ABSTRACT
Sewage sludge, in particular from the food industry, is characterized by fertilizing properties, due to the high content of organic matter and nutrients. The application of sewage sludge causes an improvement of soil parameters as well as increase in cation exchange capacity, and thus stronger binding of cations in the soil environment, which involves the immobilization of nutrients and greater resistance to contamination. In a field experiment sewage sludge has been used as an additive to the soil supporting the phytoremediation process of land contaminated with heavy metals (Cd, Zn, and Pb) using trees species: Scots pine (Pinus silvestris L.), Norway spruce (Picea abies L.), and oak (Quercus robur L.). The aim of the research was to determine how the application of sewage sludge into the soil surface improves the phytoremediation process. The conducted field experiment demonstrated that selected trees like Scots pine and Norway spruce, because of its excellent adaptability, can be used in the remediation of soil. Oak should not be used in the phytoremediation process of soils contaminated with high concentrations of trace elements in the soil, because a significant amount of heavy metals was accumulated in the leaves of oak causing a risk of recontamination.
KEYWORDS: tree species, trace elements, sewage sludge
Introduction
The areas located in the vicinity of industrial areas and communication routes with increased automobile traffic are exposed to high concentrations of pollutants and trace elements. Trace metals, called potentially toxic trace elements, cause degradation of the active surface of the earth and constitute a major threat to the food chain of plants, animals and humans. (Hirt and Shinozaki 2004; Zhang et al. 2010).
Heavy metals occurring in the ores and rocks of the formation, usually in the form of oxides, are not generally harmful for the environment. In contrast, the trace elements present in the salt solutions, which are by-products or wastes of various branches of industry and agriculture, are highly toxic. The source of heavy metals in soil is an intense mining extraction, industrial activities, transport, power engineering, and agriculture. The content of individual elements in the soil depends on the distance of the emitters of impurities and the sedimentation rate of pollutions (Fijałkowski et al. 2012; Padmavathiamma and Li 2007).
Heavy metals are initially accumulated on the soil surface without causing visible changes or they are too slow to be noticed. Changes in soil become apparent when there is a destruction of the vegetation and transformation the entire surface of the soil in the wasteland (Adriano 2001; Lynch and Moffat 2005; Nouri et al. 2009).
Trace elements emitted by industrial plants in small quantities usually do not harm the plants, but their negative effects are disclosed in the subsequent links of the trophic chain. The soil environment has limited protective capacity against the emission of heavy metals. In addition, as time passes, there is an accumulation of contaminants in the soil and an increase in the uptake of heavy metals by plants (Alkorta et al. 2004; Prasad and Freitas 2003).
Heavy metal toxicity to plants depends on the type of metal, its content in the soil and forms of occurrence. Some of them reduce the amount of chlorophyll, thereby reducing the efficiency of photosynthesis and, consequently leading to an inhibition of the growth of plants (Dimkpa et al. 2009; Liang et al. 2009). The biochemical role of the metal is mainly associated with the process of protein metabolism, transport elements and substances, both at the level of cells and organs, and enzyme activity (particularly involved in the processes of oxidation and reduction). The accumulation of trace elements occurs with an increasing trophic level in the ecosystem (Dzugan et al. 2010; Matthews et al. 2005). The moving of each element to a higher trophic level of food chain is limited by the functioning of biological barriers. However, in the case of too high a concentration of elements, the effect of these barriers is weakened, which is associated with a potential risk of negative impacts on the natural environment and especially on human health (Gang et al. 2010). Most often heavy metals get into animal and human organisms through food consumption. Trace elements cause changes in protein synthesis and disorders of production of ATP, leading to damage the cell membrane and the organelle membrane. Some of the metals cause renal dysfunction, reproductive function, and calcium metabolism (Calace et al. 2006).
Heavy metals are extremely hazardous to health due to their accumulation in human and animal organisms (An 2004).
Self-cleaning, in the case of trace elements contained in the soil, is practically disregarded due to the relatively low reactivity (e.g., time remaining of lead in the soil is estimated to be several hundred years). A quick and definitive cleaning of the soil with heavy metals is only possible through the use of so-called hard technical methods, which are usually not indifferent to the environment. The term phytoremediation is defined as a series of techniques using the plant for cleaning soil from both organic and inorganic impurities. Phytoremediation is the use of plants and associated microorganisms to immobilize (phytostabilization), to remove (phytoextraction), to evaporate (phytoporation), or to degrade (phytodegradation, rhizodegradation) contaminants from soil and water environment (Cunningham et al. 1995; Jabeen et al. 2009; Reevers and Baker 2007; Wei et al. 2008). Phytoremediation of soil is an inexpensive, socially acceptable, and eco-friendly technique for reclamation of degraded areas (Padmavathiamma and Li 2007; Grobelak et al. 2010; Wu et al. 2010).
The degraded soils, following the harmful effects of emissions from non-ferrous metal smelters, are usually arid from organic material and devoid of proper microflora. Therefore, the poor condition of the soil environment makes it impossible to carry out an effective biological remediation of the degraded area. For this purpose, some organic substances, such as sewage sludge, are used supporting the process of phytoremediation, because they are a source of biogenic elements and soil microorganisms (Blaylock and Huang 2000; Kacprzak et al. 2014; Mench et al. 2003, Sanchez-Monedero et al. 2004).
The current research study is describing changes of selected soil parameters, including the level of trace elements in the soil, occurring during the four growing seasons of the field experiment. The process of phytoremediation of degraded soils with heavy metals (Cd, Zn, and Pb) and supported with the application of sewage sludge from the food industry and with the use of forest species: Scots pine (Pinus silvestris L.), Norway spruce (Picea abies L.), and oak (Quercus robur L.) was monitored.
Materials and methods
Characteristics of the research area and soil material
The field experiment located in the zone of influence zinc smelter (18 ° 54′E, 50 °30′N) in Poland's Silesia Region (Figure 1). Soil parameters and trees biomass were determined during four growing seasons (third to sixth). As described previously (Kacprzak et al. 2014), the degraded area is dominated mainly by podsolic soil, characterized by low humidity and a low pH value. This type of soil poses a poorly formed soil profile, with high permeability and a very low content of humus. These soils are also poor in nutrients and are distinguished by a low sorption capacity and a small capacity buffer. The contamination of the study area is a result of the impact of particulate emissions from the zinc smelter and is characterized by high accumulation of heavy metals such as Cd, Pb, and Zn (Table 1).
Figure 1.
The field experiment site – Miasteczko Slaskie.
Table 1.
Chemical and physical parameters of the contaminated soil from field experiment.
| Parameter | average value±SD |
|---|---|
| humidity [%] | 18.46 ± 1.75 |
| pH in H2O | 5.28 – 5.30 |
| pH in 1 M KCl | 4.84 – 4.85 |
| CEC [cmol(+) kg−1 d.m.] | 10.90 ± 1.54 |
| humic acids [%] | 0.12 ± 0.01 |
| C total [g kg−1 d.m.] | 11.70 ± 1.32 |
| N Kjeldhal [mg kg−1 d.m.] | 658.00 ± 18.12 |
| P available [mg kg−1 d.m.] | 24.10 ± 0.86 |
| P total [mg kg−1 d.m.] | 74.53 ± 5.34 |
| Zn [mg kg −1 d.m.] | 1062.65 ± 57.49 |
| Cd [mg kg −1 d.m.] | 14.22 ± 1.63 |
| Pb [mg kg −1 d.m.] | 1422.00 ± 87.13 |
Characteristics of biosolid (sewage sludge)
In a field experiment sewage sludge was used to support the phytoremediation process of contaminated soil. Sewage sludge used in the study was collected from the sewage treatment plant at the factory of mineral waters. Used sewage sludge was characterized by a low content of heavy metals and eggs of parasites and, for this reason, can be used with both the restoration of degraded land and agriculture, having a positive effect on the increase in of biomass and the number of soil microorganisms (Table 2).
Table 2.
Chemical and physical parameters of sewage sludge.
| Parameter | average value±SD |
|---|---|
| humidity [%] | 91.3 ± 4.82 |
| pH in H2O | 7.15 – 7.17 |
| C total [g kg−1 d.m.] | 690.4 ± 23. 41 |
| N Kjeldhal [mg kg−1 d.m.] | 14560.00 ± 123.71 |
| P total [mg kg−1 d.m.] | 3479.73 ± 35.11 |
| P available [mg kg−1 d.m.] | 2461.35 ± 47.69 |
| Zn [mg kg −1 d.m.] | 288.90 ±2.8 |
| Cd [mg kg −1 d.m.] | 1.70 ± 0.31 |
| Pb [mg kg −1 d.m.] | 149.00 ± 5.9 |
Field experiment
The field study was conducted in 2010. Over the two years (third to sixth growing seasons) soil samples and samples of plant material were collected twice a year (spring and autumn). In order to perform the planned field trials experiments, two research plots were established in April 2010. The field experiment was carried out at a distance of approximately 800 meters to the north-east of the zinc smelter. On the first plot with an area of 44 m2 the sewage sludge dose of 15 Mg ha−1 d.m.per year plot was applied once (Figure 2a). Sewage sludge was applied into the original soil by mixing the sludge with the original soil to depth of 15 cm using agricultural rototillers. In contrast, the second plot with an area of 24 m2 was used as a control for the first plot, without the sewage sludge application (Figure 2b). For both research plots the three species of plants, such as Norway spruce (Picea abies L.), Scots pine (Pinus silvestris L.) and oak (Quercus robur L.) were planted. Planting was organized in rows that were spaced apart by a distance equal to 40 cm. In this experiment mycorrhizal plant seedlings were used. Two types of biopreparats were used: commercial ECTO mycorrhizae by the Mykoflor company and biopreparation containing hyphae of the fungus Hebeloma crustuliniforme produced in the State Forests (by Polish patented technology developed by Stephen Kowalski of the University of Agriculture in Krakow).
Figure 2.

The field experiment (a – field plot with sewage sludge, b – field plot without sewage sludge amendment).
Chemical and physical analysis of soil and soil solution
Prior to analysis, soil subsamples were air-dried and later passed through a 2 mm mesh screen. The following parameters were measured for the soil subsamples (five subsamples from each plot): pH in H2O and KCl deionised water suspension (PN-ISO 10390:1997), humid acids (Stevenson 1994), total Kjeldahl nitrogen (PN-ISO 11261:2002), total organic carbon using a Multi N/C H1300 Analitykjena (PN-ISO 10694:2002), available phosphorus using the Enger-Riehm method (Karczewska and Kabała 2008), CEC (Cation Exchange Capacity) according to Kappen's method (Karczewska and Kabała 2008). Soil solution samples were filtered with a Whatman 0.45 μm filter prior to analysis. Soil material and plant material (five repetitions) were digested using hot aqua regia (PN-ISO 11047:2001). Then the digested experimental material and pore soil solution were analyzed for the presence of Cd, Pb, and Zn using inductively coupled plasma optical emission spectrometry (ICP-OES; Thermo apparatus). On the basis of the content of heavy metals in shoots and roots of trees and the concentration of trace elements in the soil were calculated following indicators:
Translocation factor (TF)
The translocation process capacity of Cd, Pb, and Zn from trees shoots to roots is expressed by the translocation factor (TF), which is calculated as follows:
where:
Cshoot – metals concentration in the shoot of tree [mg kg−1],
Croot – metals concentration in the root of tree [mg kg−1].
Effective process of translocation of heavy metals from the roots of trees shoot occurs at the TF> 1 (Baker and Brooks 1989; Fayiga and Ma 2006); Rezvani and Zaefarian 2011; Zhang et al. 2002).
Bioaccumulation factor (BAF)
Bioaccumulation process capacity of Cd, Pb, and Zn in the organism of trees is expressed by the bioaccumulation factor (BAF), which is calculated as follows:
where:
Cshoot – metals concentration in the shoot of tree [mg kg−1],
Csoil – metals concentration in the soil (rhizosphere zone) [mg kg−1].
On the basis of the degree of heavy metals accumulation in the tissues of the trees and the BAF, it is possible to determine the plant belonging to hyperaccumulators, accumulator (BAF> 1) and excluder (BAF <1) (Cluis 2004; Ma et al. 2001; Rezvani and Zaefarian 2011).
Metal extraction ratio (MER)
The metal extraction ratio was used to determine the ability of the trees for the immobilization of heavy metals in their organism and to determine the suitability of a particular species of trees in the phytoremediation process of degraded soils (such as phytoextraction) (Mertens et al. 2005). The metal extraction ratio is calculated as follows:
where:
Cplant – metals concentration in the aboveground part of the tree [mg kg−1],
Csoil – metals concentration in the soil (rhizosphere zone) [mg kg−1],
Mplant – mass of the aboveground part of the tree [g],
Mrooted zone – mass of the soil rhizosphere zone) [g].
On the basis of the degree of heavy metals accumulation in the tissues of the trees and the BAF it is possible to determine the plant belonging to hyperaccumulators, accumulator (BAF> 1) and excluder (BAF <1) (Ma et al. 2001; Cluis 2004; Rezvani and Zaefarian 2011).
Statistical treatment of data
Pearson correlation coefficients were calculated between soil chemical properties(pH of soil, CEC of soil, C total, N total, and P available concentration in soil, content of heavy metals in the soil). Three levels of significance were considered: p < 0.05, p < 0.01 and p < 0.001. The level of a linear relationship between concentrations of Cd, Pb, and Z in soil samples, collected from the rhizosphere zone of Scots pine, Norway spruce and oak from plots fertilized with sewage sludge and without fertilizer, was analysed. The strong correlation between random variables was obtained (Tables 3 and 4). Furthermore, the correlations between the three indicators (TF, BAF, MER) and the content of Cd, Pb, and Zn in the trees rhizosphere zones were analysed and were expressed as the coefficient of determination R2 and the linear regression (Figs. 6–14). Analysis was carried out by using STATISTICA software.
Table 3.
Pearson's correlation coefficients between soil chemical properties (pH of soil, CEC of soil, C total, N total, and P available concentration in soil).
| ph in H2O | ph in KCl | humic acids | |||||||
|---|---|---|---|---|---|---|---|---|---|
| VP | AD | CD | Pearson | AD | CD | Pearson | AD | CD | Pearson |
| 3rd | 4,63 | 4,71 | 4,1 | 4,26 | 1,23 | 1,15 | |||
| 4th | 4,58 | 4,71 | 4,16 | 4,27 | 0,95 | 0,61 | |||
| 5th | 5,05 | 5,06 | 4,21 | 4,3 | 1,22 | 1,17 | |||
| 6th | 4,9 | 5,14 | 0,907948 | 4,3 | 4,6 | 0,894923 | 0,98 | 0,88 | 0,937819 |
| CEC | N total | P available | |||||||
| VP | AD | CD | Pearson | AD | CD | Pearson | AD | CD | Pearson |
| 3rd | 4,6 | 3,2 | 822,5 | 72,47 | 51 | 23,5 | |||
| 4th | 5,36 | 4,2 | 763 | 64,71 | 27,5 | 8,6 | |||
| 5th | 5,5 | 4,6 | 567 | 43,28 | 31 | 10 | |||
| 6th | 5,24 | 5,52 | 0,689283 | 836,5 | 80,02 | 0,983687 | 24,6 | 10,3 | 0,967567 |
| C total | |||||||||
| VP | AD | CD | Pearson | ||||||
| 3rd | 13,89 | 13,14 | |||||||
| 4th | 16,45 | 11,34 | |||||||
| 5th | 13,71 | 9,38 | |||||||
| 6th | 14,95 | 11,24 | 0,081136 | ||||||
VP - vegetation period.
AD - amended contaminated soil.
CS - contaminated soil.
Table 4.
Pearson's correlation coefficients between soil chemical properties (Cd, Pb, and Zn concentration in soil).
| Scots pine | Norway spruce | Oak | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cd concentration in soil | |||||||||
| VP | AD | CD | Pearson | AD | CD | Pearson | AD | CD | Pearson |
| 3rd | 17,87 | 22,53 | 16,45 | 22,58 | 18,76 | 19,7 | |||
| 4th | 14,45 | 23,28 | 15,4 | 22,65 | 19,07 | 18,41 | |||
| 5th | 13,5 | 23,91 | 14,35 | 22,83 | 19,22 | 18,24 | |||
| 6th | 12,34 | 24,37 | −0,973 | 13,31 | CD | −0,97884 | 19,41 | 18,01 | −0,95125 |
| Pb concentration in soil | |||||||||
| VP | AD | CD | Pearson | AD | CD | Pearson | AD | CD | Pearson |
| 3rd | 1926,5 | 1275,23 | 1739,68 | 1754,63 | 1299,8 | 1224,53 | |||
| 4th | 1915,83 | 1281,01 | 1744,19 | 1787,12 | 1294,13 | 1247,15 | |||
| 5th | 1905,17 | 1287,21 | 1748,7 | 1820,04 | 1288,47 | 1270,01 | |||
| 6th | 1894,5 | 1295,72 | −0,99558 | 1753,22 | 1852,41 | 0,999994 | 1282,81 | 1292,41 | −0,99999 |
| Zn concentration in soil | |||||||||
| VP | AD | CD | Pearson | AD | CD | Pearson | AD | CD | Pearson |
| 3rd | 802,61 | 876,98 | 686,31 | 921,93 | 818,26 | 759,13 | |||
| 4th | 789,92 | 853,86 | 786,82 | 740,5 | 772,77 | 769,5 | |||
| 5th | 777,24 | 830,75 | 887,33 | 559,03 | 727,3 | 780,97 | |||
| 6th | 764,56 | 807,64 | 0,99854 | 987,83 | 377,6 | −0,99762 | 681,83 | 790,22 | −0,99921 |
VP - vegetation period.
AD - amended contaminated soil.
CS - contaminated soil.
Figure 6.
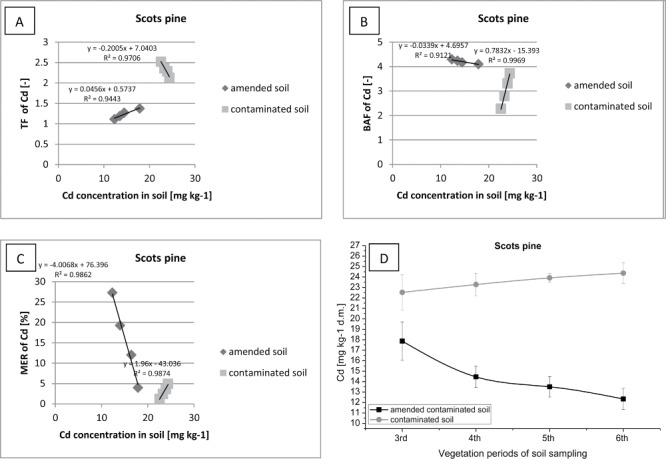
Correlation between (contaminated or amended contaminated) soil Cd concentration (D) and TF of Cd (A), BAF of Cd (B), MER of Cd (C) in Scots pine.
Figure 7.
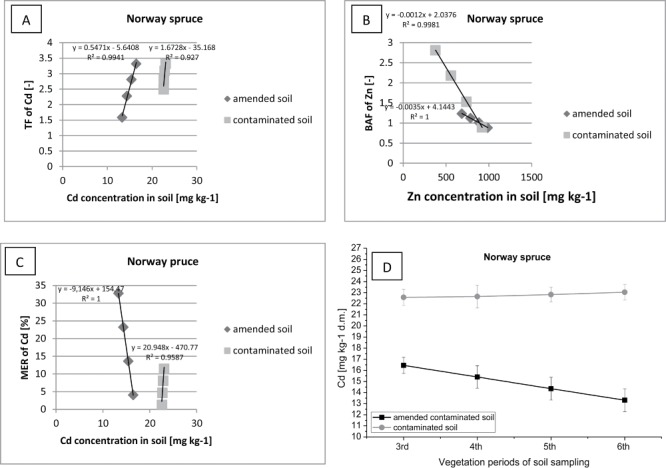
Correlation between (contaminated or amended contaminated) soil Cd concentration (D) and TF of Cd (A), BAF of Cd (B), MER of Cd (C) in Norway spruce.
Figure 8.
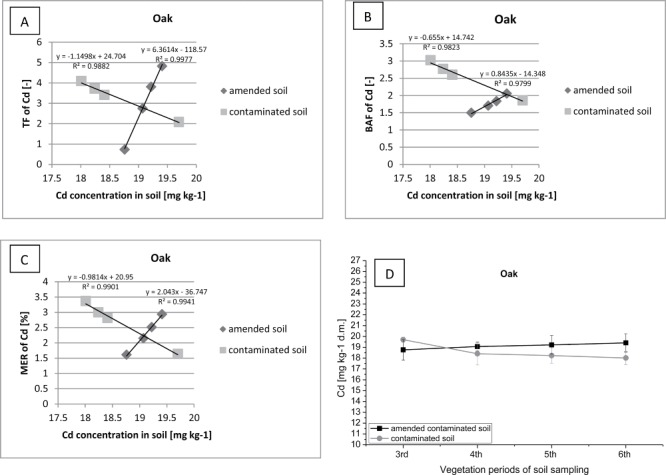
Correlation between (contaminated or amended contaminated) soil Cd concentration (D) and TF of Cd (A), BAF of Cd (B), MER of Cd (C) in oak.
Figure 9.
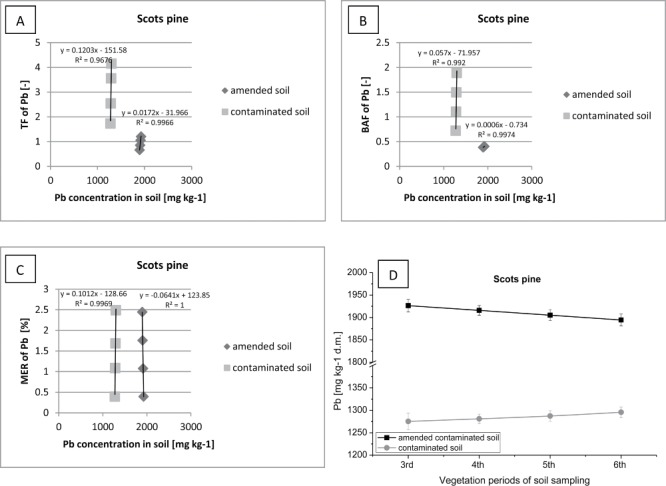
Correlation between (contaminated or amended contaminated) soil Pb concentration (D) and TF of Pb (A), BAF of Pb (B), MER of Pb (C) in Scots pine.
Figure 10.

Correlation between (contaminated or amended contaminated) soil Pb concentration (D) and TF of Pb (A), BAF of Pb (B), MER of Pb (C) in Norway spruce.
Figure 11.
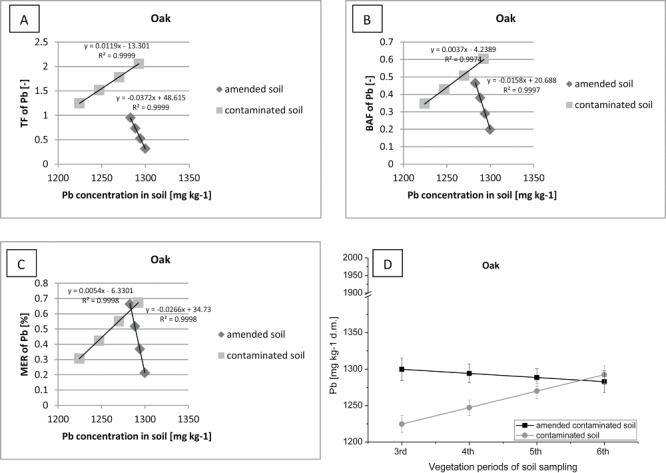
Correlation between (contaminated or amended contaminated) soil Pb concentration (D) and TF of Pb (A), BAF of Pb (B), MER of CPb (C) in oak.
Figure 12.
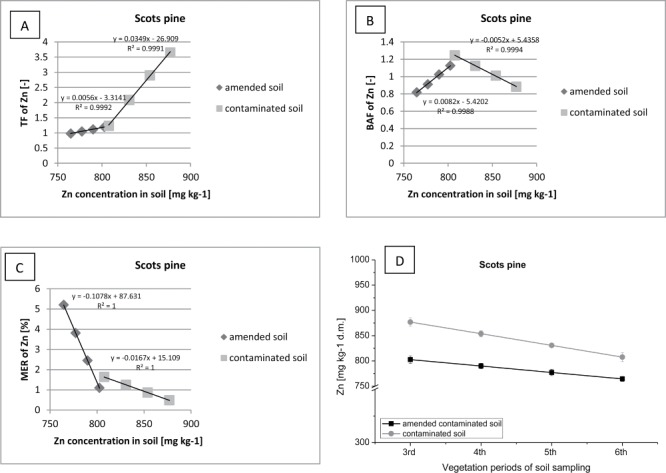
Correlation between (contaminated or amended contaminated) soil Zn concentration (D) and TF of Zn (A), BAF of Zn (B), MER of Zn (C) in Scots pine.
Figure 13.
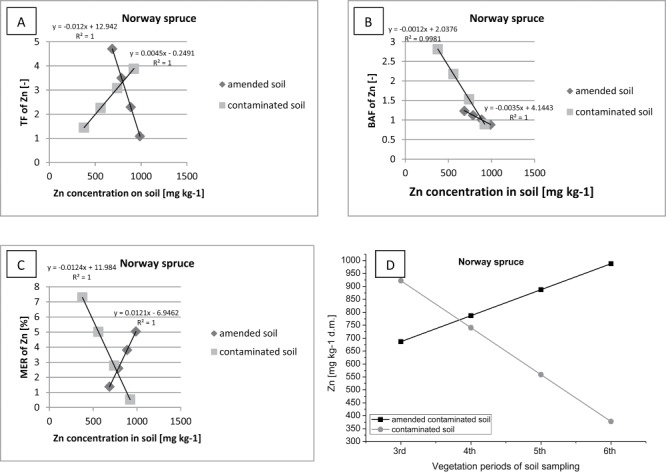
Correlation between (contaminated or amended contaminated) soil Zn concentration and TF of Zn (A), BAF of Zn (B), MER of Zn (C) in Norway spruce.
Figure 14.
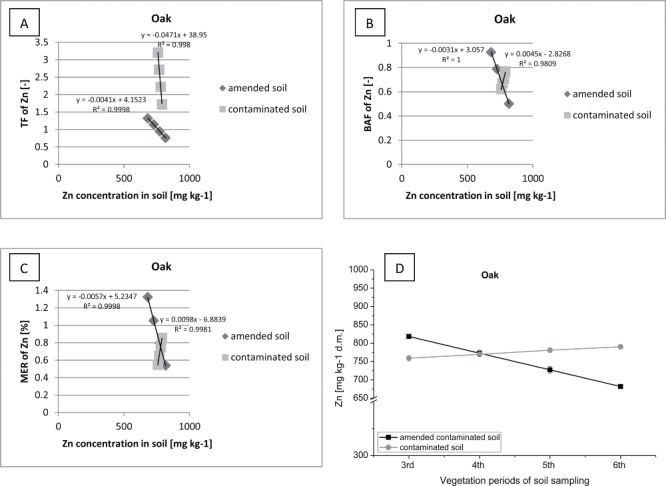
Correlation between (contaminated or amended contaminated) soil Zn concentration and TF of Zn (A), BAF of Zn (B), MER of Zn (C) in oak.
Results and discussion
Influence of trees and sewage sludge on improvement of soil chemical parameters
During the observations, (Figures 3–5) the changes of soil parameters were caused by the introduction into the degraded soil some plants (Scots pine, Norway spruce, oak) and natural fertilizers in the form of sewage sludge.
Figure 3.

Changes of the pH value in H2O (A) and in KCl (B) in contaminated soil and sewage sludge amended soil.
Figure 4.
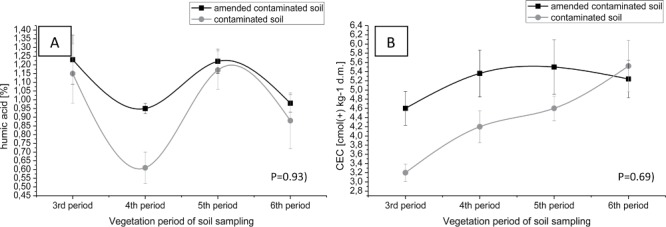
Changes of humic acids (A) and sorption capacity (B) in contaminated soil and sewage sludge amended soil.
Figure 5.
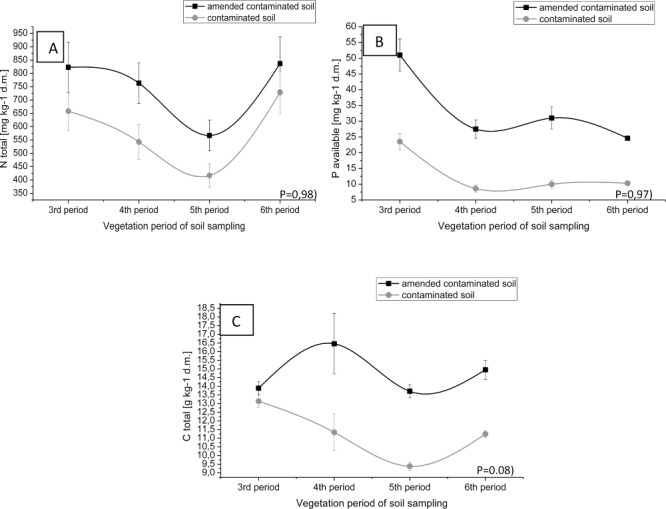
Changes of total N (A), available phosphorus (B) and total carbon (C) in contaminated soil and sewage sludge amended soil.
Initially, the application of sewage sludge to soil contaminated with heavy metals caused a decrease in pH value (Figure 3B). It was observed that the introduction of sewage sludge to soil resulted in a slight acidification of soil, which can contribute to an increase in the mobility of heavy metals in soil (Maddocks et al. 2004; Neczaj et al. 2011; Park et al. 2011). However, with time, with the increase of plant biomass, decomposition and leaching of organic matter (derived from sewage sludge) was observed, as well as a gradual increase in the pH value of the soil. It can therefore be concluded that during the time the experiment was carried out, a gradual increase in the soil pH value due to the application of the sewage sludge was recorded (Figure 1A, Figure 3B). The pH of the degraded soil, as a very important parameter, determines the solubility of many mineral compounds including heavy metals and nutrient elements. The increase in the pH value resulted in less readily soluble forms of trace elements in the ground and confined the possibility of accumulation of heavy metals by plants. Furthermore, increasing pH value prevented the leaching of biogenic components. A congruous increase in the pH value after the application of sewage sludge, was also noticed in other research (Alvarenga et al. 2009; Bramryd 2013; Ferreiro-Domínguez and Rigueiro-Rodríguez et al. 2012; Kacprzak et al. 2014).
The humic acids and the sorption capacity of soil have a protective function in the ground. These parameters are constituents of the soil and are able to form connections with heavy metals, thus influencing the solubility and migration of metallic elements in soil. Humic acids content in the degraded soil, after the addition of sewage sludge, has gradually increased within the field experiment in subsequent years as compared to their initial values in soil (Figure 4A). Moreover, to improve the sorption capacity of the soil, a reference to contaminated soil, was used sewage sludge during the field experiment (Figure 4B). Similar results during scientific experiments, obtained by many other authors (Bramryd 2013; Lo'pez-Dı'az et al. 2007; Park et al. 2011). A higher value of humic acids in soil was also noted in autumn than in spring (Figure 2A). This phenomenon can be explained by a progressive humification of organic matter in soil conditions such as: reduced oxygenation, increased hydration and at a lower soil temperature (in autumn). The addition of sewage sludge into the soil, results in a lower leaching of biogenic elements, facilitating the storage of nutrients and water in the ground. Results confirmed that used sewage sludge is a valuable source of biogenic elements (N total, P available) and C total in the soil supporting plant growth in the contaminated area with heavy metals and enables conducting remediation process of degraded land with the contribution of plants (phytoremediation process) (Figure 5A, 5B, 5C). Similar results obtained Gasco, Kacprzak, Jackson, and McBride (Gasco et al. 2004); Kacprzak et al. 2014; Jackson et al. 1999; McBride 2003). Moreover, the content of selected macroelements (N, C) in soil was higher in autumn than in spring. This phenomenon can be explained by a lower uptake of nutrients and the decrease of photosynthesis process, the accumulation of biomass from tree leaves (oak) in the soil and progressive humification of soil organic matter. However, the results of the experiments confirm the gradual release of macronutrients (N and P) of sewage sludge, fully used by plants to stimulate their growth and development.
Influence of sewage sludge amendment on the cd, pb, and zn concentration in plants
During the field experiment carried out, a considerable biomass increment in the plots fertilized with sewage sludge was observed. In addition, quite high differences in the values of the concentration of heavy metals such as Zn, Cd, and Pb in the Scots pine, Norway spruce, and oak in contaminated soil or amended contaminated soil with sewage sludge were noted.
Influence of sewage sludge amendment on the cd concentration in plants
The correlations between the content of heavy metals in the soil and the amount of Cd accumulated in the tissues of plants (factors: TF, BAF, and MER) are presented (Figure 6–8). During the four growing seasons the phenomenon of translocation and accumulation of Cd in tissues of Scots pine was observed (Figure 6). Intensive Cd translocation (TF >1) from roots to aboveground shoots of pine trees were growing on the plot fertilized with sewage sludge, but much higher value was observed for the control plot. The decrease in the value of TF with decreasing concentrations of Cd in the soil on plot with sewage sludge can be associated with immobilization of little mobile heavy metals by organic fertilizer. At the same time, the decline in the value of TF with an increasing Cd content in the soil, on the control test plot, can be an indication of the pine organism's defensive reaction to the excessive concentration of selected heavy metals in the soil environment. Scots pine can be classified as a species of plants included to the accumulators of Cd (BAF >1), where especially pines were growing on soil fertilized with sewage sludge (BAF > 4), which were characterized by a high growth of biomass. Scots pine has a greater ability for the remediation of degraded land and uptake of heavy metals, while the contaminated area (e.g., with Cd) will be amended with sewage sludge (MER = 3–27). With the increase in the MER value there was also observed a decline of Cd content in the soil, after application of organic fertilizer. In the control plot, a gradual increase in the value of the MER was recorded with increasing content of Cd in the soil. This phenomenon may be indicated as a progressive uptake and storage of the metal in the pine with the slow increasing of tree biomass. In addition, an inversely proportional correlation was observed between the TF value and the value of BAF and MER. With the decrease of the TF value the increase of BAF and MER was noted. This phenomenon has its logical explanation. Intense translocation of metals from roots to shoots of the plant was reduced when the trees stored a large amount of trace elements. The rate of metals translocation was decreased because an increase in the level of accumulation of metals in the shoots was observed. Therefore, Scots pine can be used in the phytoextraction process of degraded soil with an excessive amount of Cd.
Norway spruce has conducted more intensive Cd translocation from roots to shoots compared to the Scots pine, consequently spruce accumulated more heavy metal in the tissues (Figure 7). Simultaneously, an increase in the value of TF with acceleration of Cd content in the soil in the control plot can be linked to intense extraction of metals by plants from the soil, due to the lack of fertilizer addition and the minimum content of nutrient elements in soil, which were necessary for the growth of trees.
Furthermore spruce exhibits a high tendency for accumulation of Cd in their tissues (BAF> 5), especially on ground enriched with organic additives. Over the two years of field study an increase of Cd accumulation was reported in the shoots and needles of spruce associated with a significant decrease in the concentration of the metal in the soil on the plot with sewage sludge. At the same time, on the control plot an increase of BAF was noted with increasing concentration of Cd in the soil. The intensive uptake and immobilization of micronutrients in the organism of spruce, is associated with a deficiency of biogenic elements in the soil. In addition, a higher accumulation and immobilization of Cd was also recorded in the trees on the plot fertilized with sewage sludge (BAF> 5) compared to the control plot (BAF> 4). Differences in retention of Cd in spruce shoots can be associated with a significant increase of biomass per plot after the application of organic additives. Norway spruce, especially grown on soil with the addition of sewage sludge, can be classified as Cd accumulator, and can be use in the process of phytoremediation of soils contaminated with Cd (MER> 32). In addition there are similar relationships occurring between factors TF, BAF, and MER, as in the case of Scots pine. During the two-year period of plant growth in soil fertilised with sewage sludge, there has been a gradual increase in the Cd translocation from roots to shoots of oak with increasing concentration of the metal in soil (Figure 8). Therefore it can be concluded that the oak transfers (TF> 4) and accumulates (BAF> 2) a large amount of selected metals in an organism during the process of growth and development in the soil supplemented with organic additives. Simultaneously, a considerable quantity of Cd was also immobilized in the soil by organic matter in the amended sewage sludge. Oaks were grown on plot without sewage sludge, collected a greater amount of heavy metals from soil, because in the ground were not “competitive” microelements from fertilizers.
Oak has a high affinity for Cd translocation to the above ground parts of plants, especially at increased concentration in soil. However, the oak accumulates a significantly lower amount of Cd in the aboveground parts of the tree, and had much smaller increase in biomass from the pine and spruce (BAF≥ 3). This phenomenon can be associated with a more intense photosynthesis of deciduous trees compared to coniferous trees. However, a significant amount of Cd is accumulated in the leaves of oak, with the risk of recontamination and penetration of heavy metals into the food chain. Therefore oak, due to the showed low tendency to accumulate Cd in the shoots (MER>3.5) should not be used in the process of phytoremediation of Cd contaminated sites and remediation of degraded area. Our results were according to the results of many other authors (Baker and Brooks 1989; Liu et al. 2005; Liu et al. 2006; Marin'o and Morgan 1999; Mertens et al. 2005; Rezvani and Zaefarian 2011; Scragg 2005; Sun et al. 2009; Wu et al. 2010; Zhou and Song 2004; Zhou et al. 2004).
Influence sewage sludge amendment on the pb concentration in plants
Correlations between the content of heavy metal in the soil, and the amount of Pb accumulated in the tissues of plants, are presented in the form of factors: TF, BAF and MER (Figures 9–11). A significant amount of Pb in the soil, when introduced into the sewage sludge, was immobilized by organic matter (Figure 9). Consequently, a slight quantity of Pb, despite the high concentration of this metal in the soil environment, was translocated (Tf> 1.5) and accumulated (BAF>1) in the aboveground parts of pine trees growing on the plot supplemented with organic additive. The highest value of TF and BAF factor were noted in the 3rd vegetation period, and then the value of these factors decreased with a reduction in the concentration of Pb in the soil. In contrast, in the control plot a significant translocation (TF> 4) and accumulation of Pb in the pine tissues (BAF> 2) was observed.
Similar values of MER were recorded in both research plots (MER> 2.5). This fact confirms the thesis about the low mobility of Pb in soil and, consequently, a little extent of penetration of Pb into plant organisms. Therefore, it can be considered that Scots pine can be used in the process of biological remediation of contaminated sites.
During the two-year monitoring period a slight decrease in the value of TF with a low increase in the concentration of Pb in soil fertilized with sewage sludge was observed (Figure 10). The process of Pb uptake (TF> 1.5) from soil by spruce was the most intense in the early stage of growth and development of trees. In contrast, the level of Pb accumulation in the shoots of spruce and the value of BAF factor increased with time and with an increase in the concentration of Pb in the soil on the plot with sewage sludge. Whereas on the control plot without fertilization, the Pb translocation (TF> 7) and accumulation (BAF> 1.5) process was very intense. This phenomenon is associated with the growth of spruce and increased demand and uptake trace elements by plants. Spruce, both grown on a plot with sewage sludge (MER> 5) and on the experimental field without fertilizer (MER> 4), exhibited a high tendency for the extraction of Pb to aerial shoots of trees.
Oaks growing on soil supplemented with sewage sludge showed a slight translocation of Pb from roots to shoots (TF <1) and the accumulation of Pb (BAF> 0.5) due to the low mobility of this metal (Figure 11). However, with the increase of oak biomass and with the increasing demand trees on the trace elements, the values of TF and BAF were increased and the concentration of Pb in soil was decreased. In contrast, on the control plot without fertilization, oaks exhibited high Pb translocation to the above-ground parts of plants (TF <2). The amount of Pb absorbed by trees increased with an increase of the biomass. However, we may suppose that a significant amount of Pb was accumulated in the leaves of oak and then was penetrated into the soil environment as a result of recontamination. The degree of accumulation of Pb in oak shoots was relatively low (BAF < 1). Both oaks, were grown on plot with sewage sludge and oaks grown on the control plot showed a small biomass growth and low levels of Pb extraction to the aboveground shoots (MER < 0.7). On this basis, it can be concluded that oaks should not be used in the phytoremediation process of soils contaminated with high concentrations of Pb in the soil, similar to results reported by many researchers (Efroymson et al. 2001; Ma et al. 2001; McBride 2003; Mertens et al. 2005; Sun et al. 2008; Sun et al. 2009; Rezvani and Zaefarian 2011; Tang et al. 2009; Zhao et al. 2003; Zhao et al. 2006).
Influence of sewage sludge amendment on the zn concentration in plants
The correlation between the content of heavy metals in the soil, and the amount of Zn accumulated in the tissues of plants, are presented in the form of factors: TF, BAF and MER (Figures 12–14). Consequently, during the two years of field study, it was observed an intense translocation of Zn from roots of Scots pine to the aboveground parts (Figure 12). On both of the research plots the TF value has fallen (to a value of about Tf = 1) with a decrease in the concentration of Zn in the soil.
On the plot fertilized with sewage sludge, a decrease in the rate of accumulation of Zn (BAF> 0.8) with a decrease of concentration of this metal in the ground was observed. On the other hand, on a plot without fertilizer there was an increase of BAF factor (BAF > 1) and intensified Zn accumulation. Intensive growth of Scots pine biomass was accompanied by increased Zn uptake and storage of this metal in plants.
Used in the field experiment Scots pine was characterized by a strong ability to translocation and accumulation of Zn in the tissues. Especially plants growing on the plot supplemented with sewage sludge demonstrated a high rate of MER (MER>5). Consequently, Scots pine should be used in the phytoextraction process of soil contaminated with excessive concentration of Zn.
Moreover, the decrease of Zn in the soil, on both research plots, could be linked with leaching and infiltration of this metal into the depths of the soil profile. The mobilization process of Zn in the soil could be compounded by Scots pine root exudation (e.g., organic acids), what is more likely than the mobilization of Zn by the mineralizing organic matter (which was introduced to soil in the form of sewage sludge). Initially, Norway spruce (growing on the fertilized plot) translocated (TF> 4) and accumulated (BAF> 1) a large amount of Zn in the tissues (Figure 13). At the same time, there has been noted a gradual increase in the concentration of Zn in the soil on the plot with sewage sludge. When the process of Zn translocation was stopped, this metal was absorbed and precipitated by organic matter introduced into the soil.
On the other hand, on the research plot, which was left without fertilization, a gradual reduction in the level of translocation of Zn with time was also observed (from TF> 3 to TF> 1). The decrease of the uptake of metals from the soil by Norway spruce, can be a sign of toxic effects of Zn excess, which is a kind of defence mechanism. In contrast, the level of accumulation of Zn in the spruce was gradually increasing (BAF > 2).
Norway spruce, growing on both research plots, stands out by the ability to accumulate Zn in the tissues (MER > 5). Consequently, this tree species may be used with the process of phytoremediation and biological remediation of contaminated soils.
During the monitored phytoremediation process conducted in field conditions, an increase in the translocation of Zn in the oak tissues was observed (Figure 14). This phenomenon has been noted only on a research plot, which was introduced by the addition of sewage sludge. The large growth in the biomass of trees and increased demand for the elements, resulted in an increased translocation (TF > 1) and accumulation (BAF>0.9) of Zn in the oak tissues. The consequence of this was also a reduction in the concentration of Zn in soil on fertilized research plot.
Whereas, on the plot without sewage sludge a decline in translocation of Zn (from TF > 3 to TF > 1) in the organism of oaks and a gradual increase in the concentration (BAF > 0.7) of this metal in the ground was observed. Slowing down the process of Zn uptake by oak and a small increase in the level of immobilization of this metal in the organism of oak, despite the high demand for the elements, may suggest the initiation of a defence mechanism by this species of trees.
Compared to other species of trees, oak demonstrated the least tendency to accumulate Zn in the tissues. This species of tree was the least resistant to adverse environmental conditions and an excessive concentration of Zn in the soil. Consequently, oak is not recommended to use in the process of phytoextraction of Zn. The more that Oak is a species of deciduous trees and there is a high risk of re-contamination of the soil environment with heavy metals (autumn leaves fall). Our findings were also agreed with other authors (McBride et al. 2000; McBride 2003; Richards et al. 2000; Stacey et al. 2001).
Conclusions
On the plot fertilized with sewage sludge, the proper growth of plants and large increase in biomass were noted. Sewage sludge used in this experiment is of considerable importance in the phytoremediation process of soils contaminated with heavy metals (Cd, Zn, and Pb). The amendment of sewage sludge and use of trees species: Scots pine, Norway spruce, oak, has improved many soil properties. The increase in the pH value resulted in less readily soluble forms of trace elements in the ground and confined the possibility of accumulation of heavy metals by plants. Furthermore, increasing the pH value prevented the leaching of biogenic components. Application of sewage sludge from the food industry to soil contributed to the increase in humic acids and the sorption capacity of the soil, and improvement of the protective buffering functions of the soil, contributing in this way to reduce the leaching of trace metals and biogenic elements.
Moreover, the used amendment showed the beneficial fertilizing properties and entered some mineral compounds what effectively stimulated the plants growth and development.
Conducted studies have shown that the addition of sludge caused the sorption of metals in the soil, mainly the addition of sewage sludge to contaminated soil contributed to the sorption of heavy metals within organic matter. The application of sewage sludge to the soil creates conditions for plant cover restoration, which protects against sudden metals migrating into the soil profile as well as wind erosion, and this phenomena is confirmed by the absence of growth of roots on untreated soil. The monitoring of soil solution confirmed that the content and mobility of biogenic elements and heavy metals in the soil and from soil to soil solution is dependent on the seasonal processes of humification and mineralization of organic matter. Plants, grown on plot without sewage sludge, collected a greater amount of heavy metals from soil, because in the ground were not “competitive” microelements from fertilizers. The intensive uptake and immobilization of micronutrients in the organism of trees, is associated with a deficiency of biogenic elements in the soil. Coniferous trees have a greater ability for the remediation of degraded land and uptake of heavy metals, while the contaminated area (e.g., with Cd) will be introduced by sewage sludge (MER). With the decrease of the TF value increased value of BAF and MER. It phenomenon has its logical explanation. Intense translocation of metals from roots to shoots of the plant was reduced when the trees stored a large amount of trace elements. The rate translocation of metals was decreased, because the increasing the level of accumulation of metals in shoots was observed. Therefore, coniferous trees can be used in the phytoextraction process of degraded soil with an excessive amount of Cd.
A significant amount of Pb in the soil, when introduced into the sewage sludge, was immobilized by organic matter. Consequently, a slight quantity of Pb, despite the high concentration of this metal in the soil environment, was translocated and accumulated in the aboveground parts of pine trees growing on the plot supplemented with organic additive. Similar values of MER were recorded in both research plots. This fact confirms the thesis about the low mobility of Pb in soil and consequently a small extent of penetration of Pb into plant organisms. However, the recommended using of this plant species is phytostabilization of selected heavy metals in soil.
In particular, trees that were growing on the plot supplemented with sewage sludge from the food industry demonstrated a high rate MER. Consequently, coniferous trees should be used in the phytoextraction process and biological remediation of soil contaminated with an excessive concentration of Zn. Compared to other species of trees (Scots pine, Norway spruce), Oak demonstrated the least tendency to accumulate Zn in their tissues. This species of tree was the least resistant to adverse environmental conditions and excessive concentration of Zn in the soil. Consequently, Oak is not recommended to be used in the process of phytoextraction of Zn. Moreover, the decrease of Zn in the soil, on both research plots, could be linked with leaching and infiltration of this metal into the depths of the soil profile. Zn is a very mobile metal, therefore this hypothesis is becoming probable, especially in the plot that was left without fertilization. The escape process of Zn in the soil could be compounded by the trees roots exudation (e.g., organic acids), because there has not been recorded the immobilization of Zn by organic matter (which was introduced to soil in the form of sewage sludge). The conducted field experiment demonstrated that selected trees like Scots pine and Norway spruce, because of its excellent adaptability, can be used in the remediation of soil and of soilless devastated areas, such as pioneering plants. Oaks should not be used in the phytoremediation process of soils contaminated with high concentrations of trace elements in the soil, because a significant amount of heavy metals were accumulated in the leaves of oak and were then penetrated into the soil environment as a result of recontamination.
Acknowledgments
“The research leading to these results has received funding from the Polish-Norwegian Research Programme operated by the National Centre for Research and Development under the Norwegian Financial Mechanism 2009-2014 in the frame of Project Contract No (POL NOR/201734/76)”
References
- Adriano D. Trace elements in terrestrial environments: biogeochemistry, bioavailability and risk of metals. New York: NY: Springer Verlag; 2001. [Google Scholar]
- Alkorta I, Hernandez-Allica J, Becerril JM, Amezaga I, Albizu I, Gabisu C. Recent findings on the phytoremediation of soils contaminated with environmentally toxic heavy metals and metalloids such as zinc, cadmium, lead and arsenic. Environmental Science and Bio/Technology. 2004;3:71–90. [Google Scholar]
- Alvarenga P, Goncalves AP, Fernandes RM, de Varennes A, Duarte E, Cunha-Queda AC, Vallini G. Reclamation of a mine contaminated soil using biologically amended soil. Aus J Soil Res. 2009;48:459–469. doi: 10.1177/0734242X08091556. [DOI] [PubMed] [Google Scholar]
- An YJ. Soil ecotoxicity assessment using cadmium sensitive plants. Environmental Pollution. 2004;127:21–26. doi: 10.1016/s0269-7491(03)00263-x. [DOI] [PubMed] [Google Scholar]
- Soil quality - determination of pH. PN ISO, 10390: 1997 Polish Committee for Standardization 1997 Anonymous. [Google Scholar]
- Baker AJM, Brooks RR. Terrestrials higher plants chich hyper accumulate metallic elements. A review of their distribution, ecology and phytochemistry. Biorecovery. 1989;1:81–126. [Google Scholar]
- Blaylock MJ, Huang JW. Phytoextraction of Metals. In: Raskin I, Ensley BD, editors. Phytoremediation of Toxic Metals: Using Plants to Clean up the Environment. New York: NY: John Wiley & Sons, Inc. Publishing; 2000. pp. 53–70. [Google Scholar]
- Bramryd T. Long-term effects of sewage sludge application on the heavy metal concentrations in acid pine (Pinus sylvestris L.) forests in a climatic gradient in Sweden. Forest Ecology and Management. 2013;289:434–444. [Google Scholar]
- Calace N, Petronio BM, Pietroletti M. Metal bioavailability: how does its significance change in the time? Ann Chim. 2006;96:131–136. doi: 10.1002/adic.200690013. [DOI] [PubMed] [Google Scholar]
- Cluis C. Junk-greedy greens: phytoremediation as a new option for soil decontamination. Biotech J. 2004;2:60–67. [Google Scholar]
- Cunningham SD, Berti WR, Huang JW. Phytoremediation of contaminated soils. TIBTECH. 1995;13:393–397. [Google Scholar]
- Dimkpa CO, Merten D, Svatos A, Büchel G, Kothe E. Metal-induced oxidative stress impacting plant growth in contaminated soil is alleviated by microbial siderophores. Soil Biology & Biochemistry. 2009;41:154–162. [Google Scholar]
- Dzugan M, Pakla M, Pasternakiewicz A, Grabek-Lejko D. Proceeding Book of X International Scientific Conference “RISK FACTORS OF FOOD CHAIN.”. SUA in Nitra; Nitra: 2010. Photochemiluminescent detection of oxidate stress in Lepidum Sativum L. exsposed to cadmium and zinc; pp. 62–67. [Google Scholar]
- Efroymson RA, Sample BE, Suter GW. Uptake of inorganic chemicals from soil by plant leaves: regressions of field data. Environ Toxicol Chem. 2001;20:2561–2571. doi: 10.1897/1551-5028(2001)020<2561:uoicfs>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Fayiga AO Ma LQ. Using phosphate rock to immobilize metals in soil and increase arsenic uptake by hyperaccumulator Pteris vittata. Sci Total Environ. 2006;359(1-3):17–25. doi: 10.1016/j.scitotenv.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Ferreiro-Domínguez N, Rigueiro-Rodríguez A, Mosquera-Losada MR. Sewage sludge fertiliser use: Implications for soil and plant copper evolution in forest and agronomic soils. Science of the Total Environment. 2012;424:39–47. doi: 10.1016/j.scitotenv.2012.02.042. [DOI] [PubMed] [Google Scholar]
- Fijałkowski K, Kacprzak M, Grobelak A, Placek A. The influence of selected soil parameters on the mobility of heavy metals in soil. Engineering and Environmental Protection. 2012;15:81–92. [Google Scholar]
- Gang W, Hubiao K, Xiaoyang Z, Hongbo S, Liye C, Chengjiang R. A critical review on the Bio-removal of hazardous heavy metals from contaminated soils: Issues, progress, Eco-environmental concerns and opportunities. Journal of Hazardous Materials. 2010;174:1–8. doi: 10.1016/j.jhazmat.2009.09.113. [DOI] [PubMed] [Google Scholar]
- Gasco G., Martinez-Inigo M., Lobo M. Soil organic matter transformation after a sewage sludge application, Electronic Journal of Environmental Agricultural and Food Chemistry, 3, pp. 2004:716–722. [Google Scholar]
- Grobelak A, Kacprzak M, Fijałkowski K. Phytoremediation - the underestimated potential of plants in the environment cleaning up. J Ecol Health. 2010;6:276–280. [Google Scholar]
- Hirt H, Shinozaki K. Plant Responses to Abiotic Stress. Berlin. Heidelberg: Germany: Springer-Verlag; 2004. [Google Scholar]
- Jabeen R, Ahmad A, Iqbal M. Phytoremediation of Heavy Metals: Physiological and Molecular Mechanisms. The Botanical Review. 2009;75:339–364. [Google Scholar]
- Jackson BP, Miller WP, Schumann AW, Sumner ME. Trace element solubility from land application of fly ash/organic waste mixtures. J Environ Quali. 1999;28:639–647. [Google Scholar]
- Kacprzak M, Grobelak A, Grosser A, Prasad MNV. Efficacay of Biosolids in Assisted Phytostabilization of Metalliferous Acidic Sandy SoilS with Five Grass Species. Int J Phytoremediation. 2014;16:593–608. doi: 10.1080/15226514.2013.798625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karczewska A, Kabała C. Metodyka analiz laboratoryjnych gleb i roślin, Instytut Gleboznawstwa i Ochrony Środowiska Rolniczego, Wrocław 2008 [Google Scholar]
- Liang H-M, Lin T-H, Chiou J-M, Yeh K-C. Model evaluation of the phytoextraction potential of heavy metal hyperaccumulators and non-hyperaccumulators. Environmental Pollution. 2009;157:1945–1952. doi: 10.1016/j.envpol.2008.11.052. [DOI] [PubMed] [Google Scholar]
- Liu X, Hu C, Zhang S. Effects of earthworm activity on fertility and heavy metal bioavailability in sewage sludge. Environment International. 2005;31:874–879. doi: 10.1016/j.envint.2005.05.033. [DOI] [PubMed] [Google Scholar]
- Liu DH, Wang M, Zou JH, Jiang WS. Uptake and accumulation of cadmium and some nutrient ions by roots and shoots of maize (Zea mays L.) Pak J Bot. 2006;38:701–709. [Google Scholar]
- Lopez-Diaz ML, Riguerio-Rodri'guez A, Mosquera-Losada MR. Influence of pasture botanical composition and fertilization treatments on tree growth. Forest Ecology and Management. 2009;257:1363–1372. [Google Scholar]
- Lynch J, Moffat A. Bioremediation - prospects for the future application of innovative applied biological research. Annuals of App Biol. 2005;14:217–221. [Google Scholar]
- Ma LQ, Komar KM, Tu C, Zhang W, Cai Y, Kenelly ED. A fern that hyper accumulates arsenic. Nature. 2001;409:579–582. doi: 10.1038/35054664. [DOI] [PubMed] [Google Scholar]
- Marin'o F, Morgan AJ. The time-course of metal (Ca, Cd, Cu, Pb, Zn) accumulation from a contaminated soil by three populations of the earthworm Lumbricus rubellus . Appl Soil Ecol. 1999;12:169–177. [Google Scholar]
- Maddocks G, Lin C, McConchie D. Effects of Bauxsol and biosolids on soil conditions of acid-generating mine spoil for plant growth. Environ Pollut. 2004;127:1327–1337. doi: 10.1016/j.envpol.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Matthews DJ, Moran BM, Otte ML. Screening the wetland plant species Alisma plantago-aquatica, Carex rostrata and Phalaris arundinacea for innate tolerance to zinc and comparison with Eriophorum angustifolium and Festuca rubra Merlin. Environmental Pollution. 2005;134:343–351. doi: 10.1016/j.envpol.2004.07.023. [DOI] [PubMed] [Google Scholar]
- McBride MB, Richards BK, Steenhuis T, Spiers G. Molybdenum uptake by forage crops grown on sewage sludge-amended soils in the field and greenhouse. J Environ Qual. 2000;29:848–854. [Google Scholar]
- McBride MB. Toxic metals in sewage sludge-amended soils: has promotion of beneficial use discounted the risk? Adv Environ Res. 2003;8:5–19. [Google Scholar]
- Mench M, Bussière S, Boisson-Gruppen J, Castaing E, Vangronsveld J, Ruttens A. Progress in remediation and revegetation of the barren Jales gold mine spoil after in situ treatments. Plant Soil. 2003;249:187–202. [Google Scholar]
- Mertens J, Luyssaert S, Verheyen K. Use and abuse of trace metal concentrations in plants tissue for biomonitoring and phytoextraction. Environ Pollut. 2005;138:1–4. doi: 10.1016/j.envpol.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Neczaj E, Bień JB, Grosser A, Worwag M, Kacprzak M. Anaerobic treatment of sewage sludge and grease trap sludge in continuous co-digestion. Global Nest Journal. 2011;14:141–148. [Google Scholar]
- Nouri J, Khorasani N, Lorestani B, Karami M, Hassani AH, Yousefi N. Accumulation of heavy metals in soil and uptake by plant species with phytoremediation potential. Environ Earth Sci. 2009;59:315–323. [Google Scholar]
- Padmavathiamma PK, Li LY. Phytoremediation Technology: Hyper-accumulation Metals in Plants. Water Air Soil Pollut. 2007;184:105–126. [Google Scholar]
- Park JH, Lamb D, Paneerselvam P, Choppala G, Bolan N, Chung JW. Role of organic amendments on enhanced bioremediation of heavy metal(loid)contaminated soils. J Hazard Mater. 2011;185:549–574. doi: 10.1016/j.jhazmat.2010.09.082. [DOI] [PubMed] [Google Scholar]
- Prasad MNV, Freitas H. Metal hyperaccumulation in plants 5 Biodiversity prospecting for phytoremediation technology. Electronic Journal of Biotechnology. 2003;6:275–321. [Google Scholar]
- Reevers RD, Baker A, Becquer T, Echewvarria G, Miranda ZJG. The flora and biogeochemistry of the ultramafic soils of Goia's State. Brazil Plant and Soil. 2007;293:107–119. [Google Scholar]
- Rezvani M, Zaefarian F. Bioaccumulation and translocation factors of cadmium and lead in Aeluropus littoralis . Australian Journal of Agricultural Engineering. 2011;2:114–119. [Google Scholar]
- Richards BK, Steenhuis TS, Peverly JH, McBride MB. Effect of sludge processing mode, soil texture and soil pH on metal mobility in undisturbed soil columns under accelerated loading. Environ Pollut. 2000;109:327–346. doi: 10.1016/s0269-7491(99)00249-3. [DOI] [PubMed] [Google Scholar]
- Sanchez-Monedero MA, Mondini C, de Nobili M, Leita L, Rolg A. Land application of biosolids. Soil response to different stabilization degree of the treated organic matter. Waste Management. 2004;24:325–332. doi: 10.1016/j.wasman.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Scragg A. Environmental Biotechnology. New York: NY: Oxford University Press; 2005. [Google Scholar]
- Stacey S, Merrington G, McLaughlin MJ. The effect of aging biosolids on the availability of cadmium and zinc in soil. Eur J Soil Sci. 2001;52:313–321. [Google Scholar]
- Stevenson FJ. 1994 Humus Chemistry, Genesis, Composition, Reactions, New York: John Wiley&Sons. [Google Scholar]
- Sun YB, Zhou QX, Diao CY. Effects of cadmium and arsenic on growth and metal accumulation of Cdhyperaccumulator Solanum nigrum L. Bioresource Tech. 2008;99:1103–1110. doi: 10.1016/j.biortech.2007.02.035. [DOI] [PubMed] [Google Scholar]
- Sun YB, Zhou QX, Wang L, Liu W. Cadmium tolerance and accumulation characteristics of Bidens pilosa L. as a potential Cd-hyperaccumulator. J Hazar Mater. 2009;161:808–814. doi: 10.1016/j.jhazmat.2008.04.030. [DOI] [PubMed] [Google Scholar]
- Tang YT, Rong-Liang Qiu RL, Zenga JW, Ying RR, Yu FM, Zhou XY. Lead, zinc, cadmium hyperaccumulation and growth stimulation in Arabis paniculata Franch. Environ Exp Bot. 2009;66:126–134. [Google Scholar]
- Wei S, Teixeira da Silva JA, Zhou Q. Agro-improving method of phytoextracting heavy metal contaminated soil. Journal of Hazardous Materials. 2008;150:662–668. doi: 10.1016/j.jhazmat.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Wu G, Kang H, Zhang X, Shao H, Chu L, Ruan C. A critical review on the bio-removal of hazardous heavy metals from contaminated soils: Issues, progress, eco-environmental concerns and opportunities. Journal of Hazardous Materials. 2010;174:1–8. doi: 10.1016/j.jhazmat.2009.09.113. [DOI] [PubMed] [Google Scholar]
- Zhang WH, Cai Y, Tu C, Ma QL. Arsenic speciation and distribution in an arsenic hyperaccumulating plant. Sci Environ. 2002;300:167–177. doi: 10.1016/s0048-9697(02)00165-1. [DOI] [PubMed] [Google Scholar]
- Zhang X, Xia H, Li Z, Zhuang P, Gao B. Potential of four grasses in remediation of Cd and Zn contaminated soils. Bioresour Technol. 2010;101:2063–2066. doi: 10.1016/j.biortech.2009.11.065. [DOI] [PubMed] [Google Scholar]
- Zhao FJ, Lombi E, McGrath SP. Assessing the potential for zinc and cadmium phytoremediation with the hyperaccumulator Thlaspi caerulescens . Plant and Soil. 2003;249:37–43. [Google Scholar]
- Zhao FJ, Jiang RF, Dunham SJ, McGrath SP. Cadmium uptake, translocation and tolerance in the Hyperaccumulator Arabidopsis halleri . NewPhytol. 2006;172:646–654. doi: 10.1111/j.1469-8137.2006.01867.x. [DOI] [PubMed] [Google Scholar]
- Zhou QX, Song YF. Principles and Methods of Contaminated Soil Remediation. Bejing: China: Chin Environ Sci Press; 2004. [Google Scholar]
- Zhou QX, Wei SH, Zhang QR. Ecological Remediation. Bejing: China: Chin Environ Sci Press; 2004. [Google Scholar]


