ABSTRACT
Objectives: To understand the key characteristics of Asthma and Chronic Obstructive Pulmonary Disease Overlap Syndrome (ACOS) and to identify evidence gaps relating to the identification, treatment and management of ACOS patients.
Methods: A structured literature review and 1-hour telephone interviews with specialist respiratory physicians were conducted (n=10; China, France, Germany, Japan and the USA).
Results: All 10 physicians used the term ACOS in clinical practice. ACOS was not clearly defined in the literature. Prevalence of ACOS among adult patients with COPD or asthma ranged from 12–55%. ACOS patients had severe disease, with increased exacerbations and hospitalisations compared to some asthma and COPD patients. ACOS represented a clinical challenge due to a lack of evidence-based guidelines distinguishing between asthma, COPD and ACOS. Published data quantifying ACOS costs were limited.
Conclusions: There is a need for consensus evidence-based guidance to facilitate earlier diagnosis and to optimise the management of ACOS patients.
KEYWORDS: Asthma and Chronic Obstructive Pulmonary Disease Overlap Syndrome, ACOS, Asthma, Chronic Obstructive Pulmonary Disease, COPD, burden, health-related quality of life
Introduction
Asthma and chronic obstructive pulmonary disease (COPD) are the most common obstructive airway diseases among adults [1]. Both of these conditions cause significant disease burden and have a substantial effect on patients’ health-related quality of life (HRQoL) [2,3]. A significant proportion of patients who present with chronic airway disease have overlapping features of both asthma and COPD [2]. In the 2014 Global Initiative for Asthma (GINA) and Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, the term ‘Asthma and COPD Overlap Syndrome (ACOS)’ has been used to describe these patients [3].
ACOS patients are perceived to have a more severe disease, which is associated with a detrimental impact on patients’ HRQoL and a greater economic burden to health-care systems compared to asthma or COPD alone [4]. Although the term ‘ACOS’ is one of several that have been assigned to this group of patients, there is currently no consensus definition for this observed phenotype of asthmatic patients with COPD symptoms or COPD patients with asthmatic symptoms. There are limited data available related to ACOS, as patients are often excluded from clinical trials and their response to medications indicated for asthma or COPD is not well characterized [1].
Based on the current lack of a consensus definition and understanding of ACOS, it is likely the clinically relevant ACOS phenotype is misclassified in clinical practice. Subsequently, the impact of ACOS on patient HRQoL and the burden of ACOS to the health-care system are relatively unexplored areas that warrant further research.
The objectives of this study were to understand the key characteristics of ACOS, specifically, how ACOS is defined, the epidemiology, the patient pathway from identification to treatment, and the burden of ACOS, with respect to the impact of disease on patient HRQoL and the economic burden of ACOS to society.
To highlight the current level of understanding of the ACOS phenotype and to identify the gaps in understanding and published evidence relating to the identification, treatment, and management of ACOS patients, a structured literature review and interviews with specialist respiratory physicians were conducted. As limited evidence directly relating to the management of ACOS patients is available in the public domain, an additional search of ‘grey literature’ was conducted. Grey literature was identified from a broad internet search using key search terms, and included a search of conference proceedings. Overall, 3104 abstracts and 17 additional records identified through the ‘grey literature’ search were screened. Of these, 43 publications were identified as publications of interest. Ten interviews were conducted with respiratory physicians from different care settings, including primary, secondary (hospital-based), and/or private care setting to gather insights from different perspectives and different stages of the treatment pathway from across five countries: China (CHN), France (FRA), Germany (DEU), Japan (JPN), and the United States of America (USA). The findings of the literature review and key real-world insights from the physician interviews are presented here.
Materials and methods
Literature review
Search strategy
A structured search of the published literature was conducted electronically using the following databases in OVID (OVID Technologies, Inc.): Medline and Medline (R) in process (PubMed), Embase (OVID), EconLit (EBSCOhost), and NHS Economic Evaluation Database (NHS-EED). The search was limited to human studies and English language only. The search was also limited to a 10 year time horizon (from 2004 to September 2014) to identify the most up-to-date literature; additionally, as ACOS is a relatively new research topic, it was expected that limited data were published prior to 2004. As ACOS is poorly defined and alternative terminology may be used to define this patient population, a broad search strategy was adopted to ensure no relevant publications were missed. The search strategy investigated ‘asthma and COPD’ as two separate disease search terms, without the term ‘overlap’.
‘Grey literature’ was identified from a broad internet search and conference proceedings using the following key terms: ‘ACOS’, ‘Asthma and COPD Overlap Syndrome’, ‘Asthma-COPD Overlap’, and ‘Asthma and COPD’. The conference search was limited to a preceding 2 year time horizon (from 2012 to September 2014) and was restricted to the American Thoracic Society (ATS), European Respiratory society (ERS), Asian-pacific society of respiratory (APSR), American Academy of Allergy, Asthma and Immunology (AAAAI), and the American College of Allergy, Asthma and Immunology (ACAAI).
Search results
Overall, 3104 abstracts and 17 additional records identified through the ‘grey literature’ search were screened. Of these, 43 publications were identified as publications of interest, 3066 publications were excluded on the basis of title and abstract, and a further 12 publications were excluded at full-text review as these publications reported on asthma or COPD only (see Figure 1). Publications of interest fulfilled the following inclusion criteria: publications that reported on the treatment patterns/management of ACOS patients or publications that reported clinical or economic data on ACOS patients. The majority of publications identified were review articles; the term ‘review article’ was applied to any publications that were not primary research, guidelines, or consensus documents. These publications included current and clinical perspective articles that highlighted the lack of available evidence.
Figure 1.
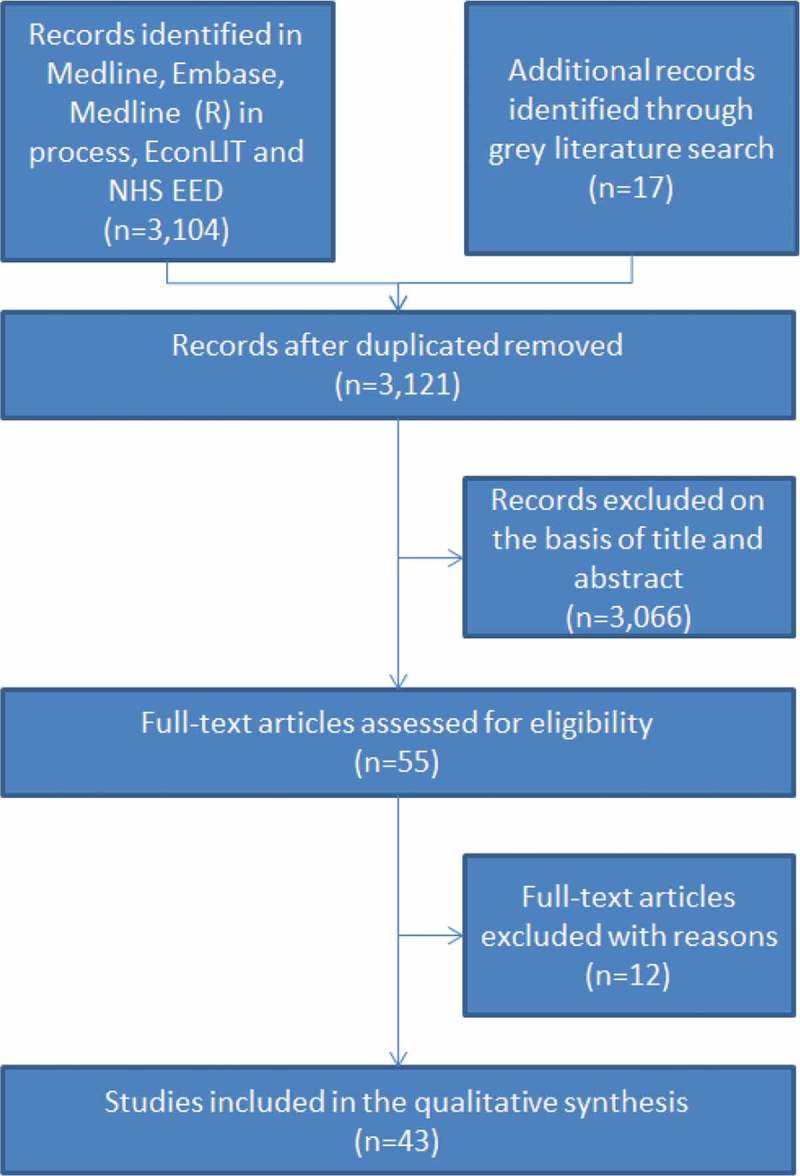
Structured literature review–attrition of identified publications.
Physician interviews
To complement the structured literature review, 10 one-hour qualitative telephone interviews were conducted with specialist respiratory physicians to draw upon real-world insights relating to the diagnosis and management of ACOS patients.
Recruitment
A recruitment agency partner was used to recruit two physicians from primary, secondary, and/or private care settings across five countries: CHN, FRA, DEU, JPN, and USA. A screener questionnaire was used to determine physician eligibility for the study. The following demographic data were collected using the screener questionnaire: gender, role, professional title, length of time in the role, specialty area (i.e. ACOS, asthma, or COPD), tasks primarily involved in (i.e. diagnosis, treatment planning, treatment delivery, and treatment monitoring), work setting (i.e. primary/secondary or private care), number of ACOS patients seen per month, and percentage of total workload dedicated to ACOS patients. Physicians who fulfilled the following inclusion criteria were recruited: ≥5 years’ experience, treat ≥10 ACOS patients per month, dedicate ≥40% of their workload to ACOS patients per month, and work in a primary, secondary, and /or private care setting. Physicians were anonymized by the recruitment agency partner. The physicians were distinguishable to the study investigators by their initials only. All of the physicians provided a signed informed consent form to the recruitment agency partner prior to the interview. All interviews and verbal consent to participant were audio-recorded.
Physician demographics
Nine of the 10 physicians were respiratory specialists, one physician was a general practitioner (GP) who saw a significant number of respiratory patients per month (n = 120, FRA), 40 of which were ACOS patients. Eight of the physicians had over 10 years experience and eight worked in a secondary care and/or private setting.
The majority of ACOS patients were cared for by respiratory specialists. This was expected as the inclusion criteria for this study specified physicians who had considerable experience in the respiratory field and who dedicate a large proportion of their workload to this patient population (>40%). Additionally, as the ACOS patient population is relatively undefined, it was perceived that there may be a lack of awareness outside of the specialist respiratory medical community.
All 10 physicians were involved in the identification, treatment planning, treatment administration, and monitoring of ACOS patients. As interviewees were required to be involved throughout the ACOS patient pathway, the majority worked within secondary care, where ACOS patients are commonly first diagnosed.
The inclusion criteria for average number of ACOS patients per month (>10) and proportion of workload dedicated to ACOS patients (>40%) were considered together and weighted by the authors, i.e. physicians who saw <10 patients but dedicated ≥40% of their workload to ACOS patients were included. Eight physicians fulfilled both criteria, i.e. on average, they saw >10 ACOS patients per month and >40% of their workload was dedicated to ACOS patients. One physician (CHN) who only saw eight ACOS patients per month was included due to lack of appropriate recruits for this market. One physician (USA) who reported that 25% of their workload was dedicated to ACOS patients was included, as the physician had a large respiratory patient population, including an average of 80 ACOS patients per month. The number of ACOS patients seen by each physician per month varied greatly by country, with physicians seeing between 8 (n = 1, CHN) and 180 (n = 1 DEU) ACOS patients per month.
Results
Prevalence and incidence
Asthma and COPD were the most common obstructive airway diseases among adults [1]. In the USA, it was estimated that one in 12 adults had asthma and one in five adults had a diagnosis of COPD, with an estimated 9.8 million adults living with undiagnosed COPD (National Health Interview Survey, 2010) [4]. Based on the published literature, the prevalence of ACOS among patients with COPD or asthma ranged from 12% to 55% (see Table 1). The majority of the physicians (n = 7) estimated that ACOS accounted for 13–30% of obstructive airway disease patients in clinical practice. However, estimates ranging from 9% (n = 1, CHN) to 50% (n = 1, FRA) were reported. This was observed within the literature as prevalence estimates varied depending on the definition used and the population studied.
Table 1.
Reported prevalence of ACOS within the published literature.
| Source | Study design | Definition of ACOS | ACOS prevalence |
|---|---|---|---|
| Andersen et al. 2013[5] | Hospital discharge registry data in Finland, covering the whole Finnish population (5.35 million, 2009). Patients >34 years of age and treatment periods from 2000 to 2009, with a primary or secondary diagnosis of COPD or asthma were identified (n = 105,122). | ICD-10 COPD or asthma plus treatment for both within the study period. | 16.1% in patients with primary or secondary diagnoses of COPD or asthma in Finland. |
| Miravitlles et al. 2013[6] | A total of 385 patients with COPD (FEV1/FVC < 0.7) identified in the cross-sectional EPI-SCAN study cohort (n = 3,885, 40–80-year-old participants, field work done from May 2006 to July 2007, Spain). | All participants with spirometric-defined COPD (defined by a post-bronchodilator FEV1/FVC ratio of <0.70) were classified as overlap COPD-asthma subjects if they confirmed that they had previously been diagnosed with asthma. | 17.4% in subjects diagnosed with COPD in Spain (n = 3885). |
| De Marco et al. 2013[7] | A screening questionnaire on respiratory symptoms, diagnoses, and risk factors was administered by mail to a random sample of the general Italian population. | Self-reported physician diagnosis of ACOS. | 1.6%, 2.1%, and 4.5% of a sample of the Italian general population, aged 20–44, 45–64, and 65–84, respectively. |
| Izquierdo-Alonso et al. 2013[8] | An observational multicenter study enrolling 331 COPD patients aged 40 or older from pulmonary outpatient centers. | Diffusion test with transfer factor of the lung for carbon monoxide (TLco) values ≥80%, absence of pulmonary emphysema demonstrated through imaging, and a history of asthma before the age of 40 without a current diagnosis of asthma. | 12.1% of COPD patients (n = 331) in Spain. |
| Fu et al. 2014[9] | A 4 year prospective cohort study in adults aged >55 years with obstructive airway diseases in Australia (n = 99, mean age = 68.8 ± 7.6 years). | Respiratory symptoms, increased airflow variability (asthma, i.e. airway hyperresponsiveness), and incompletely reversible airway obstruction (COPD, i.e. post-bronchodilator FEV1/FVC <70%). | 55.5% in patients >55 years of age with obstructive airway disease (n = 99) at baseline in Australia. |
| Milanese et al. 2014[10] | An observational multicenter survey enrolling patients >64 years old with a documented physician diagnosis of asthma between October 2012 and March 2013 in Italy (n = 350). | A diagnosis of asthma plus chronic bronchitis and/or impaired CO diffusion test. | 29% in asthma patients >64 years of age (n = 350) in Italy. |
| Yon-Lee et al. 2014[11] | Retrospective medical record review of the clinical characteristics of asthma in- and outpatients aged 41‒79 years between September 2007 and March 2012 in South Korea(n = 256). | Overlap patients were defined as patients with physician-diagnosed asthma (a positive response to bronchodilator (>200 mL FEV1 and >12% baseline) and/or positive methacholine or mannitol provocation test) and incompletely reversible airflow obstruction (post-bronchodilator FEV1/FVC <70) at admission and for ≥3 months regardless of treatment. | 38% in asthma patients aged 41–79 years (n = 256) in South Korea. |
| Marsh et al. 2008[12] | A randomized, population-based survey including questionnaires, pulmonary function tests, and chest CT scans. | Patients with COPD (post-bronchodilator FEV1/FVC <0.7) and asthma (post-bronchodilator increase in FEV1 ≥ 15% or peak flow variability ≥20% during 1 week of testing or physician-diagnosed asthma in conjunction with current symptoms). | 55.2% in COPD patients >50 years of age (n = 96) in New Zealand. |
| Zeki et al. 2011[13] | A small cohort from the academic general pulmonary/asthma referral clinic was compared to patients from the severe asthma clinic (UC Davis Asthma Network (UCAN) Clinics). | ACOS was defined as one of two clinical phenotypes:
|
Of the small cohort from the academic general pulmonary clinic/asthma referral clinic, 15.8% of patients were ACOS compared to 34.2% asthma and 43.4% COPD/emphysema. In the severe asthma clinic, 24.3% of patients were ACOS compared to 52.9% of asthma. |
ACOS = Asthma and COPD overlap syndrome; AHR = Airway hyper responsiveness; CO = Carbon monoxide; COPD = Chronic obstructive pulmonary disease; CT = computed tomography; FVC = forced vital capacity; FEV1 = forced expiratory volume in 1 second; ICD-10 = international classification of disease criteria.
Previously, studies showed that the prevalence of ACOS increases with age [7,9]. An observational cohort study, Gene Environment Interactions in Respiratory Disease (GEIRD), which investigated the prevalence of asthma, COPD, and ACOS in the Italian general population, showed that aging was associated with a marked decrease in the prevalence of asthma, i.e. from 8.2% for those aged 20–44 years to 1.6% for those aged 65–84 years. However, there was an increase in the prevalence of COPD, 3.3% for ages 20–44 to 13.3% for ages 65–84 and ACOS, 1.6% for ages 20–44, 2.1% for ages 45–64 to 4.5% for ages 65–84 [7]. These prevalence data are consistent with the ‘typical’ ACOS patient profile reported by physicians. All 10 physicians suggested that their ACOS patients tended to be ≥40 years of age, which is typically younger than COPD patients but older than asthma patients, who have had a childhood diagnosis of asthma or history of asthmatic symptoms, leading to the development of ACOS. There was no average age at diagnosis reported. Physicians also reported that the majority of ACOS patients were current or ex-smokers (≥20 years) and comorbidities included arterial hypertension, gastroesophageal reflux, osteoporosis, diabetes, and chronic heart disease.
Within the published literature, ACOS prevalence varied between asthmatic patient populations and COPD patient populations. This may be attributable to differences in age or smoking status, which are both risk factors for COPD and ACOS [11,12]. In South Korea, for an asthma clinical cohort (n = 256), the prevalence of ACOS was estimated to be 37.9%. The greatest proportion of ACOS patients within this asthma cohort were ≥70 years of age, which is consistent with studies that showed the prevalence of ACOS increases with age [11]. The ACOS patient populations were also more likely to be current or ex-smokers (31% versus 20%, ACOS and asthma only cohort, respectively, p < 0.01) [11]. Due to a lack of consensus, prevalence was likely to be underestimated [4]. The reported prevalence of ACOS within the published literature is summarized in Table 1. No data within the published literature reported on the incidence of ACOS. The perception of the incidence of ACOS was divided amongst physicians. Six physicians reported that ACOS patients were predominantly male (CHN n = 1, DEU n = 2, FRA n = 1, JPN n = 2). Two physicians reported a female predominance (FRA n = 1 and USA n = 1) and two physicians reported an equal predominance (CHN n = 1 and USA n = 1).
Defining ACOS
Several terms were identified within the literature that described the overlap phenotype of asthma and COPD: ‘Asthmatic bronchitis’, ‘COPD with a prominent asthmatic component’, ‘asthma that complicates COPD’ and ‘mixed COPD-asthma’ [2,4]. These terms referred to the overlap phenotype as predominantly COPD with an asthmatic component. This is supported by a recently published consensus document which proposed a diagnosis of COPD in addition to fulfillment of major and minor criteria (characteristics of the asthmatic component of the overlap phenotype) can be used to identify the ‘mixed COPD-asthma’ phenotype.
In 2014, GINA and GOLD collaborated to publish guidelines on the diagnosis of asthma, COPD, and ACOS. The guidelines present a descriptive criterion that can be used to differentiate ACOS from asthma and COPD [2]. Contrary to the consensus document, [14] the GINA GOLD guidelines characterize ACOS as persistent airflow limitation, with several features usually associated with asthma and several features usually associated with COPD. ACOS was identified by the features that it shares with both asthma and COPD, with equal weighting ascribed to both components [2]. Within the published literature, ACOS was the most frequently used term to define the overlap phenotype (see Figure 2).
Figure 2.
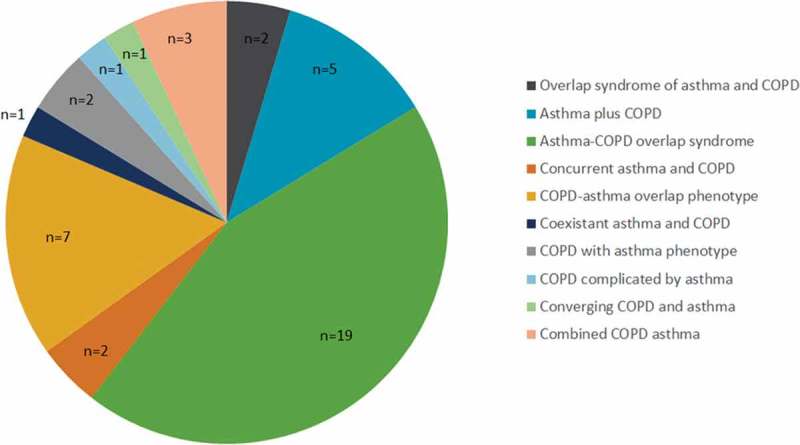
Terminology reported within the published literature to describe the overlap phenotype (n = 43).
Although different terminologies have been ascribed to the ACOS population, all 10 physicians were well acquainted with, and routinely used the term ‘ACOS’ in clinical practice; to define the patients’ phenotype and when discussing the syndrome with patients and colleagues. However, for JPN, physicians (JPN n = 2) noted that the term ‘ACOS’ was used interchangeably with ‘asthma and COPD, ACOPD’.
Two physicians (CHN n = 1 and USA n = 1) reported two ACOS patient subgroups, asthmatic patients with COPD symptoms or COPD patients with asthmatic symptoms. However, generally, when discussing ACOS, the physicians (n = 10) were referring to COPD patients with asthmatic symptoms. Primarily, this was because this patient group was associated with greater morbidity and mortality compared to asthmatic patients with COPD symptoms. The physicians were reluctant to further refine the ACOS patient population, because the definition of ACOS was newly established and a clear descriptive criterion was lacking.
Guidelines
ACOS patients are frequently excluded from clinical trials for obstructive airway diseases so there was a lack of evidence-based clinical guidelines [1]. Published respiratory consensus statements and guidelines did not capture the heterogeneity and spectrum of obstructive airway diseases and lacked data concerning the variable response of respiratory disease phenotypes to pharmacotherapies, in particular to inhaled corticosteroids (ICSs) [3,4,15,16].
Clinical guidelines from Canada, Japan, and Spain referred to the overlap phenotype; however, there was a significant lack of consensus. The Canadian Thoracic Society guidelines referred to ‘combined COPD and asthma’ and the difficulties in quantifying the relative contribution of each disease [17]. The Japanese Respiratory Society guidelines referred to difficulties in making a differential diagnosis from bronchial asthma, COPD with significant reversibility, refractory asthma with low reversibility, and COPD complicated by asthma [18]. The Spanish, Guía Española de la EPOC (GesEPOC), COPD guidelines described four COPD phenotypes: non-exacerbator with emphysema or chronic bronchitis; mixed COPD-asthma; exacerbator with emphysema; and exacerbator with chronic bronchitis [19].
In 2014, the global ‘diagnosis of diseases of chronic airflow limitation: asthma, COPD and ACOS’ guidelines were developed via a joint project by GINA and GOLD. The guidelines assist clinicians in distinguishing between asthma, COPD, and ACOS to aide diagnoses, referral decisions, management, and treatment [2]. The majority of physicians (8 of the 10 physicians, CHN, FRA, DEU, USA) spontaneously referred to the recently published GINA GOLD 2014. The Spanish GesEPOC guidelines were also referred to, as an aide in the identification and management of ACOS patients (CHN, n = 1). However, all physicians stated that there is a lack of evidence-based well-defined guidelines.
Distinguishing between asthma, COPD, and ACOS
ACOS patients represent a significant clinical challenge; in practice, it may be difficult to distinguish between asthma and COPD, particularly in older patients and smokers, due to common features across the two diseases [13] and due to the lack of evidence-based guidelines. ACOS patients may be asthmatics who smoke and subsequently develop chronic airflow obstruction with a high degree of reversibility. Alternatively, ACOS patients may be heavy smokers with a genetic background, which leads to an allergic inflammatory response to inhaled particles, characteristic of allergic asthma.
The current, proposed descriptive criteria for ACOS include [4,14,20]:
Major criteria: marked reversibility with bronchodilators (>15% and >400 ml in forced expiratory volume in 1 second (FEV1)), a history of asthma (<40 years of age), and sputum eosinophilia.
Minor criteria: reversibility on two separate occasions (>12% and >200 ml in FEV1), history of atopy, increased serum IgE.
Age and medical history, exposure to risk factors, pattern of symptom development, and history of exacerbations were also considered to be good indicators of disease. Patients >40 years of age with a history of asthma or asthmatic symptoms and COPD symptoms was indicative of ACOS [21]. Asthmatic patients who developed COPD typically had allergic rhinitis, unspecific bronchial hyperresponsiveness (BHR), wheezing, greater IgE plasma concentrations, dyspnea, chronic cough, and chronic sputum production, indicating the mixed presence of asthma and COPD symptoms [22].
Physiological manifestations were commonly used to distinguish between phenotypes (see Table 2). Spirometry, which is a reproducible, objective measure of airflow limitation, was frequently used to assess pulmonary function. An FEV1/forced vital capacity (FVC) <70% would be required for a diagnosis of COPD, and this was usually observed for ACOS patients too. FEV1 <80% predicted was an indicator of severity of airflow limitation and risk of mortality and exacerbations in both COPD and ACOS. Although an FEV1 ≥80% was suggestive of asthma, it may also be indicative of mild airflow limitation in COPD or mild ACOS, if FEV1/FVC <70% was demonstrated [2]. To recognize ACOS in COPD patient populations, provocation tests with agents that do no cause direct airway smooth muscle contraction, e.g. histamine, mannitol, adenosine, hypertonic saline, were used [23]. However, a clinically significant bronchodilator response (>15%, indicating significant reversibility) has previously been observed in COPD patient cohorts [4]. Based on the variability of spirometry response, multiple tests should be conducted; this was reflected in the descriptive criterion for ACOS, which requires demonstration of reversibility on two separate occasions [4,14].
Table 2.
| Measure | Asthma | ACOS | COPD |
|---|---|---|---|
| Symptoms | Intermittent, worse at night or in the morning | Progressively worsen | Progressively worsen |
| FEV1/FVC | ≥70% | <70% | <70% |
| FEV1 %predicted* | >80% | <80% | <80% |
| AHR, PD15^ | <12 ml | <12 ml | >12 ml |
| PB increase in FEV1 | ≥12% and 400 ml (marked reversibility) | ≥12% and ≥200 ml (reversible) | ≥12% and ≥200 ml (reversible) |
| FeNO | >50 ppb | 25–50 ppb | <25 ppb |
| DLco | Normal, although smokers may present with a lower DLco | Normal–low | <80% predicted |
| Imaging | Usually normal | Bronchial wall thickening, emphysema, gas trapping on expiratory chest CT scans, greater segmental wall area on inspiratory CT scans, fibrosis, hyperinflation | Bronchial wall thickening, emphysema, fibrosis, hyperinflation |
| Inflammation | Eosinophils > neutrophils, mast cells, CD4+ T lymphocytes | Eosinophils and neutrophils, CD4+ and CD8+ T lymphocytes | Neutrophils > eosinophils, CD4+, CD8+ T lymphocytes |
| IgE, IL-4/-5/-13, eotaxin | IgE, IL-4/-5/-13/-1β/-8/-6, TNF-α, eotaxin, proteases | IL-1β/-8/-6, TNF-α, proteases | |
| Test for atopy, (MAST) | Commonly allergic to environmental allergens | Commonly allergic to environmental allergens | Do not rule out COPD, ACOS may be more likely |
| Exacerbations | >3/year, well controlled by treatment | More frequent than asthma and COPD alone | >2/year |
*postbronchodilator, ^provocation dose of hypertonic saline that induces a 15% fall in FEV1.
AHR = airway hyperresponsiveness; COPD = Chronic obstructive pulmonary disease; CT = computed tomography; DLco = carbon monoxide diffusing capacity; FeNO = fraction of exhaled nitric oxide; FEV1 = forced expiratory volume in 1s; FVC = Forced vital capacity; IgE = immunoglobulin E; MAST= multiple allergens simultaneous test; PD = provocation dose; PB = post bronchodilator; TNF = tumour necrosis factor.
All physicians reported frequent use of pulmonary function tests, particularly spirometry (n = 10) and X-rays or computed tomography (CT) scans (n = 8). In CHN, X-rays and CT scans were not routinely used and would only be conducted if a patient did not respond to treatment. Blood tests were also used to quantify peripheral inflammation, and respiratory allergen panels were used to identify the allergic component of ACOS (FRA n = 1, DEU n = 2, JPN n = 1, and the USA n = 1). One physician (USA) highlighted the significant underuse of radioallergosorbent tests (RASTs) and multiple allergens simultaneous tests (MASTs) for identifying patients with atopy. However, overall, physicians cited that there was a lack of clear descriptive criteria to distinguish between asthma, COPD, and ACOS; a multitude of different tests were required and there was a lack of awareness of the ACOS phenotype outside of the specialist respiratory community. These were associated with a delay in referral from primary care and a delay in identification of the ACOS phenotype; physicians reported a delay of between 3 and 5 months in FRA to up to 5 years in CHN.
Treatment
Management of ACOS patients required an integrated approach, including the identification of the clinically relevant phenotype, clinical experience, and extrapolation from published literature in asthma and COPD to inform the optimum treatment for ACOS [4]. The aim of therapy was to prevent exposure to risk factors, control symptoms, reduce exacerbations, and improve patient HRQoL [25]. ACOS treatment was a combination of asthma and COPD treatments. It was not clear which condition was diagnosed first. Patients were commonly prescribed a combination of ICSs, long-acting β2-agonists (LABAs), and long-acting muscarinic antagonists (LAMAs). The ineffective treatment of ACOS through misclassification of the clinically relevant phenotype and incorrect prescribing of treatments is associated with an increased risk of adverse events without clinical benefit [26].
All 10 physicians identified smoking as a trigger of exacerbations and suggested that smoking cessation was essential for the management of ACOS patients. Physicians in the USA (n = 2) highlighted the importance of decreasing allergen exposure within the home, e.g. by removing carpets and changing sheets. Physicians in CHN described the implementation of a number of non-pharmacological treatments, including pulmonary rehabilitation, exercise/weight loss, psychological counseling, and traditional Chinese medicine.
However, there was limited evidence in the literature relating to the efficacy of pharmacological or non-pharmacological treatment in the ACOS patient population, a challenging respiratory patient group for health-care providers to manage [24,27].
Humanistic burden
Asthma and COPD are chronic conditions that have a significant impact on patient HRQoL and restrict activities of daily living [28]. ACOS was associated with more rapid disease progression, frequent exacerbations, and poorer HRQoL compared to COPD or asthma alone [7]. A retrospective review of patient-reported outcomes and medical records of patients with asthma, COPD, or ACOS (n = 1546) in Finland demonstrated that HRQoL was the poorest within the overlap patient population [29]. ACOS patients reported the lowest scores on the workability index, had ≥10 work absences/year, and a greater proportion were receiving disability pensions; however, these observed differences were only significant between the asthma and ACOS groups [29].
All 10 physicians acknowledged the impact of ACOS on patient HRQoL and five suggested that the perceived impact of ACOS on patient HRQoL was worse than asthma or COPD alone (DEU n = 1, FRA n = 2, and USA n = 2). Dyspnea, wheezing, waking in the night, and exacerbations were cited as the most burdensome symptoms (DEU n = 1, FRA n = 1, JPN n = 1, and USA n = 1) associated with severe restrictions on patients’ ability to carry out normal daily activities, such as walking a short distance.
Physicians highlighted that there were no disease-specific HRQoL measure available, but a combination of respiratory HRQoL measures were used: COPD assessment test (CAT), Clinical COPD questionnaire (CCQ), modified Medical Research Council (mMRC) dyspnea scale, Body mass, airflow Obstruction, Dyspnoea, and Exercise tolerance (BODE), DEU n = 1 and USA n = 1; Karnofsky performance status (KPS) scale, JPN n = 1; The New York Heart Association (NYHA) functional classification for dyspnea, FRA n = 1). However, implementation of these tools varied from country to country; in the USA, HRQoL tools were perceived as impractical and time-consuming in general practice (USA n = 2); conversely, in DEU, HRQoL tools were used at each patient visit (DEU n = 1).
Economic burden
In the literature, ACOS was associated with more frequent and severe exacerbations, which, in general, incurred greater health-care resource utilization and costs than asthma or COPD [4,23]. Exacerbations with hospitalization were the main cost drivers for both COPD and ACOS patients. Management and treatment of acute exacerbations accounted for 50–75% of COPD health-care costs in the USA. The typical, severe COPD patients would experience ≥2 exacerbations annually, whereas ACOS patients were expected to experience up to three times more exacerbations than COPD alone [4]. However, published data reporting the direct and indirect costs of ACOS were lacking. Two retrospective analyses were identified in the literature search that suggested an increase in resource utilization and costs compared to asthma or COPD alone [28,30].
A retrospective analysis of Medicaid patients’ medical claims in the USA (n = 9131) determined that the average annual incremental medical costs attributable to asthma, COPD, and ACOS were $2307, $4879, and $14914, respectively [28]. However, these results were not generalizable to the ACOS population, as Medicaid overrepresented females, African Americans, and low-income populations [28]. A retrospective analysis of the 2009 Korean National Health Insurance (NHI) database identified 101,004 patients with ACOS [30]. The percentage of emergency room (ER) visits, admissions, and intensive care unit (ICU) admissions was significantly higher in the ACOS cohort compared to the COPD cohort (30.5% vs. 14.1%, ACOS versus COPD, respectively, p < 0.001) [30]. Medical utilization and costs associated with claims data in 2009 were calculated, with all costs expressed as USD (exchange rate, 1 USD = 1152 Korean Won (KRW), July 2012) [30]. The cost of outpatient and inpatient services was significantly higher for the ACOS cohort compared to the COPD cohort (outpatient $78,527,082 vs. $33,961,656, and inpatient $105,259,446 vs. $36,446,055, ACOS and COPD, respectively, p < 0.001).
Limitations
All qualitative data were based on the views and practices of two physicians from each country (CHN, DEU, FRA, JPN, and USA). Therefore, the results may not be representative of general practice within each country and may not be generalizable to the wider management of ACOS patients.
The literature review was a structured search of published literature from 2004 to September 2014 and publications that were not English language were excluded. Furthermore, the authors acknowledge that a number of publications relating to ACOS have been published in 2015, following the publication of the GINA GOLD guidelines and after this literature review was conducted (January 2004 to September 2014). Therefore, this summary of literature may not be complete or representative of published literature on ACOS globally.
Discussion and conclusions
The results of the structured literature review and physician interviews highlighted significant evidence gaps, which suggest that ACOS is a poorly defined disease state. Although all 10 physicians had a good understanding of the ACOS phenotype and used the term ‘ACOS’ routinely when discussing the syndrome with patients, the definition of ACOS was not clearly defined within the published literature [4,13,14]. The prevalence estimates for ACOS were highly dependent on the definition used. The literature results showed that prevalence was approximately 15–25% of obstructive airway disease patients [4]. Similarly, the majority of physicians (n = 7) reported prevalence rates for ACOS were 13–30% of obstructive airway disease patients in clinical practice. Similar patient characteristics were described in the literature and by all 10 physicians. The majority of ACOS patients had asthma in childhood with concomitant allergic rhinitis and were smokers (≥20 years) in adulthood. This may have led to the development of an additional disease component characteristic of COPD, which contributed to the overlap syndrome. Generally, when discussing ACOS, all of the physicians were referring to COPD patients with asthmatic symptoms. The physicians were reluctant to further refine the ACOS patient population, because the definition of ACOS was newly established and a clear descriptive criterion was lacking. This perception was reflected in the published literature; the consensus document (2012) [14] suggested that COPD was the primary component of ACOS accompanied by asthmatic symptoms, and ACOS was frequently referred to as a phenotype of COPD [4,19,21,24,31].
The lack of evidence-based guidelines negatively impacts upon the identification and treatment of the ACOS patient population. The challenges associated with the identification of ACOS were highlighted in the physician interviews. Physiological manifestations were commonly used to distinguish between phenotypes; however, spirometry responses were variable in asthma, COPD, and ACOS patients. Differences in the diagnostic techniques employed were observed across countries. These findings were reflected in the literature, where there was also a lack of evidence detailing the differential classification of ACOS. The GINA GOLD guidelines (2014) describe the ACOS phenotype, aide in the identification of ACOS patients, and suggest that a totality of evidence, including medical history, age, and presence of mixed symptoms, was required to distinguish between asthma, COPD, and ACOS [2]. Although physicians were aware of the GINA GOLD guidelines, there was little reference to these guidelines within the literature, suggesting that the impact of this published guidance has not yet been fully recognized.
The importance of distinguishing ACOS patients from those with asthma or COPD was recognized both in the published literature and by the respiratory community as the clinical outcomes, functional outcomes, and the course of disease progression differ for ACOS patients when compared to patients with asthma or COPD alone [4].
In particular, there was a lack of economic evidence. Although both the literature and physician interviews suggested that ACOS was associated with a higher frequency of exacerbations and subsequent hospitalizations [25,29,30,32], only two studies were identified that quantified these costs and the wider economic impact of ACOS [28,30]. These two studies suggested that the cost of ACOS was associated with an incremental cost burden in both the USA and South Korea [28,30].
Costs were expected to increase further with disease severity due to the increased need for medication and increased hospitalizations. The societal burden of ACOS was also perceived to be great, as ACOS patients were limited in their daily activities and may require informal care (e.g. family members or neighbors). Subsequently, ACOS may impact caregiver and patient work productivity and presenteeism. All 10 physicians acknowledged the impact of ACOS on patient HRQoL and five suggested that the perceived impact of ACOS on patient HRQoL was worse than asthma or COPD alone (DEU n = 1, FRA n = 2 and USA n = 2).
With increased interest and research in the disease area, the terminology and defining features used to characterize this group of patients were expected to evolve over time [2,13]. Recognition of the ACOS phenotype and further research into the burden of ACOS may increase awareness and subsequently improve ACOS patient management globally.
Acknowledgements
The authors would like to thank the respiratory specialist physicians who were interviewed to provide real-world insight for this project.
Financial and competing interests disclosure
The study was funded by AstraZeneca and conducted by Adelphi Values. Adelphi values provided editorial support. B Ding is an employee of AstraZeneca. A Enstone is an employee of Adelphi Values. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Key issues
The results of the structured literature review and physician interviews highlighted significant evidence gaps.
There was a lack of available published evidence detailing the differential diagnosis of ACOS. A definitive diagnosis was based on a number of different measures as asthma, COPD, and ACOS all demonstrate a degree of variability, e.g. in the range of reversibility observed by spirometry.
Physicians suggested that diagnosing ACOS in primary care was difficult due to a lack of awareness and the multitude of different tests that were required for a confirmatory diagnosis.
Physician interviews indicated that the GINA GOLD guidelines were used and understood by the respiratory medical community. However, reference to these guidelines within the literature was limited, suggesting the impact of these guidelines has not yet been fully recognized.
There was a lack of economic evidence; both literature and physician interviews suggested that ACOS was associated with a higher frequency of exacerbations and subsequent hospitalizations, yet only two studies that quantify these costs were identified in the review [28,30].
References
Reference annotations
• Of interest
•• Of considerable interest
•• Global guidelines which highlight the differential diagnosis of ACOS patients.
• Consensus document acknowledging the existance of the differential clinical phenotype of ACOS. This document highlights the need to establish adequate guidelines and recommendations.
• Previously published literature review.
• An analysis of medical resource use and costs associated in 101,004 patients who were classified with the overlap syndrome. The findings of this study suggest ACOS is more costly than both COPD and asthma.
- Gibson P, Simpson J. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 2009;64(8):728–735. doi: 10.1136/thx.2008.108027. [DOI] [PubMed] [Google Scholar]
- Global Initiative for Asthma and Global Initiative for Chronic Obstructive Pulmonary Disease [cited 2014 Nov];Diagnosis of diseases of chronic airflow limitation: asthma, COPD and asthma-COPD overlap syndrome (ACOS) 2014 http://www.ginasthma.org/local/uploads/files/AsthmaCOPDOverlap.pdf Available from.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2014. [updated 2014; cited 2014 Oct]. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2014_Jan23.pdf Available from. [Google Scholar]
- Louie S, Zeki AA, Schivo M. The asthma-chronic obstructive pulmonary disease overlap syndrome: pharmacotherapeutic considerations. Expert Rev Clin Pharmacol. 2013;6(2):197–219. doi: 10.1586/ecp.13.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen H, Lampela P, Nevanlinna A. High hospital burden in overlap syndrome of asthma and COPD. Clin Respir J. 2013;7(4):342–346. doi: 10.1111/crj.12013. [DOI] [PubMed] [Google Scholar]
- Miravitlles M, Soriano JB, Ancochea J. Characterisation of the overlap COPD-asthma phenotype. Focus on physical activity and health status. Respir Med. 2013;107(7):1053–1060. doi: 10.1016/j.rmed.2013.03.007. [DOI] [PubMed] [Google Scholar]
- de Marco R, Pesce G, Marcon A. The coexistence of asthma and Chronic Obstructive Pulmonary Disease (COPD): prevalence and risk factors in young, middle-aged and elderly people from the general population. PLoS ONE. 2013;8(5):e62985. doi: 10.1371/journal.pone.0062985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Alonso JL, Rodriguez-Gonzalezmoro JM, De Lucas-Ramos P. Prevalence and characteristics of three clinical phenotypes of chronic obstructive pulmonary disease (COPD) Respir Med. 2013;107(5):724–731. doi: 10.1016/j.rmed.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Fu JJ, Gibson PG, Simpson JL. Longitudinal changes in clinical outcomes in older patients with asthma, COPD and asthma-COPD overlap syndrome. Respiration. 2014;87(1):63–74. doi: 10.1159/000352053. [DOI] [PubMed] [Google Scholar]
- Milanese M, Di Marco F, Corsico AG. Asthma control in elderly asthmatics. An Italian observational study. Respir Med. 2014;108(8):1091–1099. doi: 10.1016/j.rmed.2014.05.016. [DOI] [PubMed] [Google Scholar]
- Lee HY, Kang JY, Yoon HK. Clinical characteristics of asthma combined with COPD feature. Yonsei Med J. 2014;55(4):980–986. doi: 10.3349/ymj.2014.55.4.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh SE, Travers J, Weatherall M. Proportional classifications of COPD phenotypes. Thorax. 2008;63(9):761–767. doi: 10.1136/thx.2007.089193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki AA, Schivo M, Chan A. The asthma-COPD overlap syndrome: a common clinical problem in the elderly. J Allergy. 2011;2011:1–10. doi: 10.1155/2011/861926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler-Cataluna JJ, Cosio B, Izquierdo JL. Consensus document on the overlap phenotype COPD-asthma in COPD. Arch Bronconeumol. 2012;48(9):331–337. doi: 10.1016/j.arbres.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Qaseem A, Wilt TJ, Weinberger SE. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179–191. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- Makino S, Adachi M, Ohta K. A prospective survey on safety of sustained-release theophylline in treatment of asthma and COPD. Allergol Int. 2006;55(4):395–402. doi: 10.2332/allergolint.55.395. [DOI] [PubMed] [Google Scholar]
- O’Donnell DE, Aaron S, Bourbeau J. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease–2007 update. Can Respir J. 2007;14(Suppl B):5B. doi: 10.1155/2007/830570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee for the Third edition of the COPD guidelines of the Japanese Respiratory Society [cited 2014 Oct];Guidelines for the diagnosis and treatment of COPD 3rd edition. 2009 http://www.jrs.or.jp/uploads/uploads/files/photos/765.pdf Available from.
- Miravitlles M, Soler-Cataluna JJ, Calle M. A new approach to grading and treating COPD based on clinical phenotypes: summary of the Spanish COPD guidelines (GesEPOC) Prim Care Respir J. 2013;22(1):117–121. doi: 10.4104/pcrj.2013.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen N, He B. Personalized medicine in COPD treatment. Curr Respir Care Rep. 2014;3(3):133–139. [Google Scholar]
- Price D, Brusselle G. Challenges of COPD diagnosis. Expert Opin Med Diagn. 2013;7(6):543–556. doi: 10.1517/17530059.2013.842552. [DOI] [PubMed] [Google Scholar]
- Miravitlles M. The overlap syndrome between asthma and COPD: implications for management. Hot Topics Respir Med. 2011;6(16):15–20. [Google Scholar]
- Papaiwannou A, Zarogoulidis P, Porpodis K. Asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): current literature review. J Thorac Dis. 2014;6(SUPPL1):S146–S51. doi: 10.3978/j.issn.2072-1439.2014.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segreti A, Stirpe E, Rogliani P. Defining phenotypes in COPD: an aid to personalized healthcare. Mol Diagn Ther. 2014;18(4):381–388. doi: 10.1007/s40291-014-0100-9. [DOI] [PubMed] [Google Scholar]
- Diaz-Guzman E, Mannino DM. Airway obstructive diseases in older adults: from detection to treatment. J Allergy Clin Immunol. 2010;126(4):702–709. doi: 10.1016/j.jaci.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Price D, Yawn B, Brusselle G. Risk-to-benefit ratio of inhaled corticosteroids in patients with COPD. Prim Care Respir J. 2013;22(1):92–100. doi: 10.4104/pcrj.2012.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzola M, Segreti A, Rogliani P. Comparative effectiveness of drugs for chronic obstructive pulmonary disease. Drugs Today (Barc) 2012;48(12):785–794. doi: 10.1358/dot.2012.48.12.1860770. [DOI] [PubMed] [Google Scholar]
- Shaya FT, Dongyi D, Akazawa MO. Burden of concomitant asthma and COPD in a Medicaid population. Chest. 2008;134(1):14–19. doi: 10.1378/chest.07-2317. [DOI] [PubMed] [Google Scholar]
- Kauppi P, Kupiainen H, Lindqvist A. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma. 2011;48(3):279–285. doi: 10.3109/02770903.2011.555576. [DOI] [PubMed] [Google Scholar]
- Rhee CK, Yoon HK, Yoo KH. Medical utilization and cost in patients with overlap syndrome of chronic obstructive pulmonary disease and asthma. COPD: J Chronic Obstructive Pulm Dis. 2014;11(2):163–170. doi: 10.3109/15412555.2013.831061. [DOI] [PubMed] [Google Scholar]
- Miravitlles M, Calle M, Soler-Cataluna JJ. Clinical phenotypes of COPD: identification, definition and implications for guidelines. Arch Bronconeumol. 2012;48(3):86–98. doi: 10.1016/j.arbres.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Hardin M, Silverman EK, Barr RG. The clinical features of the overlap between COPD and asthma. Respir Res. 2011;12(1):127–132. doi: 10.1186/1465-9921-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]


