ABSTRACT
Purpose: Intravitreal injections of anti-vascular endothelial growth factor (VEGF) agents including ranibizumab and aflibercept are used to treat patients with ocular disorders such as neovascular age-related macular degeneration (nAMD); however, the injections are associated with rare instances of severe ocular inflammation. This study compared severe ocular inflammation rates in patients treated with ranibizumab versus aflibercept.
Methods: United States physician-level claims data covering an 18-month period for each therapy were analyzed. The primary analysis compared severe ocular inflammation event rates per 1000 injections. Sensitivity and subgroup analyses evaluated the impact of factors including intraocular surgery, intravitreal antibiotic administration, and previous intravitreal injections.
Results: The analysis included 432,794 injection claims (ranibizumab n = 253,647, aflibercept n = 179,147); significantly, more unique severe ocular inflammation events occurred in patients receiving aflibercept than ranibizumab (1.06/1000 injections, 95% confidence interval [CI], 0.91–1.21, vs. 0.64/1000 injections, 95% CI 0.54–0.74; p < 0.0001). Comparable results were observed for analyses of patients who had undergone glaucoma or cataract surgeries, had antibiotic-associated endophthalmitis, had non-antibiotic-associated endophthalmitis, and were non-treatment-naive. In contrast, no significant differences in severe ocular inflammation claims were recorded in treatment-naive patients who had no record of anti-VEGF treatment in the 6 months preceding the index claim. No significant change occurred in the rate of severe ocular inflammation claims over time following ranibizumab treatment.
Conclusions: Severe ocular inflammation was more frequent following intravitreal injection with aflibercept than with ranibizumab during routine clinical use in patients with nAMD. This highlights the importance of real-world, post-approval, observational monitoring of novel medicines, and may aid clinical decision-making, including choice of anti-VEGF agent.
KEYWORDS: Aflibercept, anti-VEGF therapy, claims database, endophthalmitis, intravitreal injection, neovascular age-related macular degeneration
Introduction
Neovascular (wet) age-related macular degeneration (nAMD) is the leading cause of irreversible blindness in people aged over 65 years in the developed world. 1 Anti-vascular endothelial growth factor (VEGF) therapies such as ranibizumab, aflibercept, and bevacizumab, which inhibit VEGF-A, have revolutionized the treatment of patients with nAMD and other retinal vascular conditions, 2 – 5 and are now widely used in ophthalmic care. Since its approval (in 2006 and 2007 in the United States and Europe, respectively),6, 7 ranibizumab has become the standard of care for the management of patients with nAMD in many countries. 8 – 10 The use of intravitreal anti-VEGF injection, a procedure mostly conducted by retinal specialists, has increased dramatically in recent years, 11 and blindness certifications and visual impairment resulting from nAMD have fallen by approximately half since the introduction of anti-VEGF agents. 12 – 19
Aflibercept has also been approved for the intravitreal treatment of individuals with nAMD and has non-inferior efficacy compared with ranibizumab.20,21 It is a recombinant fusion protein that, similar to ranibizumab, inhibits VEGF-A; unlike ranibizumab, it also inhibits VEGF-B and placental growth factor.6,20,22 A further distinction between the two agents is that ranibizumab possesses an antigen-binding fragment with no crystallizable fragment (Fc) component, in contrast to the immunoglobulin G1 Fc present in aflibercept. The clinical significance of these differences is not fully established, but the role of the Fc domain in inflammatory reactions, including those involved in binding complement, suggests that there may be differences in the therapeutic profiles of the two agents. 23 – 25
Bevacizumab, an anti-VEGF agent indicated for the treatment of patients with metastatic carcinomas, is also used off-label for patients with nAMD. 26 It is a full-length anti-VEGF antibody derived from the same murine VEGF monoclonal antibody as ranibizumab, and retains the Fc component. Its efficacy in nAMD treatment is non-inferior to that of ranibizumab; however, there are potential systemic safety differences. 27
The eye contains immune-privileged sites with the potential for unique immune-related responses. Severe ocular inflammation, or endophthalmitis, can occur following ophthalmic surgery or intravitreal injection and can negatively impact on vision and ocular structures. 28 – 30 Interest in treatment-related ocular disorders is high owing to recent reports of endophthalmitis clusters following intravitreal aflibercept injections.28,31 Over 40 cases of endophthalmitis occurred following approximately 800,000 aflibercept injections administered in the US in the 18 months to June 2013. 31 Signals of blindness and conditions leading to impaired visual acuity associated with aflibercept were also reported in Europe late in 2013; 32 most cases were reported in association with known adverse reactions such as endophthalmitis and intraocular inflammation. Clusters of endophthalmitis have also been reported following intravitreal bevacizumab injections. Adverse events with bevacizumab have been linked to suspected contamination, possibly arising as a consequence of vial splitting and compounding. 33 – 35
Monitoring the frequency of severe ocular inflammation associated with intraocular procedures is critical. However, rates of post-intravitreal anti-VEGF injection endophthalmitis are very low, with an estimated 0.10–0.83 cases per 1000 injections.29,30,36–44 Few studies have therefore been powered to analyze such rare events, and misclassification or clustering of events can cause confounding. Claims databases detail information relating to patient–physician interactions and generate substantial amounts of data on routine clinical treatment. They can therefore provide more data than clinical studies for analysis of rare events, and for detection of differences between drugs in routine clinical practice. 45 This in turn means that there is the potential for a more accurate assessment of the frequency of clinical events in real-world care than there is with adverse-event monitoring. The purpose of this study was to investigate the frequency of claims for the treatment of patients with severe ocular inflammation in routine clinical practice following therapy with ranibizumab or aflibercept using data from a US physician-level claims database.
Materials and methods
This retrospective study used US physician-level claims data from the Integrated Data Warehouse (IDW), which collects data on approximately 1 billion professional fee claims per year. The IDW covers about 80% of practicing eye care specialists in the US; over 16,000 ophthalmologists submit claims data to this database. The data held in the IDW are obtained in accordance with agreements with electronic claims reprocessors and with physician practices submitting claims through billing software. The data used in this study are Health Insurance Portability and Accountability Act 1996 (HIPAA) compliant, encrypted and de-identified patient level claims provided by IMS Health, comprising Centers for Medicare and Medicaid Services (CMS)-1500 health insurance claim forms completed for patients seen by private practitioners. Institutional review board/ethical approval was not required for this study as it was exempt under the US Code of Federal Regulations. The exemption is granted under Title 45 (Public Welfare) Part 46 (Protection of Human Subjects) Subpart A Section 46.101 header 2 subheader b part 4. IMS Health owns the raw data underlying this study. Novartis purchased the dataset from IMS Health for the purposes of this research. Requests for data access can be addressed to Deborah Huffnagle (dhuffnagle@us.imshealth.com).
International Classification of Diseases 9th Revision Clinical Modification (ICD-9-CM) diagnosis codes for endophthalmitis were used as a proxy for the identification of cases of severe ocular inflammation (see Supplemental Table S1 – online only). The Healthcare Common Procedure Coding System (HCPCS) codes for the identification of intravitreal antibiotic treatment and anti-VEGF injections are provided in Supplemental Tables S2 and S3 (online only), respectively. We considered all claims for injections of ranibizumab and aflibercept from 1 November 2011 to 31 August 2013 with a concomitant ICD-9-CM diagnosis code for nAMD (ICD-9-CM 362.52). The index date of each injection was defined as the date of the injection claim. To ensure stability of the data-reporting source (i.e. the practitioner or hospital) and accurate capture of clinical use and outcomes data, participating physicians were required to have submitted medical claims consistently to the IDW during the month of injection. Data relating to injections were excluded if: an additional anti-VEGF treatment (ranibizumab, aflibercept, bevacizumab, or pegaptanib) was given on the same date; the injection record was not followed by further claims in the IDW; the patient was aged younger than 18 years; or the index eye and/or sex was not specified on the claim. Each injection identified was followed up for 30 days, until another anti-VEGF injection was given or a claim for endophthalmitis was recorded, or until the study period ended (i.e. 30 September 2013, 30 days after the end of the recruitment period). Owing to the injection-level nature of the analysis, an individual patient could be represented in both treatment groups if they switched treatments.
A severe ocular inflammation event was defined as the presence of any claim for the management of endophthalmitis during the 30-day follow-up period. The time (days) from injection to occurrence of the event was recorded for each event. For all analyses, crude event rates (CERs) per 1000 injections were calculated, and a generalized linear model with a Poisson distribution was used to derive unadjusted relative risks (RRs) and 95% confidence intervals (CIs). Repeated-measures models, including generalized estimating equations, provide a more robust analysis than unadjusted calculations in circumstances such as these, in which multiple injections were administered per patient. An adjusted RR was therefore also estimated for all analyses, using a generalized estimating equation model with a Poisson distribution and a compound symmetry variance–covariance structure. Adjustments were made for both injection-level (left/right injection eye, number of prior injections, and occurrence of events with prior injections) and patient-level characteristics (age, sex, health plan type and geographical location). Given the rarity of the events, patients were expected to experience at most one endophthalmitis event; therefore, the primary analysis censored events and injections after the first claim of endophthalmitis per patient. An additional analysis including all claims per patient was also performed. In all cases, χ 2 tests were used to evaluate between-group differences.
To test robustness of the results, several sensitivity and subgroup analyses were conducted for both ranibizumab and aflibercept injection cohorts. Endophthalmitis can occur following cataract or glaucoma surgery; 46 – 48 therefore, a sensitivity analysis censoring intravitreal injections and severe ocular inflammation events occurring in the 90 days following cataract surgery or any time following glaucoma drainage/filtering surgery (as indicated by HCPCS codes) was performed to adjust for potential endophthalmitis claims that may have been due to intraocular surgery rather than to anti-VEGF treatment. Intravitreal antibiotics are usually used to treat endophthalmitis cases that are suspected to be associated with infection. 49 A subgroup analysis was performed to assess whether or not severe ocular inflammation events were followed by intravitreal antibiotic administration within 7 days (as indicated by HCPCS codes; see Table S2 for the codes used to identify intravitreal antibiotic use). To investigate the possible influence of previous treatment, subgroup analyses were performed in treatment-naive patients (i.e. those who had no record of anti-VEGF treatment in the 6 months preceding the index claim) and non-treatment-naive patients (i.e. those who had received anti-VEGF therapy in the 6 months preceding the index claim, as indicated by HCPCS codes for anti-VEGF treatments).
Results
A total of 479,123 intravitreal anti-VEGF injection claims were identified and assessed for inclusion (Figure 1). After applying exclusion/inclusion criteria, 432,794 injection claims (ranibizumab n = 253,647; aflibercept n = 179,147) were included in the analysis; the proportions of identified injections retained for analysis were similar for ranibizumab (91.4%) and aflibercept (88.9%). These injections were administered to 81,046 patients (54,551 ranibizumab and 39,389 aflibercept; individuals could be represented in both groups if they switched treatments). Patient characteristics were similar in the ranibizumab and aflibercept treatment groups (Table 1). The majority of patients (88.5%) included in the study received more than one injection, with a median of four injections per patient. Most severe ocular inflammations (85.4%) occurred in the 5 days following anti-VEGF injection (ranibizumab 83.1%; aflibercept 87.7%), and the median time from injection to a severe ocular inflammation event was similar for both ranibizumab and aflibercept (1.0 day for both).
Figure 1.
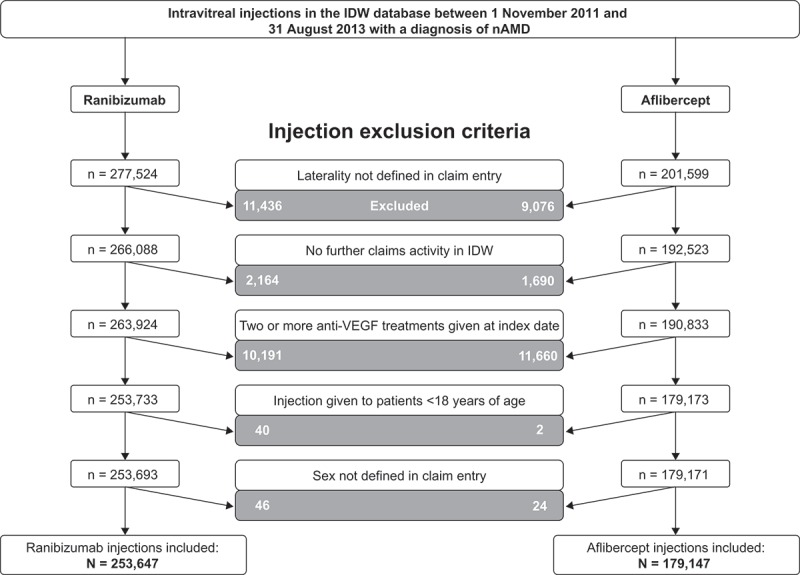
Attrition of intravitreal anti-VEGF injection claims included for analysis of severe ocular inflammation rates in the US. nAMD, neovascular age-related macular degeneration; IDW, Integrated Data Warehouse; VEGF, vascular endothelial growth factor.Note: Injections were excluded if the administering physician exhibited evidence of “physician instability” (i.e. physicians with abnormally high or low prescribing patterns).
Table 1.
Characteristics of patients receiving intravitreal aflibercept and ranibizumab injections for neovascular age-related macular degeneration, US.
| Ranibizumab injections (n = 253,647) |
Aflibercept injections (n = 179,147) |
|
|---|---|---|
| Patients receiving injections, n | 54,551 | 39,389 |
| Median age at injection, yearsa | 83.0 | 82.0 |
| Injections by age group, n (%)a | ||
| <65 years | 9138 (3.6) | 5602 (3.1) |
| 65–69 years | 12,550 (4.9) | 11,022 (6.2) |
| 70–74 years | 25,468 (10.0) | 21,569 (12.0) |
| 75–79 years | 43,081 (17.0) | 32,778 (18.3) |
| 80–84 years | 62,176 (24.5) | 45,244 (25.3) |
| ≥85 years | 101,234 (39.9) | 62,932 (35.1) |
| Injections given to women, n (%)a | 163,626 (64.5) | 111,202 (62.1) |
| Injections by prescribing physician, n (%)a | ||
| Ophthalmologist/retinal specialist/eye care doctor | 250,643 (98.8) | 176,186 (98.3) |
| Other/unknown | 3004 (1.2) | 2961 (1.7) |
| Injections by payer type, n (%)a | ||
| Commercial | 40,360 (15.9) | 26,967 (15.1) |
| Medicare | 211,399 (83.3) | 151,799 (84.7) |
| Medicaid | 1888 (0.7) | 381 (0.2) |
| Injections by injection eye, n (%) | ||
| Left | 125,791 (49.6) | 89,344 (49.9) |
| Right | 127,856 (50.4) | 89,803 (50.1) |
| Injections by geographic region, n (%)a | ||
| Northeast | 55,335 (21.8) | 46,078 (25.7) |
| Midwest | 40,871 (16.1) | 38,225 (21.3) |
| South | 112,702 (44.4) | 67,462 (37.7) |
| West | 44,739 (17.6) | 27,381 (15.3) |
| Unknown | − | 1 (0.0) |
| Injections by place of administration, n (%)a | ||
| Physician office | 244,157 (96.3) | 173,175 (96.7) |
| Ambulatory surgical center | 176 (0.1) | 146 (0.1) |
| Outpatient hospital | 1 (0.0) | – |
| Emergency department | 918 (0.4) | 321 (0.2) |
| Other | 16 (0.0) | 10 (0.0) |
| Unknown/missing | 8379 (3.3) | 5495 (3.1) |
a p < 0.0001 for ranibizumab vs. aflibercept across category.
SD, standard deviation.
The primary analysis included 162 unique events in patients receiving ranibizumab injections and 189 in those receiving aflibercept. There were significantly more severe ocular inflammation events (p < 0.0001) following treatment with aflibercept than with ranibizumab (1.06 events per 1000 injections, 95% CI 0.91–1.21, vs. 0.64 events per 1000 injections, 95% CI 0.54–0.74; adjusted RR 1.65, 95% CI 1.34–2.04; Figure 2a and 2b).
Figure 2.
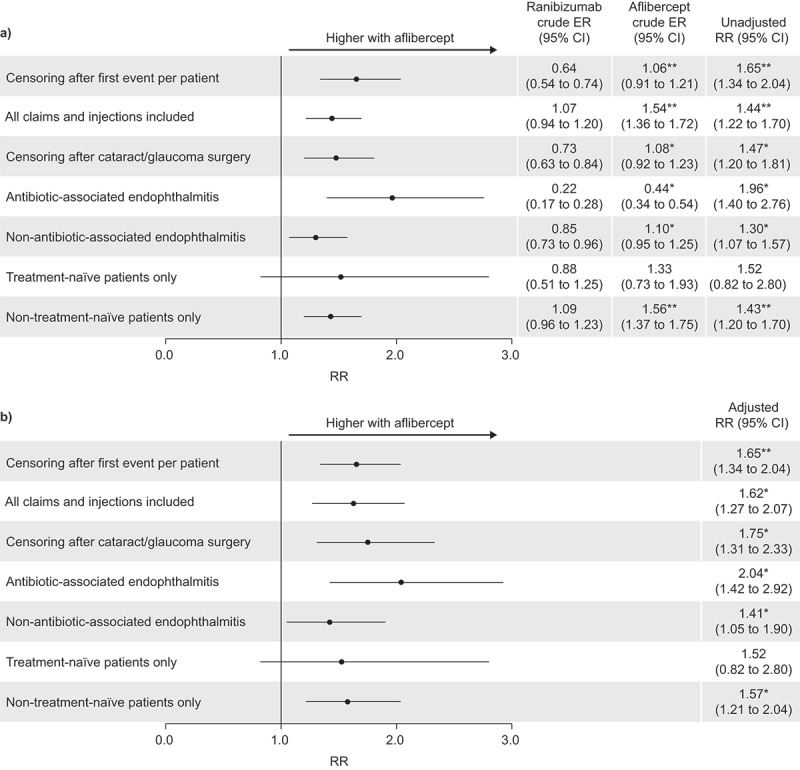
Relative risk (RR) of endophthalmitis with aflibercept injections relative to ranibizumab injections in the US; (a) unadjusted, and (b) adjusted results. CI, confidence interval; ER, event rate. Unadjusted results were calculated by Poisson regression. Adjusted results were calculated using the generalized estimating equation model with compound symmetry adjustments; adjustments were made for both injection-level (left/right injection eye, number of prior injections and occurrence of events with prior injections) and patient-level characteristics (age, sex, health plan type and geographical location). *p < 0.05; **p < 0.0001 (χ 2 test).
Comparable results to those from the primary analysis were seen for subgroup/sensitivity analyses of patients who had undergone glaucoma or cataract surgeries (adjusted RR 1.75, 95% CI 1.31–2.33, p < 0.05; ranibizumab n = 179, CER 0.73, 95% CI 0.63–0.84, aflibercept n = 186, CER 1.08, 95% CI 0.92–1.23), patients with antibiotic-associated endophthalmitis (adjusted RR 2.04, 95% CI 1.42–2.92, p < 0.05; ranibizumab n = 57, CER 0.22, 95% CI 0.17–0.28, aflibercept n = 79, CER 0.44, 95% CI 0.34–0.54), those with non–antibiotic-associated endophthalmitis (adjusted RR 1.41, 95% CI 1.05–1.90, p < 0.05; ranibizumab n = 215, CER 0.85, 95% CI 0.73–0.96, aflibercept n =197, CER 1.10, 95% CI 0.95–1.25), and non-treatment-naive patients (adjusted RR 1.57, 95% CI 1.21–2.04, p < 0.05; ranibizumab n = 250, CER 1.09, 95% CI 0.96–1.23 aflibercept n = 257, CER 1.56, 95% CI 1.37–1.75). In contrast, no significant differences in severe ocular inflammation claims were recorded for patients who were treatment-naive (adjusted RR 1.52, 95% CI 0.82–2.80; ranibizumab n = 22, CER 0.88, 95% CI 0.51–1.25 aflibercept n = 19, CER 1.33, 95% CI 0.73–1.93; Figure 2a and 2b).
A further sensitivity analysis investigated the frequency of severe ocular inflammation over time (Table 2). There was no significant change in the rate of claims over time for severe ocular inflammation following ranibizumab treatment.
Table 2.
Crude event rates for severe ocular inflammation claims over time from intravitreal anti-VEGF injections for neovascular age-related macular degeneration, US.
| Time of injection | Ranibizumab |
Aflibercept |
||||
|---|---|---|---|---|---|---|
| Events, n | Injections, n | CERa (95% CI) |
Events, n | Injections, n | CERa (95% CI) |
|
| Nov 2011–Apr 2012 | 76 | 78,164 | 0.97 (0.75–1.19) |
19 | 17,461 | 1.09 (0.60–1.58) |
| May 2012–Oct 2012 | 77 | 65,075 | 1.18 (0.92–1.45) |
48 | 48,212 | 1.00 (0.71–1.28) |
| Nov 2012–Apr 2013 | 72 | 66,078 | 1.09 (0.84–1.34) |
118 | 65,688 | 1.80b (1.47–2.12) |
| May 2013–Aug 2013 | 47 | 44,330 | 1.06 (0.76–1.36) |
91 | 47,786 | 1.90b (1.51–2.30) |
aCERs compared by t-test between treatment groups.
b p < 0.05.
CI, confidence interval; CER, crude event rate per 1000 injections; VEGF, vascular endothelial growth factor.
Discussion
This was the first study to directly compare the incidence of severe ocular inflammation following ranibizumab and aflibercept treatment in routine clinical practice in the US, which is highly relevant in light of recent reports of endophthalmitis associated with aflibercept.31,32 The primary finding of this retrospective, claims-based, injection-level database study indicated that the risk of severe ocular inflammation following treatment with ranibizumab or aflibercept was low (0.64 events per 1000 injections and 1.06 events per 1000 injections, respectively); however, the reported rates of severe ocular inflammation were significantly higher with aflibercept than with ranibizumab. In addition, despite differences in methodology, the rates reported in this study were similar to previously reported endophthalmitis rates, which were in the range 0.10–0.83 events per 1000 injections.29,30,36–44
All additional sensitivity analyses and subgroup analyses undertaken were consistent with the primary result showing a significantly higher frequency of severe ocular inflammation claims after aflibercept injections than following ranibizumab injections, with the exception of the analysis of patients who were naive to anti-VEGF treatment in the 6 months preceding the index claim. Approximately 85% of claims were recorded in the 5 days following injection, confirming the acute nature of the condition. Approximately 10% of severe ocular inflammation claims were for patients who were naive to anti-VEGF treatment in the 6 months preceding the index claim, and approximately 25% of all claims were accompanied by claims for intravitreal antibiotics. 45
Although the study shows that significantly fewer ocular events occur with ranibizumab than with aflibercept, the reasons for the differences between treatments could not be discerned. Pro-inflammatory interaction of the Fc component with intraretinal Fc receptors is a plausible pathophysiological pathway whereby aflibercept could mediate inflammation. In primate studies of intravitreal aflibercept and ranibizumab injections, 50 aflibercept was primarily taken up by the neuronal and retinal pigment epithelial cells, unlike the smaller ranibizumab molecule which permeated the retina through intercellular spaces. The putative contribution of the Fc component of aflibercept to intraretinal pro-inflammatory degeneration has been supported by recent observations of increased expression of Fc receptors in the retinas of donor eyes from patients with nAMD relative to healthy controls. 51
Although the risk of severe ocular inflammation following intravitreal anti-VEGF injection is low, the severity of this clinical event, combined with the requirement for repeated injections for patients with nAMD, makes the cumulative risk of severe ocular inflammation a key concern. 52 Thus, it is clinically relevant if small differences in the risk of severe ocular inflammation are apparent following anti-VEGF therapies of comparable efficacy.
Currently, the only proven preventive measure for postoperative infection is the application of topical povidone–iodine; evidence of the efficacy of topical antibiotics is inconclusive. 53 – 55 The use of face masks and/or prohibition of talking during the procedure may reduce the likelihood of developing severe ocular inflammation after intravitreal injection by reducing levels of airborne pathogens likely to come into contact with patients’ eyes. 56 A recent preliminary report found that fewer cases of culture-positive endophthalmitis were identified in a group of patients who received intravitreal anti-VEGFs under no-talking versus talking conditions. 57 Other factors such as eyelid hygiene and equipment and drug storage conditions could also play a role. Whether differences in these approaches or risk factors can explain differences in observed event rates between ranibizumab and aflibercept is beyond the scope of our study, and worthy of future assessment.
The present study has a number of strengths. It is the first study to directly compare the risk of severe ocular inflammation following intravitreal treatment with aflibercept and with ranibizumab in the real world. Second, it is the largest comparative study of severe ocular inflammation rates following anti-VEGF injections to date. Although a previous large US-based case series assessed endophthalmitis rates following approximately 60,000 injections of ranibizumab or bevacizumab, 42 the current study involved over 400,000 injections in more than 80,000 patients; the very large sample size ensures that the study is sufficiently powered to detect differences between alternative treatments. This study used the IDW claims database, which is particularly appropriate because it includes longitudinal claims data from a representative population of patients receiving treatment for nAMD across the US. Furthermore, claims data for IDW are submitted by the consulting/attending physician in response to observed clinical manifestations; because such data are necessary for physician compensation, under-reporting, differential reporting between drugs, and misclassification are reduced compared with chart reviews and case reports. This contrasts with analyses of adverse events, which could be confounded by under-reporting or the tendency to report an event known to be related to a specific drug, especially when dealing with rare events. The sensitivity analyses employed indicate that the primary findings are robust. Last, although the rates of severe ocular inflammation reported in retrospective claims analyses do not necessarily agree with those reported in randomized clinical trials, in which patients are followed up under more stringent protocols, the real-world nature of studies such as this provide valuable information about what occurs in clinical practice.
There are some limitations to this study, primarily relating to known issues with observational studies. 58 The claims themselves are based on physician diagnosis codes, which have an inherent risk of misclassification. The sensitivity and subgroup analyses completed in this study aimed to mitigate these confounding factors; furthermore, any misclassifications would apply equally to both treatment groups and therefore should not affect the RRs. A further potential limitation of this study was that during the time period investigated there was limited experience with aflibercept among US ophthalmologists (owing to its recent launch). This could potentially have resulted in a lower threshold for suspecting severe ocular inflammation with aflibercept than with ranibizumab. It would therefore be of interest to perform a 5-year follow-up analysis on the data to eliminate the possibility of sampling bias resulting from the possible sub-normal threshold for endophthalmitis diagnosis. Several previous studies also focused on presumed infectious endophthalmitis cases,38–41,44 whereas this study reports claims for severe ocular inflammation patient care for any reason, and thus did not differentiate sterile from infectious intraocular inflammation, because this information is not available from claims data. In addition, 25% of cases were accompanied by claims for intravitreal antibiotics; a further limitation of the claims data is that they do not specify the type of endophthalmitis, nor do they state whether a bacterial culture test was performed. Importantly, it is not clear whether the claims are for culture-positive events or not. Approximately 75% of cases were not associated with intravitreal antibiotic claims, which suggests that the majority of cases were sterile events. Last, the fact that the interventions were not randomly assigned could potentially have an impact on the results.
In conclusion, intravitreal aflibercept injections are associated with a higher risk of severe ocular inflammation than intravitreal ranibizumab injections during routine clinical use for the management of patients with nAMD. The findings show the importance of real-world, post-market approval, observational monitoring of novel medicines, and may be useful for clinical decision-making, including the decision regarding choice of anti-VEGF agent. Reasons for the treatment differences are beyond the scope of this analysis; however, further research into the physiological mechanisms of anti-VEGF therapies and study of the RRs of other ocular events during therapy with ranibizumab or aflibercept is warranted to improve understanding of clinically important differences between these therapies. It would also be of value to perform a similar analysis of claims data for other widely used anti-VEGF intravitreal injections, such as bevacizumab. As with aflibercept, bevacizumab contains a Fc fragment, and so would provide a further test of the hypothesis that the inflammatory response is mediated by Fc fragment interaction with retinal Fc receptors. Finally, data are specific to US clinical practice, and confirmation from practice in other countries will be needed.
Supplementary Material
Acknowledgments
Writing assistance was provided by Dr. Martin Bell and Dr. Robert Gillies from Oxford PharmaGenesis.
Declaration of interest: Eric H. Souied has received payment for consultancy, travel, and review activities from Allergan, Bayer AG, and Novartis Pharma AG; has been an advisory board member and consultant for Théa; and has received payment for lectures and travel from Heidelberg Pharma. Pravin U. Dugel is a consultant to Alcon, Genentech, Novartis Pharma AG, and Ophthotech. Alberto Ferreira and Ron Hashmonay are employees of Novartis Pharma AG and own shares in the company. Jingsong Lu is an employee of IMS Health, which has received funding from Novartis Pharma AG. Simon P. Kelly has received payment for consultancy and travel expenses from Bayer AG and Novartis Pharma AG, and has been an advisory board member and received payment for lectures from Novartis Pharma AG.
This study was supported by funding from Novartis Pharma AG, Basel, Switzerland.
Supplemental material
Supplemental data for this article can be accessed on the publisher’s website.
References
- Rudnicka AR, Jarrar Z, Wormald R. Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: a meta-analysis. Ophthalmology. 2012;119:571–580. doi: 10.1016/j.ophtha.2011.09.027. [DOI] [PubMed] [Google Scholar]
- Boyer DS, Hopkins JJ, Sorof J. Anti-vascular endothelial growth factor therapy for diabetic macular edema. Therapeutic Adv Endocrinol Metabolism. 2013;4:151–169. doi: 10.1177/2042018813512360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanville J, Patterson J, McCool R. Efficacy and safety of widely used treatments for macular oedema secondary to retinal vein occlusion: a systematic review. BMC Ophthalmol. 2014;14:7. doi: 10.1186/1471-2415-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho AC, Scott IU, Kim SJ. Anti-vascular endothelial growth factor pharmacotherapy for diabetic macular edema: a report by the American Academy of Ophthalmology. Ophthalmology. 2012;119:2179–2188. doi: 10.1016/j.ophtha.2012.07.058. [DOI] [PubMed] [Google Scholar]
- Pielen A, Feltgen N, Isserstedt C. Efficacy and safety of intravitreal therapy in macular edema due to branch and central retinal vein occlusion: a systematic review. PLoS One. 2013;10(8):e78538. doi: 10.1371/journal.pone.0078538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucentis summary of product characteristics. London, UK: European Medicines Agency. 2013; [Google Scholar]
- Lucentis prescribing information. Silver Spring, MD: US Food and Drug Administration. 2013; [Google Scholar]
- American Academy of Ophthalmology Retina Panel. Preferred practice pattern guidelines. Age-related macular degeneration. San Francisco, CA: American Academy of Ophthalmology. 2008; [Google Scholar]
- Age-related macular degeneration (management recommendations). San Francisco, CA: International Council of Ophthalmology. 2011; [Google Scholar]
- Royal College of Ophthalmologists. Age-related macular degeneration: guidelines for management. London, UK: Royal College of Ophthalmologists. 2013; [Google Scholar]
- Menon G, Walters G. Eye . New paradigms in the treatment of wet AMD: the impact of anti-VEGF therapy. 2009;23(Suppl. 1):S1–7. doi: 10.1038/eye.2009.13. [DOI] [PubMed] [Google Scholar]
- Belkin M, Kalter-Leibovici O, Chetrit A. Time trends in the incidence and causes of blindness in Israel. Am J Ophthalmol. 2013;155:404. doi: 10.1016/j.ajo.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Bloch SB, Larsen M, Munch IC. Incidence of legal blindness from age-related macular degeneration in Denmark: year 2000 to 2010. Am J Ophthalmol. 2012;153:209–213, e2. doi: 10.1016/j.ajo.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Rostron E, McKibbin M. Visual impairment certification secondary to ARMD in Leeds, 2005–2010: is the incidence falling? Eye. 2012;26:933–936. doi: 10.1038/eye.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaat A, Chetrit A, Belkin M. Time trends in the incidence and causes of blindness in Israel. Am J Ophthalmol. 2012;153:214–221, e1. doi: 10.1016/j.ajo.2011.08.035. [DOI] [PubMed] [Google Scholar]
- Keenan TD, Kelly SP, Sallam A. Incidence and baseline clinical characteristics of treated neovascular age-related macular degeneration in a well-defined region of the UK. Br J Ophthalmol. 2013;97:1168–1172. doi: 10.1136/bjophthalmol-2013-303233. [DOI] [PubMed] [Google Scholar]
- Claessen H, Genz J, Bertram B. Evidence for a considerable decrease in total and cause-specific incidences of blindness in Germany. Eur J Epidemiol. 2012;27:519–524. doi: 10.1007/s10654-012-9705-7. [DOI] [PubMed] [Google Scholar]
- Sloan FA, Hanrahan BW. The effects of technological advances on outcomes for elderly persons with exudative age-related macular degeneration. JAMA Ophthalmol. 2014;132:456–463. doi: 10.1001/jamaophthalmol.2013.7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cackett P, Borooah S, Gavin M. Scotland AMD study group. Intravitreal ranibizumab treatment of wet macular degeneration in SE Scotland – effect on blindness rates and 5 year follow up data. Invest Ophthalmol Vis Sci. 2013;54:372. [Google Scholar]
- Eylea summary of product characteristics. London, UK: European Medicines Agency. 2013. [Google Scholar]
- Eylea prescribing information. Silver Spring, MD: US Food and Drug Administration. 2013. [Google Scholar]
- Papadopoulos N, Martin J, Ruan Q. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15:171–185. doi: 10.1007/s10456-011-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daha NA, Banda NK, Roos A. Complement activation by (auto-) antibodies. Mol Immunol. 2011;48:1656–1665. doi: 10.1016/j.molimm.2011.04.024. [DOI] [PubMed] [Google Scholar]
- Meyer CH, Holz FG. Preclinical aspects of anti-VEGF agents for the treatment of wet AMD: ranibizumab and bevacizumab. Eye. 2011;25:661–672. doi: 10.1038/eye.2011.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar O, Yoeruek E, Szurman P. Effect of bevacizumab on inflammation and proliferation in human choroidal neovascularization. Arch Ophthalmol. 2008;126:782–790. doi: 10.1001/archopht.126.6.782. [DOI] [PubMed] [Google Scholar]
- Brechner RJ, Rosenfeld PJ, Babish JD. Pharmacotherapy for neovascular age-related macular degeneration: an analysis of the 100% 2008 medicare fee-for-service part B claims file. Am J Ophthalmol. 2011;151:887–895, e1. doi: 10.1016/j.ajo.2010.11.017. [DOI] [PubMed] [Google Scholar]
- CATT research group, Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–1398. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal S, Joshi M, Christoforidis JB. Vitreous inflammation associated with intravitreal anti-VEGF pharmacotherapy. Mediators Inflammation. 2013;2013(943409) doi: 10.1155/2013/943409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein KS, Walsh MK, Hassan TS. Ophthalmology. Endophthalmitis after anti-VEGF injections. [DOI] [PubMed] [Google Scholar]
- Shah CP, Garg SJ, Vander JF. Post-Injection Endophthalmitis Study Team. Outcomes and risk factors associated with endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents. Ophthalmology. 2011;118:2028–2034. doi: 10.1016/j.ophtha.2011.02.034. [DOI] [PubMed] [Google Scholar]
- Hahn P, Kim JE, Stinnett S. Aflibercept-related sterile inflammation. Ophthalmology. 2013;120 doi: 10.1016/j.ophtha.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Pharmacovigilance Risk Assessment Committee (PRAC). London, UK: European Medicines Agency. 2013; [Google Scholar]
- Fukami T, Kitahashi M, Sato E. [Six cases of sterile endophthalmitis developed consecutively after intravitreal injection of bevacizumab] Nihon Ganka Gakkai Zasshi. 2011;115:706–710. [PubMed] [Google Scholar]
- Goldberg RA, Flynn HW, Jr., Miller D. Streptococcus endophthalmitis outbreak after intravitreal injection of bevacizumab: one-year outcomes and investigative results. Ophthalmology. 2013;120:1448–1453. doi: 10.1016/j.ophtha.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal College of Ophthalmologists. Potential bevacizumab-related sterile endophthalmitis cases – update, 15 March. London, UK: Royal College of Ophthalmologists, 2012; 2012. [Google Scholar]
- Cavalcante LL, Cavalcante ML, Murray TG. Intravitreal injection analysis at the Bascom Palmer Eye Institute: evaluation of clinical indications for the treatment and incidence rates of endophthalmitis. Clin Ophthalmol. 2010;4:519–524. doi: 10.2147/opth.s11094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CS, Wong AW, Lui A. Incidence of endophthalmitis and use of antibiotic prophylaxis after intravitreal injections. Ophthalmology. 2012;119:1609–1614. doi: 10.1016/j.ophtha.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Diago T, McCannel CA, Bakri SJ. Infectious endophthalmitis after intravitreal injection of antiangiogenic agents. Retina. 2009;29:601–605. doi: 10.1097/IAE.0b013e31819d2591. [DOI] [PubMed] [Google Scholar]
- Fintak DR, Shah GK, Blinder KJ. Incidence of endophthalmitis related to intravitreal injection of bevacizumab and ranibizumab. Retina. 2008;28:1395–1399. doi: 10.1097/IAE.0b013e3181884fd2. [DOI] [PubMed] [Google Scholar]
- Gillies MC, Walton R, Simpson JM. Fight Retinal Blindness! Project Investigators. Prospective audit of exudative age-related macular degeneration: 12-month outcomes in treatment-naive eyes. Invest Ophthalmol Vis Sci. 2013;54:5754–5760. doi: 10.1167/iovs.13-11993. [DOI] [PubMed] [Google Scholar]
- Lyall DA, Tey A, Foot B. Post-intravitreal anti-VEGF endophthalmitis in the United Kingdom: incidence, features, risk factors, and outcomes. Eye. 2012;26:1517–1526. doi: 10.1038/eye.2012.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshfeghi AA, Rosenfeld PJ, Flynn HW., Jr. Endophthalmitis after intravitreal vascular [corrected] endothelial growth factor antagonists: a six-year experience at a university referral center. Retina. 2011;31:662–668. doi: 10.1097/IAE.0b013e31821067c4. [DOI] [PubMed] [Google Scholar]
- Pilli S, Kotsolis A, Spaide RF. Endophthalmitis associated with intravitreal anti-vascular endothelial growth factor therapy injections in an office setting. Am J Ophthalmol. 2008;145:879–882. doi: 10.1016/j.ajo.2007.12.036. [DOI] [PubMed] [Google Scholar]
- Tabandeh H, Boscia F, Sborgia A. Endophthalmitis associated with intravitreal injections: office-based setting and operating room setting. Retina. 2014;34:18–23. doi: 10.1097/IAE.0000000000000008. [DOI] [PubMed] [Google Scholar]
- Kiss S, Dugel PU, Wilson K. Treatment patterns and associated costs of anti-VEGF therapy for neovascular age-related macular degeneration. Association for Research in Vision and Ophthalmology (ARVO) annual meeting. Orlando, FL: USA, 2014; Poster; p. 5596. [Google Scholar]
- Ang GS, Varga Z, Shaarawy T. Postoperative infection in penetrating versus non-penetrating glaucoma surgery. Br J Ophthalmol. 2010;94:1571–1576. doi: 10.1136/bjo.2009.163923. [DOI] [PubMed] [Google Scholar]
- Cao H, Zhang L, Li L. Risk factors for acute endophthalmitis following cataract surgery: a systematic review and meta-analysis. PLoS One. 2013;8(e71731) doi: 10.1371/journal.pone.0071731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eifrig CW, Flynn HW, Jr., Scott IU. Acute-onset postoperative endophthalmitis: review of incidence and visual outcomes (1995–2001) Ophthalmic Surg Lasers. 2002;33:373–378. [PubMed] [Google Scholar]
- Kernt M, Endophthalmitis: Kampik A. pathogenesis, clinical presentation, management, and perspectives. Clin Ophthalmol. 2010;4:121–135. doi: 10.2147/opth.s6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien S, Biesemeier A, Taubitz T. Different effects of intravitreally injected ranibizumab and aflibercept on retinal and choroidal tissues of monkey eyes. Br J Ophthalmol. 2014;98:813–825. doi: 10.1136/bjophthalmol-2013-304019. [DOI] [PubMed] [Google Scholar]
- Murinello S, Mullins RF, Lotery AJ. Fc gamma receptor upregulation is associated with immune complex inflammation in the mouse retina and early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55:247–258. doi: 10.1167/iovs.13-11821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyman GA, Lad EM, Moshfeghi DM. Intravitreal injection of therapeutic agents. Retina. 2009;29:875–912. doi: 10.1097/IAE.0b013e3181a94f01. [DOI] [PubMed] [Google Scholar]
- Bhatt SS, Stepien KE, Joshi K. Prophylactic antibiotic use after intravitreal injection: effect on endophthalmitis rate. Retina. 2011;31:2032–2036. doi: 10.1097/IAE.0b013e31820f4b4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavsar AR, Googe JM, Jr., Stockdale CR. Diabetic Retinopathy Clinical Research Network. Risk of endophthalmitis after intravitreal drug injection when topical antibiotics are not required: the diabetic retinopathy clinical research network laser-ranibizumab-triamcinolone clinical trials. Arch Ophthalmol. 2009;127:1581–1583. doi: 10.1001/archophthalmol.2009.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey P, Dollin M, Pitcher J. Post-Injection Endophthalmitis Study Team. The role of topical antibiotic prophylaxis to prevent endophthalmitis after intravitreal injection. Ophthalmology. 2014;121:283–289. doi: 10.1016/j.ophtha.2013.08.037. [DOI] [PubMed] [Google Scholar]
- McCannel CA. Meta-analysis of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents: causative organisms and possible prevention strategies. Retina. 2011;31:654–661. doi: 10.1097/IAE.0b013e31820a67e4. [DOI] [PubMed] [Google Scholar]
- Dollin M, Storey P, Vander J. policy during intravitreal injection on post-injection endophthalmitis. Association for Research in Vision and Ophthalmology (ARVO) annual meeting. Orlando, FL: USA, 2014; Abstract; The effect of a “no talking”; p. 587. [Google Scholar]
- Dreyer NA, Velentgas P, Westrich K. The GRACE checklist for rating the quality of observational studies of comparative effectiveness: a tale of hope and caution. J Managed Care Pharmacy. 2014;20:301–308. doi: 10.18553/jmcp.2014.20.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


