Abstract
Gut microbiome is an integral part of the Gut-Brain axis. It is becoming increasingly recognized that the presence of a healthy and diverse gut microbiota is important to normal cognitive and emotional processing. It was known that altered emotional state and chronic stress can change the composition of gut microbiome, but it is becoming more evident that interaction between gut microbiome and central nervous system is bidirectional. Alteration in the composition of the gut microbiome can potentially lead to increased intestinal permeability and impair the function of the intestinal barrier. Subsequently, neuro-active compounds and metabolites can gain access to the areas within the central nervous system that regulate cognition and emotional responses. Deregulated inflammatory response, promoted by harmful microbiota, can activate the vagal system and impact neuropsychological functions. Some bacteria can produce peptides or short chain fatty acids that can affect gene expression and inflammation within the central nervous system. In this review, we summarize the evidence supporting the role of gut microbiota in modulating neuropsychological functions of the central nervous system and exploring the potential underlying mechanisms.
Keywords: Anxiety, Brain-Gut axis, Depression, Gut microbiota, Stress
Introduction
Over the past decade, experimental data has suggested a complex and bidirectional interaction between the gastrointestinal (GI) tract and the central nervous system (CNS), the so-called “Gut-Brain axis.”1 Derangements of this axis (typically in the brain-to-gut direction) have been implicated in the pathogenesis of symptoms of many functional bowel disorders such as the irritable bowel syndrome (IBS).2,3 In recent years, however, emerging knowledge about gut microbiota has compelled us to re-examine the directionality of this process.4–11 The presence of a healthy and diverse gut microbiota appears to be imperative not only for normal gastrointestinal function, but may also influence a variety of systemic and mental processes. Our understanding of the interaction between gut microbiota and the CNS is incomplete and only at its starting point. In this article, we will review the current evidence in the literature that points towards a role for gut microbiota in various developmental and psychiatric disorders such as anxiety, depression, schizophrenia and autism. We will also review the possible mechanisms through which gut microbiota might be involved in the pathogenesis of these disorders.
The gut microbiota at infancy is usually diverse and highly variable, trending towards its final composition between 6–12 months of age,12 reflecting a combination of genetic factors, maternal health, method of delivery, subsequent nutrition, and maternal and postnatal exposure to antibiotics.13–16 Germ-free mice show developmental abnormality in the GI tract that can be reversed by reconstructing the gut microbiota, suggesting a role for gut microbiota in postnatal development of the enteric nervous system (ENS).17,18 This period is also critical for the development of the CNS leading to the suggestion, based on experimental models, that gut microbiota may be an important factor participating in the development of cognitive, emotional, and behavioral processes shortly after birth.19,20 For example, germ free mice show significant alteration in the concentration of the key neurotransmitters such as serotonin in the hypothalamus.21 Alterations in serotonin concentration can in turn affect several aspects of the development of central nervous system, including synapse formation and connectivity between various regions in the central nervous system and their plasticity.22 The picture becomes more complicated because serotonin is also a key factor in the development of the ENS, and alteration of its concentration in the blood may modulate ENS structure and function23; in turn this can affect the composition of gut microbiota, thus potentially providing a closed loop system for mutual regulation of the 2 nervous systems.
Microbiota and Modulation of the Central Nervous System—General Mechanisms
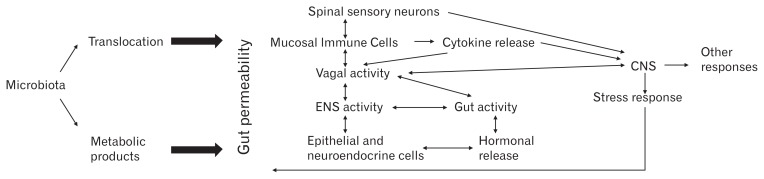
A central issue in any discussion on this topic relates to the question of how microbes that live in the colon can influence a remote organ such as the brain. We are just beginning to scratch the surface of this problem, but theoretically there are multiple, possible overlapping mechanisms, that amplify each other in short as well as long loops (Figure). With the exception of the microbe-epithelial interface, all these mechanisms imply some degree of access of either the microorganism itself or its products to the deeper layers of the gut, in turn activating a myriad of factors. Thus, as is being increasingly recognized, gut permeability is perhaps the most important factor in initiating microbial interactions with the rest of the body. These factors will now be briefly described.
Figure.
Bidirectional interactions between gut microbiota, gut permeability and central nervous system (CNS). Increased gut permeability can lead to translocation of gut microbiota or metabolic products such as lipopolysaccharides through the intestinal barrier. Exposure of epithelial cells or mucosal immune cells to bacterial or metabolic products can lead to activation of an immune response and release of pro-inflammatory cytokines. Additionally, metabolic products can directly affect the function of enteric neurons, spinal sensory neurons and vagus nerve through activation to Toll-like receptors or translocation and release of neuroactive peptides and hormones. On the other hand, stress can lead to activation of the hypothalamus-pituitary axis and excessive release of the corticotropin-releasing factor. This hormone along with altered vagal activity can modulate the local activation of mast cells in the intestinal wall and release of cytokines, causing increased gut permeability. ENS, enteric nervous system.
Effect of Gut Microbiota on Intestinal Permeability
The normal intestinal barrier consists of multiple layers that includes gut flora and external mucus layer, epithelial layer, and lamina propria, to name them from outside to inside.24 Mucus is secreted by goblet cells and acts as a mechanical protective layer that also contains digestive and antibacterial enzymes and antibodies, and will hydrate the epithelial layer and helps it regenerate.25 The epithelial layer, in addition to playing an important part in absorption of the nutrients, also serves as a physical barrier due to the tight junctions between the epithelial cells. Furthermore, enteroendocrine cells are distributed through the epithelial layer.26 This layer along with lamina propria is also the host of the largest repository of immune cells in the body which is known as mucosa-associated immune cells. The population of immune cells in the epithelial layer is mostly CD8+ lymphocytes, while the immune cells in the lamina propria are more diverse and consisted of macrophages, plasma cells, antigen presenting cells, and mast cells in addition to lymphocytes.27
Normal gut microbiota is essential in preventing colonization of the harmful bacteria by competing with them for vital resources such as food and growth factors. If the population of normal gut microbiota is reduced, for example due to antibiotic therapy, pathogenic organisms find the opportunity to colonize the gut epithelium. Toxins produced by pathogenic microorganisms and the focal inflammation created by immune responses to them can increase gut permeability.28 For example, Clostridium difficile that can colonize the gut in the absence of normal gut flora produces an enterotoxin that increase the gut permeability by impairing epithelial tight junctions through damaging aggregation of actin filaments.29 Another way that gut microbiota can enhance the function of the intestinal barrier is through protecting and improving epithelial tight junctions. Most of the evidence that supports this role of microbiota in the normal function of the intestinal barrier comes from studies that have shown that probiotic treatment can reduced gut permeability in models of GI tract disorders. For example, in experimental models of colitis, several species of probiotics including Lactobacillus, Escherichia coli, and Bifidobacterium can reduce gut permeability by upregulating trans-membrane proteins that are important in preserving tight junctions between epithelial cells.30–33 It has also been shown that treatment with these probiotics can enhance mucus production and consequently improve the physical barrier protecting the epithelial layer.34,35 Products of bacterial fermentation can also play an important role in maintaining the intestinal barrier. It has been shown that short-chain fatty acids can act as trophic factors for mucosal and epithelial layers. Also, normal bacteria can produce trophic peptides such as glucagon-like peptide-2 (GLP-2) that can enhance the proliferation of crypt cells and villi.36,37
Impaired intestinal barrier function and consequent increased gut permeability can lead to increased translocation of gut bacteria across the intestinal wall and into the mesenteric lymphoid tissue.34 Increased exposure of the ENS or mucosal immune cells to bacteria can provoke an immune response that can lead to release of inflammatory cytokines and activation of the vagus nerve and spinal afferent neurons. Inflammatory cytokines and the vagal system in turn can modulate the activity of the CNS and ENS.38,39 Furthermore, increased permeability of the gut can also increase the translocation of metabolic products such as lipopolysaccharide (LPS) or neuro-active peptides created by the bacteria that can alter the activity of the ENS and CNS.40 For example, LPS can activate Toll-Like receptors that are present on epithelial cells, enteric neurons, sensory afferent neurons in the spine, and various cells in the brain, modulating their activity and affecting the function of both ENS and CNS.41–44
As mentioned above, the interaction between the gut and brain is bidirectional- the CNS can affect gut permeability and increased gut permeability in turn can alter CNS function. In both animal models of stress and human subjects who were exposed to stress, the intestinal barrier is impaired. It has been shown that both acute and chronic stress can reduce water secretion and increase ion secretion in the intestine, and therefore impair the physical protection of the epithelial layer and lamina propria against adhesion of harmful bacteria and nociceptive chemicals.45–47 Activation of the hypotha-lamic-pituitary-adrenal (HPA) axis and increased production of corticotropin-releasing factor (CRF), altered activation of the vagal system, mast cell activation, and release of certain cytokines such as IFN-γ, TNF-α, and IL-4 are suggested culprits in this interaction.48–54 Additionally, stress can change the function of mucosal-associated immune cells and cause increased antigenic and bacterial uptake.55,56 Multiple studies have been published that have shown that the composition of gut microbiota is changed in the face of acute or chronic stress, and this in turn can subsequently change the function of intestinal barrier as explained above.57–60 There is limited data regarding the changes in intestinal barrier or GI physiology and the underlying mechanisms of it in neuropsychiatric disorders. It has been reported that the frequency of GI symptoms is increased in children with autism but the mechanism is not known.61 In patients with schizophrenia, there are increased intestinal permeability and change in intestinal function.62 Emotional stress and depression have been shown to increase prevalence of disorders of the digestive system.63
Effect of Bacterial Metabolites on the Central Nervous System
Theoretically, bacterial products like other luminal contents, can be absorbed into the blood stream and affect remote sites in the brain. Alternatively, or in addition, bacteria can interact with local elements in the gut such as nerves or endocrine cells that then in turn signal to the brain. Experimental data suggest that a variety of biologically active products derived from gut microbiota can directly or indirectly influence the brain. These include well known, although non-specific, factors such as LPS, which can influence the CNS directly by activating Toll-like receptor 4 on microglial cells causing release of inflammatory cytokines by them within the CNS, or indirectly by inducing release of inflammatory cytokines from the GI tract.64,65 LPS can cause behavioral changes during an acute illness or cause a delayed change in mood after sickness.66,67 IgA and IgM against LPS of gut bacteria are found in the blood of patients with depression or chronic fatigue syndrome, suggesting a potential role for LPS in the pathogenesis of these diseases.66 Other bacterial products reflect the role of colonic microbiota in the fermentation of undigested carbohydrates to short chain fatty acids (SCFA).68 SC-FAs can act as signaling molecules by binding to G protein-coupled receptors, Gpr41, and Gpr43.69–71 It has been shown that Gpr41 and Gpr43 receptors are abundantly present on the surface of gut epithelial and immune cells and are activated by SCFAs. This activation can provoke an inflammatory and immune response that can be helpful in the setting of an acute infection, but dysregulation can produce an exaggerated response leading to increased gut permeability and increased absorption of neuro-active metabolites.72,73 SCFAs can also directly activate the sympathetic nervous system through Gpr41 receptors that are found on sympathetic ganglionic neurons.74 Furthermore, it has been shown that SCFAc can pass through the blood-brain barrier and influence behavior, neural signaling, the production of neurotransmitters and, ultimately, behavior.75–77
Change in Central Nervous System Neurotransmitters
Studies have reported on CNS neurotransmitter changes in response to more specific biological factors that may be restricted to certain types of bacteria, thus providing a mechanistic link to changes in microbial metabolism. Germ free mice have elevated levels of dopamine and tryptophan in striatum, but not serotonin or gama-amino butyric acid (GABA).78 Another study has reported increased levels of serotonin in the hippocampus of germ free mice.79 It has been recently shown that indigenous bacteria from gut of mice and humans can induce serotonin production in entrochromaffin cells and increase the level of serotonin in blood.80 Histaminergic pathways are found in areas of the limbic system and also areas in the brain heavily involved in cognitive functions.81 Lactobacillus reuteri, a commensal in the human gut, expresses histidine decar-boxylase that converts histidine to histamine. Therefore, a change in the population of this gut microbe can potentially modulate the levels of circulating histidine and histamine,82 which in turn can affect the concentration of CNS histamine.83 In another example, rats that are given Bifidobacterium infantis for 14 days showed increased concentrations of the serotonin precursor tryptophan in plasma, an effect that may be mediated by the altered expression of indoleamine-2,3-dioxygenase, a key enzyme in the kynurenine pathway of tryptophan degradation.84,85 In addition to pathogen-specific alterations in neurotransmitters within the CNS, specific structural microbial molecules that are often referred to as microbe-associated molecular patterns (MAMPs) are found in the brain. The profile of MAMPs has been shown to be significantly influenced by the composition of gut microbiota.21,86,87
Vagus Nerve as a Mediator of the Effect of Gut Microbiota on the Central Nervous System
One potential unifying mechanism through which these various processes can influence the activity of CNS is via vagal nerve activity. In animal models, administration of Campylobacter jejuni into the gut can induce anxiety like behavior. These animals show increased fos activity in vagal sensory nucleus and other areas in brain stem related to this nucleus.88 Furthermore, intraduodenal administration of a non-pathogenic bacterium, Bifidobacterium longum, is anxiolytic but also requires an intact vagus.89 Another anxiolytic probiotic, Lactobacillus rhamnosus, results in region specific change in the expression of GABA receptor subunits. GABA type B subunit 1 isoform b (GABAB1b) mRNA was decreased in the amygdala and hippocampus, while increased in cortical areas. On the other hand, GABAAα 2 receptor mRNA showed the opposite changes.90 These effects were abolished by subdiaphragmatic vagotomy.90 The vagus nerve might also be involved in behavioral effects of microbial LPS. It is known that LPS can induce depressive-like and anxious behavior in animal models.91 Studies have shown that rat or mice that undergo vagotomy before exposure to LPS, do not show the expected cytokine profile changes in the CNS92 or the same depressive or anxious behavior.93,94 However, the role of the vagus may be restricted to specific models or pathogenic processes. Thus, mice infected with the noninvasive parasite Trichuris muris exhibit anxiety-like behavior, associated with colitis and decreased hippocampal brain derived neurotrophic factor (BDNF) expression, along with increases in circulating TNF-α and IFN-γ, as well as the kynurenine and kyn-urenine/tryptophan ratio. Although anxiety behavior was normalized by both anti-inflammatory agents and the probiotic B. longum, it persisted in infected animals after a vagotomy.95
Gut Microbiota and Specific Neuropsychological Processes and Phenotypes
Stress Response and Anxiety
It has been shown that stress can alter gut permeability as well as the composition of gut microbiota.46,57 In a mice model of stress due to social disruption, Bacteroids are reduced while Clostridia are increased, resulting in a pro-inflammatory change in the profile of cytokines produced by gut microbiota.58 More recently, the interaction between stress and gut microbiome has been shown to be bidirectional, and that gut microbes can modulate the stress response and the activity of the corticosterone pathway orchestrated by the HPA, a key stress regulatory system in the CNS. Germ-free mice show an exaggerated HPA response to stress and the amount of CRF released in response to stress.96 Introduction of B. infantis corrects the abnormal response of the HPA to stress in this model, but only if administered within the first 6 weeks of age in this model.96 This finding is in line with the idea that the effect of microbes on the host is confined to a window of opportunity during neonatal life, and the presence or absence of any specific microbe during that window might have a durable and long-life effect.97 Germ-free mice also show reduced anxiety in behavioral tasks78 which can be reversed by re-introduction of gut microbiota.98 An anxiety prone behavioral phenotype is also seen in germ-free rats, along with elevated CRF expression in the hypothalamus and reduced glu-cocorticoid receptor (GR) expression in the hippocampus, (GR in this region regulates the CRF response in a negative feedback loop) and along with a lower dopaminergic turnover rate in specific CNS regions.99
Exposing rats in the early postnatal period to stress by maternal separation also leads to a change in composition of gut microbiota, which is linked to a long-term increase in anxiety-like behavior.100–102 Introducing probiotics containing Lactobacillus in early stages of separation can ameliorate the effects of separation on HPA and reduce the corticosterone release.60 Other events that lead to a change in the composition of gut microbiota such as infection or use of probiotics can also change the level of anxiety.89,103–105
Further, it is shown that the behavioral phenotype of anxietyprone strains of mice is also dependent on their existing microbiota. For example, BALB/c mice exhibit a highly anxious phenotype that does not show much exploratory locomotion in a new environment, while NIH Swiss mice show less anxiety and more exploratory motions in the same environment. Transferring gut microbiota from one of the species to another can change their behavior to the one typical of the donor.106 In another study, described above, infecting mice with Trichuris led to anxiety related behavior in mice, an effect that was reversed by administration of B. longum but not lactobacillus showing a species specific effect for gut microbiota.95 The underlying mechanisms, however, remain unclear as this was not vagally mediated. Further, anti-inflammatory agents normalized behavior and reduced cytokine and kynurenine levels without an effect on BDNF expression, whereas B. longum normalized behavior and BDNF mRNA but did not affect cytokine or kynurenine levels.
It has also been suggested that the modulatory effects of gut microbiota on the level of anxiety are exerted through alterations in serotonin signaling.84 This idea is in part based on the finding that reduced anxiety-like behavior in germ free mice is associated with increased expression of serotonin receptor 1A in the hippocampus.78 However, experimental data from mice showed that while reintroduction of gut microbiota to germ free mice can normalize the anxious behavior, it fails to reverse the changes in serotonin levels in the hypothalamic-pituitary pathway.79 Another mechanism that has been described is increased release of the adreno-corticotropin hormone from the HPA axis in response to stress.96 A link between hypersensitivity of the HPA axis and reduced BDNF expression in the prefrontal cortex and hippocampus, and subsequently reduced N-methyl-d-aspartate receptor expression in germ-free mice is observed and was thought to play a role in regulation of HPA activity.78 Alteration of BDNF expression in the hippocampus was seen in mice that were treated with non-absorbable antibiotics such as neomycin, but not in mice treated with systemic intraperitoneal injection of antibiotics, suggesting that this effect is a result of elimination of gut microbiota, not the antibiotic treatment itself.106
Gut Microbiota and Depression
In animal models of depression, it has been reported that the composition of gut microbiota has been changed.107,108 These data however, have not been validated in patients with depression. In one study on human subjects with depression, no significant difference in the composition of gut microbiota was found between depressed patients and a control group.109 However, another recent study examined the composition of fecal microbiota in 46 patients with depression and 30 healthy controls, and reported significant differences with increased population of Bacteroidetes, Proteobacteria, and Actinobacteria, and decreased population of Frimicutes in patients with depression.110 Other evidence that might suggest a role for gut microbiota in the pathogenesis of depression is from studies that have shown certain probiotics can alleviate depressive symptoms in rodent models. Rats that are exposed to stress in early stages of life show behavior traits that are consistent with mood disorder that persists through their adulthood. Treatment of these rats with probiotics containing B. infantis can reduce the mood disturbance and correct the abnormalities in the concentration of norepinephrine in the brain.111 L. rhamnosus and L. helveticus strains have also been reported to ameliorate maternal separation-induced depression through a corticosterone and GABA mediated mechanism.60,90 In a model of depression post myocardial infarction, treatment with probiotics including L. helveticus and B. longum has been reported to reduce the depression, presumably by reducing the pro-inflammatory cytokines and gut permeability.112,113 Some antibiotics such as minocycline have been shown to be effective in treatment of depression. Their mechanism of action is not exactly understood, and a potential role for changes in gut microbiota has been proposed, although not studied in detail.114,115
Gut Microbiota and Cognition
In mice, elimination of gut microbiota can alter performance in tasks that require intact spatial memory, hippocampal function or working memory.116 Similarly, altering the composition of gut microbiota in mice by infection or dietary modifications also can change the performance of the animal in memory tasks.117 For example, adding lean beef to the mice diet will alter composition of gut microbiota and will improve their performance in cognitive tasks. In this experiment, a temporal relationship with dietary induced changes in gut microbiota and working memory performance was reported.118 Mice infected with Citrobacter rodentium show impairment of cognitive function that can be reversed with probiotics.117 Diabetic rats are known to have impaired memory and learning due to impaired long-term potentiation and long-term depression in hippocampal synapses.119 It has been reported that treating the diabetic rats with a mixture of Lactobacillus acidophilus, Bifidobacterium lacti, and Lactobacillus fermentum, can ameliorate this effect of diabetes on memory and behavior, as well as electrophysiological changes.120 Evidence of similar effects in humans is limited but it has been shown that in normal human subjects, consumption of probiotics can alter the functional activity of the areas in the brain that are involved in cognitive functions.121
Emerging Areas
Given the explosion of interest in the microbiota and the gut-brain axis, it is not surprising that investigators are moving beyond more traditional phenotypes such as anxiety/depression to other neuropsychological syndromes including schizophrenia and autism. Increased gut permeability and translocation of gut bacteria has been shown in schizophrenic patients.122 The fundamental cause of this is unknown and could include both the controversial association with gluten sensitivity and celiac disease123 as well as primary changes in gut microbiota.62,124 These theories may not be mutually exclusive as it is possible that certain compositions of gut microbiota can lead to changed metabolism of certain food products such as gluten, and subsequent production of neuroactive peptides, increased absorption of these products due to local inflammation, and alteration of dopaminergic and serotonergic pathways in individuals who are genetically susceptible to schizophrenia.62 Germ-free mice tend to show a schizoid type behavior, not spending more time in a chamber with another mice in it when put in a 3-chamber sociability test.125 In a mice model that shows behavioral changes that resemble schizophrenia, treatment with Bacteroides fragilis can ameliorate the symptoms.126 However, a randomized clinical trial of a probiotic regimen containing Lactobacillus and Bifidobacterium strains failed to show any significant change in the psychiatric outcome measures was observed.127
Another area in which information is rapidly evolving is that of autism spectrum disorders (ASD). In a study in rodent model, it was found that the composition of gut microbiota in animals with ASD-like behavior is significantly changed compared with control animals. These changes were similar to those found in human patients with most changes observed in Clostridia and Bacterioidia species (see below). Treatment of these animals with B. fragilis restored the altered composition of gut microbiota and significantly reduced the stereotypical behavior.128 It has been hypothesized that these effects are mediated by specific chemical metabolites produced by gut microbes. For example, in the same rodent model of autism, elevated circulating levels of 4-ethylphenylsulfate normalized after probiotic treatment. However, systemic administration of this metabolite did not cause autistic behavior and only created anxiety-related behavior. Other mechanisms involve changes in the availability of tryptophan and histidine, and consequent alterations in serotonin and histamine in the CNS.129 Propionic acid, a SFCA that is produced by gut microbiota can also induce significant changes in social development and behavior and create a similar picture to ASD.130,131 Intraventricular administration of propionic acids to rats can cause structural abnormalities similar to those found in patients with ASD.132,133
Some of these findings have parallels in humans. Children with ASD also show altered composition of gut microbiota with a reduced population of Bacteroides and increased levels of Clostridium species.134–136 It has been postulated that altered gut microbiota in children with ASD can lead to potential imbalances in metabolism of carbohydrates and amino acids in the gut, and altered levels of metabolites in the blood and urine. To test this hypothesis, a few studies of metabolic products have been performed in patients with ASD. For example, studies using metabolomics techniques have reported a different urinary amino acid profile in patients with ASD compared to healthy subjects with lower anti-oxidant levels in the urine, and abnormal levels of hippurate and N-methyl nicotinic acid in children with ASD.137,138 Hippurate is an end-product of the metabolism of dietary proteins and phenolic acids, and is formed by the liver from benzoic acid.139 Benzoic acid is a product of protein metabolism by gut microbiota, and altered hippurate level points towards altered metabolism in protein, potentially caused by altered gut microbiota.140 Another study has reported significant difference in children with autism and normal children in the fatty acid composition of phospholipids, with autistic children having an increased level of most of the saturated fatty acids, except for propionic acid, and a decreased level of polyunsaturated fatty acids.141 This change in composition of fatty acids can lead to abnormalities in oxidative stress, or cause mitochondrial dysfunction that might play a role in pathogenesis of ASD.142
Another bacterial genus that has been linked to autism is Desulfovibrio. This microbe is found with significantly higher prevalence in children with autism compared to developmentally normal children, and has sulfur-reducing properties that can explain the known sulfur deficiency in children with autism.143 Sulfate deficiency can potentially lead to inefficient detoxification through sulfation, leading to accumulation of neurotoxins. Increased gut permeability and elevated level of bacterial metabolic products such as LPS leading to increased proinflammatory cytokines such as IL-6 have also been shown in children with ASD.144
A few small clinical trials have shown beneficial effects for gluten free and casein free diets on symptoms of children with ASD145,146 that could potentially be attributed to the change in gut microbiota.4,8,19,61,128,147 Furthermore, in children with autism, the frequency of GI symptoms is increased148,149 and has been attributed to a low-grade chronic inflammation in the GI tract caused by altered gut microbiota. In a clinical study, oral vancomycin was used as a minimally absorbed antibiotic to treat the GI problems, based on this theory. Interestingly, in addition to improvement in GI symptoms, autistic behavior was also improved in these children.150
Conclusions
The influence of gut microbiota on several aspects of CNS function is increasingly supported by a growing body of experimental data. The mechanism of this influence is complex and involves multiple direct and indirect pathways. Increased gut permeability appears to be the cornerstone of the microbiome-gut-brain interaction. This provides a pathway for gut bacteria and their metabolic products to access the immune system, ENS, the blood stream, and centripetal neural pathways. Much of this evidence comes from rodent studies, and considerable work has to be done to validate these findings in humans before we can understand how best, if at all, to modulate the gut microbiota for clinical benefit.
Footnotes
Financial support: None.
Conflicts of interest: None.
Author contributions: Shadi Yarandi and Pankaj J Pasricha performed the review of the literature, reviewed the manuscripts and wrote the manuscript; and Daniel A Peterson, Glenn J Tries-man, and Timothy H Moran participated in reviewing the data, discussion on interpreting the data, and writing the manuscript.
References
- 1.Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology. 2009;136:2003–2014. doi: 10.1053/j.gastro.2009.01.075. [DOI] [PubMed] [Google Scholar]
- 3.Mayer EA, Savidge T, Shulman RJ. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology. 2014;146:1500–1512. doi: 10.1053/j.gastro.2014.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Asmakh M, Anuar F, Zadjali F, Rafter J, Pettersson S. Gut microbial communities modulating brain development and function. Gut Microbes. 2012;3:366–373. doi: 10.4161/gmic.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borre YE, Moloney RD, Clarke G, Dinan TG, Cryan JF. The impact of microbiota on brain and behavior: mechanisms & therapeutic potential. Adv Exp Med Biol. 2014;817:373–403. doi: 10.1007/978-1-4939-0897-4_17. [DOI] [PubMed] [Google Scholar]
- 6.Bravo JA, Julio-Pieper M, Forsythe P, et al. Communication between gastrointestinal bacteria and the nervous system. Curr Opin Pharmacol. 2012;12:667–672. doi: 10.1016/j.coph.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10:735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 8.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 9.Cryan JF, O’Mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil. 2011;23:187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- 10.Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K. Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci. 2014;34:15490–15496. doi: 10.1523/JNEUROSCI.3299-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azad MB, Konya T, Maughan H, et al. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. 2013;185:385–394. doi: 10.1503/cmaj.121189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Penders J, Thijs C, Vink C, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 16.Zivkovic AM, German JB, Lebrilla CB, Mills DA. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc Natl Acad Sci USA. 2011;108(suppl 1):4653–4658. doi: 10.1073/pnas.1000083107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McVey Neufeld KA, Mao YK, Bienenstock J, Foster JA, Kunze WA. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol Motil. 2013;25:183, e188. doi: 10.1111/nmo.12049. [DOI] [PubMed] [Google Scholar]
- 18.Saulnier DM, Ringel Y, Heyman MB, et al. The intestinal microbiome, probiotics and prebiotics in neurogastroenterology. Gut Microbes. 2013;4:17–27. doi: 10.4161/gmic.22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med. 2014;20:509–518. doi: 10.1016/j.molmed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Clarke G, O’Mahony SM, Dinan TG, Cryan JF. Priming for health: gut microbiota acquired in early life regulates physiology, brain and behaviour. Acta Paediatr. 2014;103:812–819. doi: 10.1111/apa.12674. [DOI] [PubMed] [Google Scholar]
- 21.Wikoff WR, Anfora AT, Liu J, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavdas AA, Blue ME, Lincoln J, Parnavelas JG. Serotonin promotes the differentiation of glutamate neurons in organotypic slice cultures of the developing cerebral cortex. J Neurosci. 1997;17:7872–7880. doi: 10.1523/JNEUROSCI.17-20-07872.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu MT, Kuan YH, Wang J, Hen R, Gershon MD. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci. 2009;29:9683–9699. doi: 10.1523/JNEUROSCI.1145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farhadi A, Banan A, Fields J, Keshavarzian A. Intestinal barrier: an interface between health and disease. J Gastroenterol Hepatol. 2003;18:479–497. doi: 10.1046/j.1440-1746.2003.03032.x. [DOI] [PubMed] [Google Scholar]
- 25.Liévin-Le Moal V, Servin AL. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clin Microbiol Rev. 2006;19:315–337. doi: 10.1128/CMR.19.2.315-337.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ménard S, Cerf-Bensussan N, Heyman M. Multiple facets of intestinal permeability and epithelial handling of dietary antigens. Mucosal Immunol. 2010;3:247–259. doi: 10.1038/mi.2010.5. [DOI] [PubMed] [Google Scholar]
- 27.Gill N, Wlodarska M, Finlay BB. Roadblocks in the gut: barriers to enteric infection. Cell Microbiol. 2011;13:660–669. doi: 10.1111/j.1462-5822.2011.01578.x. [DOI] [PubMed] [Google Scholar]
- 28.Lyte M. Microbial endocrinology in the microbiome-gut-brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog. 2013;9:e1003726. doi: 10.1371/journal.ppat.1003726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hecht G, Pothoulakis C, LaMont JT, Madara JL. Clostridium difficile toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J Clin Invest. 1988;82:1516–1524. doi: 10.1172/JCI113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin HL, Zheng JJ, Tong DN, et al. Effect of Lactobacillus plantarum enteral feeding on the gut permeability and septic complications in the patients with acute pancreatitis. Eur J Clin Nutr. 2008;62:923–930. doi: 10.1038/sj.ejcn.1602792. [DOI] [PubMed] [Google Scholar]
- 31.Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, Schmidt MA. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCzeta redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol. 2007;9:804–816. doi: 10.1111/j.1462-5822.2006.00836.x. [DOI] [PubMed] [Google Scholar]
- 32.Patel RM, Myers LS, Kurundkar AR, Maheshwari A, Nusrat A, Lin PW. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am J Pathol. 2012;180:626–635. doi: 10.1016/j.ajpath.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin PW, Nasr TR, Berardinelli AJ, Kumar A, Neish AS. The probiotic Lactobacillus GG may augment intestinal host defense by regulating apoptosis and promoting cytoprotective responses in the developing murine gut. Pediatr Res. 2008;64:511–516. doi: 10.1203/PDR.0b013e3181827c0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dicksved J, Schreiber O, Willing B, et al. Lactobacillus reuteri maintains a functional mucosal barrier during DSS treatment despite mucus layer dysfunction. PLoS One. 2012;7:e46399. doi: 10.1371/journal.pone.0046399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ukena SN, Singh A, Dringenberg U, et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One. 2007;2:e1308. doi: 10.1371/journal.pone.0001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cani PD, Possemiers S, Van de Wiele T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caricilli AM, Picardi PK, de Abreu LL, et al. Gut microbiota is a key modulator of insulin resistance in TLR 2 knockout mice. PLoS Biol. 2011;9:e1001212. doi: 10.1371/journal.pbio.1001212. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Maes M. The cytokine hypothesis of depression: inflammation, oxidative & nitrosative stress (IO&NS) and leaky gut as new targets for adjunctive treatments in depression. Neuro Endocrinol Lett. 2008;29:287–291. [PubMed] [Google Scholar]
- 39.Gareau MG, Silva MA, Perdue MH. Pathophysiological mechanisms of stress-induced intestinal damage. Curr Mol Med. 2008;8:274–281. doi: 10.2174/156652408784533760. [DOI] [PubMed] [Google Scholar]
- 40.Holzer P, Danzer M, Schicho R, Samberger C, Painsipp E, Lippe IT. Vagal afferent input from the acid-challenged rat stomach to the brainstem: enhancement by interleukin-1beta. Neuroscience. 2004;129:439–445. doi: 10.1016/j.neuroscience.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 41.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 42.Barajon I, Serrao G, Arnaboldi F, et al. Toll-like receptors 3, 4, and 7 are expressed in the enteric nervous system and dorsal root ganglia. J Histochem Cytochem. 2009;57:1013–1023. doi: 10.1369/jhc.2009.953539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anitha M, Vijay-Kumar M, Sitaraman SV, Gewirtz AT, Srinivasan S. Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling. Gastroenterology. 2012;143:1006–1016. e4. doi: 10.1053/j.gastro.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Noort JM, Bsibsi M. Toll-like receptors in the CNS: implications for neurodegeneration and repair. Prog Brain Res. 2009;175:139–148. doi: 10.1016/S0079-6123(09)17509-X. [DOI] [PubMed] [Google Scholar]
- 45.Saunders PR, Kosecka U, McKay DM, Perdue MH. Acute stressors stimulate ion secretion and increase epithelial permeability in rat intestine. Am J Physiol. 1994;267(5 Pt 1):G794–G799. doi: 10.1152/ajpgi.1994.267.5.G794. [DOI] [PubMed] [Google Scholar]
- 46.Barclay GR, Turnberg LA. Effect of psychological stress on salt and water transport in the human jejunum. Gastroenterology. 1987;93:91–97. doi: 10.1016/0016-5085(87)90319-2. [DOI] [PubMed] [Google Scholar]
- 47.Barclay GR, Turnberg LA. Effect of cold-induced pain on salt and water transport in the human jejunum. Gastroenterology. 1988;94:994–998. doi: 10.1016/0016-5085(88)90558-6. [DOI] [PubMed] [Google Scholar]
- 48.Santos J, Benjamin M, Yang PC, Prior T, Perdue MH. Chronic stress impairs rat growth and jejunal epithelial barrier function: role of mast cells. Am J Physiol Gastrointest Liver Physiol. 2000;278:G847–G854. doi: 10.1152/ajpgi.2000.278.6.G847. [DOI] [PubMed] [Google Scholar]
- 49.Vicario M, Guilarte M, Alonso C, et al. Chronological assessment of mast cell-mediated gut dysfunction and mucosal inflammation in a rat model of chronic psychosocial stress. Brain Behav Immun. 2010;24:1166–1175. doi: 10.1016/j.bbi.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 50.Meddings JB, Swain MG. Environmental stress-induced gastrointestinal permeability is mediated by endogenous glucocorticoids in the rat. Gastroenterology. 2000;119:1019–1028. doi: 10.1053/gast.2000.18152. [DOI] [PubMed] [Google Scholar]
- 51.Saunders PR, Santos J, Hanssen NP, Yates D, Groot JA, Perdue MH. Physical and psychological stress in rats enhances colonic epithelial permeability via peripheral CRH. Dig Dis Sci. 2002;47:208–215. doi: 10.1023/A:1013204612762. [DOI] [PubMed] [Google Scholar]
- 52.Ferrier L, Mazelin L, Cenac N, et al. Stress-induced disruption of colonic epithelial barrier: role of interferon-gamma and myosin light chain kinase in mice. Gastroenterology. 2003;125:795–804. doi: 10.1016/S0016-5085(03)01057-6. [DOI] [PubMed] [Google Scholar]
- 53.Wallon C, Yang PC, Keita AV, et al. Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut. 2008;57:50–58. doi: 10.1136/gut.2006.117549. [DOI] [PubMed] [Google Scholar]
- 54.Vanuytsel T, van Wanrooy S, Vanheel H, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014;63:1293–1299. doi: 10.1136/gutjnl-2013-305690. [DOI] [PubMed] [Google Scholar]
- 55.Kiliaan AJ, Saunders PR, Bijlsma PB, et al. Stress stimulates transepithelial macromolecular uptake in rat jejunum. Am J Physiol. 1998;275(5 Pt 1):G1037–G1044. doi: 10.1152/ajpgi.1998.275.5.G1037. [DOI] [PubMed] [Google Scholar]
- 56.Velin AK, Ericson AC, Braaf Y, Wallon C, Soderholm JD. Increased antigen and bacterial uptake in follicle associated epithelium induced by chronic psychological stress in rats. Gut. 2004;53:494–500. doi: 10.1136/gut.2003.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bailey MT, Coe CL. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev Psychobiol. 1999;35:146–155. doi: 10.1002/(SICI)1098-2302(199909)35:2<146::AID-DEV7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 58.Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eutamene H, Bueno L. Role of probiotics in correcting abnormalities of colonic flora induced by stress. Gut. 2007;56:1495–1497. doi: 10.1136/gut.2007.124040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gareau MG, Jury J, MacQueen G, Sherman PM, Perdue MH. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut. 2007;56:1522–1528. doi: 10.1136/gut.2006.117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mayer EA, Padua D, Tillisch K. Altered brain-gut axis in autism: comorbidity or causative mechanisms? Bioessays. 2014;36:933–939. doi: 10.1002/bies.201400075. [DOI] [PubMed] [Google Scholar]
- 62.Severance EG, Yolken RH, Eaton WW. Autoimmune diseases, gastrointestinal disorders and the microbiome in schizophrenia: more than a gut feeling. Schizophr Res. doi: 10.1016/j.schres.2014.06.027. Published Online First: 14 Jul 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee SP, Sung IK, Kim JH, Lee SY, Park HS, Shim CS. The effect of emotional stress and depression on the prevalence of digestive diseases. J Neurogastroenterol Motil. 2015;21:273–282. doi: 10.5056/jnm14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim KA, Gu W, Lee IA, Joh EH, Kim DH. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One. 2012;7:e47713. doi: 10.1371/journal.pone.0047713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maes M, Twisk FN, Kubera M, Ringel K, Leunis JC, Geffard M. Increased IgA responses to the LPS of commensal bacteria is associated with inflammation and activation of cell-mediated immunity in chronic fatigue syndrome. J Affect Disord. 2012;136:909–917. doi: 10.1016/j.jad.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 67.Flierl MA, Rittirsch D, Nadeau BA, et al. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature. 2007;449:721–725. doi: 10.1038/nature06185. [DOI] [PubMed] [Google Scholar]
- 68.Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62:67–72. doi: 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- 69.Brown AJ, Goldsworthy SM, Barnes AA, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 70.Kimura I, Ozawa K, Inoue D, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xiong Y, Miyamoto N, Shibata K, et al. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci USA. 2004;101:1045–1050. doi: 10.1073/pnas.2637002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145:396–406. e1–e10. doi: 10.1053/j.gastro.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 73.Milo LA, Reardon KA, Tappenden KA. Effects of short-chain fatty acid-supplemented total parenteral nutrition on intestinal pro-inflammatory cytokine abundance. Dig Dis Sci. 2002;47:2049–2055. doi: 10.1023/A:1019676929875. [DOI] [PubMed] [Google Scholar]
- 74.Kimura I, Inoue D, Maeda T, et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41) Proc Natl Acad Sci USA. 2011;108:8030–8035. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Frost G, Sleeth ML, Sahuri-Arisoylu M, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5:3611. doi: 10.1038/ncomms4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mitchell RW, On NH, Del Bigio MR, Miller DW, Hatch GM. Fatty acid transport protein expression in human brain and potential role in fatty acid transport across human brain microvessel endothelial cells. J Neurochem. 2011;117:735–746. doi: 10.1111/j.1471-4159.2011.07245.x. [DOI] [PubMed] [Google Scholar]
- 77.DeCastro M, Nankova BB, Shah P, et al. Short chain fatty acids regulate tyrosine hydroxylase gene expression through a cAMP-dependent signaling pathway. Brain Res Mol Brain Res. 2005;142:28–38. doi: 10.1016/j.molbrainres.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 78.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neuro-gastroenterol Motil. 2011;23:255–264. e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 79.Clarke G, Grenham S, Scully P, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 80.Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Panula P, Nuutinen S. The histaminergic network in the brain: basic organization and role in disease. Nat Rev Neurosci. 2013;14:472–487. doi: 10.1038/nrn3526. [DOI] [PubMed] [Google Scholar]
- 82.Thomas CM, Hong T, van Pijkeren JP, et al. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One. 2012;7:e31951. doi: 10.1371/journal.pone.0031951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- 84.O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 85.Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepres-sant properties in the rat. J Psychiatr Res. 2008;43:164–174. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 86.Tremaroli V, Bäckhed F. Functional interactions between the gut micro-biota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 87.Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 88.Goehler LE, Gaykema RP, Opitz N, Reddaway R, Badr N, Lyte M. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav Immun. 2005;19:334–344. doi: 10.1016/j.bbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 89.Bercik P, Park AJ, Sinclair D, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 2011;23:1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bluthe RM, Walter V, Parnet P, et al. Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. C R Acad Sci III. 1994;317:499–503. [PubMed] [Google Scholar]
- 92.Laye S, Bluthé RM, Kent S, et al. Subdiaphragmatic vagotomy blocks induction of IL-1 beta mRNA in mice brain in response to peripheral LPS. Am J Physiol. 1995;268(5 Pt 2):R1327–R1331. doi: 10.1152/ajpregu.1995.268.5.R1327. [DOI] [PubMed] [Google Scholar]
- 93.Luheshi GN, Bluthé RM, Rushforth D, et al. Vagotomy attenuates the behavioural but not the pyrogenic effects of interleukin-1 in rats. Auton Neurosci. 2000;85:127–132. doi: 10.1016/S1566-0702(00)00231-9. [DOI] [PubMed] [Google Scholar]
- 94.Konsman JP, Luheshi GN, Bluthé RM, Dantzer R. The vagus nerve mediates behavioural depression, but not fever, in response to peripheral immune signals; a functional anatomical analysis. Eur J Neurosci. 2000;12:4434–4446. doi: 10.1046/j.0953-816X.2000.01319.x. [DOI] [PubMed] [Google Scholar]
- 95.Bercik P, Verdu EF, Foster JA, et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102–2112. e1. doi: 10.1053/j.gastro.2010.06.063. [DOI] [PubMed] [Google Scholar]
- 96.Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558(Pt 1):263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zeissig S, Blumberg RS. Life at the beginning: perturbation of the microbiota by antibiotics in early life and its role in health and disease. Nat Immunol. 2014;15:307–310. doi: 10.1038/ni.2847. [DOI] [PubMed] [Google Scholar]
- 98.Matthews DM, Jenks SM. Ingestion of Mycobacterium vaccae decreases anxiety-related behavior and improves learning in mice. Behav Processes. 2013;96:27–35. doi: 10.1016/j.beproc.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 99.Crumeyrolle-Arias M, Jaglin M, Bruneau A, et al. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology. 2014;42:207–217. doi: 10.1016/j.psyneuen.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 100.Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci. 2007;25:3091–3098. doi: 10.1111/j.1460-9568.2007.05522.x. [DOI] [PubMed] [Google Scholar]
- 101.O’Mahony SM, Hyland NP, Dinan TG, Cryan JF. Maternal separation as a model of brain-gut axis dysfunction. Psychopharmacology (Berl) 2011;214:71–88. doi: 10.1007/s00213-010-2010-9. [DOI] [PubMed] [Google Scholar]
- 102.O’Mahony SM, Marchesi JR, Scully P, et al. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65:263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 103.Goehler LE, Park SM, Opitz N, Lyte M, Gaykema RP. Campylobacter jejuni infection increases anxiety-like behavior in the holeboard: possible anatomical substrates for viscerosensory modulation of exploratory behavior. Brain Behav Immun. 2008;22:354–366. doi: 10.1016/j.bbi.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lyte M, Varcoe JJ, Bailey MT. Anxiogenic effect of subclinical bacterial infection in mice in the absence of overt immune activation. Physiol Behav. 1998;65:63–68. doi: 10.1016/S0031-9384(98)00145-0. [DOI] [PubMed] [Google Scholar]
- 105.Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes. 2011;2:256–261. doi: 10.4161/gmic.2.4.16108. [DOI] [PubMed] [Google Scholar]
- 106.Bercik P, Denou E, Collins J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609. 609, e1–e3. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 107.Park AJ, Collins J, Blennerhassett PA, et al. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol Motil. 2013;25:733, e575. doi: 10.1111/nmo.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dinan TG, Cryan JF. Melancholic microbes: a link between gut microbiota and depression? Neurogastroenterol Motil. 2013;25:713–719. doi: 10.1111/nmo.12198. [DOI] [PubMed] [Google Scholar]
- 109.Naseribafrouei A, Hestad K, Avershina E, et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 2014;26:1155–1162. doi: 10.1111/nmo.12378. [DOI] [PubMed] [Google Scholar]
- 110.Jiang H, Ling Z, Zhang Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 111.Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 112.Arseneault-Breard J, Rondeau I, Gilbert K, et al. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. Br J Nutr. 2012;107:1793–1799. doi: 10.1017/S0007114511005137. [DOI] [PubMed] [Google Scholar]
- 113.Gilbert K, Arseneault-Bréard J, Flores Monaco F, et al. Attenuation of post-myocardial infarction depression in rats by n-3 fatty acids or probiotics starting after the onset of reperfusion. Br J Nutr. 2013;109:50–56. doi: 10.1017/S0007114512003807. [DOI] [PubMed] [Google Scholar]
- 114.Mello BS, Monte AS, McIntyre RS, et al. Effects of doxycycline on depressive-like behavior in mice after lipopolysaccharide (LPS) administration. J Psychiatr Res. 2013;47:1521–1529. doi: 10.1016/j.jpsychires.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 115.Soczynska JK, Mansur RB, Brietzke E, et al. Novel therapeutic targets in depression: minocycline as a candidate treatment. Behav Brain Res. 2012;235:302–317. doi: 10.1016/j.bbr.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 116.Gareau MG. Microbiota-gut-brain axis and cognitive function. Adv Exp Med Biol. 2014;817:357–371. doi: 10.1007/978-1-4939-0897-4_16. [DOI] [PubMed] [Google Scholar]
- 117.Gareau MG, Wine E, Rodrigues DM, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60:307–317. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- 118.Li W, Dowd SE, Scurlock B, Acosta-Martinez V, Lyte M. Memory and learning behavior in mice is temporally associated with diet-induced alterations in gut bacteria. Physiol Behav. 2009;96:557–567. doi: 10.1016/j.physbeh.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 119.Kamal A, Biessels GJ, Ramakers GM, Hendrik Gispen W. The effect of short duration streptozotocin-induced diabetes mellitus on the late phase and threshold of long-term potentiation induction in the rat. Brain Res. 2005;1053:126–130. doi: 10.1016/j.brainres.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 120.Davari S, Talaei SA, Alaei H, Salami M. Probiotics treatment improves diabetes-induced impairment of synaptic activity and cognitive function: behavioral and electrophysiological proofs for microbiome-gut-brain axis. Neuroscience. 2013;240:287–296. doi: 10.1016/j.neuroscience.2013.02.055. [DOI] [PubMed] [Google Scholar]
- 121.Tillisch K, Labus J, Kilpatrick L, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394–1401. e4. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Severance EG, Gressitt KL, Stallings CR, et al. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophr Res. 2013;148:130–137. doi: 10.1016/j.schres.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dohan FC, Harper EH, Clark MH, Rodrigue RB, Zigas V. Is schizophrenia rare if grain is rare? Biol Psychiatry. 1984;19:385–399. [PubMed] [Google Scholar]
- 124.Morgan C, Charalambides M, Hutchinson G, Murray RM. Migration, ethnicity, and psychosis: toward a sociodevelopmental model. Schizophr Bull. 2010;36:655–664. doi: 10.1093/schbul/sbq051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Mol Psychiatry. 2014;19:146–148. doi: 10.1038/mp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dinan TG, Borre YE, Cryan JF. Genomics of schizophrenia: time to consider the gut microbiome? Mol Psychiatry. 2014;19:1252–1257. doi: 10.1038/mp.2014.93. [DOI] [PubMed] [Google Scholar]
- 127.Dickerson FB, Stallings C, Origoni A, et al. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion CNS Disord. 2014;16 doi: 10.4088/PCC.13m01579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hsiao EY, McBride SW, Hsien S, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.de Theije CG, Wopereis H, Ramadan M, et al. Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain Behav Immun. 2014;37:197–206. doi: 10.1016/j.bbi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 130.MacFabe DF, Cain NE, Boon F, Ossenkopp KP, Cain DP. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: relevance to autism spectrum disorder. Behav Brain Res. 2011;217:47–54. doi: 10.1016/j.bbr.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 131.Thomas RH, Meeking MM, Mepham JR, et al. The enteric bacterial metabolite propionic acid alters brain and plasma phospholipid molecular species: further development of a rodent model of autism spectrum disorders. J Neuroinflammation. 2012;9:153. doi: 10.1186/1742-2094-9-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shultz SR, Macfabe DF, Martin S, et al. Intracerebroventricular injections of the enteric bacterial metabolic product propionic acid impair cognition and sensorimotor ability in the Long-Evans rat: further development of a rodent model of autism. Behav Brain Res. 2009;200:33–41. doi: 10.1016/j.bbr.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 133.Shultz SR, MacFabe DF, Ossenkopp KP, et al. Intracerebroventricular injection of propionic acid, an enteric bacterial metabolic end-product, impairs social behavior in the rat: implications for an animal model of autism. Neuropharmacology. 2008;54:901–911. doi: 10.1016/j.neuropharm.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 134.Finegold SM, Dowd SE, Gontcharova V, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16:444–453. doi: 10.1016/j.anaerobe.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 135.Parracho HM, Bingham MO, Gibson GR, McCartney AL. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol. 2005;54(Pt 1):987–991. doi: 10.1099/jmm.0.46101-0. [DOI] [PubMed] [Google Scholar]
- 136.Song Y, Liu C, Finegold SM. Real-time PCR quantitation of clostridia in feces of autistic children. Appl Environ Microbiol. 2004;70:6459–6465. doi: 10.1128/AEM.70.11.6459-6465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ming X, Stein TP, Barnes V, Rhodes N, Guo L. Metabolic perturbance in autism spectrum disorders: a metabolomics study. J Proteome Res. 2012;11:5856–5862. doi: 10.1021/pr300910n. [DOI] [PubMed] [Google Scholar]
- 138.Emond P, Mavel S, Aïdoud N, et al. GC-MS-based urine metabolic profiling of autism spectrum disorders. Anal Bioanal Chem. 2013;405:5291–5300. doi: 10.1007/s00216-013-6934-x. [DOI] [PubMed] [Google Scholar]
- 139.Schwab AJ, Tao L, Yoshimura T, Simard A, Barker F, Pang KS. Hepatic uptake and metabolism of benzoate: a multiple indicator dilution, perfused rat liver study. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1124–G1136. doi: 10.1152/ajpgi.2001.280.6.G1124. [DOI] [PubMed] [Google Scholar]
- 140.Yap IK, Angley M, Veselkov KA, Holmes E, Lindon JC, Nicholson JK. Urinary metabolic phenotyping differentiates children with autism from their unaffected siblings and age-matched controls. J Proteome Res. 2010;9:2996–3004. doi: 10.1021/pr901188e. [DOI] [PubMed] [Google Scholar]
- 141.Bell JG, MacKinlay EE, Dick JR, MacDonald DJ, Boyle RM, Glen AC. Essential fatty acids and phospholipase A2 in autistic spectrum disorders. Prostaglandins Leukot Essent Fatty Acids. 2004;71:201–204. doi: 10.1016/j.plefa.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 142.El-Ansary AK, Bacha AG, Al-Ayahdi LY. Impaired plasma phospholipids and relative amounts of essential polyunsaturated fatty acids in autistic patients from Saudi Arabia. Lipids Health Dis. 2011;10:63. doi: 10.1186/1476-511X-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Finegold SM. Desulfovibrio species are potentially important in regressive autism. Med Hypotheses. 2011;77:270–274. doi: 10.1016/j.mehy.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 144.Emanuele E, Orsi P, Boso M, et al. Low-grade endotoxemia in patients with severe autism. Neurosci Lett. 2010;471:162–165. doi: 10.1016/j.neulet.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 145.Elder JH, Shankar M, Shuster J, Theriaque D, Burns S, Sherrill L. The gluten-free, casein-free diet in autism: results of a preliminary double blind clinical trial. J Autism Dev Disord. 2006;36:413–420. doi: 10.1007/s10803-006-0079-0. [DOI] [PubMed] [Google Scholar]
- 146.Millward C, Ferriter M, Calver S, Connell-Jones G. Gluten- and casein-free diets for autistic spectrum disorder. Cochrane Database Syst Rev. 2008;(2):CD003498. doi: 10.1002/14651858.CD003498.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Diaz Heijtz R, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Coury DL, Ashwood P, Fasano A, et al. Gastrointestinal conditions in children with autism spectrum disorder: developing a research agenda. Pediatrics. 2012;130(suppl 2):S160–S168. doi: 10.1542/peds.2012-0900N. [DOI] [PubMed] [Google Scholar]
- 149.McElhanon BO, McCracken C, Karpen S, Sharp WG. Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics. 2014;133:872–883. doi: 10.1542/peds.2013-3995. [DOI] [PubMed] [Google Scholar]
- 150.Sandler RH, Finegold SM, Bolte ER, et al. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol. 2000;15:429–435. doi: 10.1177/088307380001500701. [DOI] [PubMed] [Google Scholar]