Abstract
Objective: Correct inhaler technique is central to effective delivery of asthma therapy. The study aim was to identify factors associated with serious inhaler technique errors and their prevalence among primary care patients with asthma using the Diskus dry powder inhaler (DPI). Methods: This was a historical, multinational, cross-sectional study (2011–2013) using the iHARP database, an international initiative that includes patient- and healthcare provider-reported questionnaires from eight countries. Patients with asthma were observed for serious inhaler errors by trained healthcare providers as predefined by the iHARP steering committee. Multivariable logistic regression, stepwise reduced, was used to identify clinical characteristics and asthma-related outcomes associated with ≥1 serious errors. Results: Of 3681 patients with asthma, 623 (17%) were using a Diskus (mean [SD] age, 51 [14]; 61% women). A total of 341 (55%) patients made ≥1 serious errors. The most common errors were the failure to exhale before inhalation, insufficient breath-hold at the end of inhalation, and inhalation that was not forceful from the start. Factors significantly associated with ≥1 serious errors included asthma-related hospitalization the previous year (odds ratio [OR] 2.07; 95% confidence interval [CI], 1.26–3.40); obesity (OR 1.75; 1.17–2.63); poor asthma control the previous 4 weeks (OR 1.57; 1.04–2.36); female sex (OR 1.51; 1.08–2.10); and no inhaler technique review during the previous year (OR 1.45; 1.04–2.02). Conclusions: Patients with evidence of poor asthma control should be targeted for a review of their inhaler technique even when using a device thought to have a low error rate.
Keywords: Asthma therapy, cross-sectional, Diskus inhaler, inhalation devices, multinational
Introduction
The prevalence of uncontrolled asthma has remained at approximately 50% in recent European surveys despite the availability of effective therapies [1–3], with patient errors in using their inhaler devices likely playing a role [3,4]. Proper inhaler technique is an important component of effective asthma therapy: bronchodilators and inhaled corticosteroids (ICS) are delivered via a variety of inhaler devices, each with its specific dose preparation and handling technique, advantages and disadvantages [4–7].
Data derived from randomized controlled trials (RCTs) indicate that clinical outcomes do not differ significantly among inhaler devices; however, these studies typically include only highly trained patients with correct inhalation technique [6–9]. Indeed, meta-analyses of observational studies and RCTs report that any inhaler device type is similarly effective as long as the patient is able to use it correctly and there is drug remaining in the device [7,10]. However, findings from observational studies indicate that patients with obstructive lung disease commonly misuse inhalers in clinical practice [11–16], and poor inhaler technique is associated with worse clinical outcomes [16–20].
The correct use of an inhaler involves device-specific dose preparation steps followed by the inhalation pattern appropriate to the device. The ideal pattern with a dry powder inhaler (DPI) is a full exhalation (ideally to residual volume), then a rapid and forcible inhalation (also described as “deep and hard as you can”), followed by a breath-hold for 10 s or as long as possible [5]. Unlike standard pressurized metered-dose inhalers (pMDIs), DPIs are breath-actuated so they obviate the need to co-ordinate actuation and inhalation [5]. It has been suggested that some patients experiencing an asthma exacerbation could be unable to generate the forcible inhalation required with DPIs [21]. However, the Diskus DPI (also known as the Accuhaler) is designed to facilitate ease of use, and there is some evidence to suggest that, of the DPIs, this device is associated with fewest inhaler technique errors [14,22–24]. In a randomized study of patients with obstructive lung disease who were hospitalized for an exacerbation, the highest number of optimum inhalation profiles were observed with the Diskus (100%), followed by a pMDI with a Volumatic spacer (87%), and the lowest number with MDIs alone (14%) [24].
The global Helping Asthma in Real People (HARP) project seeks to understand and address the reasons for uncontrolled asthma [25]. The HARP inhaler technique assessment initiative (iHARP) is an extension of HARP that incorporates an assessment of potential handling errors and an appraisal of patient inhalation profile against device-specific thresholds in addition to the standard review [26,27]. Inhaler use has been reviewed for 5000 patients since the launch of iHARP in June 2011. The aim of this study was to use iHARP data to identify factors associated with serious inhaler technique errors, as observed by healthcare providers (HCPs), and their prevalence among primary care patients with asthma using the Diskus dry powder inhaler.
Methods
Data source
This cross-sectional observational study used anonymized patient data from the iHARP database, an international database administered by the Respiratory Effectiveness Group [28] comprising anonymized data from practices receiving the iHARP asthma review service [26,27]. Data for the study were collected from June 2011 to November 2013; permission to use the data for research was obtained from primary care practices in Australia and seven European countries (the United Kingdom [UK], Italy, Spain, the Netherlands, France, Norway and Sweden). The data included patient demographic characteristics, respiratory reviews by trained HCPs and results of asthma questionnaires completed by patients on the day of the iHARP asthma review. For patients assessed within the UK, these data were linked to anonymized clinical, diagnostic and prescribing information from electronic medical records.
Each of the participating centers obtained appropriate ethics approval for the iHARP asthma review service based on their country-specific requirements. This study was registered with the European Network of Centers for Pharmacoepidemiology and Pharmacovigilance (as ENCePP/SDPP/8020) [29].
Cohort definition
The cohort drawn from the iHARP database for the current study was restricted to adult patients (≥18 years old) who were receiving fixed-dose combination ICS/LABA therapy delivered by a Diskus device.
Patients eligible for iHARP respiratory review, an ongoing international initiative, are 16 years and older, have a current diagnosis of asthma and have received two or more prescriptions for fixed-dose combination ICS/long-acting beta-agonist (LABA) therapy in the year before the review [26,27]. Patients are excluded from iHARP if they have a diagnosis of chronic obstructive pulmonary disease (COPD) or any chronic respiratory disease other than asthma, or if they are prescribed separate ICS in addition to fixed-dose combination ICS/LABA, are receiving long-term systemic treatment for asthma (e.g. maintenance oral corticosteroids, theophylline, leukotriene receptor antagonists or anti-IgE therapy), or had received oral corticosteroids and/or antibiotics for a lower respiratory condition in the 2 weeks before respiratory review.
Study data and definitions
Demographic and clinical characteristics were obtained from the iHARP database (see Supplementary material for further details). All patients were asked whether their inhaler technique had been reviewed in the previous year by an HCP, and patients were asked to self-assess their inhaler technique using a Likert scale with scores ranging from 1 (“I think my inhaler technique is very poor”) to 6 (“I think my inhaler technique is excellent”).
Self-reported adherence to asthma therapy was assessed for patients from the UK, Italy, Spain, Australia, Sweden, France and Norway using the Medication Adherence Rating Scale (MARS) [30]. Adherence to asthma therapy for patients from the Netherlands was assessed using patients’ answers to the question, “Do you ever forget your prevention inhalation medication?” Adherence was categorized as (1) poor for responses of “very often” or “always”; (2) borderline for responses of “now and then” or “regularly” and (3) good for responses of “never” and “rarely.”
The Global INitiative for Asthma (GINA) criteria [31] were used to assess asthma control during 1 week preceding the clinic visit; and the Asthma Therapy Assessment Questionnaire (ATAQ) [32] was used to assess asthma control during the preceding 4 weeks (see Supplementary material for details).
The numbers of patient-reported acute courses of oral corticosteroids, asthma-related hospitalizations and severe asthma exacerbations in the prior year were recorded during the iHARP review on the patient-completed questionnaire. Severe exacerbations were defined as patients having had either an asthma-related hospitalization or a course of oral corticosteroids (as defined above) in the prior year.
Study endpoint: serious inhaler technique errors
Serious inhaler technique errors identified by the HCPs were defined as errors potentially limiting drug uptake to the lungs, as enumerated by the iHARP steering committee before commencing the study. Table 1 depicts the checklist of pre-defined serious errors for the Diskus device.
Table 1. Checklist of predefined serious errors for the Diskus device.
| Does not slide cover as far as possible | |
| Does not slide lever fully to open mouthpiece | |
| Holds in a downward position after dose preparation (before an inhalation) | |
| Shakes after dose preparation | |
| Blowing into the device | |
| Failure to exhale before inhalation | |
| Failure to put in mouth and seal lips around mouthpiece | |
| Failure to inhale through mouthpiece | |
| Inhalation through the nose | |
| Inhalation is not forceful from the start | |
| No breath-hold for at least 3 s | |
| Does not prepare second dose correctly | |
| Does not correctly inhale second dose as above | |
| Does not know when his or her device is empty |
The study HCPs – physicians in Italy, Norway and Spain, pharmacists in Australia, and nurses in the UK, the Netherlands, Sweden and France – were trained and tested to identify serious inhaler errors by watching a standardized instructional video. Eligible patients were invited to respiratory reviews at their primary care practices, during which the HCPs observed patients using their inhaler once, or twice if a second dose were required, and recorded serious errors against the checklist (Table 1).
Statistical analyses
Analyses were performed with SPSS Statistics versions 19 and 21 (IBM SPSS Statistics, Feltham, Middlesex, UK), SAS version 9.3 (SAS Institute, Marlow, Buckinghamshire, UK) and Microsoft Excel 2007 (Microsoft Corporation, Redmond, Washington). Statistically significant results were defined as a p value of ≤0.05.
Characteristics of patients who made no serious errors using a Diskus device were compared with those of patients making one or more (≥1) serious errors. Variables measured on the interval or ratio scale were compared using Student’s t-test for normally distributed data and the Mann–Whitney U-test for skewed data. Categorical variables were compared using the χ 2 test.
Univariable logistic regression models, with a dichotomous indicator variable for serious errors made (yes/no) as the dependent variable and each patient characteristic as an explanatory (or predictor) variable, were first used to identify characteristics associated with making serious errors. Demographic and clinical characteristics associated with making ≥1 serious errors in the univariable model (p < 0.05) were then entered into a multivariable model, which was stepwise reduced to produce a final list of non-collinear independently associated variables. Significant interactions were defined as p value <0.10. A list of demographic and clinical characteristics included in the univariable model is in the Supplementary material.
Results
Patients
There were 3681 patients with asthma in the iHARP database who had a respiratory review between June 2011 and November 2013. Of these patients, 627 (17%) were using a Diskus inhaler. After excluding four (0.5%) patients aged <18 years, the final study cohort included 623 adult patients with asthma using Diskus who were from the UK (232 [37%]), Italy (149 [24%]), Spain (102 [16%]), the Netherlands (83 [13%]), Australia (37 [6%]), France (8 [1%]), Norway (5 [1%]) and Sweden (7 [1%]). The final study cohort comprised 61% women and had a mean (SD) age of 51 (14) years (Table 2). Approximately three quarters of patients had recorded lung function results; of these, 69% had a percent predicted forced expiratory volume in 1 s (FEV1) or peak expiratory flow (PEF) that was >80% (Supplemental Table S1).
Table 2. Patient characteristics grouped by frequency of serious inhaler technique error (0 versus ≥1).
| Characteristic | Total N (%) (n = 623) | 0 errors (n = 282) | ≥1 errors (n = 341) | p value |
|---|---|---|---|---|
| Age, mean (SD) | 51 (14) | 50 (15) | 51 (14) | 0.84a |
| 18–30 years, n (%) | 66 (11) | 36 (13) | 30 (9) | |
| 31–50 years, n (%) | 219 (35) | 94 (33) | 125 (37) | |
| 51–70 years, n (%) | 313 (50) | 140 (50) | 173 (51) | |
| >70 years, n (%) | 25 (4) | 12 (4) | 13 (4) | |
| Sex, female, n (%) | 380 (61) | 159 (56) | 221 (65) | 0.032b |
| male | 243 (39) | 123 (44) | 120 (35) | |
| Body mass indexc, n (%) | ||||
| Underweight | 8 (1) | 4 (1) | 4 (1) | 0.036b |
| Normal | 214 (34) | 107 (38) | 107 (31) | |
| Overweight | 212 (34) | 102 (36) | 110 (32) | |
| Obese | 189 (30) | 69 (25) | 120 (35) | |
| Smoking status, n (%) | ||||
| Current smoker | 78 (13) | 28 (10) | 50 (15) | 0.179b |
| Ex-smoker | 189 (30) | 91 (32) | 98 (29) | |
| Non-smoker | 356 (57) | 163 (58) | 193 (57) | |
| Educationd, known status, n (%) | (n = 499 [80]) | (n = 216) | (n = 283) | |
| PG or professional degree | 11 (2) | 4 (2) | 7 (3) | 0.006b |
| University degreee | 157 (31) | 80 (37) | 77 (27) | |
| Secondary educatione | 207 (41) | 88 (41) | 119 (42) | |
| Primary educatione | 100 (20) | 39 (18) | 61 (22) | |
| None | 24 (5) | 5 (2) | 19 (7) | |
Percentages are column percentages. BMI, body mass index; PG, postgraduate; SD, standard deviation.
aMann–Whitney U test.
b χ 2 test.
cUnderweight BMI < 18.5 kg/m2; normal BMI = 18.5–24.99 kg/m2; overweight BMI = 25–29.99 kg/m2; obese BMI ≥ 30 kg/m2.
dErrors by education reported as n (%) of known status.
eCompleted or some education.
Serious errors and characteristics of patients making serious errors
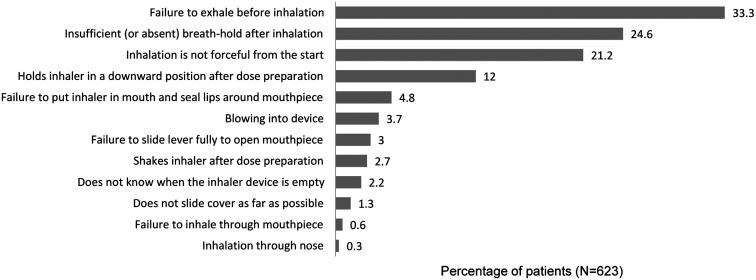
Fifty-five percentage of patients made from 1 to 10 serious errors, most commonly one (157 [25%]), two (92 [15%]) or three errors (55 [9%]); 37 patients (6%) made four or more errors. The most frequent errors were the failure to exhale before inhalation, insufficient (or absent) breath-hold at the end of inhalation and inhalation that was not forceful from the start (Figure 1).
Figure 1.
Percentage of patients making each type of serious inhaler error with the Diskus.
Patients making ≥1 serious errors were significantly more likely to be female, obese or lacking a university degree than those making no error (Table 2). Age, smoking status, the duration of asthma, the prevalence and severity of rhinitis and lung function test results were not significantly different between patients making 0 and ≥1 serious errors (Table 2, Supplemental Table S1). Patient-reported adherence to therapy did not differ between patients making 0 and ≥1 serious errors (Table 3).
Table 3. Clinical characteristics grouped by serious inhaler error category (0 versus ≥ 1).
| Clinical characteristic | Total N (%) (n = 623) | 0 error (– = 282) | ≥1 errors (n = 341) | p value | |
|---|---|---|---|---|---|
| Patient-reported prior inhaler review by HCPb, n (%) | 297 (48) | 150 (53) | 147 (43) | 0.012 | |
| Patient self-assessment of inhaler technique, n (%), known | (n = 588 [94]) | 270 (96) | 318 (93) | ||
| Very poor to poor | 24 (4) | 11 (4) | 13 (4) | 0.024 | |
| Fair to average | 122 (21) | 43 (16) | 79 (25) | ||
| Good to excellent | 442 (75) | 216 (80) | 226 (71) | ||
| ATAQ asthma control, n (%), known | (n = 622 [100]) | 282 (100) | 340 (99.7) | ||
| Poor control | 137 (22) | 47 (17) | 90 (27) | 0.012 | |
| Partial control | 469 (75) | 228 (81) | 241 (71) | ||
| Good control | 16 (3) | 7 (3) | 9 (3) | ||
| Adherence to therapyc, n (%) | |||||
| Poor | 202 (37) | 84 (34) | 118 (40) | 0.087 | |
| Borderline | 18 (3) | 5 (2) | 13 (4) | ||
| Good | 320 (59) | 156 (64) | 164 (56) | ||
| Adherence to therapy in the Netherlandsd, n (%), known | (n = 59 [71]) | 26 (70.3) | 33 (72) | ||
| Poor | 2 (3) | 2 (7.7) | 0 (0) | 0.12 | |
| Borderline | 0 | 0 (0) | 0 (0) | ||
| Good | 57 (97) | 24 (92.3) | 33 (100) | ||
| Acute courses of oral corticosteroidsb, n (%) | |||||
| 0 | 380 (61) | 183 (65) | 197 (58) | 0.13 | |
| 1 | 139 (22) | 58 (21) | 81 (24) | ||
| 2 | 59 (10 | 27 (10) | 32 (9) | ||
| ≥3 | 45 (7) | 14 (5) | 31 (9) | ||
| Severe exacerbationsb,e, n (%), known | (n = 622 [100]) | 282 (100) | 340 (99.7) | ||
| 0 | 355 (57) | 176 (62) | 179 (53) | 0.044 | |
| 1 | 132 (21) | 55 (20) | 77 (23) | ||
| ≥2 | 135 (22) | 51 (18) | 84 (25) | ||
| Hospitalizationsb, n (%) | |||||
| 0 | 531 (85) | 255 (90) | 276 (81) | 0.008 | |
| 1 | 42 (7) | 10 (4) | 32 (9) | ||
| 2 | 21 (3) | 8 (3) | 13 (4) | ||
| ≥3 | 28 (5) | 9 (3) | 19 (6) | ||
Abbreviations: ATAQ, Asthma Therapy Assessment Questionnaire; FEV1, forced expiratory volume in 1 second; HCP, health care practitioner; PEF, peak expiratory flow.
a χ 2 test.
bIn the year before an iHARP respiratory consultation.
cNumber (%) calculated as percentage of patients from the UK, Italy, Spain, Australia, Sweden, France and Norway (n = 245 for 0 serious errors and n = 295 for ≥1 serious error).
dNumber (%) calculated as percentage of known patients from the Netherlands (n = 37 for 0 serious errors and n = 46 for ≥1 serious error).
eNumber (%) calculated as percentage of known.
Patients making ≥1 serious errors were significantly less likely to report that they had their inhaler technique reviewed by an HCP in the prior year or to report good to excellent inhaler technique proficiency (Table 3). In addition, those making ≥1 serious errors were significantly more likely to have poor asthma control in the previous 1–4 weeks, as assessed using the GINA criteria and/or ATAQ score (Table 3 and Supplemental Table S1).
Severe exacerbations and asthma-related hospitalizations during the previous year were significantly more frequent among patients making ≥1 serious inhaler errors than among those making no errors. The number of acute courses of oral corticosteroids was not significantly different between these two patient groups overall (Table 3) or as a dichotomized variable (0 versus ≥1 oral corticosteroid course: p = 0.070 for the comparison between patients making 0 versus ≥ 1 serious error).
Risk factors for making serious inhaler technique errors: multivariable model
The univariable logistic regression results for the risk of a serious error are reported in the online Supplementary material (Supplemental Tables S2–S4).
In the multivariable logistic regression analysis, the most significant association with making ≥1 serious error was having experienced ≥1 asthma-related hospitalizations (inpatient or emergency department attendance) in the previous year (Table 4). Other factors significantly associated with making ≥1 serious errors included being female, being obese (versus underweight/normal weight), having no inhaler technique review in the prior year, and having poor asthma control in the prior 4 weeks (via ATAQ) versus having good/partial control.
Table 4. Demographic and clinical factors associated with making ≥1 serious error versus 0 (multivariable results).
| Reference category | Category | Odds ratio (95% CI) | p value | Overall p value | |
|---|---|---|---|---|---|
| Sex | Male | Female | 1.51 (1.08–2.10) | 0.017 | |
| BMI | Underweight/Normal weight | Overweight | 1.18 (0.80–1.74) | 0.401 | 0.024 |
| Obese | 1.75 (1.17–2.63) | 0.007 | |||
| Inhaler review | Yes | No | 1.45 (1.04–2.02) | 0.027 | |
| ATAQ | Good control/Partial control | Poor control | 1.57 (1.04–2.36) | 0.031 | |
| Inpatient admission or ED attendance | 0 | ≥1 | 2.07 (1.26–3.40) | 0.004 |
Abbreviations: ATAQ, Asthma Therapy Assessment Questionnaire; BMI, body mass index; ED, emergency department.
There was a significant interaction between body mass index (BMI) and inhaler technique review. Among overweight patients, those who had no inhaler technique review in the previous year were more likely to make a serious error compared with patients who had their inhaler technique reviewed (Table 5). Among patients who had no inhaler technique review in the previous year, overweight and obese patients were more likely to make a serious error compared with underweight/normal weight patients. (The proportions of patients reporting a previous inhaler technique review were 41% of those underweight/normal weight, 52% of those overweight and 50% of those obese; χ 2 test p = 0.059).
Table 5. Odds ratios of recording a serious error (vs. not) for categorical variables – multivariable analyses (including interaction).
| Reference category | Category | Odds ratio (95% CI) | p value | ||
|---|---|---|---|---|---|
| Sex | Male | Female | 1.50 (1.07, 2.11) | 0.018 | |
| BMI by Inhaler check | Inhaler check = YES | Underweight/Normal weight | Overweight | 0.75 (0.43, 1.32) | 0.090 |
| Obese | 1.26 (0.70, 2.27) | ||||
| Inhaler check = NO | Overweight | 1.77 (1.03, 3.03) | |||
| Obese | 2.26 (1.28, 3.99) | ||||
| Inhaler check by BMI | Underweight/Normal | Inhaler check = Yes | No | 0.91 (0.53, 1.57) | 0.090 |
| Overweight | No | 2.14 (1.22, 3.76) | |||
| Obese | No | 1.63 (0.88, 3.01) | |||
| ATAQ | Good control/Partial control | Poor control | 1.60 (1.06, 2.41) | 0.026 | |
| Inpatient admission or ED attendance | 0 | 1+ | 2.00 (1.22, 3.29) | 0.006 |
Abbreviations: ATAQ, Asthma Therapy Assessment Questionnaire; BMI, body mass index; ED, emergency department.
Discussion
We found that errors in Diskus inhaler technique are common among patients with asthma, with over half of patients in this study making ≥1 serious errors. The incidence of serious errors was significantly higher among female patients, obese patients and those who had not had their inhaler technique reviewed within the previous year, who were of lower educational level and who assessed their own technique as poor. We found that asthma-related hospitalizations and severe exacerbations during the previous year, and poor asthma control during the previous 4 weeks as measured on the ATAQ, were more common among patients who made ≥1 serious errors. In the multivariable model, risk factors identified for making ≥1 serious errors were having experienced ≥1 asthma-related hospitalizations during the previous year, being obese, being female, having poor asthma control during the previous 4 weeks (as measured by the ATAQ), and no patient-reported review of inhaler technique by an HCP during the previous year.
Results of this study support the consensus that incorrect inhaler technique is a common issue rather than an exception among patients with asthma [4,8,9]. The two errors most frequently reported in our study (failure to exhale before inhalation and insufficient [or absent] breath-hold at the end of inhalation) are in agreement with previously published reports of the most frequently observed errors with the Diskus being “no exhalation before inhalation” and “no breath-holding after inhalation” [13,15,23,33]. The third most common error in our study (inhalation that was not forceful from the start) has also been reported [13,15,33,34]. Specific dose preparation errors with the Diskus (does not slide cover as far as possible and does not slide lever fully to open mouthpiece) were found to be low (4.3% total), consistent with previous reports in which 7.3% of patients incorrectly loaded the dose [16] and 2.5% did not slide the lever as far as possible [23]. Other errors reported as common with the Diskus in previous observational studies include incorrect dose metering [15], failure to prime the device correctly [33,34] and exhaling into the device [13,23].
Our findings suggest that some patient characteristics may potentially predict incorrect inhalation technique, specifically female sex and obesity. One alternative explanation for these findings could be that suboptimally controlled asthma is more common in obese patients [35], and the phenotype of obese non-eosinophilic asthma responds poorly to ICS [36,37]. Moreover, we cannot rule out the possibility of these being statistically significant findings that are of no clinical significance or relevance.
We found that patients with a lower level of education, as previously reported [13,16,38], were more likely to make a serious error, although education was not included in the multivariable model because it was confounded by the BMI and inhaler review status. An association between inhaler misuse and older age has been described previously [13,16,38,39]. We found no significant association with age, although only 4% of patients in the study were older than 70 years. The incidence of errors was also not significantly associated with lung function in this study, perhaps because of our broad study population that was not limited to patients with severe asthma [21,38]. Instead, we found that sex and BMI may be more important factors to consider when selecting inhaler device.
The association between incorrect inhaler technique and lack of prior inhaler technique instruction by HCPs has been described previously [13,16,33,38]. However, in practice it is relevant not only whether inhaler technique instruction is given but also the nature of that instruction, as not all methods of instruction are equally effective. Furthermore, the action of checking to see what the patient is actually doing incorrectly and the associated feedback/correction from the HCP may be critical and needs to be considered [40]. Inhaler training has been shown to significantly improve the proportion of patients with proper inhaler technique and to produce improved asthma control scores [17,41–43]. That said, many HCPs are not skilled in using inhalation devices and could also benefit from training [44]; consequently, patients may not receive appropriate (if any) inhaler instruction.
We also found that a patient self-assessment of inhaler technique as being poor was associated with increased likelihood of inhaler errors, suggesting that a simple query to patients could be an easy, fast and inexpensive screening method to identify patients who would benefit from inhaler training. These data are actually inconsistent with other research showing that individuals who demonstrate errors may think they are using their devices correctly [9,45]. While it is easy to address inhaler technique for those who feel there is a problem, being able to identify those who have incorrect technique but do not realize it is more challenging. We need to look at other factors to detect those individuals, and the findings of this study have identified some of those factors.
Strengths of this study are its observational design and the inclusion of data from more than 600 patients undergoing inhaler technique review in a routine clinical setting in eight different countries. We intentionally applied minimal exclusion criteria in order to study a heterogeneous, representative population of patients with asthma to maximize generalizability of results to primary care practice; this was in contrast to enrolment in RCTs, which typically include only patients who demonstrate ideal inhaler technique in order to minimize potential bias. In addition, this study included patient-reported feedback, providing important insight into patient perspectives.
The use of real-life datasets presents a set of limitations for which adjustments are not always possible, particularly the issue of missing data, which in the required anonymized form was not feasible to retrieve. As the missing data in our study appeared randomly distributed (data not shown), we believe this limitation is unlikely to substantially bias the results. Another potential confounder is the possibility of inter-individual observation bias among the iHARP HCPs. While iHARP nurses and doctors receive the same training for observing and evaluating inhaler technique, different interpretations of observed technique are still possible, particularly with regard to the more subjective errors such as inhalation being “not forceful”. Subsequent studies would benefit from incorporating objective appraisals of patient inhalation profiles using specific thresholds with devices such as the In-Check™ DIAL (Alliance Tech Medical, Granbury, TX), the Aerosol Inhalation Monitor® (Vitalograph), or any other system that measures inhalation profile [46]. Furthermore, medical staff were not blinded to the questionnaire results, which could potentially have influenced their observations. Data such as frequency of hospitalizations and exacerbations in the year preceding the patient review were obtained via a patient-completed questionnaire; hence, recall bias may be a further limitation of the study. Finally, the design of this study does not enable us to address the issue of cause and effect.
Using an international dataset enabled us to compare patient inhaler technique in several countries and to identify incorrect technique as a widespread problem. However, a limitation of this approach is possible confounding caused by inter-country differences in how disease outcomes are measured and recorded (e.g. the Netherlands does not use the MARS to measure adherence).
Nonetheless, although observational studies such as this may lack the internal validity achievable in RCTs, they provide a valuable insight into patient behavior and health outcomes in clinical practice. As such our findings should be considered in conjunction with those arising from other study designs including RCTs.
Our study focused on a single dry powder device, the Diskus inhaler, with which a rather low rate of errors has previously been reported [14,22–24]. Additional areas for further investigation include the characteristics of patients who made more than one serious error (quantity) and the impact of error type (quality) in order to clarify the importance and clinical relevance of each type of error. Further study is also needed to identify factors associated with making serious errors with other devices. The importance of choosing the right inhaler device for the individual patient is widely recognized [8,9,47,48], and selecting devices based on patient characteristics and preferences could be an important step for improving asthma control and along with assessing adherence should be the first step before increasing therapy. Patient preference for an inhaler device combined with ease of inhaler instructions is associated with an increased likelihood of correct use [14,34]. Personalized prescribing of inhaler devices could provide a safe and effective method of achieving better asthma control, fewer exacerbations, fewer hospital admissions and ultimately lower healthcare costs.
Conclusions
We found that inhaler technique errors are common among patients with asthma using the Diskus inhaler. The most frequent error in this study, the failure to exhale before inhalation, was observed in one third of patients. We found that errors were more common among female patients and obese patients. In addition to these two patient characteristics, factors significantly associated with making ≥1 serious errors were an asthma-related hospitalization or emergency department attendance the prior year, lack of an inhaler technique review within 1 year and poor asthma control over the previous 4 weeks. These findings highlight the importance of regular monitoring of inhaler technique and can aid in identifying patients who could benefit from a review of their Diskus inhaler technique. Patients with evidence of poor asthma control should be targeted for a review of their inhaler technique even when using a device thought to have a low error rate.
Acknowledgements
The iHARP database was funded by unrestricted grants from Mundipharma International Ltd and Research in Real-Life Ltd; these analyses were funded by an unrestricted grant from Teva Pharmaceuticals. Mundipharma and Teva played no role in study conduct or analysis and did not modify or approve the manuscript. The authors wish to direct a special appreciation to all the participants of the iHARP group who contributed data to this study and to Mundipharma, sponsors of the iHARP group. In addition, we thank Julie von Ziegenweidt for assistance with data extraction and Anna Gilchrist and Valerie L. Ashton, PhD, for editorial assistance. Elizabeth V. Hillyer, DVM, provided editorial and writing support, funded by Research in Real-Life, Ltd.
Declaration of interest
Janine A. M. Westerik declares that she has no conflicts of interest in relation to this article. Victoria Carter, Anne Burden, Samantha L. Thompson and Catherine Hutton were employees of Research in Real-Life (RiRL), which conducted this study and which has conducted paid research in respiratory disease on behalf of the following other organizations in the past 5 years: Aerocrine, AKL Ltd, Almirall, Astra Zeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, Mundipharma, Napp, Novartis, Orion, Sanofi, Takeda and Teva. Henry Chrystyn has no shares in any pharmaceutical companies. He has received sponsorship to carry out studies, together with Board Membership, consultant agreements and honoraria for presentation, from several pharmaceutical companies that market inhaled products. These include Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Innovata Biomed, Meda, Napp Pharmaceuticals, Mundipharma, NorPharma, Novartis, Orion, Sanofi, Teva, Truddell Medical International, UCB and Zentiva. Research sponsorship has also been received from grant awarding bodies (EPSRC and MRC). He owns 50% of Inhalation Consultancy Ltd. Dermot Ryan has provided consultancy to or lectured on behalf of AstraZeneca, GlaxoSmithKline, Novartis, Almirall, Teva, Meda, Uriach, Stallergenes, Chiesi, Boehringer-Ingelheim, Orion. Kevin Gruffydd-Jones has spoken on behalf of and acted as a consultant for AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mundipharma/NAPP. John Haughney has received reimbursements for attending symposia, fees for speaking, organizing educational events, funds for research or fees for consulting from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Merck Sharp & Dohme, Mundipharma, Novartis and Teva. Nicolas Roche has received over the past 3 years (i) fees for speaking, organizing education, participation in advisory boards or consulting from Aerocrine, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, MSD-Chibret, Mundipharma, Novartis, Pfizer, Stallergenes, Teva; (ii) research grants from Novartis, Boehringer Ingelheim and Pfizer. Federico Lavorini has received in the past 5 years honoraria for consultancy and presentations from the following pharmaceutical companies that market inhaled products: Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, TEVA and Zentiva. Alberto Papi has received grants, personal fees and non-financial support from AstraZeneca, Chiesi Farmaceutici, GlaxoSmithKline, Boehringer Ingelheim, Merck Sharp & Dohme, Menarini, Novartis, Zambon, TEVA, Pfizer, Takeda and Mundipharma. Antonio Infantino has no conflicts of interest to declare in relation to this article. Miguel Román-Rodríguez has provided consultancy to or lectured on behalf of AstraZeneca, GlaxoSmithKline, Novartis, Almirall, Chiesi, Mundipharma, Boehringer-Ingelheim, Rovi and Teva. Sinthia Bosnic-Anticevich has received reimbursements for attending meetings, fees for speaking or consultancy from Mundipharma and Teva. Karin Lisspers has received payment for advisory board from Meda, Novartis, scientific committee from AstraZeneca, Novartis and for lectures from Meda, AstraZeneca, Novartis, GlaxoSmithKline, Nycomed and Boehringer Ingelheim. Björn Ställberg has received honoraria for educational activities and lectures from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Meda, MSD, Novartis and TEVA, and has served on advisory boards arranged by AstraZeneca, Novartis and Boehringer Ingelheim. Svein Høegh Henrichsen has no conflicts of interest to declare. Thys van der Molen has received grants for research, travel fees, reimbursement for presentations and advisory boards from AstraZeneca, GlaxoSmithKline, Almirall, Mundipharma, Boehringer Ingelheim, Chiesi, Teva and Novartis. David B. Price has Board Membership with Aerocrine, Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis and Teva. Consultancy: Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, Mundipharma, Napp, Novartis, Pfizer, Teva and Zentiva; Grants/Grants Pending with UK National Health Service, British Lung Foundation, Aerocrine, AstraZeneca, Boehringer Ingelheim, Chiesi, Eli Lilly, GlaxoSmithKline, Meda, Merck, Mundipharma, Novartis, Orion, Pfizer, Respiratory Effectiveness Group, Takeda, Teva and Zentiva; Payments for lectures/speaking: Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Meda, Merck, Mundipharma, Novartis, Pfizer, SkyePharma, Takeda and Teva; Payment for manuscript preparation: Mundipharma and Teva; Patents (planned, pending or issued): AKL Ltd.; Payment for the development of educational materials: GlaxoSmithKline, Novartis; Stock/Stock options: Shares in AKL Ltd which produces phytopharmaceuticals and owns 80% of Research in Real Life Ltd and its subsidiary social enterprise Optimum Patient Care; received Payment for travel/accommodations/meeting expenses from Aerocrine, Boehringer Ingelheim, Mundipharma, Napp, Novartis and Teva; Funding for patient enrolment or completion of research: Almirral, Chiesi, Teva and Zentiva; Peer reviewer for grant committees: Medical Research Council (2014), Efficacy and Mechanism Evaluation programme (2012), HTA (2014); and Received unrestricted funding for investigator-initiated studies from Aerocrine, AKL Ltd, Almirall, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis, Orion, Takeda, Teva and Zentiva.
Supplementary material available online Supplementary Tables S1–S4
References
- Rabe KF, Adachi M, Lai CK, Soriano JB, Vermeire PA, Weiss KB, Weiss ST. Worldwide severity and control of asthma in children and adults: the global asthma insights and reality surveys. J Allergy Clin Immunol. 2004;114:40–47. doi: 10.1016/j.jaci.2004.04.042. [DOI] [PubMed] [Google Scholar]
- Demoly P, Gueron B, Annunziata K, Adamek L, Walters RD. Update on asthma control in five European countries: results of a 2008 survey. Eur Respir Rev. 2010;19:150–157. doi: 10.1183/09059180.00002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D, Fletcher M, van der Molen T. Asthma control and management in 8 000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014;24:14009. doi: 10.1038/npjpcrm.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D, Bosnic-Anticevich S, Briggs A, Chrystyn H, Rand C, Scheuch G, Bousquet J. Inhaler competence in asthma: common errors, barriers to use and recommended solutions. Respir Med. 2013;107:37–46. doi: 10.1016/j.rmed.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Laube BL, Janssens HM, de Jongh FH, Devadason SG, Dhand R, Diot P, Everard ML, et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J. 2011;37:1308–1331. doi: 10.1183/09031936.00166410. [DOI] [PubMed] [Google Scholar]
- Dolovich MB, Ahrens RC, Hess DR, Anderson P, Dhand R, Rau JL, Smaldone GC, et al. Device selection and outcomes of aerosol therapy: evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127:335–371. doi: 10.1378/chest.127.1.335. [DOI] [PubMed] [Google Scholar]
- Brocklebank D, Ram F, Wright J, Barry P, Cates C, Davies L, Douglas G, et al. Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature. Health Technol Assess. 2001;5:1–149. doi: 10.3310/hta5260. [DOI] [PubMed] [Google Scholar]
- Haughney J, Price D, Barnes NC, Virchow JC, Roche N, Chrystyn H. Choosing inhaler devices for people with asthma: current knowledge and outstanding research needs. Respir Med. 2010;104:1237–1245. doi: 10.1016/j.rmed.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Papi A, Haughney J, Virchow JC, Roche N, Palkonen S, Price D. Inhaler devices for asthma: a call for action in a neglected field. Eur Respir J. 2011;37:982–985. doi: 10.1183/09031936.00150910. [DOI] [PubMed] [Google Scholar]
- Wright J, Brocklebank D, Ram F. Inhaler devices for the treatment of asthma and chronic obstructive airways disease (COPD) Qual Saf Health Care. 2002;11:376–382. doi: 10.1136/qhc.11.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzella BA, Brooks CM, Richards JM, Jr., Windsor RA, Soong S, Bailey WC. Assessing the use of metered dose inhalers by adults with asthma. J Asthma. 1989;26:223–230. doi: 10.3109/02770908909073253. [DOI] [PubMed] [Google Scholar]
- van Beerendonk I, Mesters I, Mudde AN, Tan TD. Assessment of the inhalation technique in outpatients with asthma or chronic obstructive pulmonary disease using a metered-dose inhaler or dry powder device. J Asthma. 1998;35:273–279. doi: 10.3109/02770909809068218. [DOI] [PubMed] [Google Scholar]
- Melani AS, Zanchetta D, Barbato N, Sestini P, Cinti C, Canessa PA, Aiolfi S, et al. Inhalation technique and variables associated with misuse of conventional metered-dose inhalers and newer dry powder inhalers in experienced adults. Ann Allergy Asthma Immunol. 2004;93:439–446. doi: 10.1016/s1081-1206(10)61410-x. [DOI] [PubMed] [Google Scholar]
- Schulte M, Osseiran K, Betz R, Wencker M, Brand P, Meyer T, Haidl P. Handling of and preferences for available dry powder inhaler systems by patients with asthma and COPD. J Aerosol Med Pulm Drug Deliv. 2008;21:321–328. doi: 10.1089/jamp.2007.0634. [DOI] [PubMed] [Google Scholar]
- Lavorini F, Magnan A, Dubus JC, Voshaar T, Corbetta L, Broeders M, Dekhuijzen R, et al. Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir Med. 2008;102:593–604. doi: 10.1016/j.rmed.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Melani AS, Bonavia M, Cilenti V, Cinti C, Lodi M, Martucci P, Serra M, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930–938. doi: 10.1016/j.rmed.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Giraud V, Allaert FA, Roche N. Inhaler technique and asthma: feasability and acceptability of training by pharmacists. Respir Med. 2011;105:1815–1822. doi: 10.1016/j.rmed.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Giraud V, Roche N. Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stability. Eur Respir J. 2002;19:246–251. doi: 10.1183/09031936.02.00218402. [DOI] [PubMed] [Google Scholar]
- Al-Jahdali H, Ahmed A, Al-Harbi A, Khan M, Baharoon S, Bin Salih S, Halwani R, et al. Improper inhaler technique is associated with poor asthma control and frequent emergency department visits. Allergy Asthma Clin Immunol. 2013;9:8. doi: 10.1186/1710-1492-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy ML, Hardwell A, McKnight E, Holmes J. Asthma patients' inability to use a pressurised metered-dose inhaler (pMDI) correctly correlates with poor asthma control as defined by the global initiative for asthma (GINA) strategy: a retrospective analysis. Prim Care Respir J. 2013;22:406–411. doi: 10.4104/pcrj.2013.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melani AS. Inhalatory therapy training: a priority challenge for the physician. Acta Biomed. 2007;78:233–245. [PubMed] [Google Scholar]
- Chrystyn H. The Diskus: a review of its position among dry powder inhaler devices. Int J Clin Pract. 2007;61:1022–1036. doi: 10.1111/j.1742-1241.2007.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molimard M, Raherison C, Lignot S, Depont F, Abouelfath A, Moore N. Assessment of handling of inhaler devices in real life: an observational study in 3811 patients in primary care. J Aerosol Med. 2003;16:249–254. doi: 10.1089/089426803769017613. [DOI] [PubMed] [Google Scholar]
- Broeders ME, Molema J, Hop WC, Vermue NA, Folgering HT. The course of inhalation profiles during an exacerbation of obstructive lung disease. Respir Med. 2004;98:1173–1179. doi: 10.1016/j.rmed.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Haughney J, Sims E, Holohan J, Ryan D, Price D. Improving clinician-patient communication in asthma: the HARP project. Allergy. 2010;65:413–414. doi: 10.1111/j.1398-9995.2009.02127.x. [DOI] [PubMed] [Google Scholar]
- Carter V. iHARP Database. Respiratory Effectiveness Group, 2015 Winter Summit; Rotterdam. 2015. http://www.effectivenessevaluation.org/reg-2015-winter-summit/ [Google Scholar]
- Inhaler technique assessment initiative Helping Asthma in Real-life Patients (iHARP): Web resource. https://iharp.org/ [Google Scholar]
- Respiratory Effectiveness Group. http://www.effectivenessevaluation.org/ [Google Scholar]
- Study registration, European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) 2014. http://www.encepp.eu/encepp/viewResource.htm?id=8020 [Google Scholar]
- Horne R, Weinman J. Self-regulation and self-management in asthma: exploring the role of illness perceptions and treatment beliefs in explaining non-adherence to preventer medication. Psychol Health. 2002;17:17–32. [Google Scholar]
- Global Initiative for Asthma. GINA report, Global Strategy for Asthma Management and Prevention. http://www.ginasthma.org/ [Google Scholar]
- Vollmer WM, Markson LE, O'Connor E, Sanocki LL, Fitterman L, Berger M, Buist AS. Association of asthma control with health care utilization and quality of life. Am J Respir Crit Care Med. 1999;160:1647–1652. doi: 10.1164/ajrccm.160.5.9902098. [DOI] [PubMed] [Google Scholar]
- Lee SM, Chang YS, Kim CW, Kim TB, Kim SH, Kwon YE, Lee JM, et al. Skills in handling turbuhaler, diskus, and pressurized metered-dose inhaler in korean asthmatic patients. Allergy Asthma Immunol Res. 2011;3:46–52. doi: 10.4168/aair.2011.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenney J, Innes JA, Crompton GK. Inappropriate inhaler use: assessment of use and patient preference of seven inhalation devices. EDICI. Respir Med. 2000;94:496–500. doi: 10.1053/rmed.1999.0767. [DOI] [PubMed] [Google Scholar]
- O'Byrne PM, Pedersen S, Schatz M, Thoren A, Ekholm E, Carlsson LG, Busse WW. The poorly explored impact of uncontrolled asthma. Chest. 2013;143:511–523. doi: 10.1378/chest.12-0412. [DOI] [PubMed] [Google Scholar]
- Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J. 2006;27:495–503. doi: 10.1183/09031936.06.00077205. [DOI] [PubMed] [Google Scholar]
- Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, Wardlaw AJ, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestini P, Cappiello V, Aliani M, Martucci P, Sena A, Vaghi A, Canessa PA, et al. Prescription bias and factors associated with improper use of inhalers. J Aerosol Med. 2006;19:127–136. doi: 10.1089/jam.2006.19.127. [DOI] [PubMed] [Google Scholar]
- Kamin WE, Genz T, Roeder S, Scheuch G, Cloes R, Juenemann R, Trammer T. The inhalation manager: a new computer-based device to assess inhalation technique and drug delivery to the patient. J Aerosol Med. 2003;16:21–29. doi: 10.1089/089426803764928329. [DOI] [PubMed] [Google Scholar]
- Basheti IA, Reddel HK, Armour CL, Bosnic-Anticevich SZ. Counseling about turbuhaler technique: needs assessment and effective strategies for community pharmacists. Respir Care. 2005;50:617–623. [PubMed] [Google Scholar]
- Basheti IA, Armour CL, Bosnic-Anticevich SZ, Reddel HK. Evaluation of a novel educational strategy, including inhaler-based reminder labels, to improve asthma inhaler technique. Patient Educ Couns. 2008;72:26–33. doi: 10.1016/j.pec.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Basheti IA, Reddel HK, Armour CL, Bosnic-Anticevich SZ. Improved asthma outcomes with a simple inhaler technique intervention by community pharmacists. J Allergy Clin Immunol. 2007;119:1537–1538. doi: 10.1016/j.jaci.2007.02.037. [DOI] [PubMed] [Google Scholar]
- Takemura M, Kobayashi M, Kimura K, Mitsui K, Masui H, Koyama M, Itotani R, et al. Repeated instruction on inhalation technique improves adherence to the therapeutic regimen in asthma. J Asthma. 2010;47:202–208. doi: 10.3109/02770900903581692. [DOI] [PubMed] [Google Scholar]
- Self TH, Arnold LB, Czosnowski LM, Swanson JM, Swanson H. Inadequate skill of healthcare professionals in using asthma inhalation devices. J Asthma. 2007;44:593–598. doi: 10.1080/02770900701554334. [DOI] [PubMed] [Google Scholar]
- Ho SF, OMahony MS, Steward JA, Breay P, Burr ML. Inhaler technique in older people in the community. Age Ageing. 2004;33:185–188. doi: 10.1093/ageing/afh062. [DOI] [PubMed] [Google Scholar]
- Azouz W, Chetcuti P, Hosker HS, Saralaya D, Stephenson J, Chrystyn H. The inhalation characteristics of patients when they use different dry powder inhalers. J Aerosol Med Pulm Drug Deliv. 2015;28:35–42. doi: 10.1089/jamp.2013.1119. [DOI] [PubMed] [Google Scholar]
- Virchow JC, Crompton GK, Dal Negro R, Pedersen S, Magnan A, Seidenberg J, Barnes PJ. Importance of inhaler devices in the management of airway disease. Respir Med. 2008;102:10–19. doi: 10.1016/j.rmed.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Broeders ME, Sanchis J, Levy ML, Crompton GK, Dekhuijzen PN. The ADMIT series – issues in inhalation therapy. 2. Improving technique and clinical effectiveness. Prim Care Respir J. 2009;18:76–82. doi: 10.4104/pcrj.2009.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]