7) | – | 462 (10.6) |
| 80–84 years | 1294 (10.3) | 1 (7.1) | 342 (9.5) | 887 (26.4) | – | 964 (22.2) |
| 85 + years | 585 (4.6) | – | 120 (3.3) | 1335 (39.7) | – | 2268 (52.2) |
| Gender (%) | ||||||
| Men | 8765 (69.6) | 12 (85.7) | 2437 (67.8) | 1769 (52.7) | – | 2043 (47.0) |
| Women | 3831 (30.4) | 2 (14.3) | 1157 (32.2) | 1590 (47.3) | – | 2301 (53.0) |
| Qualifying ACS event (index event) (%) | ||||||
| NSTE ACS (Unstable angina pectoris, NSTEMI) | 6871 (54.5) | 5 (35.7) | 2617 (72.8) | 1892 (56.3) | – | 2362 (54.4) |
| ST segment elevation myocardial infarction | 3508 (27.9) | 8 (57.1) | 430 (12.0) | 306 (9.1) | – | 260 (6.0) |
| Myocardial infarction not sub classified | 2217 (17.6) | 1 (7.1) | 547 (15.2) | 1161 (34.6) | 1722 (39.6) | |
| Invasively treated (%) | ||||||
| PCI | 10211 (81.1) | 14 (100.0) | 675 (18.8) | – | – | – |
| CABG | 244 (1.9) | – | 1721 (47.9) | – | – | – |
| Comorbidity
a
(%) | ||||||
| Previous myocardial infarction(s) | 2097 (16.6) | 4 (28.6) | 590 (16.4) | 1263 (37.6) | – | 1531 (35.2) |
| Previous unstable angina pectoris | 1207 (9.6) | 2 (14.3) | 358 (10.0) | 494 (14.7) | – | 560 (12.9) |
| Previous PCI | 1961 (15.6) | 3 (21.4) | 463 (12.9) | 504 (15.0) | – | 400 (9.2) |
| Previous CABG | 530 (4.2) | – | 130 (3.6) | 221 (6.6) | – | 249 (5.7) |
| Heart failure | 912 (7.2) | 1 (7.1) | 439 (12.2) | 942 (28.0) | – | 1547 (35.6) |
| Peripheral arterial disease | 134 (1.1) | 1 (7.1) | 48 (1.3) | 410 (12.2) | – | 511 (11.8) |
| Stroke (ischeamic and non-ischaemic) | 1028 (8.2) | 1 (7.1) | 424 (11.8) | 752 (22.4) | – | 1060 (24.4) |
| Atrial fibrillation | 918 (7.3) | 1 (7.1) | 486 (13.5) | 624 (18.6) | – | 100 (2.3) |
| Chronic renal dysfunction | 674 (1.4) | 18 (0.9) | 303 (2.0) | 69 (2.1) | – | 476 (3.2) |
| Diabetes | 2229 (17.7) | 4 (28.6) | 829 (23.1) | 940 (28.0) | – | 1114 (25.6) |
| Major bleeding | 546 (4.3) | 1 (7.1) | 216 (6.0) | 298 (8.9) | – | 637 (14.7) |
| Moderate and severe liver disease | 54 (0.4) | – | 20 (0.6) | 21 (0.6) | – | 53 (1.2) |
| Bleeding diathesis/coagulation disease | 85 (0.7) | – | 31 (0.9) | 38 (1.1) | – | 89 (2.0) |
| Cancer | 1484 (11.8) | 1 (7.1) | 457 (12.7) | 709 (21.1) | – | 1003 (23.1) |
| Drugs at discharge (%) | ||||||
| ACE inhbitor/ARB | 10094 (80.1) | 13 (92.9) | 2424 (67.4) | 2506 (74.6) | – | 2715 (62.5) |
| Acetylsalicylic acid | 12548 (99.6) | 14 (100.0) | 2682 (74.6) | 3328 (99.1) | – | 3353 (77.2) |
| Beta-blocker | 11726 (93.1) | 14 (100.0) | 2979 (82.9) | 3006 (89.5) | – | 3512 (80.8) |
| Statins | 12085 (95.9) | 14 (100.0) | 2916 (81.1) | 2412 (71.8) | – | 2106 (48.5) |
| Calcium channel blocker | 3444 (27.3) | 6 (42.9) | 1147 (31.9) | 1244 (37.0) | – | 1396 (32.1) |
| Antidiabetic drugs | 2183 (17.3) | 4 (28.6) | 1573 (43.8) | 1449 (43.1) | – | 940 (21.6) |
| Proton pump inhibitor | 3670 (29.1) | 5 (35.7) | 1224 (34.1) | 1296 (38.6) | – | 1893 (43.6) |
| Warfarin/new OAC | 685 (5.4) | – | 651 (18.1) | 147 (4.4) | – | 769 (17.7) |
SD: standard deviation; ACS: acute coronary syndrome; NSTE: non-ST-segment elevation; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; CABG: coronary artery bypass graft; ACE: angiotensin-converting enzyme; ARB: angiotensin receptor blocker; OAC: oral anticoagulant.
Data prior index ACS episode.
Table 2.
Baseline demographic and clinical characteristics for the 01 January–30 November 2013 acute coronary syndrome population.
| Angiography performed (n = 13 429, 78%) |
No angiography performed (n = 3796, 22%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Clopidogrel (n = 3488, 26%) | Prasugrel (n = 193, 1%) | Ticagrelor (n = 7311, 54%) | No DAPT (n = 2437, 18%) | Clopidogrel (n = 1346, 35%) | Prasugrel (n = 1, <1%) | Ticagrelor (n = 548, 14%) | No DAPT (n = 1901, 50%) | |
| Age (years) | ||||||||
| Mean (SD) | 70.5 (11.2) | 62.9 (10.4) | 66.3 (11.1) | 69.6 (10.5) | 83.0 (9.3) | 65.0 (–) | 75.8 (13.0) | 82.8 (10.3) |
| Median | 71 | 64 | 67 | 70 | 85 | 65 | 79 | 85 |
| Age groups (%) | ||||||||
| 18–49 years | 146 (4.2) | 20 (10.4) | 554 (7.6) | 102 (4.2) | 6 (0.4) | – | 21 (3.8) | 18 (0.9) |
| 50–64 years | 844 (24.2) | 85 (44.0) | 2505 (34.3) | 596 (24.5) | 59 (4.4) | – | 85 (15.5) | 109 (5.7) |
| 65–69 years | 531 (15.2) | 35 (18.1) | 1301 (17.8) | 435 (17.8) | 63 (4.7) | 1 (100.0) | 56 (10.2) | 81 (4.3) |
| 70–74 years | 570 (16.3) | 31 (16.1) | 1201 (16.4) | 434 (17.8) | 90 (6.7) | – | 59 (10.8) | 136 (7.2) |
| 75–79 years | 567 (16.3) | 13 (6.7) | 865 (11.8) | 434 (17.8) | 139 (10.3) | – | 68 (12.4) | 189 (9.9) |
| 80–84 years | 506 (14.5) | 8 (4.1) | 578 (7.9) | 325 (13.3) | 255 (18.9) | – | 90 (16.4) | 339 (17.8) |
| 85 + years | 324 (9.3) | 1 (0.5) | 307 (4.2) | 111 (4.6) | 734 (54.5) | – | 169 (30.8) | 1029 (54.1) |
| Gender (%) | ||||||||
| Men | 2314 (66.3) | 142 (73.6) | 5346 (73.1) | 1623 (66.6) | 665 (49.4) | 1 (100.0) | 321 (58.6) | 871 (45.8) |
| Women | 1174 (33.7) | 51 (26.4) | 1965 (26.9) | 814 (33.4) | 681 (50.6) | – | 227 (41.4) | 1030 (54.2) |
| Qualifying ACS event (index event) (%) | ||||||||
| NSTE ACS (Unstable angina pectoris, NSTEMI) | 2419 (69.4) | 71 (36.8) | 3998 (54.7) | 1797 (73.7) | 886 (65.8) | – | 340 (62.0) | 1154 (60.7) |
| ST segment elevation myocardial infarction | 626 (17.9) | 109 (56.5) | 2452 (33.5) | 336 (13.8) | 109 (8.1) | 1 (100.0) | 94 (17.2) | 119 (6.3) |
| Myocardial infarction not sub classified | 443 (12.7) | 13 (6.7) | 861 (11.8) | 304 (12.5) | 351 (26.8) | – | 114 (20.8) | 628 (33.4) |
| Invasively treated (%) | ||||||||
| PCI | 2547 (73.0) | 187 (96.9) | 6468 (88.5) | 619 (25.4) | – | – | – | – |
| CABG | 177 (5.1) | – | 209 (2.9) | 1028 (42.2) | – | – | – | – |
| Comorbidity
a
(%) | ||||||||
| Previous myocardial infarction(s) | 518 (14.9) | 24 (12.4) | 667 (9.1) | 279 (11.4) | 288 (21.4) | – | 76 (13.9) | 428 (22.5) |
| Previous unstable angina pectoris | 313 (9.0) | 15 (7.8) | 396 (5.4) | 191 (7.8) | 136 (10.1) | – | 47 (8.6) | 182 (9.6) |
| Previous PCI | 521 (14.9) | 31 (16.1) | 730 (10.0) | 233 (9.6) | 174 (12.9) | – | 69 (12.6) | 174 (9.2) |
| Previous CABG | 190 (5.4) | 6 (3.1) | 241 (3.3) | 77 (3.2) | 96 (7.1) | – | 24 (4.4) | 146 (7.7) |
| Heart failure | 348 (10.0) | 5 (2.6) | 265 (3.6) | 299 (12.3) | 326 (24.2) | – | 79 (14.4) | 615 (32.4) |
| Peripheral arterial disease | 281 (8.1) | 13 (6.7) | 295 (4.0) | 164 (6.7) | 173 (12.9) | – | 44 (8.0) | 233 (12.3) |
| Stroke (Ischaemic and non-ischaemic) | 426 (12.2) | 9 (4.7) | 495 (6.8) | 281 (11.5) | 338 (25.1) | – | 93 (17.0) | 482 (25.4) |
| Atrial fibrillation | 432 (12.4) | 7 (3.6) | 263 (3.6) | 413 (16.9) | 246 (18.3) | – | 59 (10.8 | 604 (31.8) |
| Chronic renal dysfunction | 81 (2.3) | 2 (1.0) | 74 (1.0) | 76 (3.1) | 64 (4.8) | – | 16 (2.9) | 82 (4.3) |
| Diabetes | 796 (22.8) | 38 (19.7) | 1229 (16.8) | 614 (25.2) | 361 (26.8) | – | 124 (22.6) | 492 (25.9) |
| Major bleeding | 270 (7.7) | 12 (6.2) | 319 (4.4) | 189 (7.8) | 138 (10.3) | – | 41 (7.5) | 293 (15.4) |
| Moderate and severe liver disease | 235 (0.5) | 9 (0.5) | 75 (0.6) | 108 (0.7) | 71 (0.6) | – | 5 (0.6) | 152 (1.0) |
| Bleeding diathesis/coagulation disease | 362 (0.8) | 14 (0.7) | 65 (0.5) | 188 (1.3) | 138 (1.1) | – | 6 (0.7) | 358 (2.4) |
| Cancer | 637 (18.3) | 22 (11.4) | 1015 (13.9) | 430 (17.6) | 350 (26.0) | – | 122 (22.3) | 547 (28.8) |
| Drugs at discharge (%) | ||||||||
| ACE inhbitor/ARB | 2875 (82.4) | 178 (92.2) | 6238 (85.3) | 1771 (72.7) | 963 (71.5) | 1 (100.0) | 399 (72.8) | 1170 (61.5) |
| Acetylsalicylic acid | 3457 (99.1) | 192 (99.5) | 7288 (99.7) | 1592 (65.3) | 1326 (98.5) | 1 (100.0) | 548 (100.0) | 1347 (70.9) |
| Clopidogrel a | 261 (7.5) | 9 (4.7) | 185 (2.5) | 79 (3.2) | 157 (11.7) | – | 18 (3.3) | 89 (4.7) |
| Beta-blocker | 3136 (89.9) | 180 (93.3) | 6700 (91.6) | 2004 (82.2) | 1153 (85.7) | 1 (100.0) | 484 (88.3) | 1480 (77.9) |
| Statins | 3311 (94.9) | 190 (98.4) | 7114 (97.3) | 1933 (79.3) | 901 (66.9) | 1 (100.0) | 435 (79.4) | 943 (49.6) |
| Calcium channel blocker | 1173 (33.6) | 54 (28.0) | 1902 (26.0) | 840 (34.5) | 518 (38.5) | – | 196 (35.8) | 651 (34.2) |
| Antidiabetic drugs | 721 (20.7) | 42 (21.8) | 1247 (17.1) | 590 (24.2) | 317 (23.6 | – | 117 (21.4) | 403 (21.2) |
| Proton pump inhibitor | 1380 (39.6) | 63 (32.6) | 2311 (31.6) | 892 (36.6) | 613 (45.5) | – | 215 (39.2) | 925 (48.7) |
| Warfarin/new OAC | 546 (15.7) | 8 (4.1) | 193 (2.6) | 657 (27.0) | 102 (7.6) | – | 15 (2.7) | 483 (25.4) |
SD: standard deviation; ACS: acute coronary syndrome; NSTE: non-ST-segment elevation; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; CABG: coronary artery bypass graft; ACE: angiotensin-converting enzyme; ARB: angiotensin receptor blocker; OAC: oral anticoagulant.
aData prior index ACS episode.
Mean DAPT duration increased from 225 to 298 days in patients investigated with angiography, and from 155 to 208 days in patients not investigated with angiography (Figure 3) from 2009 until 2013. Median DAPT duration increased from 201 to 354 days in patients investigated with angiography, and from 101 to 201 days in patients not investigated with angiography (Figure 3). In patients undergoing PCI, mean DAPT duration increased from 240 to 313 days, and from 159 to 213 days in patients not undergoing PCI. Median DAPT duration increased from 202 to 359 days in patients undergoing PCI, and from 101 to 201 days in patients not undergoing PCI.
When restricting the patient population to only patients with their first time ACS episode, a similar invasive and DAPT treatment pattern was observed as for the whole study population (Supplementary data, Figure 1).
Patients hospitalized in 2009
In 2009, the DAPT duration prescribed varied substantially depending on geographical region. This pattern was harmonized during the study period (see Supplementary data, Figure 1). Patients investigated with angiography were younger, more likely to be male, had less co-morbidities and were more likely to be prescribed DAPT than patients not investigated with angiography (Tables 1 and 2). Among the patients not prescribed DAPT, approximately 70% of patients were prescribed acetylsalicylic acid and 20% oral anticoagulation therapy. There was no tendency that patients receiving DAPT were more commonly treated with a proton pump inhibitor.
Patients hospitalized in 2013
The proportion of patients prescribed DAPT with ticagrelor increased quickly after its introduction in 2011. In 2013, ticagrelor was the most common P2Y12 inhibitor in patient investigated with angiography, whereas clopidogrel was still remaining more common in patients not investigated with angiography (Figure 2). Compared to clopidogrel-treated patients, ticagrelor-treated patients were younger more often men and more investigated with angiography and revascularized with PCI (Tables 1 and 2). A minority (2%) of patients was treated with prasugrel and almost all these patients underwent PCI and were diagnosed with STEMI (Tables 1 and 2).
Approximately 10% of the ticagrelor patients were switched to clopidogrel (mean time to switch was 112 and 130 days for those undergoing or not undergoing angiography, respectively) during the first year after the ACS episode (Table 3).
Table 3.
Switch pattern for patients discharged with different dual antiplatelet treatment.
| |
Dual antiplatelet treatment |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Clopidogrel + ASA Angio (N = 44 361) | Clopidogrel + ASA non-Angio (N = 9356) | Ticagrelor + ASA Angio (N = 11 045) | Ticagrelor + ASA non-Angio (N = 635) | Prasugrel + ASA Angio (N = 1888) | Prasugrel + ASA non-Angio (N = 37) | Clopidogrel Angio (N = 1106) | Total (N = 68 428) | ||
| No of switches within 30 days a | n (%) | 37 (0.1%) | 1 (0.0%) | 83 (0.8%) | 9 (1.4%) | 17 (0.9%) | - | 1 (0.1%) | 148 (0.2%) |
| No of switches within 31–365 days a | n (%) | 542 (1.2%) | 64 (0.7%) | 1003 (9.1%) | 58 (9.1%) | 147 (7.8%) | 4 (10.8%) | 20 (1.8%) | 1838 (2.7%) |
| Time to switch | |||||||||
| Mean (SD) | 147.0 (103.1) | 168.9 (105.2) | 113.0 (85.4) | 127.3 (103.9) | 128.6 (99.1) | 100.3 (28.7) | 137.0 (86.6) | 126.8 (94.7) | |
| Median | 118 | 147 | 86 | 95 | 91 | 106 | 119 | 95 | |
| Switch to Clopidogrel | n (%) | – | – | 1014 (9.2%) | 63 (9.9%) | 142 (7.5%) | 4 (10.8%) | – | 1223 (1.8%) |
| Switch to Ticagrelor | n (%) | 318 (0.7%) | 47 (0.5%) | – | – | 22 (1.2%) | – | 16 (1.4%) | 403 (0.6%) |
| Switch to Prasugrel | n (%) | 261 (0.6%) | 18 (0.2%) | 72 (0.7%) | 4 (0.6%) | – | – | 5 (0.5%) | 360 (0.5%) |
Angio: angiography; ASA: acetylsalicylic acid; SD: standard deviation.
aDays after qualifying event.
Discussion
This is the first nationwide study describing the prescription patterns of DAPT after ACS following the introduction of the novel P2Y12 inhibitors ticagrelor and prasugrel. The results from this observational study show major changes in the treatment of patients with ACS in Sweden during the observation period from 2009 to 2013. At the end of the study period more patients were undergoing invasive procedures (angiography and PCI) and a larger proportion of patients were prescribed DAPT for 12 months according to current guidelines.[6,7] The latter is most apparent in patients undergoing coronary angiography and patients treated with ticagrelor and prasugrel, but the tendency is also present in patients treated non-invasively with clopidogrel. Furthermore, persistence with clopidogrel, prasugrel and ticagrelor therapy was seen to be in line with previously reported data from randomized, controlled clinical trials.[12,13]
The overall medical treatment of patients with ACS in Sweden, with more and older patients undergoing angiography and PCI, follows the same trend as seen in other Western countries. The quality of care in Sweden is monitored by the collection of a variety of variables and a constantly ongoing reporting of data from the extensive cardiovascular nationwide quality registry, SWEDEHEART.[19] This monitoring of quality can lead to a more rapid and widespread uptake of guideline recommendations and a more homogeneous care across the nation. The finding in our study is an example of rapid changes in the treatment: ticagrelor was launched in 2010, implemented in national/regional guidelines in late 2011 and by the end of 2012 was already the main P2Y12 antagonist for patients undergoing angiography. In contrast, the uptake of prasugrel in Sweden was slow, with only a few centers in Sweden using it for NSTEMI and STEMI patients undergoing PCI according to the prescribing indication.[20] The reasons for the difference in the uptake and use of ticagrelor and prasugrel are not clear, but the broader prescribing indication for ticagrelor, both for NSTEMI and STEMI patients who are invasively treated or managed medically might be a simplifying factor that facilitates the use in a daily setting.[21] The potential advantage for a P2Y12 antagonist that can be used for a broader ACS population might be further enhanced by the publications of the ESC guidelines on non-ST-segment elevation (NSTE)-ACS in 2011 and on STEMI in 2012.[6,7] Furthermore, many hospitals in Sweden were involved in the PLATO trial, and thus many clinicians had a broad experience with ticagrelor before it was launched.[13]
To our knowledge, there are few comparable studies in which the use of contemporary DAPT is described in unselected patients with ACS at a national level. A similar observational study from Denmark reported the same treatment patterns for post-MI patients including patients from 2000 to 2005, with shorter duration of treatment with clopidogrel for patients not undergoing PCI versus patients undergoing PCI.[14] Patients in that study were in general, treated longer with clopidogrel than patients observed during 2009 in the present study. The overall treatment pattern of clopidogrel in 2009, showing a great variation in treatment length depending on region in Sweden, deserves some additional comments (Supplementary data, Figure 1). The duration of DAPT in the Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) trial,[11] which studied patients with NSTE-ACS, varied from 3 to 12 months and the median duration of DAPT in the Clopidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT),[22] which studied patients with ST-segment elevation (STE)-ACS, was only 15 days. Hence, the initial interpretation of these studies to decide the optimal duration of DAPT was not consistent throughout Sweden, and 2008 guidelines stated a DAPT duration of 3–12 months after ACS.[23]
However, a recent report from Sweden found that the effect of DAPT duration of more than 3 months, compared with a shorter duration, was associated with a lower risk of death, stroke or re-infarction, thus supporting a longer treatment duration with clopidogrel.[24] The more recent randomized studies with ticagrelor and prasugrel had a DAPT duration of 12 and 15 months, respectively,[12,13] and current guidelines recommend a DAPT duration of 12 months unless there are contraindications, such as excessive risk of bleeding.[6,7]
In our study, the conservatively treated patients, patients not undergoing angiography, were older and had a higher cardiovascular risk. There was an increase in the proportion of non-angiography patients discharged with DAPT during the observational period. Despite the elevated risk, these patients were in general prescribed a shorter DAPT duration than patients undergoing angiography. Patients with ACS in whom coronary angiography is not performed are likely to be a selected as high-risk group with increased risk of bleeding and a high risk of procedural complications and limited perceived benefit of revascularization. Only about half of the conservatively treated patients with ACS received DAPT treatment in our study. Partly, this probably reflects concomitant use of oral anticoagulants, such as warfarin, which are perhaps used more frequently in this population. It is also possible that a portion of these patients were treated only with a single antiplatelet agent because of their high-risk profile. The treatment pattern, in general, with a large proportion of patients ending clopidogrel treatment after 3 and 6 months following initiation, most likely reflects the DAPT duration intended by the treating physician and is probably less likely due to an adverse event, e.g., bleeding. The use of ticagrelor in conservatively treated patients has been increasing since 2012, and by mid-2013, almost half of the DAPT-treated patients with a conservative strategy received ticagrelor. Interestingly, the duration of ticagrelor treatment in these patients was longer, compared with that in clopidogrel-treated patients.
In our study, approximately 16% of the patients undergoing angiography did not receive DAPT (Supplementary data, Table 1). However, there was a marked overall decrease in patients not receiving DAPT, from 33% in 2009 to 25% 2013. Approximately 42% of non-DAPT-treated patients undergo CABG, and the practice of using DAPT after CABG is highly variable. Furthermore, around 10–15% of non-DAPT-treated patients have a history of oral anticoagulation use, and the practice to handle concomitant DAPT in this patient category also varies between centers. A proportion of these patients will also be prescribed an oral anticoagulant because of newly identified atrial fibrillation subsequent to the index event. Some of these patients might also be considered too high risk to be prescribed DAPT after the angiography has been performed.
The uptake of ticagrelor has been rapid in Sweden. Ticagrelor is used primarily for patients undergoing angiography and PCI. In the present study, approximately 80% of the patients receiving ticagrelor were still on treatment after 10 months. Approximately 10% of the patients were switched to clopidogrel after about 4 months of treatment. When examining the proportion of patients still on treatment over time (Figure 3), we see a smooth and steady decrease in patients on treatment over time. Thus, we have no reason to believe that there is an underlying specific treatment pattern shorter than 12 months. It is complex to compare the persistence with treatment observed in real-life clinical settings with observations in randomized clinical trials because of the selection of patients and protocol-driven follow-up. However, we observe a persistence pattern with treatment that is similar to what has been observed in the large outcome trials.[12,13]
The patients treated with prasugrel are younger, mostly men with STEMI. Approximately 80% of the prasugrel patients were still on treatment after 10 months. The use of prasugrel peaked in 2011, and the use has gradually decreased since then; in 2013, only 1% of the patients with ACS received prasugrel.
A major strength of the present study is that it was conducted in a large national cohort including all patients in Sweden hospitalized for ACS within the observation period. This study design diminishes potential problems that could arise from selection bias. However, the study also has its limitations. Because this was a registry analysis, we were reliant on ICD-10 codes for morbidity data, and thus, the possibility of coding errors cannot be completely ruled out, although previous data show a >98% correct coding of Swedish IPR entries.[25] The sensitivity and specificity rates of ICD-10 codes for MI have been shown elsewhere to exceed 93%.[17] However, ICD codes still lack specificity regarding important patient population descriptors, and the precise division of the qualifying ACS episode into NSTE-ACS or STEMI might be questioned. In the study, we included patients with ACS as only main reason for hospitalization and report the percentage of patients where the ACS diagnosis was not further specified. The proportion of non-sub-classified diagnoses varies between the different patient populations, with the lowest proportion among patients undergoing angiography and included at the end of observation period.
Further, patients were included based on their first ACS episode during the observational period. Thus, the patient population may change over time, and patients included early may have a recent ACS event history prior inclusion, while patients included later had to be event-free for a longer time. However, only a limited number of patients would be expected to have had a recent ACS episode prior inclusion as approximately only 10% of patients surviving MI in Sweden have a recurrent MI during the first year.[8] Nevertheless, the effect of a recent prior ACS episode on selection for invasive treatment and DAPT is difficult to predict. A higher attention on these patients would however be expected, and thus a higher likelihood of receiving appropriate guideline treatment. We could not observe any difference in persistence to DAPT in first time ACS patients compared to the study population (Supplementary data, Table 1).
Furthermore, the diagnostic criteria for MI have changed during the observation period, including a gradual introduction of high-sensitivity troponins, which might have a potential effect on yearly incidence of ACS patients. However, it is not likely to affect patient eligibility for DAPT in our study as we only include patients with a primary ACS diagnosis.
Another limitation of our study was the lack of available data on clinical risk factors, e.g., smoking, lipids, body weight, blood pressure and socio-economic status.[26] Moreover, we have not taken into account events after the qualifying ACS event (recurrent MI, bleeding, stroke or revascularizations) that might influence the treatment length. Our study included only patients with ACS as primary diagnosis and we cannot rule out that patient with ACS as a secondary diagnosis might be treated differently.
Conclusions
The results from this study show that the treatment pattern of patients with ACS in Sweden underwent major changes from 2009 to 2013. More patients are investigated and treated with invasive procedures (angiography and PCI), and there is a shift in DAPT pattern from merely clopidogrel to different treatments for selected patient populations. A larger proportion of patients are discharged with DAPT, and the overall treatment length of DAPT showed a marked increase during the observation period. Still, there is a significant proportion of patients with ACS, especially elderly patients >80 years of age, who are discharged without guideline-recommended DAPT. The persistence with clopidogrel, prasugrel and ticagrelor therapy was in line with what has been observed in randomized, controlled clinical trials.
Disclosure statement
This work was supported by AstraZeneca. Project management was provided by AstraZeneca. The statistical analysis was agreed on by the study steering committee, and data analysis was performed by the study database owner in collaboration with AstraZeneca. AstraZeneca took part, as members of the study steering committee, in the interpretation of the data and the drafting of the manuscript.
References
- Mathers C, Fat DM, Boerma JT. The global burden of disease: 2004 update. World Health Organization; 2008. [Google Scholar]
- Tofield A. European cardiovascular disease statistics 2012 summary. Eur Heart J. 2013;34:1086–1086. [Google Scholar]
- Rivera J, Lozano ML, Navarro-Nunez L, et al. Platelet receptors and signaling in the dynamics of thrombus formation. Haematol Hematol J. 2009;94:700–711. doi: 10.3324/haematol.2008.003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furie B, Furie BC. Mechanisms of disease: mechanisms of thrombus formation. N Engl J Med. 2008;359:938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- Damman P, Woudstra P, Kuijt WJ, et al. P2y12 platelet inhibition in clinical practice. J Thromb Thrombolysis. 2012;33:143–153. doi: 10.1007/s11239-011-0667-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm CW, Bassand JP, Agewall S. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- Steg G, James SK, Atar D, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- Jernberg T, Hasvold P, Henriksson M, et al. Cardiovascular risk in post-myocardial infarction patients: nationwide real world data demonstrate the importance of a long-term perspective. Eur Heart J. 2015;36:1163–1170. doi: 10.1093/eurheartj/ehu505. [DOI] [PubMed] [Google Scholar]
- Briffa TG, Hobbs MS, Tonkin A, et al. Population trends of recurrent coronary heart disease event rates remain high. Circ Cardiovasc Qual Outcomes. 2011;4:107–113. doi: 10.1161/CIRCOUTCOMES.110.957944. [DOI] [PubMed] [Google Scholar]
- Gerschutz GP, Bhatt DL. The clopidogrel in unstable angina to prevent recurrent events (cure) study: to what extent should the results be generalizable? Am Heart J. 2003;145:595–601. doi: 10.1067/mhj.2003.180. [DOI] [PubMed] [Google Scholar]
- Yusuf S. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation (vol. 345, p. 494, 2001). N Engl J Med. 2001;345:1716–1716. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- Sorensen R, Gislason GH, Fosbol EL, et al. Initiation and persistence with clopidogrel treatment after acute myocardial infarction – a nationwide study. Br J Clin Pharmacol. 2008;66:875–884. doi: 10.1111/j.1365-2125.2008.03284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijns W, Kolh P. Guidelines on myocardial revascularization the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2010;31:2501–2555. doi: 10.1093/eurheartj/ehq277. [DOI] [PubMed] [Google Scholar]
- Hambraeus K, Held C, Johansson P, et al. Swedeheart annual report 2012. Scand Cardiovasc J. 2014;48:1–129. [Google Scholar]
- Linnersjo A, Hammar N, Gustavsson A, et al. Recent time trends in acute myocardial infarction in Stockholm, Sweden. Int J Cardiol. 2000;76:17–21. doi: 10.1016/s0167-5273(00)00366-1. [DOI] [PubMed] [Google Scholar]
- Gislason GH, Rasmussen JN, Abildstrøm SZ, et al. Long-term compliance with beta-blockers, angiotensin-converting enzyme inhibitors, and statins after acute myocardial infarction. Eur Heart J. 2006;27:1153–1158. doi: 10.1093/eurheartj/ehi705. [DOI] [PubMed] [Google Scholar]
- Jernberg T, Attebring MF, Hambraeus K, et al. The Swedish web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart. 2010;96:1617–1621. doi: 10.1136/hrt.2010.198804. [DOI] [PubMed] [Google Scholar]
- EMEA (2014-02-17) Prasugrel (Efient) Summary of product characteristics; [cited 2015 Jun 1]. Available from: http://www.ema. europa.eu/docs/en_GB/document_library/EPAR_-_Product_Informa-tion/human/000984/WC500021971.pdf.
- EMA (2015-08-11) Ticagrelor (Brilique) Summary of product characteristics. European Medicines Agency; [cited 2015 Sep 1]. Avail-able from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001241/WC500100494.pdf.
- Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366:1607–1621. doi: 10.1016/S0140-6736(05)67660-X. [DOI] [PubMed] [Google Scholar]
- Asplund K, Kärvinge C. Nationella riktlinjer för hjärtsjukvård 2008-beslutsstöd för prioriteringar. Swedish National Board of Health and Welfare (Socialstyrelsen); 2008. [Google Scholar]
- Varenhorst C, Jensevik K, Jernberg T, et al. Duration of dual antiplatelet treatment with clopidogrel and aspirin in patients with acute coronary syndrome. Eur Heart J. 2014;35:969–978. doi: 10.1093/eurheartj/eht438. [DOI] [PubMed] [Google Scholar]
- Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish National Inpatient Register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamala S, Merlo J, Bostrom G, et al. Socioeconomic disadvantage and primary non-adherence with medication in Sweden. Int J Qual Health Care. 2007;19:134–140. doi: 10.1093/intqhc/mzm011. [DOI] [PubMed] [Google Scholar]