Abstract
Irritable bowel syndrome (IBS) is traditionally defined as a functional disorder since it lacks demonstrable pathological abnormalities. However, in recent years, low grade inflammatory infiltration, often rich in mast cells, in both the small and large bowel, has been observed in some patients with IBS. The close association of mast cells with major intestinal functions, such as epithelial secretion and permeability, neuroimmune interactions, visceral sensation, and peristalsis, makes researchers and gastroenterologists to focus attention on the key roles of mast cells in the pathogenesis of IBS. Numerous studies have been carried out to identify the mechanisms in the development, infiltration, activation, and degranulation of intestinal mast cells, as well as the actions of mast cells in the processes of mucosal barrier disruption, mucosal immune dysregulation, visceral hypersensitivity, dysmotility, and local and central stress in IBS. Moreover, therapies targeting mast cells, such as mast cell stabilizers (cromoglycate and ketotifen) and antagonists of histamine and serotonin receptors, have been tried in IBS patients, and have partially exhibited considerable efficacy. This review focuses on recent advances in the role of mast cells in IBS, with particular emphasis on bridging experimental data with clinical therapeutics for IBS patients.
Keywords: Innate immunity, Irritable bowel syndrome, Mast cells, Mucosal barrier, Visceral pain
Introduction
Irritable bowel syndrome (IBS) is one of the most common functional gastrointestinal disorders worldwide, with the prevalence ranging from 1.1% to 29.2% in the general population diagnosed by the Rome III criteria.1 Multiple factors contribute to the pathogenesis of IBS, such as increased intestinal permeability, visceral hypersensitivity and dysmotility, intestinal dysbacteriosis, food intolerance, brain-gut axis dysregulation, and psychological stress, which are important biomarkers of IBS.2 However, the generation and association of these biomarkers are incompletely understood.
In recent years, IBS is increasingly viewed as a low grade inflammatory disorder,3 and attentions have been focused on the roles of mast cells (MCs) in the gut wall, accounting for the close relations of MCs with major intestinal functions, such as epithelial secretion, epithelial permeability, blood flow, neuroimmune interactions, visceral sensation, and peristalsis.4 MCs hyperplasia and activation lead to abnormal gastrointestinal sensitivity, motility, and secretion, which in turn contribute to the hallmark symptoms of IBS—abdominal pain and/or discomfort, bloating, and abnormal bowel function (diarrhea and/or constipation).5 Moreover, therapies targeting mast cells, such as mast cell stabilizers (cromoglycate and ketotifen) and antagonists of histamine and serotonin receptors, have been tried in IBS patients in several studies, and partially exhibited good efficacy in symptom improvement.6,7 It further supported the key role of MCs in the process of IBS. In this review, it discussed the mechanisms of MCs in the gut dysfunctions of IBS. Most importantly, it also summarized potential drugs targeting MCs, including development and migration, activation and secretion, and mediators, for the clinical therapy of IBS patients.
Mast Cells in the Human Gut
MCs are the progeny of CD34+ hematopoietic stem cells and have a stringent requirement for stem cell factor, the ligand for the c-kit receptor (CD117), for differentiation.8 These cells widely distribute in tissues that form host barriers such as the skin, respiratory and intestinal mucosa, peritoneum, and meninges.9,10 In the human gastrointestinal tract, there is the highest density of MCs in the lamina propria mucosae (2–3% of all cells), and slightly less (about 1% of all cells) in the submucosa,4,8–10 but MCs can be recruited in large numbers in response to an array of stimuli.8 Furthermore, sporadical MCs also exist in the muscle layers and in the serosa.9 Most MCs in the gastrointestinal mucosa are MCT (containing only tryptase), whereas the dominating phenotype in submucosa is MCTC (containing both tryptase and chymase).11
Generally, MCs can be activated by an IgE dependent way via the high-affinity receptor FcɛRI which play a key role in allergic reactions. Besides this classical and most effective way, IgG, IgA, Ig-free-light chains (IgFLC) and even complements (C3a and C5a) might also play a role via binding to the related receptors expressed on MCs.4 Moreover, amounts of immune-independent stimulus could trigger MCs activation, such as neuro-hormonal stimuli including neurotransmitters, neuropeptides, hormones and growth factors, particularly substance P (SP) or calcitonin gene-related peptide (CGRP) released from nociceptive endings and corticotropin releasing factor (CRF) induced by psychological stress, which are important in the regulation of gut functions.9,11,12 Other biological factors such as bacteria components (via Toll-like receptors or other pattern recognition receptors), and some physiochemical factors also could lead to MCs activation.11,13 Upon activation, MCs release bioactive substances preformed in granules (histamine, enzymes, and heparin) and newly synthesized cytokine, chemokines, and lipid metabolites (Table 1).4,9,10,14 These mediators contribute to the broad spectrum of regulating effects of MCs in the gut, and participate in multiple pathophysiological processes and diseases beyond allergy, which including IBS, functional dyspepsia, inflammatory bowel disease, and intestinal infections. Particularly, the mechanisms of MCs involved in the process of IBS are discussed in this review (Fig. 1).
Table 1.
Bioactive Substances Released by Human Mast Cells upon Activation (Adapted from Data Summarized in References4,9,10,14)
| Type of molecule | Human mast cell mediators |
|---|---|
| Preformed in granules | |
| Biogenic amines | Histamine, Serotonin |
| Enzymes | Tryptase (α, β, γ), Chymase, Carboxypeptidase-A, Cathepsin, Acid hydrolases, Phospholipases |
| Proteoglycans | Heparin, Chondroitin sulfates |
| Others | TNF-α, IL-6, VEGF |
| De novo synthesized | |
| Cytokines | Interleukins (IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-8, IL-9, IL-10, IL-11, IL-12, IL-13, IL-14, IL-15, IL-16, IL-18), TNF-α, INF-γ |
| Chemokines | CCL1, MCP-1 (CCL2), MIP-1α (CCL3), MIP-1β (CCL4), CCL5, CCL7, CCL8, CCL11, CCL13, CCL16, CCL17, CCL20, CCL22, CXCL1, CXCL2, CXCL3, CXCL10, MIF, LIF |
| Growth factors | VEGF, TGF-β, basic FGF, NGF, NT-3, GM-CSF, M-CSF, SCF, EGF, PDGF |
| Eicosanoids | Leukotrienes (LTB4, LTC4, LTD4, LTE4) Prostaglandins (PGD2, PGE2), PAF |
| Other mediators | Nitric oxide, SP, VIP, ATP, CRF, Urocortin |
TNF-α, tumor necrosis factor-α; IL, interleukins; VEGF, vascular endothelial growth factor; INF-γ;, interferon-γ; CCL, chemokine (C-C motif) ligand; MCP, monocyte chemoattractant protein; MIP, macrophage inflammation protein; CXCL, chemokine (C-X-C motif) ligand; MIF, macrophage migration inhibitory factor; LIF, leukemia inhibitor factor; TGF-β, transforming growth factor-β; FGF, fibroblast growth factor; NGF, nerve growth factor; NT-3, neurotrophin-3; GM-CSF, granulocyte-macrophage colony stimulating factor; M-CSF, macrophage colony-stimulating factor; SCF, stem cell factor; EGF, epidermal growth factor; PDGF, platelet derived growth factor; LT, leukotrienes; PG, prostaglandins; PAF, platelet-activating factor; SP, substance P; VIP, vasoactive intestinal peptide; ATP, adenosine triphosphate; CRF, corticotropin releasing factor.
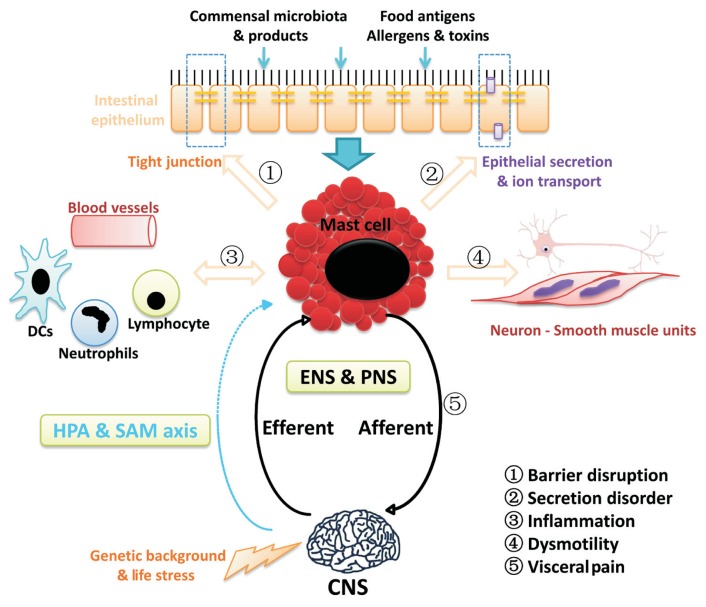
Figure 1.
Mechanisms of mast cells involved in the process of irritable bowel syndrome (IBS). On one hand, local microenvironment in the gut lumen including commensal bacteria and products, food antigens, allergens and toxin play key roles in regulating mast cell activation and secretion. Central stress such as psychological distress and negative life events may also contribute to the activation and degranulation of mast cells via the direct pathway (peripheral nervous innervations) and the indirect pathways (the hypothalamic-pituitary-adrenal [HPA] axis and the sympathetic-adrenal-medullary [SAM] axis). On the other hand, activation of mast cell participates in multiple pathophysiological processes in the gut of IBS: ➀ regulating epithelial permeability via acting on tight junction; ➁ regulating epithelial water and ion transport; ➂ regulating blood flow and endothelial functions, as well as immunomodulation, inflammation, and defense against microbes; ➃ regulating intestinal peristalsis; and ➅ regulating function of visceral afferent and sensation via neuroimmune mechanisms. DCs, dendritic cells; ENS, enteric nervous system; PNS, peripheral nervous system; CNS, central nervous system.
Mast Cells and Irritable Bowel Syndrome: The Present
MCs hyperplasia has been considered a common feature of IBS patients.11,13 The density of MCs in both the small and large bowel may be increased in patients with IBS, but the results are somewhat inconsistent. Some studies demonstrated the number of MCs or the area of mucosa occupied by MCs was increased in IBS patients,5 moreover, it varies in different genders,15 in distinct bowel segments16 and in subgroups of IBS with higher MCs counts in diarrhea-predominant IBS (D-IBS) patients than constipation-predominant IBS (C-IBS) patients.17,18 However, others suggested there was no significant change in MCs density in IBS.19–21 Further data confirmed the hypothesis that the function but not numbers of the mast cell is more valuable to be assessed,22 since the presence of MCs per se does not necessarily imply they are pathogenic unless they are activated.17 Although no difference in the number of MCs was observed in IBS, the degranulation or activation rate of MCs was significantly higher,19 the mucosal content and spontaneous release of tryptase and histamine were increased in IBS subjects.23 Therefore, a unified and effective standard should be established to assess the role of MCs in these subjects.
Numerous studies have focused on the relationship between MCs infiltration and the clinical aspects of IBS.17,23,24 The severity of IBS significantly correlated with colonic MCs counts and spontaneous release of tryptase.24 The MCs in close proximity to nerves significantly correlated with the severity and frequency of abdominal pain/discomfort.23 In addition, MCs stabilizers (chromones), which act primarily by stabilizing the plasma membrane of MCs, significantly improved abdominal pain and stool consistency in patients with D-IBS.25 Similarly, ketotifen increased the rectal threshold for discomfort, markedly reduced the severity of abdominal pain, and improved quality of life in IBS patients.25 These promising results further support the hypothesis that MCs participate in IBS symptom generation, and provide alternative interventions for some IBS patients.
Mast Cells Activation and Gastrointestinal Functions in Irritable Bowel Syndrome
The Mast Cell–Neuronal Units
The gastrointestinal tract possesses both the largest neural network outside the brain and the most extensive immune systems, which brings about the opportunity for crosstalk between neurons and immune cells, including MCs.14 Plenty of evidence has confirmed that endings of postganglionic sympathetic, peptidergic and vagal fibers, and enteric neurons are in close proximity to MCs.10,11 An estimated 70% of intestinal mucosal MCs are in direct contact with nerves, and another 20% are within 2 μm.10 Furthermore, neuro-anatomical studies have provided strong evidence for the direct innervation of MCs, including the intrinsic and extrinsic nerves, particularly, the nonadrenergic noncholinergic nerves.14 This direct action may be via the synaptic-like connections (distance between nerves and MCs is 20–200 nm).11 Adhesion molecules such as cell adhesion molecule-1 and N-cadherin may also participate in the communication between MCs and nerves.10,26 The anatomically MC–neuronal units make it easy for the crosstalk between nerves and MCs, which is crucial for maintaining intestinal homeostasis.9
The functionally MC–neuronal units consist of 2 pathways: the nerve to MCs signaling and the MCs to nerve signaling (Fig. 2).9,14 MCs respond to various neurotransmitters and neuropeptides, such as SP, adenosine triphosphate, vasoactive intestinal polypeptide, CGRP and corticotropin releasing hormone (CRH) which mediate mast cell degranulation,9,14,27 or somatostatin which has an inhibitory influence on MCs.9 SP is considered one of the most important mediators in nociceptive sensation, which could be expressed by both human intestinal MCs and enteric neurons or extrinsic afferents nerves.9 The classical concept suggested that SP plays a role in the signal transmission form enteric neurons to MCs, which may be mediated by the high-affinity neurokinin receptor 1 on MCs.27 Notably, integral functionality of neuron to MCs crosstalk in the gut probably requires primed resident MCs, such as IgE-sensitized and interleukin (IL)-4 or stem cell factor-primed, since non-activated MCs do not respond to SP or other neuropeptides.11,28 Upon activation, MCs release a variety of mediators which in turn sensitize the senso-secreto-motor neurons, and form a positive feedback loop to amplify the neurogenic inflammation.9
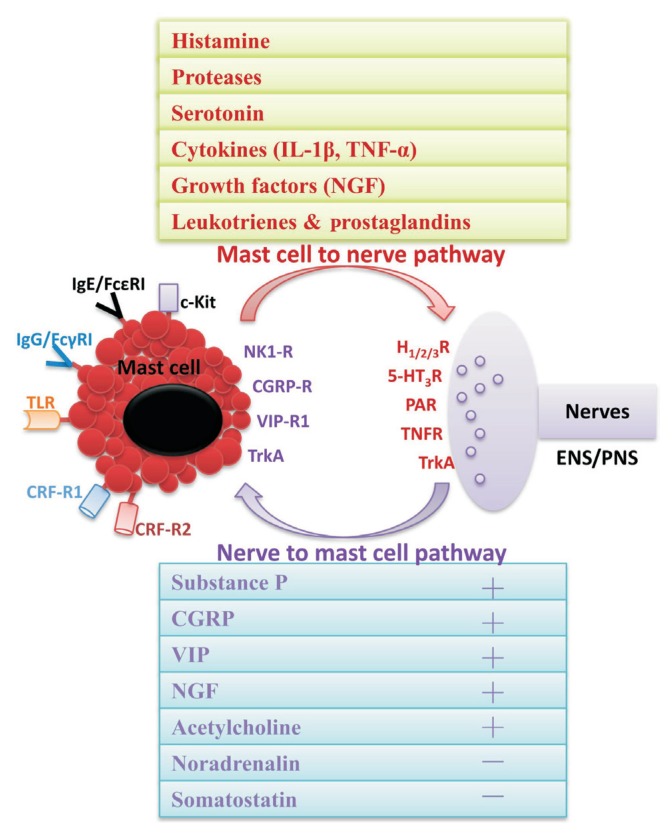
Figure 2.
The functionally mast cell (MC)–neuronal units consist of 2 pathways: the nerve to MC signaling and the MC to nerve signaling. In the MC to nerve signaling, MC activate and release bioactive substances preformed in granules (histamine, serotonin, and enzymes) and newly synthesized (cytokines, growth factors, and lipid metabolites), which act on the relevant receptors expressed on the nerve endings, and result in a series of neuronal effects, especially visceral pain. In the nerve to MC signaling, both intrinsic and extrinsic nerves may respond to a variety of mechanical, biological, or chemical stimuli and release a high number of neuropeptides, such as substance P (SP), vasoactive intestinal polypeptide (VIP), calcitonin gene-related peptide (CGRP), nerve growth factor (NGF), and somatostatin, which in turn regulate the activation of MCs. IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α; FcɛRI, Fc epsilon receptor I; FcγRI, Fc gamma receptor I; TLR, Toll-like receptor; CRF-R1, corticotropin releasing factor receptor 1; CRF-R2, corticotropin releasing factor receptor 2; NK1-R, neurokinin receptor 1; TrkA, tropomyosin receptor kinase A; HR, histamine receptor; 5-HT3, 5-hydroxytryptamine receptor 3; PAR, protease-activated receptor; TNFR, TNF receptor; ENS, enteric nervous system; PNS, peripheral nervous system.
Mast Cells Modulate Visceral Sensation
Low grade mucosal inflammation could be responsible for the peripheral sensitization and visceral hypersensitivity, the hallmark of IBS associated with abdominal pain and discomfort.29,30 A large number of data show that MCs play a role in chronic pain, particularly at the visceral level.10 Indeed, MCs in patients with IBS have been shown to be in closer proximity to colonic nerve endings, including the SP-positive afferents and endings expressing transient receptor potential vanilloid type 1 (TRPV1), and correlated strongly with severity and frequency of pain.31,32 MC degranulation increases excitability of vagal, splanchnic, and mesenteric afferents, contributing to nociceptive processes associated with visceral pain.10,14
Supernatants from mucosal biopsies from IBS patients are more likely to activate intestinal nerves than those from healthy subjects.31,33 Histamine and proteases are the main sensitizing mediators to nerves, which increased in spontaneous release in IBS patients without consideration of different subtypes.33 Histamine H1 and H2 receptors (H1R and H2R) may mediate the activation of visceral afferents and enteric neurons, while H3R mediate suppression of fast synaptic transmission.9,24 Tryptase causes long-lasting neuronal hyperexcitability through cleaving and activating protease-activated receptor 2 (PAR2) located on enteric nerves and visceral afferents.23 It is also well recognized that submucous neurons would respond with a transient excitation to serotonin, mediated primarily by 5-hydroxytryptamine receptor 3 (5-HT3) and 5-HT1A.34–36 Moreover, MC mediators, eg, prostaglandins, leukotrienes, and inflammatory cytokines usually are algogenic, and may promote the formation of peripheral sensitization.37,38 Taken together, MCs activation, along with the MC to nerve signaling, may have an important role in the pathophysiology of visceral hypersensitivity. This concept is further supported by evidence that MC stabilizers such as ketotifen and doxantrazole increased the threshold of pain and improved abdominal discomfort in IBS patients,35 and decreased the colorectal distension-induced mechanical excitability of colonic C-fibers in IBS-like animals.10
Neural plasticity is the basis of persistent functioning enhancement of nerves and may play a role in the visceral hypersensitivity.35 Several studies are now providing initial evidence suggesting the involvement of neuroplastic changes in the enteric nervous system or afferent pathways in functional gastrointestinal disorders, including IBS.35,39 Infiltration of MCs in the colonic mucosa of patients with IBS promote neuronal sprouting and neuroplastic changes owing to the release of nerve growth factor.40 Nerve growth factor acting on tropomyosin receptor kinase A (TrkA) receptors could also up-regulate of TRPV1 expression.41,42 Moreover, sustained inflammation, including MCs mediators, have the common effect of reducing the transduction threshold of ion channels on the peripheral terminals of nociceptors, such as TRPV1.41 In this sense, MCs play an important role in neural plasiticity in the gut and maintain the hyper-excitability of nociceptors.
Mast Cells Regulate Gastrointestinal Motility
In the sensitized gut, MCs infiltration and release of mediators have been approved to be associated with disturbed motility, for instance, increasing in colonic and intestinal myoelectric spike activity,43 contraction of circular and longitudinal smooth muscle,11 and intense duodenal clusters of contraction.44 To a certain extent, IBS is related to food intolerance and/or allergy,2,13,24 hence it may at least partly possess the common physiopathological mechanisms with allergic dysmotility. Although MCs only sporadically distribute in the inner and outer muscle layers, they may play a significant role in modulating the function of myenteric neurons and smooth muscles.45 A recent study suggests that MCs may also be increased in the external muscle layer in patients with slow transit constipation.46 Colonic mucosal mediators from IBS patients, which exert excitatory action on human submucosal neuron,34 could also excite myenteric cholinergic motor neurons.47 It correlated with MCs counts and mediated by activation of prostanoid receptors, TRPV1 and P2X receptors.48 This supported the concept that MCs can impact on smooth muscle contractility or on intrinsic motor neurons.
Most attention has been focused on D-IBS in which the MCs infiltration and activation are more common, and are well identified to be associated with visceral pain and diarrhea.17,23 The major MCs mediators such as histamine, proteases, serotonin, and prostaglan-din E2 are known to excite smooth muscle cell and enteric nervous activity.48 For example, H1R and H2R were upregulated within the mucosa in food allergy and IBS,44 which could evoke the activation of enteric nerve cells. Whereas, presynaptic H3R suppress release of noradrenalin from sympathetic terminals, and thereby enhance the neurogenic secretomotor responses that underlie diarrhea.49,50 PAR1 and PAR2 expressed on myenteric neurons have been reported to modulate gut motility in different ways, for example, promote contraction of gastric smooth muscle but reduce contractility of both circular and longitudinal colonic smooth muscles.51,52 But changed expression ratio of PAR1 to PAR2 in the colon is connected with D-IBS patients,53 which may partly associated with increased motility. Relatively, MCs have been less extensively studied in patients complaining of constipation, including slow transit constipation and C-IBS patients.46 It is speculated that the increase of MCs activity in severely constipated patients might be a mechanism trying to compensate for the impaired propulsive activity of these patients.46
Mast Cells Regulate Epithelial Secretion
Apart from abnormal peristalsis, altered intestinal fluid and electrolyte transport may also play a vital role,54 with fluid secretion increased in patients with D-IBS and reduced in those with C-IBS.55,56 In vitro studies have clearly demonstrated that MC activation alters intestinal epithelial ion transport directly or indirectly (via intrinsic or extrinsic nerves),57,58 through recording the transepithelial short-circuit current of the intestinal mucosal tissues or epithelial cell monolayers. In sensitized mast cell-deficient mice, secretory response to serosal antigen was reduced by 70% in comparison to normal congenic mice, but fully restored after reconstitution of intestinal mast cell population by injecting bone marrow-derived mast cell precursors.11,59 Others demonstrated that the compound 48/80-induced and anti-IgE-induced secretory response could be reduced by ketotifen and H1R antagonist in chicken ileum60 and human colon,57 respectively. Furthermore, SP stimulated net ion secretion could be reduced partly by H1R and H2R antagonists and a mast cell stabilizer (doxantrazole).59 All of these suggest that MCs are important in regulating the epithelial secretion, and histamine may play a key role. Besides histamine, MC tryptase may stimulate chloride ion secretion via activating PAR2, which is strongly expressed in both basolateral and apical membranes of enterocytes.52
Mast Cells Modulate Epithelial Permeability and Mucosal Barrier
The gut permeability, both paracellular and transcellular permeability, plays a pivotal role in maintaining the immune balance between the internal and external milieu.51 Abnormal small intestinal and colonic permeability, which facilitates enhanced antigen exposure that may activate the intestinal immune system, has been implicated in the pathogenesis of IBS.61,62 Numerous evidence showed a positive relationship between the number of mucosal MCs and intestinal permeability,61 and the MC-derived tryptase was well identified as a key factor disrupts the intestinal barrier.63 MC tryptase cleaves PAR2 on colonocytes to increase paracellular permeability by acting on intercellular apical junction complex, which mainly consists of the tight junctions such as claudins, occludin, zonula occludens, junctional adhesion molecule, and the adherens junction such as E-cadherin.63,64 Furthermore, PAR2 may induce the activation of extracellular signal-related kinase 1/2 (ERK1/2) and phosphorylation of myosin light chain kinase, which regulates reorganization of F-actin and cytoskeleton and redistribution of tight junction, to increase epithelial permeability.63,65 Other MC mediators such as interferon-γ, tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-4, IL-13, and prostaglandin E2 also have destructive effects on both trans- and paracellular permeability.65
More recently, further investigations were carried out to map the regulation of permeability in follicle-associated epithelium (FAE), which is a specialized epithelium that covers Peyer’s patches, and contain microfold cells that are dedicated to sampling antigens and bacteria from the lumen and transporting them to the underlying immune aggregate in the intestinal mucosa.66,67 With a water avoidance stress rat model, it was suggested that stress disrupts the FAE barrier with decreased transepithelial resistance and increased transepithelial flux of macromolecules by mechanisms involving nerve-mast cell interaction, including CRF, SP, vasoactive intestinal peptide, and acetylcholine.66,67 It prompts us that MC-mediated barrier disruption is common in the gut, which is not only in the villus epithelium, but also in the immune-related FAE.
Mast Cells in Gut Immunomodulation and Inflammation
MCs are proposed as key players in the initiation and maintenance of the inflammatory circuitry in the intestine.68 As a low grade inflammation condition, IBS patients had significantly higher colorectal mucosal immune cell counts than healthy controls, including MCs, intraepithelial lymphocytes, and lamina propria lymphocytes.69 Besides, increased MC and lymphocyte numbers have also been detected in the ileum and duodenum.12 MCs release of inflammatory mediators contribute to the recruitment of other immune cells, for instance, interleukin-6, interleukin-8 and TNF-α recruit neutrophils, while IL-3, IL-5, IL-13, and granulocyte-macrophage colony-stimulating factor (GM-CSF) recruit eosinophils and basophils, chemokines such as monocyte chemotactic protein 1, and macrophage inflammatory protein 1α and 1β also play important roles.4,10,11 The increased inflammatory infiltration in the murine intestine, induced by MC degranulation after intestinal manipulation could be prevented by MC stabilizers such as ketotifen and doxantrazole, and could not be elicited in MC deficient KitWsh/Wsh mice, but in MC reconstituted ones.70 Notably, the MC-mediated mucosal barrier dysfunction may also be blamed, because the epithelial function and morphology were unchanged and no inflammatory cell infiltration was observed in MC-deficient rats by chronic stress.71
On the other hand, MCs have also been documented to have a protective role in colonic colitis, since deletion of MCs in IL-10-deficient mice resulted in enhanced T helper 1 type (Th1) pro-inflammatory cytokines and inflammatory signaling.72 It was concluded that MCs may suppress Th1 immune response and inflammation. Moreover, MC-derived proteases also contributed to the immune regulation on Th2 polarization, by acting on PAR2 expressed on Th2 cells and increasing the expression of B-cell lymphoma 6 protein in Th2 cells. The latter suppressed the expression of Th2 cytokines and increased the expression of Foxp3 genes in Th2 cells, which contributed to the immune regulatory properties similar to regulatory T cells.73 Although MCs play an immunomodulatory role in the gut, how this mechanism action works in IBS is unknown, and needs more interpretation.
Stress Induced Mast Cells Activation in Irritable Bowel Syndrome
Local Stress: Diet, Allergen, and Infections
The role of MCs in allergic inflammation has been well established. Allergens generally induce a shift in the Th1/Th2 balance toward Th2 immunity which produce allergen-specific IgE and sensitizes the MCs by binding to FcɛRI on the surface.74,75 Once subsequently exposed to the same allergen, MCs activate and produce both immediate and delayed allergic effects.4,75,76 IBS is usually disturbed by dietary factors,77 including either immunologic (food allergy) or nonimmunologic (food intolerance), in which MCs involved. The latter being much more common since many patients with IBS link their symptoms to specific foods, although a real IgE-dependent food allergy can be found infrequently in these patients.77 Food elimination based on specific serum IgG antibodies has been found to result in a significant decrease in IBS symptoms.24,77,78 Moreover, in IBS patients, the presence of an allergic background correlates with a more severe disease and diarrhea predominance, possibly by enhancing mucosal MC activation and paracellular permeability.24 It should be emphasized that increased intestinal MCs activation could be a consequence of local hypersensitivity to food antigens.
MCs also act as gatekeepers to protect the host against bacterial infections.76,79 MC-deficient models showed impaired function of bacterial clearance, while specific reconstitution of MCs contributed to surviving bacterial infections in peritonitis.76 Differing from Th2-mediated process in allergies, most bacterial infections induce a Th1-skewed response,76 in which MCs may provide a link between innate and adaptive immunity.74 For example, MC-derived TNF-α not only recruited neutrophil into the infection side, but also induced hypertrophy of draining lymph nodes and T-cell recruitment at sites of infection with Escherichia coli.4 MCs are also present in regulating the migration, maturation, and activation of dendritic cells in the infected sites by releasing TNF-α, IL-1β, and GM-CSF in response to Toll-like receptors-mediated stimulation.76 Infectious gastroenteritis is at increased risk for IBS, however, the mechanisms underlying the sustained entities after cure of infection remain unknown. Whether acute infection induced MC activation and subsequently immune response take part in the process of post infectious IBS remains to be established.
In contrast, growing evidence support the view that intestinal commensal or probiotic bacteria suppress MC activation.74,80,81 For example, commensal bacteria, particularly Enterococcus faecalis, showed strong suppression of MCs degranulation through partial inhibition of Ca2+ signaling upon the FcɛRI cross-linking.80 It seemed that gut microbiota might be involved in allergic diseases. Recent clinical trials have demonstrated that supplementation of lactobacilli and bifidobacteria improved the balance of intestinal microbiota, which was beneficial for the prevention of allergic diarrhea.74 Indeed, beneficial microbes may interact directly (via Toll-like receptors or other pattern recognition receptors) and/or indirectly (via modulation of other host cells) with MCs, and play a crucial role in modulating the Th1-Th2-T regulatory balance, which is a major factor controlling the development of allergic disease.74,80,81 In recent years, wide progress has been made evidencing dysbiosis in IBS. It is easy to speculate that flora imbalance may affect the stabilities of MCs which participate in the pathogenesis of IBS.
Central Stress: Psychological Distress and Negative Life Events
Psychological distress or stressful life events, as well as various types of early adverse life events, have been associated with the onset or exacerbation of IBS symptoms, especially in women.82,83 Acute or chronic stress exposure has widely impacted on intestinal functions and MC activation.62,84 Plenty of work has been done to verify the concept that MCs contribute to stress induced gut dysfunction. For example, MCs mediate stress induced intestinal barrier disruption85–87 and visceral hypersensitivity82,88,89 due to the release of tryptase, TNF-α,86 and histamine.82 Peripheral administration of H1R antagonists fexofenadine and ebastine are capable of reversing post stress visceral hypersensitivity in rats.90 The observation that stress-induced increase in permeability does not exhibit in MC-deficient rodents, but in MC-reconstituted ones, which highlights the importance of MCs in stress-related colonic barrier dysfunction.91 Recently, evidence supported that stress induced CRF, acting on the CRF1 and CRF2 receptors, should be the key factors in MCs degranulation.62,85,86 Stress triggers the increase in intestinal paracellular permeability via mast cell dependent release of proteases, which could be reproduced by peripheral administration of CRF, and blocked by CRF1/2 receptor antagonist, mast cell stabilizers, and protease inhibitors.86
Mast Cells as Potential Therapeutic Target for Irritable Bowel Syndrome
Recent discoveries regarding MCs in the pathophysiology of IBS have revealed numerous potential therapeutic targets. The candidate drugs targeting MC maturation, development and homing to the gut as well as MC activation and major mediators are under development, some of which show benefit to IBS symptoms improvement (Table 2).92–102
Table 2.
Mast Cell as Potential Therapeutic Target for Irritable Bowel Syndrome
| Potential targets | Typical drugs | Pharmacological mechanisms | Clinical researches |
|---|---|---|---|
| Targeting MC maturation and development | |||
| SCF/c-Kit signaling | Sunitinib | C-kit signaling blocked | No data for IBS92,93 |
| Targeting MC activation and degranulation/secretion | |||
| IgE/FcɛRI signaling | Omalizumab | Anti-IgE Mab, neutralize soluble IgE and downregulate FcɛRI density on MCs and basophils | Case report, significantly improve overall IBS symptoms in IBS with antihistamine resistant chronic spontaneous urticaria94 |
| Mast cell stabilizers | Cromoglycate | Inhibit MCs degranulation | Reduced MC mediators in biopsies, and clinical improvement of bowel function in IBS-D6 |
| Ketotifen | Antagonize H1R and stabilize MCs | Improve IBS symptoms and quality of life, increase the threshold of discomfort7 | |
| CRF receptors | α-helical CRH9-41 | Non-selective CRF-R antagonist | Improve distension-induced gut visceral perception in IBS patients95 |
| GW876008 | CRF1 receptor antagonist | Pre-clinical data show direct improvement of IBS symptoms, as well as co-existing anxiety/depression96 | |
| Neurokinin receptors | Ibodutant | NK2 antagonist | Dose-dependently reduce pain and benefit on global relief in D-IBS with a 8 weeks-therapy97 |
| DNK333 | NK1/2/3 antagonist | Effective and well tolerated in women with IBS-D98 | |
| Syk tyrosine kinase | R406 | Block downstream signals of FcɛRI for MC activation | No data for IBS99,100 |
| Targeting MC mediators and receptors | |||
| Tryptase | FUT-175 | PAR2 antagonist | Inhibit protease-induced hypersensitivity symptoms101 |
| Histamine | Ebastin | H1R antagonist | Obviously improve overall well-being, visceral pain and QoL of IBS with a 12 weeks-therapy102 |
MC, master cell; SCF, stem cell factor; IBS, irritable bowel syndrome; FcɛRI, Fc epsilon receptor I; IBS-D, diarrhea-predominant IBS; H1R, histamine H1 receptor; CRF, corticotropin releasing factor; CRH, corticotropin releasing hormone; CRF-R, CRF receptor; NK, Neurokinin; Syk, spleen tyrosine kinase; PAR, protease-activated receptor; QoL, quality of life.
Disodium cromoglycate (DSCG) and ketotifen are 2 kinds of classical MC stabilizers and are available for IBS. Preliminary clinical data indicated that a 6 month of DSCG treatment significantly reduced release of tryptase from jejunal biopsies, and increased clinical improvement of bowel function in D-IBS.6 A placebo-controlled trail in 60 IBS patients showed that 8 weeks of treatment with ketotifen obviously increased the threshold for discomfort in IBS patients with visceral hypersensitivity, reduced IBS symptoms, and improved the health-related quality of life.7 Furthermore, a prior study of 120 D-IBS patients with one or more food intolerance indicated that dietary exclusion along with oral DSCG was effective in these patients, and had a prolonged symptomatic benefit.6 However, these studies had several limitations, such as poor design, small sample size, and selection bias. In addition, natural drugs including flavonoids, such as quercetin and fisetin, has been proved to be effective in MC stabilization, which may be potential selections for IBS.99 The clinical benefit of MC-stabilizers for IBS need to be further established, and which group of subjects will benefit from MC-stabilizers should be confirmed.
Humanized monoclonal anti-IgE antibody represents a new class of MC-stabilizing agents, which could neutralize soluble IgE and downregulate FcɛRI density on MCs and basophils which lead them to be insensitive to allergens.99 It was reported that omalizumab significantly improved the overall IBS symptoms in a case with concomitant antihistamine resistant chronic spontaneous urticaria.94 This may be an attractive way for the treatment of complicated and refractory IBS. Other drugs targeting MC activation, CRF receptors (CRF-R) and neurokinin receptors antagonists, may be an innovative treatment of IBS in the near future. Alpha-helical CRH9-41, a non-selective CRF-R antagonists, could improve the distension-induced changes of motor and perception in IBS patients.95 Notably, differential, even opposite, roles between CRF1 and CRF2 receptors are being increasingly recognized,95 so selective CRF-R antagonists are necessary. Encouragingly, a series of CRF-R1 antagonists, such as pexacerfont, antalarmin, and GW876008, have been developed, and some of which have been tested in treating IBS patients.96,103,104 Recently, a double-blind, randomized, placebo-controlled, phase II study showed that ibodutant, a selective neurokinin receptor 2 antagonist, dose-dependently relieved overall IBS symptoms and abdominal pain/discomfort in D-IBS with satisfactory safety and tolerability.97 However, these 2 receptors have broad actions, apart from MC activation, so the benefit and safety remains to be assessed.
In addition, interventions that block the effects of MC mediators should be considered. A double blind, randomized controlled trial evaluated the effects of the H1R-antagonist ebastin in IBS patients, in which 12 weeks of ebastin treatment resulted in a significant improvement in global symptom relief, abdominal pain, and quality of life.102 These findings indicated H1R blockade may turn to be an effective treatment for IBS. Several studies also demonstrated that serine protease inhibitors and PAR2 antagonist could control the mucosal inflammation and block the pro-nociceptive effects in mice colon.101 Accordingly, antagonizing PAR2 may be a highly compelling means for IBS treatment.
Other potential targets associated with MC activation such as microbes and foods, or mediators released from MCs such as the nerve growth factor, TNF-α, chemokines and lipid metabolites, are undoubtedly of great value. However, too much work needs to be done in the future in this field.
Conclusions
Taken together, previous findings strongly argue in favor of MCs as remarkable players in the pathogenesis and pathophysiology of IBS. MC activation and mediator release contribute to the development of major IBS symptoms, such as abdominal pain, constipation and diarrhea. Future trials from the bench to the bedside may help to substantiate the mechanisms of MCs in IBS and develop potential drugs targeting MCs for the management of IBS.
Footnotes
Financial support: This study was in part supported by a grant from the National Natural Science Foundation of China (NSFC), China (Grant No. 81330014).
Conflicts of interest: None.
Author contributions: Lei Zhang collected the references, adapted the figures, and wrote the manuscript; and Jun Song and Xiaohua Hou provided ideas, designed and supervised the manuscript.
References
- 1.Oshima T, Miwa H. Epidemiology of functional gastrointestinal disorders in Japan and in the world. J Neurogastroenterol Motil. 2015;21:320–329. doi: 10.5056/jnm14165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whorwell PJ. IBS in 2014: developments in pathophysiology, diagnosis and management. Nat Rev Gastroenterol Hepatol. 2015;12:72–74. doi: 10.1038/nrgastro.2014.225. [DOI] [PubMed] [Google Scholar]
- 3.Ohman L, Simrén M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol. 2010;7:163–173. doi: 10.1038/nrgastro.2010.4. [DOI] [PubMed] [Google Scholar]
- 4.Bischoff SC, Krämer S. Human mast cells, bacteria, and intestinal immunity. Immunol Rev. 2007;217:329–337. doi: 10.1111/j.1600-065X.2007.00523.x. [DOI] [PubMed] [Google Scholar]
- 5.O’Sullivan M, Clayton N, Breslin NP, et al. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil. 2000;12:449–457. doi: 10.1046/j.1365-2982.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- 6.Camilleri M. Current and future pharmacological treatments for diarrhea-predominant irritable bowel syndrome. Expert Opin Pharmacother. 2013;14:1151–1160. doi: 10.1517/14656566.2013.794223. [DOI] [PubMed] [Google Scholar]
- 7.Klooker TK, Braak B, Koopman KE, et al. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. 2010;59:1213–1221. doi: 10.1136/gut.2010.213108. [DOI] [PubMed] [Google Scholar]
- 8.Shea-Donohue T, Stiltz J, Zhao A, Notari L. Mast cells. Curr Gastroenterol Rep. 2010;12:349–357. doi: 10.1007/s11894-010-0132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buhner S, Schemann M. Mast cell-nerve axis with a focus on the human gut. Biochim Biophys Acta. 2012;1822:85–92. doi: 10.1016/j.bbadis.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Héron A, Dubayle D. A focus on mast cells and pain. J Neuroimmunol. 2013;264:1–7. doi: 10.1016/j.jneuroim.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Santos J, Guilarte M, Alonso C, Malagelada JR. Pathogenesis of irritable bowel syndrome: the mast cell connection. Scand J Gastroenterol. 2005;40:129–140. doi: 10.1080/00365520410009410. [DOI] [PubMed] [Google Scholar]
- 12.Barbara G, Cremon C, Carini G, et al. The immune system in irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:349–359. doi: 10.5056/jnm.2011.17.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wouters MM, Vicario M, Santos J. The role of mast cells in functional GI disorders. Gut. 2016;65:155–168. doi: 10.1136/gutjnl-2015-309151. [DOI] [PubMed] [Google Scholar]
- 14.Rijnierse A, Nijkamp FP, Kraneveld AD. Mast cells and nerves tickle in the tummy: implications for inflammatory bowel disease and irritable bowel syndrome. Pharmacol Ther. 2007;116:207–235. doi: 10.1016/j.pharmthera.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Cremon C, Gargano L, Morselli-Labate AM, et al. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol. 2009;104:392–400. doi: 10.1038/ajg.2008.94. [DOI] [PubMed] [Google Scholar]
- 16.Song J, Zhang L, Bai T, Qian W, Li R, Hou X. Mast cell-dependent mesenteric afferent activation by mucosal supernatant from different bowel segments of guinea pigs with post-infectious irritable bowel syndrome. J Neurogastroenterol Motil. 2015;21:236–246. doi: 10.5056/jnm14095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Philpott H, Gibson P, Thien F. Irritable bowel syndrome - an inflammatory disease involving mast cells. Asia Pac Allergy. 2011;1:36–42. doi: 10.5415/apallergy.2011.1.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goral V, Kucukoner M, Buyukbayram H. Mast cells count and serum cytokine levels in patients with irritable bowel syndrome. Hepatogastro-enterology. 2010;57:751–754. [PubMed] [Google Scholar]
- 19.La JH, Kim TW, Sung TS, Kim HJ, Kim JY, Yang IS. Role of mucosal mast cells in visceral hypersensitivity in a rat model of irritable bowel syndrome. J Vet Sci. 2004;5:319–324. [PubMed] [Google Scholar]
- 20.Celik AF, Demirkesen C, Pamuk ON, Pamuk GE, Uzunismail H. Mast cells: do they really have a role in disturbed bowel habits of IBS patients? Am J Gastroenterol. 2001;96:927–929. doi: 10.1111/j.1572-0241.2001.03655.x. [DOI] [PubMed] [Google Scholar]
- 21.Theoharides TC, Asadi S, Chen J, Huizinga JD. Irritable bowel syndrome and the elusive mast cells. Am J Gastroenterol. 2012;107:727–729. doi: 10.1038/ajg.2012.61. [DOI] [PubMed] [Google Scholar]
- 22.Theoharides TC. Mast cells in irritable bowel syndrome and ulcerative colitis: function not numbers is what makes all the difference. Dig Dis Sci. 2014;59:897–898. doi: 10.1007/s10620-013-2988-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 24.Vivinus-Nébot M, Dainese R, Anty R, et al. Combination of allergic factors can worsen diarrheic irritable bowel syndrome: role of barrier defects and mast cells. Am J Gastroenterol. 2012;107:75–81. doi: 10.1038/ajg.2011.315. [DOI] [PubMed] [Google Scholar]
- 25.Barbara G, Stanghellini V, Cremon C, et al. Aminosalicylates and other anti-inflammatory compounds for irritable bowel syndrome. Dig Dis. 2009;27(suppl 1):115–121. doi: 10.1159/000268131. [DOI] [PubMed] [Google Scholar]
- 26.Ito A, Hagiyama M, Oonuma J. Nerve-mast cell and smooth muscle-mast cell interaction mediated by cell adhesion molecule-1, CADM1. J Smooth Muscle Res. 2008;44:83–93. doi: 10.1540/jsmr.44.83. [DOI] [PubMed] [Google Scholar]
- 27.Bischoff SC, Schwengberg S, Lorentz A, et al. Substance P and other neuropeptides do not induce mediator release in isolated human intestinal mast cells. Neurogastroenterol Motil. 2004;16:185–193. doi: 10.1111/j.1365-2982.2004.00502.x. [DOI] [PubMed] [Google Scholar]
- 28.van der Kleij HP, Ma D, Redegeld FA, Kraneveld AD, Nijkamp FP, Bienenstock J. Functional expression of neurokinin 1 receptors on mast cells induced by IL-4 and stem cell factor. J Immunol. 2003;171:2074–2079. doi: 10.4049/jimmunol.171.4.2074. [DOI] [PubMed] [Google Scholar]
- 29.Kanazawa M, Hongo M, Fukudo S. Visceral hypersensitivity in irritable bowel syndrome. J Gastroenterol Hepatol. 2011;26(suppl 3):119–121. doi: 10.1111/j.1440-1746.2011.06640.x. [DOI] [PubMed] [Google Scholar]
- 30.Adam B, Tsopelas C, Liebregts T, Bartholomeusz FD, Holtmann G. Host immune response determines visceral hyperalgesia in a rat model of post-inflammatory irritable bowel syndrome. J Gastroenterol. 2013;48:1119–1127. doi: 10.1007/s00535-012-0729-2. [DOI] [PubMed] [Google Scholar]
- 31.Hughes PA, Zola H, Penttila IA, Blackshaw LA, Andrews JM, Krumbiegel D. Immune activation in irritable bowel syndrome: can neuroimmune interactions explain symptoms? Am J Gastroenterol. 2013;108:1066–1074. doi: 10.1038/ajg.2013.120. [DOI] [PubMed] [Google Scholar]
- 32.Schemann M, Camilleri M. Functions and imaging of mast cell and neural axis of the gut. Gastroenterology. 2013;144:698–704. doi: 10.1053/j.gastro.2013.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbara G, Wang B, Stanghellini V, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 34.Farmer AD, Aziz Q. Visceral pain hypersensitivity in functional gastrointestinal disorders. Br Med Bull. 2009;91:123–136. doi: 10.1093/bmb/ldp026. [DOI] [PubMed] [Google Scholar]
- 35.Barbara G, Cremon C, De Giorgio R, et al. Mechanisms underlying visceral hypersensitivity in irritable bowel syndrome. Curr Gastroenterol Rep. 2011;13:308–315. doi: 10.1007/s11894-011-0195-7. [DOI] [PubMed] [Google Scholar]
- 36.Wood JD. Visceral pain: spinal afferents, enteric mast cells, enteric nervous system and stress. Curr Pharm Des. 2011;17:1573–1575. doi: 10.2174/138161211796196918. [DOI] [PubMed] [Google Scholar]
- 37.Buhner S, Li Q, Vignali S, et al. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology. 2009;137:1425–1434. doi: 10.1053/j.gastro.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Peiris M, Bulmer DC, Baker MD, et al. Human visceral afferent recordings: preliminary report. Gut. 2011;60:204–208. doi: 10.1136/gut.2010.221820. [DOI] [PubMed] [Google Scholar]
- 39.Yang X, Sheng L, Guan Y, Qian W, Hou X. Synaptic plasticity: the new explanation of visceral hypersensitivity in rats with Trichinella spiralis infection? Dig Dis Sci. 2009;54:937–946. doi: 10.1007/s10620-008-0444-2. [DOI] [PubMed] [Google Scholar]
- 40.Dothel G, Barbaro MR, Boudin H, et al. Nerve fiber outgrowth is increased in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2015;148:1002–1011. doi: 10.1053/j.gastro.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 41.Knowles CH, Aziz Q. Visceral hypersensitivity in non-erosive reflux disease. Gut. 2008;57:674–683. doi: 10.1136/gut.2007.127886. [DOI] [PubMed] [Google Scholar]
- 42.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/S0896-6273(02)00908-X. [DOI] [PubMed] [Google Scholar]
- 43.Gasbarrini A, Lauritano EC, Garcovich M, Sparano L, Gasbarrini G. New insights into the pathophysiology of IBS: intestinal microflora, gas production and gut motility. Eur Rev Med Pharmacol Sci. 2008;12(suppl 1):111–117. [PubMed] [Google Scholar]
- 44.Murch S. Allergy and intestinal dysmotility--evidence of genuine causal linkage? Curr Opin Gastroenterol. 2006;22:664–668. doi: 10.1097/01.mog.0000245546.18279.7e. [DOI] [PubMed] [Google Scholar]
- 45.Barbara G, Stanghellini V, De Giorgio R, Corinaldesi R. Functional gastrointestinal disorders and mast cells: implications for therapy. Neurogastroenterol Motil. 2006;18:6–17. doi: 10.1111/j.1365-2982.2005.00685.x. [DOI] [PubMed] [Google Scholar]
- 46.Bassotti G, Villanacci V, Nascimbeni R, et al. Colonic mast cells in controls and slow transit constipation patients. Aliment Pharmacol Ther. 2011;34:92–99. doi: 10.1111/j.1365-2036.2011.04684.x. [DOI] [PubMed] [Google Scholar]
- 47.Schemann M, Michel K, Ceregrzyn M, Zeller F, Seidl S, Bischoff SC. Human mast cell mediator cocktail excites neurons in human and guinea-pig enteric nervous system. Neurogastroenterol Motil. 2005;17:281–289. doi: 10.1111/j.1365-2982.2004.00591.x. [DOI] [PubMed] [Google Scholar]
- 48.Balestra B, Vicini R, Cremon C, et al. Colonic mucosal mediators from patients with irritable bowel syndrome excite enteric cholinergic motor neurons. Neurogastroenterol Motil. 2012;24:1118, e570. doi: 10.1111/nmo.12000. [DOI] [PubMed] [Google Scholar]
- 49.Wood JD. Neuropathophysiology of irritable bowel syndrome. J Clin Gastroenterol. 2002;35(suppl 1):S11–S22. doi: 10.1097/00004836-200207001-00004. [DOI] [PubMed] [Google Scholar]
- 50.Breunig E, Michel K, Zeller F, Seidl S, Weyhern CW, Schemann M. Histamine excites neurones in the human submucous plexus through activation of H1, H2, H3 and H4 receptors. J Physiol. 2007;583(Pt 2):731–742. doi: 10.1113/jphysiol.2007.139352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bueno L. Protease activated receptor 2: a new target for IBS treatment. Eur Rev Med Pharmacol Sci. 2008;12(suppl 1):95–102. [PubMed] [Google Scholar]
- 52.Gao C, Liu S, Hu HZ, et al. Serine proteases excite myenteric neurons through protease-activated receptors in guinea pig small intestine. Gastroenterology. 2002;123:1554–1564. doi: 10.1053/gast.2002.36581. [DOI] [PubMed] [Google Scholar]
- 53.Bian ZX, Li Z, Huang ZX, et al. Unbalanced expression of protease-activated receptors-1 and -2 in the colon of diarrhea-predominant irritable bowel syndrome patients. J Gastroenterol. 2009;44:666–674. doi: 10.1007/s00535-009-0058-2. [DOI] [PubMed] [Google Scholar]
- 54.Heuvelin E, Lebreton C, Bichara M, Cerf-Bensussan N, Heyman M. A Bifidobacterium probiotic strain and its soluble factors alleviate chloride secretion by human intestinal epithelial cells. J Nutr. 2010;140:7–11. doi: 10.3945/jn.109.114553. [DOI] [PubMed] [Google Scholar]
- 55.Camilleri M. Intestinal secretory mechanisms in irritable bowel syndrome-diarrhea. Clin Gastroenterol Hepatol. 2015;13:1051–1107. doi: 10.1016/j.cgh.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thayalasekeran S, Ali H, Tsai HH. Novel therapies for constipation. World J Gastroenterol. 2013;19:8247–8251. doi: 10.3748/wjg.v19.i45.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crowe SE, Luthra GK, Perdue MH. Mast cell mediated ion transport in intestine from patients with and without inflammatory bowel disease. Gut. 1997;41:785–792. doi: 10.1136/gut.41.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Castro GA, Harari Y, Russell D. Mediators of anaphylaxis-induced ion transport changes in small intestine. Am J Physiol. 1987;253(4 Pt 1):G540–G548. doi: 10.1152/ajpgi.1987.253.4.G540. [DOI] [PubMed] [Google Scholar]
- 59.Wang L, Stanisz AM, Wershil BK, Galli SJ, Perdue MH. Substance P induces ion secretion in mouse small intestine through effects on enteric nerves and mast cells. Am J Physiol. 1995;269(1 Pt 1):G85–G92. doi: 10.1152/ajpgi.1995.269.1.G85. [DOI] [PubMed] [Google Scholar]
- 60.Collins CB, McGrath J, Baird AW, Campion DP. Effect of mast cell degranulation on chicken ileal ion transport in vitro. Poult Sci. 2007;86:843–849. doi: 10.1093/ps/86.5.843. [DOI] [PubMed] [Google Scholar]
- 61.Lee H, Park JH, Park DI, et al. Mucosal mast cell count is associated with intestinal permeability in patients with diarrhea predominant irritable bowel syndrome. J Neurogastroenterol Motil. 2013;19:244–250. doi: 10.5056/jnm.2013.19.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taché Y, Kiank C, Stengel A. A role for corticotropin-releasing factor in functional gastrointestinal disorders. Curr Gastroenterol Rep. 2009;11:270–277. doi: 10.1007/s11894-009-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jacob C, Yang PC, Darmoul D, et al. Mast cell tryptase controls para-cellular permeability of the intestine. Role of protease-activated receptor 2 and beta-arrestins. J Biol Chem. 2005;280:31936–31948. doi: 10.1074/jbc.M506338200. [DOI] [PubMed] [Google Scholar]
- 64.Wilcz-Villega EM, McClean S, O’Sullivan MA. Mast cell tryptase reduces junctional adhesion molecule-A (JAM-A) expression in intestinal epithelial cells: implications for the mechanisms of barrier dysfunction in irritable bowel syndrome. Am J Gastroenterol. 2013;108:1140–1151. doi: 10.1038/ajg.2013.92. [DOI] [PubMed] [Google Scholar]
- 65.Keita AV, Söderholm JD. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol Motil. 2010;22:718–733. doi: 10.1111/j.1365-2982.2010.01498.x. [DOI] [PubMed] [Google Scholar]
- 66.Keita AV, Carlsson AH, Cigéhn M, Ericson AC, McKay DM, Söderholm JD. Vasoactive intestinal polypeptide regulates barrier function via mast cells in human intestinal follicle-associated epithelium and during stress in rats. Neurogastroenterol Motil. 2013;25:e406–e417. doi: 10.1111/nmo.12127. [DOI] [PubMed] [Google Scholar]
- 67.Keita AV, Söderholm JD, Ericson AC. Stress-induced barrier disruption of rat follicle-associated epithelium involves corticotropin-releasing hormone, acetylcholine, substance P, and mast cells. Neurogastroenterol Motil. 2010;22:770–778. e221–e222. doi: 10.1111/j.1365-2982.2010.01471.x. [DOI] [PubMed] [Google Scholar]
- 68.De Winter BY, van den Wijngaard RM, de Jonge WJ. Intestinal mast cells in gut inflammation and motility disturbances. Biochim Biophys Acta. 2012;1822:66–73. doi: 10.1016/j.bbadis.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 69.Ahn JY, Lee KH, Choi CH, et al. Colonic mucosal immune activity in irritable bowel syndrome: comparison with healthy controls and patients with ulcerative colitis. Dig Dis Sci. 2014;59:1001–1011. doi: 10.1007/s10620-013-2930-4. [DOI] [PubMed] [Google Scholar]
- 70.Stenton GR, Vliagoftis H, Befus AD. Role of intestinal mast cells in modulating gastrointestinal pathophysiology. Ann Allergy Asthma Immunol. 1998;81:1–11. doi: 10.1016/S1081-1206(10)63105-5. [DOI] [PubMed] [Google Scholar]
- 71.Söderholm JD, Yang PC, Ceponis P, et al. Chronic stress induces mast cell-dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology. 2002;123:1099–1108. doi: 10.1053/gast.2002.36019. [DOI] [PubMed] [Google Scholar]
- 72.Zhang H, Xue Y, Wang H, et al. Mast cell deficiency exacerbates inflammatory bowel symptoms in interleukin-10-deficient mice. World J Gastroenterol. 2014;20:9106–9115. doi: 10.3748/wjg.v20.i27.9106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu ZQ, Song JP, Liu X, et al. Mast cell-derived serine proteinase regulates T helper 2 polarization. Sci Rep. 2014;4:4649. doi: 10.1038/srep04649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang JH, Fan SW, Zhu WY. Development of gut microbiota in a mouse model of ovalbumin-induced allergic diarrhea under sub-barrier system. Asian-Australas J Anim Sci. 2013;26:545–551. doi: 10.5713/ajas.2012.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bischoff SC. Mucosal allergy: role of mast cells and eosinophil granulocytes in the gut. Baillieres Clin Gastroenterol. 1996;10:443–459. doi: 10.1016/S0950-3528(96)90052-4. [DOI] [PubMed] [Google Scholar]
- 76.Wesolowski J, Paumet F. The impact of bacterial infection on mast cell degranulation. Immunol Res. 2011;51:215–226. doi: 10.1007/s12026-011-8250-x. [DOI] [PubMed] [Google Scholar]
- 77.Kalliomäki MA. Food allergy and irritable bowel syndrome. Curr Opin Gastroenterol. 2005;21:708–711. doi: 10.1097/01.mog.0000181712.81657.0a. [DOI] [PubMed] [Google Scholar]
- 78.Bischoff SC, Mayer JH, Manns MP. Allergy and the gut. Int Arch Allergy Immunol. 2000;121:270–283. doi: 10.1159/000024340. [DOI] [PubMed] [Google Scholar]
- 79.Kurashima Y, Goto Y, Kiyono H. Mucosal innate immune cells regulate both gut homeostasis and intestinal inflammation. Eur J Immunol. 2013;43:3108–3115. doi: 10.1002/eji.201343782. [DOI] [PubMed] [Google Scholar]
- 80.Kasakura K, Takahashi K, Itoh T, et al. Commensal bacteria directly suppress in vitro degranulation of mast cells in a MyD88-independent manner. Biosci Biotechnol Biochem. 2014;78:1669–1676. doi: 10.1080/09168451.2014.930327. [DOI] [PubMed] [Google Scholar]
- 81.Rodriguez B, Prioult G, Bibiloni R, et al. Germ-free status and altered caecal subdominant microbiota are associated with a high susceptibility to cow’s milk allergy in mice. FEMS Microbiol Ecol. 2011;76:133–144. doi: 10.1111/j.1574-6941.2010.01035.x. [DOI] [PubMed] [Google Scholar]
- 82.Bradesi S, Eutamene H, Garcia-Villar R, Fioramonti J, Buéno L. Acute and chronic stress differently affect visceral sensitivity to rectal distension in female rats. Neurogastroenterol Motil. 2002;14:75–82. doi: 10.1046/j.1365-2982.2002.00305.x. [DOI] [PubMed] [Google Scholar]
- 83.Bradford K, Shih W, Videlock EJ, et al. Association between early adverse life events and irritable bowel syndrome. Clin Gastroenterol Hepatol. 2012;10:385–390. e1–e3. doi: 10.1016/j.cgh.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim YS, Lee MY, Ryu HS, et al. Regional differences in chronic stress-induced alterations in mast cell and protease-activated receptor-2-positive cell numbers in the colon of Ws/Ws rats. J Neurogastroenterol Motil. 2014;20:54–63. doi: 10.5056/jnm.2014.20.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vanuytsel T, van Wanrooy S, Vanheel H, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014;63:1293–1299. doi: 10.1136/gutjnl-2013-305690. [DOI] [PubMed] [Google Scholar]
- 86.Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-α. PLoS One. 2012;7:e39935. doi: 10.1371/journal.pone.0039935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wallon C, Yang PC, Keita AV, et al. Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut. 2008;57:50–58. doi: 10.1136/gut.2006.117549. [DOI] [PubMed] [Google Scholar]
- 88.Larauche M. Novel insights in the role of peripheral corticotropin-releasing factor and mast cells in stress-induced visceral hypersensitivity. Neurogastroenterol Motil. 2012;24:201–205. doi: 10.1111/j.1365-2982.2011.01867.x. [DOI] [PubMed] [Google Scholar]
- 89.Taché Y, Million M. Role of corticotropin-releasing factor signaling in stress-related alterations of colonic motility and hyperalgesia. J Neurogastroenterol Motil. 2015;21:8–24. doi: 10.5056/jnm14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stanisor OI, van Diest SA, Yu Z, et al. Stress-induced visceral hypersensitivity in maternally separated rats can be reversed by peripherally restricted histamine-1-receptor antagonists. PLoS One. 2013;8:e66884. doi: 10.1371/journal.pone.0066884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kiank C, Taché Y, Larauche M. Stress-related modulation of inflammation in experimental models of bowel disease and post-infectious irritable bowel syndrome: role of corticotropin-releasing factor receptors. Brain Behav Immun. 2010;24:41–48. doi: 10.1016/j.bbi.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Afrin LB, Cichocki FM, Patel K, Molderings GJ. Successful treatment of mast cell activation syndrome with sunitinib. Eur J Haematol. 2015;95:595–597. doi: 10.1111/ejh.12606. [DOI] [PubMed] [Google Scholar]
- 93.Maniu CM, Ribi C, Spertini F. Systemic mastocytosis--new therapeutic strategies. Rev Med Suisse. 2013;9:17–21. [French] [PubMed] [Google Scholar]
- 94.Magen E, Chikovani T. Case report of irritable bowel syndrome responding to omalizumab. Georgian Med News. 2015;243:42–45. [PubMed] [Google Scholar]
- 95.Santos J, Alonso C, Guilarte M, Vicario M, Malagelada JR. Targeting mast cells in the treatment of functional gastrointestinal disorders. Curr Opin Pharmacol. 2006;6:541–546. doi: 10.1016/j.coph.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 96.Hubbard CS, Labus JS, Bueller J, et al. Corticotropin-releasing factor receptor 1 antagonist alters regional activation and effective connectivity in an emotional-arousal circuit during expectation of abdominal pain. J Neurosci. 2011;31:12491–12500. doi: 10.1523/JNEUROSCI.1860-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tack JF, Dochev YS, Bochenek A, et al. Efficacy of ibodutant, a selective antagonist of neurokinin 2 receptors, in irritable bowel syndrome with diarrhoea (IBS-D): the results of a double-blind, randomised, placebo-controlled, parallel-group phase II study (the IRIS-2) Gastroenterology. 2013;144(suppl 1):S92–S93. doi: 10.1016/S0016-5085(13)60340-6. [DOI] [Google Scholar]
- 98.Zakko S, Barton G, Weber E, Dunger-Baldauf C, Rühl A. Randomised clinical trial: the clinical effects of a novel neurokinin receptor antagonist, DNK333, in women with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2011;33:1311–1321. doi: 10.1111/j.1365-2036.2011.04656.x. [DOI] [PubMed] [Google Scholar]
- 99.Zhang T, Finn DF, Barlow JW, Walsh JJ. Mast cell stabilisers. Eur J Pharmacol. doi: 10.1016/j.ejphar.2015.05.071. Published Online First: 27 Jun 2015. [DOI] [PubMed] [Google Scholar]
- 100.Lee JH, Kim TH, Kim HS, et al. An indoxyl compound 5-bromo-4-chloro-3-indolyl 1,3-diacetate, CAC-0982, suppresses activation of Fyn kinase in mast cells and IgE-mediated allergic responses in mice. Toxicol Appl Pharmacol. 2015;285:179–186. doi: 10.1016/j.taap.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 101.Cenac N, Andrews CN, Holzhausen M, et al. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117:636–647. doi: 10.1172/JCI29255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van Wanrooij S, Wouters MM, van Oudenhove L, Vermeire S, Rutgeerts PJ, Boeckxstaens GE. Effect of the H1-receptor antagonist ebastin on visceral perception and clinical symptoms in IBS. Gastroenterology. 2013;144(suppl 1):S160. doi: 10.1016/S0016-5085(13)60576-4. [DOI] [Google Scholar]
- 103.Labus JS, Hubbard CS, Bueller J, et al. Impaired emotional learning and involvement of the corticotropin-releasing factor signaling system in patients with irritable bowel syndrome. Gastroenterology. 2013;145:1253–1261. e1–e3. doi: 10.1053/j.gastro.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sweetser S, Camilleri M, Linker Nord SJ, et al. Do corticotropin releasing factor-1 receptors influence colonic transit and bowel function in women with irritable bowel syndrome? Am J Physiol Gastrointest Liver Physiol. 2009;296:G1299–G1306. doi: 10.1152/ajpgi.00011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]