Endocrine disrupting properties require specific evaluation under the European regulation on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH; 1907/2006) and the regulations on plant protection (Regulation [EC] 1107/2009) and biocidal (Regulation [EC] 528/2012) products. The development of specific criteria to “identify endocrine disrupting properties” is underway to enable hazard‐based regulation in the European Union (EU). In the United States and Japan, scientific, risk‐based approaches are being developed.
Regardless of the regulatory process, most geographies use the World Health Organisation International Programme on Chemical Safety (WHO IPCS 2002) definition of an endocrine disrupter or variants thereof. These definitions require that a substance is demonstrated to cause a change in endocrine function that consequently leads to an adverse effect in an intact organism to identify it as an endocrine disrupter. Such a definition is very broad, and at its most cautious, might capture many mechanisms that in general would not specifically be considered endocrine disruption. For instance, stress is a nonspecific, neuroendocrine response that can lead to adverse outcomes. In addition, other toxic mechanisms (e.g., liver toxicity) may also secondarily impact the endocrine system and tissues. Such factors should therefore be considered when screening and testing substances for potential endocrine activity or disruption, respectively. In fact, following the large scale screening of pesticides and pesticide inerts under the US Environmental Protection Agency's (USEPA) Endocrine Disruptor Screening Program (EDSP), practical experience with screening assays has highlighted some of these factors as important to data interpretation and future study design (Coady et al. 2014).
The misidentification of indirect effects as truly endocrine disrupting can have serious consequences in terms of triggering unnecessary higher tier testing, resulting in additional vertebrate animal use, and can be generally resource intensive. Additionally, misidentification of indirect effects as endocrine disruption could also result in product deselection by consumers and/or severe regulatory consequences in the EU, such as removal from the market. Thus, the ability to distinguish nonendocrine from endocrine modes of action is extremely important when operating in a purely hazard‐based regulatory environment.
All organisms can experience systemic toxicity or stress at some level of exposure to any substance. These stressors are ultimately reflected in organismal responses—from reallocation of energy from nonessential processes such as growth, development, and reproduction to detoxification mechanisms. Ultimately, if the stressor is severe enough, the response will lead to death. Stress responses are a neuroendocrine cascade that has been well described in both mammalian and fish models. Stress leads to catecholamine release, corticotropin releasing factor (from the hypothalamus) causing pituitary synthesis and secretion of corticotropic hormone, which stimulates the synthesis and secretion of glucocorticoid hormones (cortisol in teleost fish or corticosterone in rats). Together, catecholamines and glucocorticoids initiate secondary and tertiary stress response factors (Figure 1).
Figure 1.
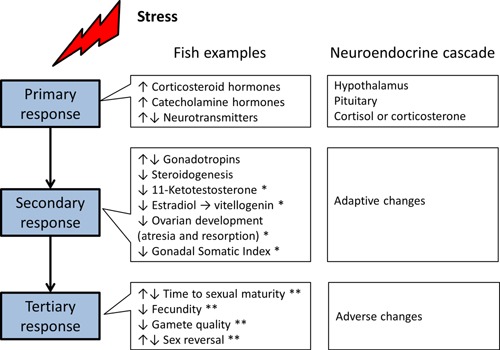
Generalized stress response highlighting the neuroendocrine cascade leading to both adaptive and adverse effects. Effects from the stress literature on fish indicate that responses are also endpoints in endocrine screening (*) and higher tier (**) studies.
The stress response in fish includes a number of endpoints that are also measured in screening studies that are designed to assess sexual endocrine activity and disruption. For instance, 11‐ketotestosterone, estradiol and vitellogenin, female gonad histopathology, and Gonadal Somatic Index are key endpoints in the fish endocrine screening studies (guidelines OECD 229, 230 and OPPTS 890.1350) that are also known to be responsive to a generalized stress response (Aluru and Vijayan 2009; Milla et al. 2009). Adverse effects documented to be derived from stress, such as time to sexual maturity, fecundity, gamete quality, and sex reversal are also measured in higher tier fish studies, such as the fish full lifecycle and fish sexual development test (guidelines OECD 240 or OSCPP 890.2200 and OECD 234, respectively). Therefore, in screens and tests designed specifically to detect sexual endocrine activity and/or disruption, “endocrine responses” can be detected from broader, more generalized stress responses that are not specific to a particular endocrine mode‐of‐action.
This example with fish highlights that the stress response as a neuroendocrine cascade meets the requirements of the WHO/IPCs definition of an endocrine disrupter because both an altered endocrine function and adverse effect can be causally related. Because “the dose makes the poison,” at a certain dose or concentration any chemical could meet the endocrine disruption definition. Clearly, screening and testing chemicals for endocrine activity or disruption needs careful consideration in regards to study design, interpretation, and regulatory decision‐making.
It is important to separate the “generalized stress endocrine response” from those of direct endocrine interaction for which there may be a higher regulatory concern (e.g., due to particular hazards during sensitive windows of exposure with subsequent organizational effects on organism development). When assessing chemistries at the screening level for their potential to interact with specific aspects of the endocrine system (i.e., estrogen, androgen, and thyroid hormone pathways), it is important to test at concentrations or doses that are as high as possible to maximize the chances of finding a true endocrine effect if it occurs. However, it is also necessary to avoid testing at concentrations that are confounded with systemic toxicity. Therefore, it is imperative to have an operationalized approach to determine the maximal tolerable dose or concentration and sufficient data and interpretation tools to separate general toxicity responses from specific endocrine interactions (Wheeler et al. 2013).
Other specific toxicities can also have indirect effects on the endocrine system that could potentially be mistaken for endocrine activity or disruption. Liver toxicity is one clear example common to both mammalian toxicological and ecotoxicological models. Liver toxicity modes of action have been described (Moslen 1996), and 2 of these mechanisms may be particularly influential in affecting endocrine endpoints: direct liver damage or degenerative changes leading to reduced functional capacity, and induction of biotransformation enzymes leading to increased hormone clearance. Because the liver plays a primary role in the metabolism of hormones, “interference” can lead to secondary effects on circulating hormone levels. This can lead to indirect effects on thyroid and sex steroid hormones, leading to impacts on endpoints related to such things as development, metamorphosis, vitellogenesis, and/or fecundity. Several of these endpoints are clearly relevant adverse effects that should be (and are) included in risk assessment. However, it would be unfortunate and potentially detrimental socio‐economically if they were misidentified as primary endocrine responses that would be regulated on hazard alone in the EU.
Broad definitions of endocrine disruption are being used in different global regulatory programs. There are a number of stress‐related and/or specific, but nonendocrine‐mediated, toxicities that can lead to responses in endocrine screening and higher tier testing and that could be mistaken for primary endocrine effects. Misinterpretation could lead to unnecessary higher tier testing and have severe regulatory implications under the hazard‐based regulations being finalized in the EU. By using hazard‐based regulation alone, there is an implicit shift toward authorizations that are based solely on mode‐of‐action (in this case endocrine) that do not take into account the dose–concentration at which a particular effect occurs. Consequently, to avoid misidentification of a large host of chemicals as endocrine disrupters, it is extremely important that decisions are made on known primary endocrine effects that are not consequent to generalized stress responses or indirect toxicities.
Acknowledgment
We are grateful for discussions with Sue Marty, Ellen Mihiach, Leah Zorrilla, and Lisa Ortego.
REFERENCES
- Aluru N, Vijayan MM. 2009. Stress transcriptomics in fish: A role for genomic cortisol signaling. Gen Comp Endocrinol 164:142–150. [DOI] [PubMed] [Google Scholar]
- Coady KK, Lehman CM, Currie RJ, Marino TA. 2014. Challenges and approaches to conducting and interpreting the amphibian metamorphosis assay and the fish short‐term reproduction assay. Birth Defects Res B Dev Repro Toxicol 101:80–89. [DOI] [PubMed] [Google Scholar]
- Milla S, Wang N, Mandiki SNM, Kestemont P. 2009. Corticosteroids: Friends or foes of teleost fish reproduction? Comp Biochem Physiol A 153:242–251. [DOI] [PubMed] [Google Scholar]
- Moslen MT. 1996. Toxic responses of the liver. In: Klaassen CD. editor. Casarett & Doull's toxicology: The basic science of poisons. New York (NY): McGraw‐Hill: 1111 p. [Google Scholar]
- Wheeler JR, Panter G, Weltje L, Thorpe KL. 2013. Test concentration setting for fish in vivo endocrine screening assays. Chemosphere 92:1067–1076. [DOI] [PubMed] [Google Scholar]
- [WHO IPCS] World Health Organisation International Programme on Chemical Safety. 2002. Global assessment of the state‐of‐the‐science of endocrine disruptors. Geneva (CH): World Health Organization. [Google Scholar]


