Abstract
Objectives
To compare the efficacy and safety of canagliflozin, a sodium glucose co‐transporter 2 inhibitor developed to treat type 2 diabetes mellitus (T2DM), in individuals younger than 75 and those aged 75 and older.
Design
Randomized Phase 3 studies.
Setting
International study centers.
Participants
Adults with T2DM.
Measurements
Changes from baseline in glycosylated hemoglobin (HbA1c), fasting plasma glucose (FPG), blood pressure (BP), and body weight were measured. Efficacy was evaluated using pooled data from six randomized, double‐blind, placebo‐controlled studies (N = 4,158; n = 3,975 aged <75, n = 183 aged ≥75). Safety was assessed based on adverse event (AE) reports from eight randomized, double‐blind, placebo‐ and active‐controlled studies (N = 9,439; n = 8,949 aged <75, n = 490 aged ≥75).
Results
Canagliflozin 100 and 300 mg were associated with placebo‐subtracted mean reductions in HbA1c in participants younger than 75 (−0.69% and −0.85%, respectively) and aged 75 and older (−0.65% and −0.55%, respectively). Dose‐related reductions in FPG, body weight, and BP were seen with canagliflozin 100 and 300 mg in participants in both age groups. Overall AE incidence was 67.1% with canagliflozin 100 mg, 68.6% with canagliflozin 300 mg, and 65.9% with non‐canagliflozin (pooled group of comparators in all studies) in participants younger than 75, and 72.4%, 79.1%, and 72.3%, respectively, in those aged 75 and older, with a similar safety profile in both groups. The incidence of volume depletion–related AEs was 2.2%, 3.1%, and 1.4% in participants younger than 75 with canagliflozin 100 and 300 mg and non‐canagliflozin, respectively, and 4.9%, 8.7%, and 2.6%, respectively, in those aged 75 and older.
Conclusion
Canagliflozin improved glycemic control, body weight, and BP in participants aged 75 and older. The overall incidence of AEs was high across treatment groups in participants aged 75 and older and higher than in those younger than 75. The safety profile of canagliflozin was generally similar in both age groups, with a higher incidence of AEs related to volume depletion observed with canagliflozin in participants aged 75 and older than in those younger than 75. These findings support canagliflozin, starting with the 100‐mg dose, as an effective therapeutic option for older adults with T2DM.
Keywords: Phase 3 study, SGLT2 inhibitor, canagliflozin, type 2 diabetes mellitus, older adults
Treatment of type 2 diabetes mellitus (T2DM) in older adults requires careful consideration because the risk of comorbidities, macrovascular and microvascular complications, and hypoglycemic events increases with age.1, 2 Older adults are also prone to cognitive impairment, depression, frailty, and adverse events (AEs) from polypharmacy that can further complicate the management of T2DM.1, 2, 3, 4, 5 Individuals aged 75 and older with T2DM generally have higher rates of complications than younger individuals and are more likely to require emergency treatment for hypoglycemic events.1 Current guidelines recommend an individualized approach to treating T2DM in older adults that balances achieving glycemic control with minimal risks to the individual's safety.2, 6, 7, 8
Canagliflozin is a sodium glucose co‐transporter 2 (SGLT2) inhibitor developed for the treatment of adults with T2DM.9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 By inhibiting SGLT2 in the kidneys, canagliflozin lowers the renal threshold for glucose and increases urinary glucose excretion (UGE) in individuals with T2DM, thereby decreasing plasma glucose.9, 22, 23, 24 Greater UGE with canagliflozin is associated with a net loss of calories, which leads to weight loss, and a mild osmotic diuresis that may contribute to blood pressure (BP) reductions. Because UGE depends on glomerular filtration rate (GFR), canagliflozin is expected to be less efficacious in individuals with impaired renal function or lower estimated GFR (eGFR), as is sometimes seen in older adults.25 Although previous studies have demonstrated that canagliflozin increases glycemic control in individuals with renal impairment (eGFR ≥30 and <50 mL/min per 1.73 m2)13, 19, 26 and older adults,10, 27 these changes are generally smaller than changes seen in younger individuals and those with normal renal function.
In Phase 3 studies, canagliflozin improved glycemic control, reduced body weight and systolic BP (SBP), and was generally well tolerated in a broad range of individuals with T2DM whose current treatment regimens provide inadequate glycemic control,12, 13, 14, 15, 16, 17 including a longer‐term study in individuals with T2DM aged 55 to 8010 and a pooled analysis of individuals aged 65 and older.27 Canagliflozin was associated with greater incidences of specific AEs that may be related to the mechanism of SGLT2 inhibition, including genital mycotic infections, urinary tract infections (UTIs), and AEs related to osmotic diuresis and volume depletion.28 In particular, individuals aged 75 and older have been shown to be at increased risk of AEs related to volume depletion with canagliflozin treatment.29
To further evaluate the effects of canagliflozin in older adults with T2DM, the efficacy and safety of canagliflozin was assessed in subgroups of individuals younger than 75 and those aged 75 and older based on pooled data from Phase 3 studies.
Methods
Study Design, Participant Populations, and Treatments
Efficacy analyses were performed using pooled data from individuals with T2DM enrolled in six double‐blind, placebo‐controlled Phase 3 studies and substudies of 18 or 26 weeks duration (N = 4,158; efficacy population), including canagliflozin as monotherapy,11 add‐on to metformin,16 add‐on to metformin plus sulfonylurea,17 and add‐on to metformin plus pioglitazone20 and the CANagliflozin cardioVascular Assessment Study (CANVAS) add‐on to sulfonylurea30 and add‐on to insulin31 substudies, which included 183 participants aged 75 and older (Table 1). In each study, participants were randomized to receive canagliflozin 100 or 300 mg or placebo once daily. A high‐glycemic substudy (baseline glycosylated hemoglobin (HbA1c) >10.0–12.0%) of the monotherapy study11 was not placebo controlled and therefore not included in this analysis. Safety and tolerability were assessed in a broader population of participants with T2DM enrolled in eight double‐blind, placebo‐ and active‐controlled Phase 3 studies (N = 9,439; safety population), which included 490 participants aged 75 and older (Table 1). The safety population included the four 26‐week studies above,11, 16, 17, 20 a 52‐week study of canagliflozin as add‐on to metformin vs glimepiride,15 a 26‐week study of individuals aged 55 to 80,14 and a 26‐week study of individuals with moderate renal impairment (baseline eGFR 30 to <50 mL/min per 1.73 m2).19 Safety analyses also included all participants enrolled in CANVAS (an ongoing event‐driven study), not just the prespecified substudies included in the efficacy population, through a cutoff date of September 15, 2011.
Table 1.
Number of Participants Contributing to Pooled Efficacy and Safety Populations, According to Age
| Study | Efficacy Population | Safety Population | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <75 | ≥75 | <75 | ≥75 | |||||||||
| Placebo | Canagliflozin 100 mg | Canagliflozin 300 mg | Placebo | Canagliflozin 100 mg | Canagliflozin 300 mg | Non‐canagliflozin | Canagliflozin 100 mg | Canagliflozin 300 mg | Non‐canagliflozin | Canagliflozin 100 mg | Canagliflozin 300 mg | |
| Monotherapy | 186 | 189 | 192 | 6 | 6 | 5 | 186 | 189 | 192 | 6 | 6 | 5 |
| Add‐on to metformin | 183 | 364 | 363 | 0 | 4 | 4 | 543 | 364 | 363 | 6 | 4 | 4 |
| Add‐on to metformin and sulfonylurea | 154 | 150 | 154 | 2 | 7 | 2 | 154 | 150 | 154 | 2 | 7 | 2 |
| Add‐on to metformin and pioglitazone | 108 | 109 | 108 | 7 | 4 | 6 | 108 | 109 | 108 | 7 | 4 | 6 |
| CANVAS add‐on to insulin substudy | 531 | 530 | 539 | 34 | 36 | 48 | — | — | — | — | — | — |
| CANVAS add‐on to sulfonylurea substudy | 42 | 38 | 35 | 3 | 4 | 5 | — | — | — | — | — | — |
| Add‐on to metformin vs glimepiride | — | — | — | — | — | — | 475 | 469 | 476 | 7 | 14 | 9 |
| Older adults (55–80) | — | — | — | — | — | — | 225 | 221 | 222 | 12 | 20 | 14 |
| Moderate renal impairment (estimated glomerular filtration rate 30 to <50 mL/min per 1.73 m2) | — | — | — | — | — | — | 68 | 66 | 70 | 22 | 24 | 19 |
| CANVAS (all) | — | — | — | — | — | — | 1,348 | 1,361 | 1,328 | 93 | 84 | 113 |
| Total | 1,204 | 1,380 | 1,391 | 52 | 61 | 70 | 3,107 | 2,929 | 2,913 | 155 | 163 | 172 |
CANVAS = CANagliflozin cardioVascular Assessment Study.
Participation in the studies was restricted to adults with inadequately controlled T2DM at screening while on the protocol‐specified background antihyperglycemic agent (AHA) therapy. Inclusion criteria for most studies included HbA1c between 7.0% and 10.5% at screening and repeated fasting plasma glucose (FPG) less than 270 mg/dL during the pretreatment phase. The age range for most studies was 18 to 80, with the following exceptions: the study in older adults aged 55 to 80; CANVAS, which enrolled participants aged 30 and older (with cardiovascular history) or aged 50 and older (with presence of cardiovascular risk factors); and the study in people with moderate renal impairment, which enrolled participants aged 25 and older. Details of study design, including exclusion criteria, randomization and blinding, and glycemic rescue therapy, have previously been reported for the individual studies.11, 14, 16, 17, 19, 20, 32
All studies included in this analysis were conducted in accordance with ethical principles that comply with the Declaration of Helsinki and were consistent with Good Clinical Practices and applicable regulatory requirements. Institutional review boards and independent ethics committees at participating institutions approved the study protocols and amendments. All participants provided written informed consent before participation in each of the studies.
Study Endpoints and Assessments
Changes from baseline in HbA1c, FPG, body weight, SBP, and diastolic BP (DBP) were evaluated in the efficacy population at Week 18 or 26 in subgroups of participants younger than 75 (n = 3,975) and aged 75 and older (n = 183). Safety and tolerability were assessed based on AE reports in the safety population in participants younger than 75 (n = 8,949) and those aged 75 and older (n = 490) through Week 26 or 52 or the cutoff date for CANVAS (mean duration of exposure, 45.3 weeks). The overall incidence of AEs and the incidence of specific AEs, including genital mycotic infections, UTIs, osmotic diuresis–related AEs (e.g., pollakiuria (abnormally frequent urination), polyuria (production of abnormally large volumes of dilute urine)), and volume depletion–related AEs (e.g., orthostatic hypotension, postural dizziness) were evaluated. Documented hypoglycemia episodes included biochemically confirmed episodes (concurrent fingerstick or plasma glucose ≤70 mg/dL, with or without symptoms) and severe episodes (those requiring the assistance of another individual or resulting in seizure or loss of consciousness).
Statistical Analyses
Efficacy analyses were conducted using the modified intention‐to‐treat population, which included all randomized participants who received one or more doses of double‐blind study drug. The last observation carried forward approach was used to impute missing data; for participants who received glycemic rescue therapy, the last postbaseline value before initiation of rescue was used for analysis. An analysis of covariance model, with treatment and study as fixed effects and the corresponding baseline value for each endpoint as a covariate, was used to assess primary endpoints. The least squares (LS) mean differences between groups and two‐sided 95% confidence intervals (CIs) were estimated. P‐values were calculated for difference in HbA1c with canagliflozin vs placebo; statistical testing was not prespecified for other post hoc efficacy analyses. Safety analyses included all reported AEs, regardless of rescue therapy, and included all randomized participants who received one or more doses of double‐blind study drug.
Results
Participant Disposition and Baseline Characteristics
In the efficacy (Table 2) and safety (Table 3) populations, baseline characteristics were generally similar across groups in each age subgroup. Participants aged 75 and older in the efficacy population had a lower baseline eGFR (mean 65.0 mL/min per 1.73 m2 vs 82.9 mL/min per 1.73 m2; median 65.0 mL/min per 1.73 m2 vs 82.0 mL/min per 1.73 m2) and longer mean duration of T2DM (18.3 years vs 10.9 years) than participants younger than 75; similar results were seen in the safety population. The majority of participants in both age groups in the efficacy and safety populations were taking medications at baseline that were not part of the study assessment (data available from corresponding author). In the efficacy population, 11.1% of participants younger than 75 and 10.9% of those aged 75 and older discontinued before the primary endpoint; in the safety population, 15.0% of participants younger than 75 and 18.5% of those aged 75 and older discontinued.
Table 2.
Baseline Demographic and Disease Characteristics of Participants According to Age: Efficacy Population
| Characteristic | <75 | ≥75 | ||||
|---|---|---|---|---|---|---|
| Placebo, n = 1,204 | Canagliflozin 100 mg, n = 1,380 | Canagliflozin 300 mg, n = 1,391 | Placebo, n = 52 | Canagliflozin 100 mg, n = 61 | Canagliflozin 300 mg, n = 70 | |
| Sex, n (%) | ||||||
| Male | 704 (58) | 773 (56) | 760 (55) | 36 (69) | 38 (62) | 50 (71) |
| Female | 500 (42) | 607 (44) | 631 (45) | 16 (31) | 23 (38) | 20 (29) |
| Age, mean ± SD | 58.6 ± 8.8 | 57.9 ± 9.2 | 58.2 ± 8.9 | 76.8 ± 1.8 | 77.0 ± 1.7 | 77.1 ± 2.4 |
| Race, n (%) | ||||||
| White | 894 (74) | 1,015 (74) | 1,037 (75) | 49 (94) | 51 (84) | 62 (89) |
| Black | 42 (3) | 53 (4) | 66 (5) | 2 (4) | 1 (2) | 1 (1) |
| Asian | 174 (14) | 182 (13) | 181 (13) | 0 | 2 (3) | 5 (7) |
| Othera | 94 (8) | 130 (9) | 107 (8) | 1 (2) | 7 (11) | 2 (3) |
| Glycosylated hemoglobin, %, mean ± SD | 8.2 ± 0.9 | 8.1 ± 0.9 | 8.1 ± 1.0 | 8.0 ± 0.9 | 8.1 ± 0.9 | 7.9 ± 0.7 |
| Fasting plasma glucose, mg/dL, mean ± SD | 168.3 ± 44.5 | 170.6 ± 44.2 | 170.3 ± 47.2 | 172.4 ± 48.6 | 161.2 ± 39.9 | 163.5 ± 44.1 |
| Estimated glomerular filtration rate, mL/min per 1.73 m2, mean ± SD | 81.3 ± 20.6 | 84.1 ± 19.6 | 83.0 ± 20.1 | 66.2 ± 16.9 | 63.9 ± 15.8 | 65.0 ± 16.0 |
| Body mass index, kg/m2, mean ± SD | 32.8 ± 6.5 | 32.9 ± 6.5 | 32.7 ± 6.4 | 31.1 ± 5.0 | 29.7 ± 4.6 | 31.0 ± 5.0 |
| Duration of type 2 diabetes mellitus, years, mean ± SD | 11.3 ± 8.0 | 10.8 ± 7.8 | 10.8 ± 7.8 | 19.1 ± 10.5 | 17.9 ± 8.9 | 18.0 ± 8.5 |
Percentages may not total 100% because of rounding.
American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, multiple, not reported, other, unknown.
SD = standard deviation.
Table 3.
Baseline Demographic and Disease Characteristics According to Age: Safety Population
| Characteristic | <75 | ≥75 | ||||
|---|---|---|---|---|---|---|
| Non‐canagliflozin, n = 3,107 | Canagliflozin 100 mg, n = 2,929 | Canagliflozin 300 mg, n = 2,913 | Non‐canagliflozin, n = 155 | Canagliflozin 100 mg, n = 163 | Canagliflozin 300 mg, n = 172 | |
| Sex, n (%) | ||||||
| Male | 1,828 (59) | 1,700 (58) | 1,649 (57) | 96 (62) | 103 (63) | 117 (68) |
| Female | 1,279 (41) | 1,229 (42) | 1,264 (43) | 59 (38) | 60 (37) | 55 (32) |
| Age, mean ± SD | 58.8 ± 8.5 | 59.0 ± 8.7 | 58.9 ± 8.6 | 77.6 ± 2.8 | 77.5 ± 2.2 | 77.4 ± 2.9 |
| Race, n (%) | ||||||
| White | 2,239 (72) | 2,095 (72) | 2,090 (72) | 143 (92) | 144 (88) | 146 (85) |
| Black | 114 (4) | 112 (4) | 124 (4) | 4 (3) | 3 (2) | 2 (1) |
| Asian | 501 (16) | 494 (17) | 480 (16) | 5 (3) | 2 (1) | 11 (6) |
| Othera | 253 (8) | 228 (8) | 219 (8) | 3 (2) | 14 (9) | 13 (8) |
| Glycosylated hemoglobin, %, mean ± SD | 8.0 ± 0.9 | 8.1 ± 0.9 | 8.0 ± 0.9 | 7.9 ± 0.9 | 7.9 ± 0.8 | 7.8 ± 0.8 |
| Fasting plasma glucose, mg/dL, mean ± SD | 166.2 ± 42.8 | 167.7 ± 42.9 | 166.0 ± 44.1 | 164.0 ± 43.7 | 158.7 ± 39.5 | 160.0 ± 43.7 |
| Estimated glomerular filtration rate, mL/min per 1.73 m2, mean ± SD | 82.0 ± 20.4 | 82.6 ± 20.0 | 82.2 ± 20.6 | 62.5 ± 17.3 | 62.7 ± 17.7 | 63.8 ± 18.5 |
| Body mass index, kg/m2, mean ± SD | 32.0 ± 6.1 | 32.0 ± 6.1 | 32.0 ± 6.1 | 30.2 ± 5.1 | 29.7 ± 4.4 | 30.0 ± 4.7 |
| Duration of type 2 diabetes mellitus, years, mean ± SD | 10.0 ± 7.2 | 10.4 ± 7.4 | 10.4 ± 7.4 | 16.8 ± 9.2 | 16.0 ± 8.6 | 16.3 ± 8.7 |
| Background use of diuretics, n (%) | ||||||
| Any | 1,095 (35) | 994 (34) | 989 (34) | 74 (48) | 82 (50) | 87 (51) |
| Loop | 227 (7) | 189 (7) | 215 (7) | 29 (19) | 27 (17) | 35 (20) |
| Nonloop | 949 (31) | 869 (30) | 858 (30) | 53 (34) | 63 (39) | 64 (37) |
Percentages may not total 100% because of rounding.
American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, multiple, not reported, other, unknown.
SD = standard deviation.
Efficacy
Glycemic Parameters
Participants younger than 75 and aged 75 and older taking canagliflozin 100 and 300 mg had greater reductions in HbA1c compared with those taking placebo (Figure 1A). Canagliflozin 100 and 300 mg were associated with placebo‐subtracted LS mean reductions from baseline in HbA1c in participants younger than 75 (−0.69% and −0.85%, respectively) and aged 75 and older (−0.65% and −0.55%, respectively). Median changes in HbA1c were −0.70% with canagliflozin 100 mg, −0.90% with canagliflozin 300 mg, and −0.10% with placebo in participants younger than 75 and −0.50%, −0.50%, and 0%, respectively, in those aged 75 and older. Canagliflozin also reduced FPG more than placebo in both age groups. Greater placebo‐subtracted reductions in FPG from baseline were seen with canagliflozin 100 and 300 mg in participants younger than 75 (−26.9 and −35.4 mg/dL, respectively) than in those aged 75 and older (−14.6 and −21.7 mg/dL, respectively) (Figure 1B).
Figure 1.
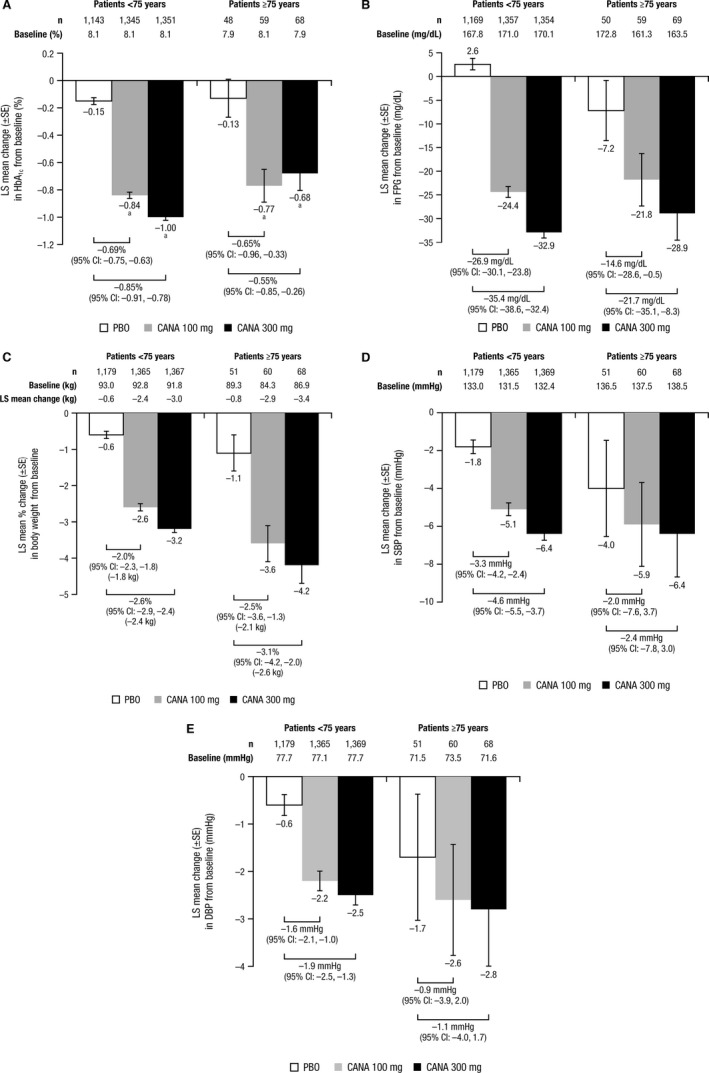
Changes from baseline in (A) glycosylated hemoglobin (HbA1c), (B) fasting plasma glucose (FPG), (C) body weight, (D) systolic blood pressure (SBP), and (E) diastolic blood pressure (DBP) in participants younger than 75 and aged 75 and older (efficacy population). LS = least squares; SE = standard error; CI = confidence interval; CANA = canagliflozin. a P < .001 vs placebo (PBO). Statistical testing was not prespecified for post hoc analyses of FPG, body weight, SBP, and DBP.
Body Weight and BP
Participants younger than 75 and those aged 75 and older taking canagliflozin 100 and 300 mg lost weight (Figure 1C); placebo‐subtracted LS mean percent changes were −2.0% and −2.6%, respectively, in participants younger than 75 and −2.5% and −3.1%, respectively, in those aged 75 and older. Both canagliflozin doses were associated with BP reductions in both age groups, although the 95% CIs for the placebo‐subtracted differences with canagliflozin 100 and 300 mg in participants aged 75 and older included 0 (Figure 1D,E). Placebo‐subtracted changes in SBP with canagliflozin 100 and 300 mg were −3.3 and −4.6 mmHg, respectively, in participants younger than 75 and −2.0 and −2.4 mmHg, respectively, in those aged 75 and older. Placebo‐subtracted changes in DBP with canagliflozin 100 and 300 mg were −1.6 and −1.9 mmHg, respectively, in participants younger than 75 and −0.9 and −1.1 mmHg, respectively, in those aged 75 and older.
Safety and Tolerability
The overall incidence of AEs with canagliflozin 100 and 300 mg and non‐canagliflozin was 67.1%, 68.6%, and 65.9%, respectively, in participants younger than 75 and 72.4%, 79.1%, and 72.3%, respectively, in those aged 75 and older (Table 4). The incidence of serious AEs with canagliflozin 100 and 300 mg and non‐canagliflozin was 7.2%, 7.8%, and 7.6%, respectively, in participants younger than 75 and 17.2%, 12.2%, and 23.2%, respectively, in those aged 75 and older. In participants younger than 75, the incidence of AEs leading to discontinuation of canagliflozin 100 and 300 mg and non‐canagliflozin was 3.9%, 5.6%, and 3.5%, respectively. In participants aged 75 and older, the incidence of AEs leading to discontinuation of canagliflozin 100 and 300 mg and non‐canagliflozin was 9.8%, 5.8%, and 7.7%, respectively; the increase in AEs leading to discontinuation of canagliflozin 100 mg was not associated with any specific AEs. Both canagliflozin doses were associated with a numerically higher incidence of AEs related to study drug than non‐canagliflozin regardless of age; this higher incidence was related to increases in specific AEs associated with canagliflozin treatment (e.g., genital mycotic infections, UTIs, osmotic diuresis–related AEs, volume depletion–related AEs).
Table 4.
Summary of Overall Safety and Selected Adverse Events (AEs) According to Age: Safety Population
| Parameter | <75 | ≥75 | ||||
|---|---|---|---|---|---|---|
| Non‐canagliflozin, n = 3,107 | Canagliflozin 100 mg, n = 2,929 | Canagliflozin 300 mg, n = 2,913 | Non‐canagliflozin, n = 155 | Canagliflozin 100 mg, n = 163 | Canagliflozin 300 mg, n = 172 | |
| Any AE | 2,048 (65.9) | 1,965 (67.1) | 1,997 (68.6) | 112 (72.3) | 118 (72.4) | 136 (79.1) |
| AE leading to discontinuation | 109 (3.5) | 113 (3.9) | 163 (5.6) | 12 (7.7) | 16 (9.8) | 10 (5.8) |
| AE related to study druga | 553 (17.8) | 716 (24.4) | 850 (29.2) | 32 (20.6) | 49 (30.1) | 62 (36.0) |
| Serious AE | 235 (7.6) | 211 (7.2) | 228 (7.8) | 36 (23.2) | 28 (17.2) | 21 (12.2) |
| Death | 14 (0.5) | 10 (0.3) | 13 (0.4) | 4 (2.6) | 2 (1.2) | 0 |
| Selected AE | ||||||
| Urinary tract infection | 131 (4.2) | 158 (5.4) | 163 (5.6) | 10 (6.5) | 13 (8.0) | 12 (7.0) |
| Genital mycotic infection | ||||||
| Menb , c | 20 (1.1) | 98 (5.8) | 130 (7.9) | 0 | 6 (5.8) | 10 (8.5) |
| Womend , e | 29 (2.3) | 153 (12.4) | 154 (12.2) | 1 (1.7) | 8 (13.3) | 7 (12.7) |
| Osmotic diuresis–related AEf | 55 (1.8) | 203 (6.9) | 202 (6.9) | 7 (4.5) | 7 (4.3) | 17 (9.9) |
| Volume depletion–related AEg | 45 (1.4) | 63 (2.2) | 90 (3.1) | 4 (2.6) | 8 (4.9) | 15 (8.7) |
| Hypoglycemia episode | ||||||
| Not taking insulin, sulfonylurea, or meglitinide, n | 1,592 | 1,386 | 1,390 | 45 | 47 | 42 |
| Documented hypoglycemiah | 192 (12.1) | 73 (5.3) | 68 (4.9) | 7 (15.6) | 2 (4.3) | 2 (4.8) |
| Severe hypoglycemia | 14 (0.9) | 6 (0.4) | 4 (0.3) | 1 (2.2) | 0 | 1 (2.4) |
| Taking insulin, sulfonylurea, or meglitinide, n | 1,515 | 1,543 | 1,523 | 110 | 116 | 130 |
| Documented hypoglycemiah | 492 (32.5) | 617 (40.0) | 646 (42.4) | 43 (39.1) | 61 (52.6) | 55 (42.3) |
| Severe hypoglycemia | 31 (2.0) | 34 (2.2) | 39 (2.6) | 6 (5.5) | 4 (3.4) | 5 (3.8) |
Possibly, probably, or very likely related to the study drug, as assessed by investigator.
<75: non‐canagliflozin, n = 1,828; canagliflozin 100 mg, n = 1,700; canagliflozin 300 mg, n = 1,649; ≥75: non‐canagliflozin, n = 96; canagliflozin 100 mg, n = 103; canagliflozin 300 mg, n = 117.
Balanitis, balanitis candida, balanoposthitis, balanoposthitis infective, genital candidiasis, genital infection fungal, penile infection, posthitis.
<75: non‐canagliflozin, n = 1,279; canagliflozin 100 mg, n = 1,229; canagliflozin 300 mg, n = 1,264; ≥75: non‐canagliflozin, n = 59; canagliflozin 100 mg, n = 60; canagliflozin 300 mg, n = 55.
Genital candidiasis, genital infection fungal, vaginal infection, vulvitis, vulvovaginal candidiasis, vulvovaginal mycotic infection, vulvovaginitis.
Dry mouth, dry throat, micturition disorder, micturition urgency, nocturia, pollakiuria, polydipsia, polyuria, thirst, high urine output.
Low blood pressure, dehydration, postural dizziness, hypotension, orthostatic hypotension, orthostatic intolerance, presyncope, syncope.
Biochemically documented (≤70 mg/dL) or severe (requiring assistance of another individual or resulting in seizure or loss of consciousness) episodes.
The incidence of male genital mycotic infections with canagliflozin 100 and 300 mg and non‐canagliflozin was 5.8%, 7.9%, and 1.1%, respectively, in participants younger than 75, and 5.8%, 8.5%, and 0%, respectively, in participants aged 75 and older (Table 4). The incidence of female genital mycotic infections was 12.4%, 12.2%, and 2.3%, respectively, in participants younger than 75 and 13.3%, 12.7%, and 1.7%, respectively, in participants aged 75 and older. None of these events was considered to be serious, and few (<2% across groups) led to study discontinuation. Genital mycotic infections responded to standard treatments, which included oral and topical antifungal agents that were self‐initiated or given at the discretion of the treating physician. Across treatment groups, 12 of 16 men and 12 of 16 women aged 75 and older with a genital mycotic infection AE had a single event; four men (1 with canagliflozin 100 mg, 3 with canagliflozin 300 mg) and four women (3 with canagliflozin 100 mg, 1 with canagliflozin 300 mg) reported more than one genital mycotic infection AE.
The incidence of UTIs with canagliflozin 100 and 300 mg and non‐canagliflozin was 5.4%, 5.6%, and 4.2%, respectively, in participants younger than 75, and 8.0%, 7.0%, and 6.5%, respectively, in those aged 75 and older (Table 4). There were 13 participants younger than 75 (6 with canagliflozin 100 mg, 4 with canagliflozin 300 mg, 3 with non‐canagliflozin) and two aged 75 and older (1 with canagliflozin 100 mg, 1 with non‐canagliflozin) who discontinued because of UTI AEs. There were 18 participants younger than 75 (7 with canagliflozin 100 mg, 5 with canagliflozin 300 mg, 6 with non‐canagliflozin) and two aged 75 and older (1 with canagliflozin 100 mg, 1 with non‐canagliflozin) with serious UTIs. Twenty participants younger than 75 (8 with canagliflozin 100 mg, 5 with canagliflozin 300 mg, 7 with non‐canagliflozin) and three aged 75 and older (2 with canagliflozin 100 mg, 1 with non‐canagliflozin) reported upper UTIs (e.g., pyelonephritis, urosepsis). UTIs were effectively treated using standard antimicrobial therapies. Twenty‐nine of 35 participants aged 75 and older with a UTI experienced one UTI AE; the six who reported more than one UTI were all taking canagliflozin 100 mg.
The incidence of osmotic diuresis–related AEs with canagliflozin 100 and 300 mg and non‐canagliflozin was 6.9%, 6.9%, and 1.8%, respectively, in participants younger than 75, and 4.3%, 9.9%, and 4.5%, respectively, in those aged 75 and older (Table 4). There were 16 (7 with canagliflozin 100 mg, 8 with canagliflozin 300 mg, 1 with non‐canagliflozin) osmotic diuresis–related AEs that led to discontinuation in participants younger than 75 and one in a participant aged 75 and older with canagliflozin 300 mg; none was considered serious. Twenty‐six of 31 participants aged 75 and older with AEs related to osmotic diuresis experienced one AE; of the five that reported more than one osmotic diuresis–related AE, two were taking canagliflozin 100 mg, two were taking canagliflozin 300 mg, and one was taking non‐canagliflozin.
The incidence of volume depletion–related AEs with canagliflozin 100 and 300 mg and non‐canagliflozin was 2.2%, 3.1%, and 1.4%, respectively, in participants younger than 75 and 4.9%, 8.7%, and 2.6%, respectively, in those aged 75 and older (Table 4). The proportion of participants taking background loop diuretics, which are associated with these AEs, was numerically higher in participants aged 75 and older across treatment groups. Most volume depletion–related AEs were mild or moderate in intensity, as assessed according to the investigator. Six volume depletion–related AEs led to discontinuation (2 in each group) in participants younger than 75. No volume depletion–related AEs led to discontinuation in participants aged 75 and older. There were 16 serious volume depletion–related AEs (5 with canagliflozin 100 mg, 3 with canagliflozin 300 mg, 8 with non‐canagliflozin) in participants younger than 75 and three in those aged 75 and older (1 in each group). Twenty‐one of 27 participants aged 75 and older with AEs related to volume depletion experienced one AE; of the six that reported more than one volume depletion–related AE, two were taking canagliflozin 100 mg, three were taking canagliflozin 300 mg, and one was taking non‐canagliflozin.
In participants who were not taking AHAs associated with hypoglycemia, the incidence of documented hypoglycemia episodes with canagliflozin 100 and 300 mg and non‐canagliflozin was 5.3%, 4.9%, and 12.1%, respectively, in those younger than 75 and 4.3%, 4.8%, and 15.6%, respectively, in those aged 75 and older (Table 4). In participants who were taking AHAs associated with hypoglycemia (insulin, sulfonylurea, meglitinide), the incidence of documented hypoglycemia was 40.0%, 42.4%, and 32.5% with canagliflozin 100 and 300 mg and non‐canagliflozin, respectively, in participants younger than 75, and 52.6%, 42.3%, and 39.1%, respectively, in those aged 75 and older. The incidence of severe hypoglycemia episodes was low across treatment groups regardless of age and background AHA treatment.
Discussion
Findings from this pooled analysis show that canagliflozin 100 and 300 mg improved glycemic control and reduced body weight and BP more than placebo in participants with T2DM younger than 75 and aged 75 and older. Greater numerical reductions in HbA1c and FPG were seen with canagliflozin in participants younger than 75 than in those aged 75 and older. The poorer glycemic efficacy of canagliflozin in participants aged 75 and older might have been related to their lower mean baseline eGFR than that of those younger than 75 (65.0 mL/min per 1.73 m2 vs 82.9 mL/min per 1.73 m2). Because of its mechanism of action (lowering blood glucose by increasing UGE), the efficacy of canagliflozin has been shown to depend on renal function.26 Consistent with this, canagliflozin had poorer glycemic efficacy in participants with T2DM aged 55 to 8010 and in a subgroup of participants aged 65 and older27 than in younger participants with higher baseline eGFR in other studies.11, 12, 15, 16, 17, 18, 20, 21 An inverse dose response was seen for mean changes in HbA1c with canagliflozin 100 and 300 mg in participants aged 75 and older, although an inverse dose response was not seen for median changes in HbA1c, suggesting the possible influence of outliers in a small sample size. A dose response was also seen in FPG, another measure of glycemic control. Dose‐dependent decreases in HbA1c were seen with canagliflozin in the subgroup of participants younger than 75 and in the overall pooled population, consistent with previous Phase 3 studies.9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21
Reductions in body weight were observed with both canagliflozin doses compared with placebo in participants younger than 75 and aged 75 and older. Canagliflozin was associated with reductions in BP relative to placebo in both age groups, but 95% CIs included 0 for both canagliflozin doses in participants aged 75 and older; numerically smaller placebo‐subtracted BP reductions were seen with canagliflozin 100 and 300 mg in participants aged 75 and older than in younger participants.
The incidence of overall AEs was higher across treatment groups in participants aged 75 and older than in those younger than 75. AEs leading to discontinuation were numerically higher with canagliflozin 100 mg than with canagliflozin 300 mg and non‐canagliflozin in participants aged 75 and older; this difference was not related to any pattern of specific AEs. Furthermore, baseline characteristics were balanced between treatment groups, suggesting that the higher incidence of these AEs with canagliflozin 100 mg in participants aged 75 and older may have been because of the small sample size.
The safety profile in the current analysis was consistent with those of previous studies,9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 with a higher incidence of AEs related to the mechanism of action of canagliflozin (UTIs, genital mycotic infections, osmotic diuresis– and volume depletion–related AEs) observed with canagliflozin in both age subgroups; these AEs were generally considered mild or moderate in intensity, and few led to study discontinuations. Current labeling for canagliflozin indicates that participants aged 75 and older are more susceptible to volume depletion–related AEs as adverse drug reactions to canagliflozin.29 Similar AE profiles were seen with canagliflozin in a study in individuals aged 55 to 80 and in a pooled analysis of Phase 3 studies in participants aged 65 and older,27 although AE rates in the current analysis were generally higher in participants aged 75 and older, which may be related to the higher mean age of participants in this subgroup and the longer duration of studies included in this analysis. Furthermore, this analysis was based on a larger pooled population that included participants with moderate renal impairment (eGFR 30 to <50 mL/min per 1.73 m2) and participants from CANVAS with a history of or at high risk of cardiovascular disease, which may have contributed to the greater incidence of AEs in participants aged 75 and older.
Although these data in older adults are novel, a limitation of this analysis is the small number of participants aged 75 and older and the lack of active comparators, which would help compare the efficacy of canagliflozin with that of other AHAs recommended for older adults with T2DM. Future research in this area will require assessments over a longer treatment period to determine the benefits and risks associated with canagliflozin treatment, especially given the limited representation of older adults in AHA clinical trials. Although long‐term improvement in glycemic control and a generally favorable safety profile were observed in a study of older adults over 104 weeks,10 longer‐term analyses including more participants aged 75 and older would be useful in evaluating the efficacy and safety of canagliflozin in this important population of patients with T2DM.
Because individuals with T2DM aged 75 and older have more comorbidities and greater risk of complications and hypoglycemia, it is important to evaluate canagliflozin in very old individuals to guide clinical decision‐making. The American Diabetes Association and the American Geriatrics Society recommend customized HbA1c goals for older adults that take into account the comorbidities, functional status, and life expectancy of the individual.1 An HbA1c target of less than 7.5% is recommended for healthy older adults with few comorbidities and good cognitive and functional status. Higher HbA1c targets (<8.0% or <8.5%) may be appropriate if the risks of intensive glycemic control outweigh potential benefits, particularly in frail older adults with multiple comorbidities, high risk of hypoglycemia, and limited life expectancy. This analysis demonstrates that canagliflozin may offer multiple benefits for older adults with T2DM, including favorable glycemic efficacy and low risk of hypoglycemia. As in the general population with T2DM, a starting dose of canagliflozin 100 mg once daily is recommended for all individuals, including those aged 75 and older. In individuals tolerating canagliflozin 100 mg with an eGFR of 60 mL/min per 1.73 m2 or greater who require additional glycemic control, the dose can be increased to 300 mg once daily. Clinicians should be aware of potential safety concerns in older adults. For example, canagliflozin was associated with a higher incidence of genital mycotic infections, but most were not serious and responded well to standard treatments; with monitoring and prompt treatment, these AEs may be efficiently managed. Clinicians should also closely monitor participants aged 75 and older, particularly those taking loop diuretics, and advise them of potential symptoms related to volume depletion. Careful management of antihypertensive medications may help to reduce the risk of these AEs. Increased risk of fracture AEs has been seen with canagliflozin in CANVAS participants but not in the general population.34 The observed difference in fracture AEs did not depend on age.
Overall, canagliflozin improved glycemic control, body weight, and BP in participants younger than 75 and in those aged 75 and older. A numerically higher incidence of UTIs, genital mycotic infections, and AEs related to osmotic diuresis and volume depletion was seen with canagliflozin in participants aged 75 and older; however, these AEs were generally not considered serious and few led to discontinuation. Participants aged 75 and older should be closely monitored for AEs related to volume depletion, and care should be taken when increasing canagliflozin dose. Together, these findings support canagliflozin, starting with the 100‐mg dose, as a safe and efficacious therapeutic option for individuals aged 75 and older with T2DM.
Acknowledgments
The studies described in this manuscript were sponsored by Janssen Research & Development, LLC.
Conflict of Interest: A.S. has received lecture and/or advisory board fees from Takeda, Novartis, Eli Lilly and Company, and Merck Sharp & Dohme. B.B. has served as investigator using research and grant support received by his institution from Janssen Research & Development, LLC. S.H. has served on advisory boards for Sanofi‐Aventis, Janssen, Novo Nordisk, AstraZeneca, Bristol‐Myers Squibb, Eli Lilly and Company, and Boehringer Ingelheim; has served as a speaker for Sanofi‐Aventis, AstraZeneca, Bristol‐Myers Squibb, Eli Lilly and Company, Boehringer Ingelheim, and Novo Nordisk; and has received research support from Sanofi‐Aventis, Novo Nordisk, and AstraZeneca. U.V., W.S., M.D., and G.M. are current full‐time employees of Janssen Research & Development, LLC.
Author Contributions: Sinclair, Bode, Harris: data interpretation; drafted, reviewed, and approved manuscript. Shaw, Desai, Meininger: design and conduct of analysis; data acquisition, analysis, and interpretation; drafted, reviewed, and approved manuscript. Vijapurkar: data analysis and interpretation; drafted, reviewed, and approved manuscript.
Sponsor's Role: The sponsor had a role in the study design and conduct and in data collection, analysis, and interpretation. The authors prepared the report with editorial assistance funded by the sponsor. All authors had full access to study data; were responsible for the integrity of the data and the accuracy of the data analysis; and reviewed, edited, and approved the report for publication. Editorial support was provided by Kimberly Fuller, PhD, of MedErgy, and was funded by Janssen Global Services, LLC. Canagliflozin has been developed by Janssen Research & Development, LLC, in collaboration with Mitsubishi Tanabe Pharma Corporation.
The authors thank all investigators, study teams, and participants for participating in these studies.
J Am Geriatr Soc 64:543–552, 2016.
References
- 1. Kirkman MS, Briscoe VJ, Clark N et al. Diabetes in older adults. Diabetes Care 2012;35:2650–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Inzucchi SE, Bergenstal RM, Buse JB et al. Management of hyperglycemia in type 2 diabetes, 2015: A patient‐centered approach: Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140–149. [DOI] [PubMed] [Google Scholar]
- 3. Booth GL, Kapral MK, Fung K et al. Relation between age and cardiovascular disease in men and women with diabetes compared with non‐diabetic people: A population‐based retrospective cohort study. Lancet 2006;368:29–36. [DOI] [PubMed] [Google Scholar]
- 4. Meneilly GS, Knip A, Tessier D. Diabetes in the elderly. Can J Diabetes 2013;37:S184–S190. [DOI] [PubMed] [Google Scholar]
- 5. Sinclair A, Morley J. Frailty and diabetes. Lancet 2013;382:1386–1387. [DOI] [PubMed] [Google Scholar]
- 6. American Diabetes Association . Standards of medical care in diabetes—2015. Diabetes Care 2015;38:S1–S93. [DOI] [PubMed] [Google Scholar]
- 7. Sinclair A, Morley JE, Rodriguez‐Manas L et al. Diabetes mellitus in older people: Position statement on behalf of the International Association of Gerontology and Geriatrics (IAGG), the European Diabetes Working Party for Older People (EDWPOP), and the International Task Force of Experts in Diabetes. J Am Med Dir Assoc 2012;13:497–502. [DOI] [PubMed] [Google Scholar]
- 8. Morley JE, Sinclair A. Individualising treatment for older people with diabetes. Lancet 2013;382:378–380. [DOI] [PubMed] [Google Scholar]
- 9. Rosenstock J, Aggarwal N, Polidori D et al. Dose‐ranging effects of canagliflozin, a sodium‐glucose cotransporter 2 inhibitor, as add‐on to metformin in subjects with type 2 diabetes. Diabetes Care 2012;35:1232–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bode B, Stenlöf K, Harris S et al. Long‐term efficacy and safety of canagliflozin over 104 weeks in patients aged 55 to 80 years with type 2 diabetes. Diabetes Obes Metab 2015;17:294–303. [DOI] [PubMed] [Google Scholar]
- 11. Stenlöf K, Cefalu WT, Kim K‐A et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab 2013;15:372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schernthaner G, Gross JL, Rosenstock J et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: A 52‐week, randomized trial. Diabetes Care 2013;36:2508–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yale JF, Bakris G, Cariou B et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab 2013;15:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bode B, Stenlöf K, Sullivan D et al. Efficacy and safety of canagliflozin treatment in older subjects with type 2 diabetes mellitus: A randomized trial. Hosp Pract 2013;41:72–84. [DOI] [PubMed] [Google Scholar]
- 15. Cefalu WT, Leiter LA, Yoon K‐H et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA‐SU): 52 week results from a randomised, double‐blind, phase 3 non‐inferiority trial. Lancet 2013;382:941–950. [DOI] [PubMed] [Google Scholar]
- 16. Lavalle‐González FJ, Januszewicz A, Davidson J et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: A randomised trial. Diabetologia 2013;56:2582–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilding JP, Charpentier G, Hollander P et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: A randomised trial. Int J Clin Pract 2013;67:1267–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stenlöf K, Cefalu WT, Kim KA et al. Long‐term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes inadequately controlled with diet and exercise: Findings from the 52‐week CANTATA‐M study. Curr Med Res Opin 2014;30:163–175. [DOI] [PubMed] [Google Scholar]
- 19. Yale JF, Bakris G, Cariou B et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab 2014;16:1016–1027. [DOI] [PubMed] [Google Scholar]
- 20. Forst T, Guthrie R, Goldenberg R et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab 2014;16:467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leiter LA, Yoon K‐H, Arias P et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: A randomized, double‐blind, Phase 3 study. Diabetes Care 2015;38:355–364. [DOI] [PubMed] [Google Scholar]
- 22. Polidori D, Sha S, Ghosh A et al. Validation of a novel method for determining the renal threshold for glucose excretion in untreated and canagliflozin‐treated subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab 2013;98:E867–E871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Devineni D, Morrow L, Hompesch M et al. Canagliflozin improves glycaemic control over 28 days in subjects with type 2 diabetes not optimally controlled on insulin. Diabetes Obes Metab 2012;14:539–545. [DOI] [PubMed] [Google Scholar]
- 24. Devineni D, Curtin CR, Polidori D et al. Pharmacokinetics and pharmacodynamics of canagliflozin, a sodium glucose co‐transporter 2 inhibitor, in subjects with type 2 diabetes mellitus. J Clin Pharmacol 2013;53:601–610. [DOI] [PubMed] [Google Scholar]
- 25. Glassock RJ, Winearls C. Ageing and the glomerular filtration rate: Truths and consequences. Trans Am Clin Climatol Assoc 2009;120:419–428. [PMC free article] [PubMed] [Google Scholar]
- 26. Yamout HM, Perkovic V, Davies M et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes and stage 3 nephropathy. Am J Nephrol 2014;40:64–74. [DOI] [PubMed] [Google Scholar]
- 27. Sinclair A, Bode B, Harris S et al. Efficacy and safety of canagliflozin compared with placebo in older patients with type 2 diabetes mellitus: A pooled analysis of clinical studies. BMC Endocr Disord 2014;14:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Usiskin K, Kline I, Fung A et al. Safety and tolerability of canagliflozin in patients with type 2 diabetes: Pooled analysis of phase 3 study results. Postgrad Med 2014;126:16–34. [DOI] [PubMed] [Google Scholar]
- 29. INVOKANA (Canagliflozin) Tablets, for Oral Use [Package Insert]. Titusville, NJ: Janssen Pharmaceuticals, 2014. [Google Scholar]
- 30. Fulcher G, Matthews D, Perkovic V et al. Efficacy and safety of canagliflozin used in conjunction with sulfonylurea in patients with type 2 diabetes mellitus: A randomized, controlled trial. Diabetes Ther 2015;6:289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Neal B, Perkovic V, de Zeeuw D et al. Efficacy and safety of canagliflozin, an inhibitor of sodium glucose co‐transporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care 2015;38:403–411. [DOI] [PubMed] [Google Scholar]
- 32. Neal B, Perkovic V, de Zeeuw D et al. Rationale, design, and baseline characteristics of the canagliflozin cardiovascular assessment study (CANVAS)—A randomized placebo‐controlled trial. Am Heart J 2013;166:217–223. [DOI] [PubMed] [Google Scholar]
- 33. INVOKANA (Canagliflozin) 100‐mg Film‐Coated Tablets, for Oral Use [Summary of Product Characteristics]. Beerse, Belgium: Janssen‐Cilag International NV, 2014. [Google Scholar]
- 34. Watts N, Bilezikian J, Usiskin K et al. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2016;101:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]


