Supplemental Digital Content is available in the text
Keywords: Africa, HIV infections, HIV testing, male, review, screening, south of the Sahara, systematic
Abstract
Objective:
This systematic review summarizes evidence on the effectiveness of strategies to increase men's HIV-testing in sub-Saharan Africa.
Methods:
Medline, EmBase, Africa-Wide Information and Global Health were searched. Cluster and individually randomized trials evaluating interventions to increase the proportion of adults (≥15 years) testing for HIV were eligible if they were conducted in sub-Saharan Africa, included men in the study population, and reported HIV-testing data by sex. References were independently screened.
Results:
Of the 1852 references, 15 papers including 16 trials were eligible. Trials were judged too heterogeneous to combine in meta-analysis. Three interventions invited men to attend antenatal care-based HIV-testing via pregnant partners, of which two showed a significant effect on partner-testing. One intervention invited men to HIV-test through pregnant partners and showed an increase in HIV-testing when it was offered in bars compared with health facilities. A trial of notification to partners of newly diagnosed HIV-positive patients showed an increase in testing where notification was by healthcare providers compared with notification by the patient. Three interventions reached men already at health facilities and eight reported the effects of community-based HIV-testing. Mobile-testing had a significant effect on HIV-testing compared with standard voluntary counselling and testing. Home-based testing also had a significant effect, but reached smaller numbers of men than mobile-testing.
Discussion:
Interventions to encourage HIV-testing can increase men's levels of HIV-testing. Community-based programmes in particular had a large effect on population levels of HIV-testing. More data on costs and potential population impact of these approaches over different time-horizons would aid policy-makers in planning resource allocation to increase male HIV-testing.
Introduction
Sub-Saharan Africa remains the region most affected by HIV, with 70% of the infections in 2012 occurring in the region [1]. The proportion of individuals aware of their HIV status remains low, despite the scale-up of standalone voluntary counselling and testing (VCT) sites and provider-initiated HIV-testing and counselling (PITC) in health facilities [2–5]. A 2006–2011 study in four sub-Saharan African countries highlights that declines in the proportion of HIV patients enrolling into care with advanced illness have been moderate [6]. Antiretroviral therapy (ART) is less effective when accessed at later stages of immune suppression [7]. In light of their findings, the authors emphasize the need to increase efforts to expand HIV-testing and improve linkage to care for HIV-positive individuals [6]. Furthermore, opportunities to provide HIV-negative individuals and sero-discordant couples with access to prevention services are limited in settings where HIV-testing is low.
Whereas women's HIV-testing and counselling (HTC) has increased since 2004, partly due to PITC implementation in antenatal care (ANC) [3,8], men's HIV-testing rates remain lower than women's across sub-Saharan Africa [2,9–11]. ART uptake is lower among HIV-infected men; men access ART at later stages of immune suppression and experience higher mortality once initiated on ART [12,13]. Approaches to increase HIV-testing rates among men are urgently needed. To date, no systematic review of interventions to increase men's HIV-testing in sub-Saharan Africa exists. Consolidated evidence of strategies that effectively reach men with HIV-testing services would support resource allocation in countries where men's testing is low. We systematically reviewed randomized controlled trials (RCTs) of interventions to increase HIV-testing among men in sub-Saharan Africa.
Methods
Medline, EmBase, Africa-Wide Information and Global Health were searched using combinations of search terms (Annex 2, http://links.lww.com/QAD/A567). Final searches were conducted on 25 October 2013. RCTs of interventions aimed at increasing the proportion of adults, including men testing for HIV, were included. Data on the proportion of men tested after intervention implementation and on the effect size of interventions compared with control are reported.
This systematic review is reported in line with the 2009 ‘Preferred Reporting Items for Systematic reviews and Meta-Analyses’ (PRISMA) guidelines where applicable (Annex 1, http://links.lww.com/QAD/A567) [14]. No protocol exists.
Screening references
References were independently screened for eligibility (B.H./S.T.) and removed after abstract or full-text review. Where reviewers did not agree on eligibility, a third author (J.H.) was consulted.
Inclusion criteria
Studies were eligible if they evaluated interventions to increase HTC uptake by adults (≥15 years), described the proportion of individuals testing for HIV after intervention implementation, were cluster (CRT) or individually RCTs, included men in the study population and HIV-testing data were stratified by sex, and were conducted in sub-Saharan Africa. If sex-disaggregated data were unavailable, up to three attempts were made to contact authors via e-mail. If authors confirmed that sex-disaggregated data were not available, trials were included if more than 40% of the population in all trial arms were men (n = 1) [15], but excluded when less than 40% were men (n = 1) [16]. Conference abstracts, commentaries and letters were excluded due to lack of detailed information on trial design. No language, date or other restrictions were applied.
Data extraction and analysis
Data on the proportion of men HIV-testing after intervention in all arms were extracted (B.H.). Crude and adjusted risk ratios (RR) and 95% confidence intervals (95% CIs) were extracted when reported. Unadjusted RR were calculated from raw data using EpiInfo or Stata 12.0 with 95% CI reported for individually randomized trials. Odds ratios (ORs) or prevalence ratios were extracted if reported.
Two reviewers (B.H./S.T.) independently appraised trials for potential risk of bias using the Cochrane Collaboration risk of bias tool [17]. Risk of bias assessments were relative judgements made by two reviewers that relied primarily on reporting. Five domains were assigned: ‘low’, ‘high’ or ‘unclear’ risk of bias (Table 2) [17]. For CRTs, recruitment bias, baseline imbalances, missing outcome data on clusters and whether analyses adjusted for clustering were also assessed. Selective reporting bias was not assessed as eligibility included reporting on HIV-testing outcomes. Disagreements were resolved through consultation. Trials were not excluded based on risk of bias.
Table 2.
Assessment of risk of bias in individual trials.

Results
The literature search identified 1852 titles. Abstract review removed 1796 titles and full-text review excluded 40 (Fig. 1). Of the 16 remaining trials, one was removed as authors confirmed that data by sex were unavailable and less than 40% of the study population were men [16]. Fifteen papers were eligible. One paper presented data for trials conducted in two sub-Saharan African countries, and a second reported on a trial conducted in four countries of which one was in sub-Saharan Africa [18,19] (Table 1).
Fig. 1.

Flowchart of study inclusion.
Table 1.
Characteristics of the trials eligible for inclusion.
| First author, publication year | Country and setting | Study period | Study design | Study objectives | Study population | Recruitment | Sample size | Intervention or policy | Comparison group |
| Invitations for ANC-based HTC provided through pregnant women | |||||||||
| Mohlala, 2011 [20] | South Africa, urban ANC clinic | Nov 2006–Dec 2007 | RCT | To compare women's acceptance of written invitations for VCT and pregnancy information sessions (PIS) for male sexual partner (MSP) and uptake of VCT by MSP | Pregnant women at <30 weeks gestation and their MSP | Consecutive women attending ANC without MSP; MSP recruited through women | 1000 pregnant women; 500 intervention 500 in control | Written invitation for MSP to attend ANC the for VCT along with community sensitisation 9 months before the intervention during follow-up and recruitment. | Written invitation for MSP to attend PIS and offered VCT 12 weeks after the initial visit |
| Byamugisha, 2011 [21] | Uganda, ANC at a referral hospital | Oct 2009–Feb 2010 | RCT | To evaluate the effect of an invitation letter on couples attendance to ANC and VCT uptake by MSP within a 4-week follow-up period | Pregnant women (≥15 years) attending their first ANC visit and their MSP | Women attending ANC without MSP who were willing to return within 4 weeks identified at reception and approached by research assistant; MSP recruited through women | 1060 pregnant women; 530 intervention 530 control | Invitation letter addressed to MSP to attend subsequent ANC visit | A leaflet containing information on the services available at the ANC |
| Orne-Gliemann, 2013 [19] | Cameroon, urban health centre | 26 Feb–15 Oct 2009 | RCT | To determine the impact of couple-oriented post-test counselling (COC) on partner HIV-testing | Pregnant women aged ≥15 years attending their first prenatal visit who agreed to 6 months of follow-up and their male partners | Women who were interested in participating and were eligible asked for written informed consent; male partners identified through women | 484 women; 239 intervention arm 245 control arm | COC: develops women's communication skills and self-efficacy; empowering women and encouraging HTC-related discussion with partners | Standard post-test counselling |
| Invitations for community-based HTC provided through pregnant women | |||||||||
| Ditekemena, 2011 [22] | DRC, urban NHC, bar and church | 1 Sept 2006–31 Jan 2007 | RCT | To identify alternative strategies to increase participation in VCT by men whose pregnant female partner received HIV-testing | Male partners of pregnant women (≥18 years) who received VCT at ANC in maternity hospital | All women attending an ANC centre were provided information about the study and asked for consent | 2706 pregnant women; 906 church arm, 891 bar arm, 909 NHC arm | Written invitation to attend VCT at a church or in a bar | Invitation to attend VCT in a neighbourhood health centre |
| Partner notification to invite individuals for HTC in health facilities | |||||||||
| Brown, 2011 [23] | Malawi, STI clinics in two urban hospitals | Oct 2008–Sept 2009 | RCT | To determine the effectiveness of different methods of partner notification on notification rates and partner HTC uptake | Partners of STI clinic patients with newly diagnosed HIV infection | Selection of hospitals not reported. All patients (aged ≥18 years) testing positive for first time and sexually active within last 90 days invited to participate | Provider referral: 48 female index patients 52 male partners Contract referral: 46 female index patients 50 male partners Passive referral: 46 female index patients 48 male partners | Provider: newly diagnosed patients given 48 h before provider initiated partner contact Contract: newly diagnosed patients given 7 days to notify partners of their status | Passive referral to notify sexual partners and refer for HTC |
| Reaching men attending health facilities | |||||||||
| Simbayi, 2004 [15] | South Africa, STI clinic | Aug–Nov 2003 | RCT | To test the efficacy of a brief theory-based HIV prevention counselling intervention for STI patients | Repeat STI patients | Repeat STI patients being treated for multiple STIs referred by nurse or physician | 228 recruited; 151 (66%) male. 114 motivational/skills counselling 114 information, education | 60 min theory-based information-motivation-behavioural skills risk reduction counselling to change knowledge, attitudes and behaviours and increase self-efficacy | 20 min information and education session |
| Pope, 2008 [25] | South Africa, 20 primary care TB clinics | 12 Aug–10 Nov 2005 | CRT | To determine whether opt-out PITC increases the proportion of TB patients HIV-tested | Newly registered TB patients (≥18 years) who remained in care for ≥14 days | Clinics selected from 44 PHCs based on presence of TB nurse and min of 3 newly registered TB patients per month | 10 intervention and 10 control clinics; 194 males intervention 238 males control | PITC, including training for nurses on the offer of HTC | Opt-in HIV testing |
| Wanyenze, 2011 [24] | Uganda, urban hospital | 2004–2005 | RCT | To compare the impact of inpatient HTC on HTC uptake, linkage to care and survival among inpatients compared with referral for VCT | Medical inpatients aged ≥18 years with unknown HIV status, residing within 20 km of hospital | Participants identified in consultation with medical teams; potential participants randomly selected from list of hospitalized patients | 500 inpatients 109 males intervention 96 males control | PITC with next day results | Referral for HTC at the hospital 1 week after discharge |
| Reaching men in community settings | |||||||||
| Corbett, 2006 [31] | Zimbabwe, 22 urban businesses | 2 years follow-up/site; last site completed July 2004 | CRT | To estimate the impact of on-site HTC on HTC uptake compared with referral to off-site VCT | Employees expected to remain employed for at least 3 months | Businesses identified with an HIV Prevention Project. Eligible if they had: 100–600 employees; a first aid clinic; individual based absenteeism records | 11 businesses and 2981 males intervention arm 11 businesses and 2474 males control arm | Counselling and on-site rapid HIV testing | Counselling and vouchers for off-site VCT at standalone centre. Two week appointment to discuss results |
| Burnett, 2011 [30] | Swaziland, secondary school | 2006–2007 | RCT | To evaluate the effect of an HIV education intervention on HIV-related behaviours including HIV-testing | Secondary students in form 2 (grade 9) or form 4 (grade 11) | All students eligible, 204 enrolled on a first-come first-served basis | 93 students intervention group 84 control group Data provided for 115 male students aged ≥15 years with complete outcome data postintervention | A 13-week life skills-based HIV education programmes to increase HIV knowledge, change attitudes and behaviours. Mobile HTC available at one session | No education programme (delayed intervention) |
| Sweat, 2011 [18] | Tanzania, 10 rural communities Zimbabwe, eight rural communities | Mar 2006–Apr 2009 Jan 2006–July 2009 | CRT | To examine whether mobile testing in combination with community mobilisation and post-test support increases HTC uptake compared with standard VCT | Adult populations (16–32 years) residing in selected communities | Ethnographic mapping used to select community pairs matched on access to health services, economic activity, population density, civic organization | Tanzania: 6250 individuals in intervention and 6733 in control communities Zimbabwe: 10 700 individuals intervention, 12 150 control communities | Community-based HTC service delivery combined with community mobilisation and availability of post-test support | Standard clinic-based VCT |
| Lugada, 2010 [26] | Uganda, 44 clusters defined by geographical area in five districts | Feb 2005–Feb 2007 | CRT | To compare HTC uptake among household members of index ART-patient offered home-based HTC to uptake among those offered vouchers for VCT | Household members of index ART-patients (results presented for males aged ≥15 years) | Cluster selection not defined. Index Patients aged ≥18 years recruited from an ART clinic | 22 clusters intervention and 22 control arm: 947 male household members ≥15 years intervention 484 males household members ≥15 years control arm | Home-based HTC provided to household members of index ART-patients | Vouchers for free VCT given to index ART-patients to provide to household members |
| Doherty, 2013 [27] | South Africa, geographically similar rural clusters | Intervention: Sept 2009–Nov 2010 Survey: Feb –May 2011 | CRT | To determine the effectiveness of home-based HTC compared with facility based testing | Household members aged 18 years and older; 14–17-year-olds also eligible with guardian/parental consent | Geographical clusters randomized, all households in intervention clusters targeted | 16 clusters; 8 intervention clusters, 484 men surveyed postintervention 8 control clusters, 578 men surveyed after intervention | HBHTC with extensive community mobilization | Standard of care: HTC services at local clinics and NGO outreach teams. Mobile HTC was implemented halfway through the study |
| Fylkesnes, 2013 [29] | Zambia, rural villages | Intervention: March–May 2010 survey: Nov 2010–Jan 2011 | CRT | To evaluate the acceptance of HBHTC compared with standard HTC services | Household members aged 18 years or older | Villages randomized, all households visited | 36 clusters; 18 intervention clusters, 255 men surveyed postintervention 18 control clusters, 261 men surveyed postintervention | HBHTC with community mobilisation, radio spots and drama | Standard of care: VCT in health facilities and outreach by NGOs |
| Low, 2013 [28] | Kenya, administrative regions | Intervention: 2009 Survey: 2011 | CRT | To evaluate the effects of HBHTC on HIV testing compared with no HBHTC | All households in intervention and control regions | Administrative regions randomized, randomly selected households surveyed | 18 clusters; 9 intervention clusters, 626 men ≥15 years surveyed; 9 control clusters, 655 men ≥15 years surveyed | HBHTC | No offer of HBHTC |
ANC, antenatal care; ART, antiretroviral therapy; COC, couple-oriented counselling; CRT, cluster randomized trial; DRC, Democratic; HBHTC; home-based HTC, Republic of Congo; M, moderate; MSP, male sexual partner; NGO; non-government organisation; NHC, neighbourhood health centre; PHC, primary healthcare; PIS, pregnancy information sessions; PITC, provider-initiated HIV-testing and counselling; QA, quality; RCT, randomized controlled trial; S, strong; VCT, voluntary counselling and testing.
Trials included in the review were heterogeneous (Table 1). Four evaluated interventions targeting men through pregnant partners [19–22], one targeted partners of newly diagnosed HIV-positive individuals [23], three evaluated interventions to increase HIV-testing among men attending healthcare facilities [15,24,25]. The remainder offered HIV-testing outside of the facility settings [18,26–31]. Target populations also differed. A meta-analysis was therefore not performed. The proportion of men testing in intervention arms was plotted against the proportion testing in control arms (Fig. 2).
Fig. 2.
Proportion of men testing in intervention versus control arm.
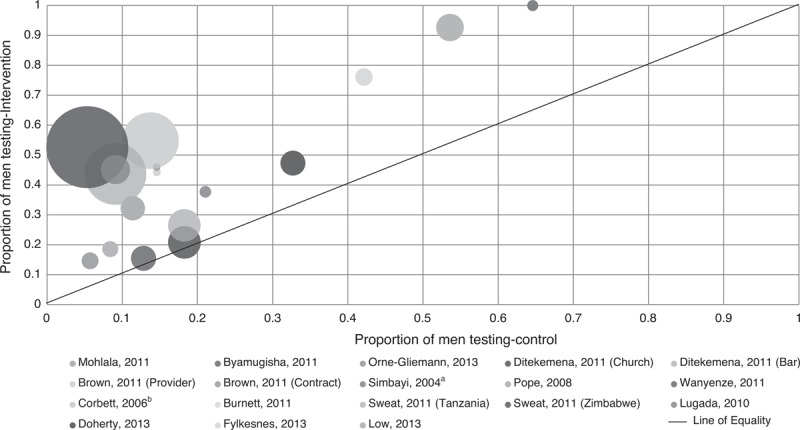
aOutcomes for men and women. bControl is mens uptake of voucher for off-site VCT.
Risk of bias
An assessment of the risk of bias across all trials was not performed due to heterogeneity. Six trials were assigned an unclear risk of selection bias as details of sequence generation and concealment were considered insufficient (Table 2). Blinding of intervention personnel/participants was reported in four trials with additional detail obtained from supporting information for two trials. Six trials were considered to have a low risk of performance bias and three a potential high risk. In one CRT, though matched, it was unclear whether baseline imbalances had been explored and adjustment for clustering made, though the latter would not bias the effect estimate.
Invitations through pregnant partners
Four interventions aimed to increase HIV-testing among men with a pregnant partner attending ANC [19–22] (Tables 1 and Tables 3; Fig. 2). In a South African trial, pregnant women in the intervention arm received invitations for their partners to attend ANC-based VCT. The control arm received an invitation for partners to attend pregnancy information sessions [20]. In the intervention arm, 32% (n = 161/500) of partners tested for HIV compared with 11% (n = 57/500) in the control arm (RR 2.82, 95% CI 2.14–3.72).
Table 3.
Proportion of men testing for HIV post-intervention in intervention and control arms.
| Study reference | Outcome measurement | Proportion HIV-testing intervention (%, n) (A) | Proportion HIV-testing control (%, n) (B) | Difference (%) (A − B) | RR (unless otherwise reported) (95% CI) | Adjusted RR (unless otherwise reported) (95% CI) |
| Invitations for ANC-based HTC provided through pregnant women | ||||||
| Mohlala et al., 2011 | Num: # of men HIV-tested in study period Denom: # invited to ANC through pregnant partners | 32.2 (161/500) | 11.4 (57/500) | 20.8 | 2.82 (2.14–3.72) | NR |
| Byamugisha et al., 2011 | Num: # of men HIV-tested in study period Denom: # of men invited through pregnant partners | 15.5 (82/530) | 12.8 (68/530) | 2.7 | 1.21 (0.90–1.62)d | OR: 1.6 (0.4, 6.8)a |
| Orne-Gliemann et al., 2013 | Num: # of men HIV-tested in study period Denom: # pregnant women recruited | Logbook only: 14.6 (35/239) Combined: 24.7 (59/239) | Logbook only: 5.7 (14/245) Combined: 14.3 (35/245) | 8.9 10.4 | Logbook only: 2.56 (1.42–4.64)d Combined: 1.73 (1.18–2.52)d | Combined OR: 2.38 (1.41–4.02)b |
| Invitations for community-based HTC provided through pregnant women | ||||||
| Ditekemena et al., 2011 | Num: # of men HIV-tested in study period Denom: # of men invited to HTC site | Church: 20.9 (189/906) Bar: 26.5 (236/891) | 18.3 (166/909) | Church: 2.6 Bar: 8.2 | Church: 1.14 (0.95–1.38)d Bar: 1.45 (1.22–2.03)d | OR Church: 1.10 (0.87–1.39)c OR Bar: 1.50(1.19–1.89)c |
| Invitations for facility-based HTC through notification that partner is newly diagnosed HIV-positive | ||||||
| Brown et al., 2011o | Num: # of male partners tested Denom: # of male partners identified by index patient | Provider: 44.2 (23/52) Contract: 46 (23/50) | Passive: 14.6 (7/48) | Provider: 29.6 Contract: 31.4 | Provider: 3.03 (1.43–6.42)d Contract: 3.15 (1.49–6.66)d | NR |
| Num: # of male partners tested Denom: # of locatable male partners identified by index patient | Provider: 47.9 (23/48) Contract: 48.9 (23/47) | Passive: 16.0 (7/45) | Provider: 31.9 Contract: 32.9 | Provider: 3.08 (1.47–6.47)d Contract: 3.15 (1.50–6.60) | NR | |
| Health facility-based strategies | ||||||
| Simbayi et al., 2004 | Num: # of individuals reporting testing 1 month postcounselling Denom: # of individuals in study arm | 37.7 (43/114) | 21.9 (25/114) | 15.8 | 1.72 (1.13–2.62)d | OR: 2.4e |
| Num: # of individuals reporting testing 1 month before 3 months follow-up Denom: # of individuals in study arm | 30.7 (35/114) | 25.4 (29/114) | 5.3 | 1.21 (0.79–1.83)d | OR: 1.2e | |
| Pope et al., 2008 | Num: # of men HIV-tested in study period Denom: # of male patients | 18.6 (36/194) | 8.4 (20/238) | 10.2 | OR: 3.7g OR: 2.40 (1.05–5.50)f | NR |
| Wanyenze et al., 2011p | Num: # of male patients tested and receiving results Denom: # of male patients offered PITC/voucher | 100 (109/109) | 64.6 (62/96) | 35.4 | 1.55 (1.34–1.80)d | NR |
| Community-based strategies | ||||||
| Corbett et al., 2006h | Num: # of males accepting on-site VCT/voucher for off-site VCT in study period Denom: # of male employees | 54.8 (1634/2981) | 13.7 (340/2474) | 41.1 | 2.7 (1.8–3.9)i 3.99d | 2.8 (1.8–3.8)j |
| Burnett et al., 2011 | Num: # of males reporting ever-testing postintervention Denom: # of males with complete data postintervention | 48.4 (15/31) | 6.5 (2/31) | 41.9 | 7.50 (1.87–30.08)d | OR: 10.96 (4.59–26.15)k |
| Sweat et al., 2011 (Tanzania) | Num: # of men testing at least once in study period Denom: 50% of target populationl | 43.7 (1365/3125) | 9.1 (306/3367) | 34.6 | 4.81d | NR |
| Sweat et al., 2011 (Zimbabwe) | Num: # of men testing at least once in study period Denom: 50% of target populationl | 52.6 (2816/5350) | 5.3 (323/6075) | 47.3 | 9.90d | NR |
| Lugada et al., 2010 | Num: # of male HH members tested in study period Denom: # of male HH members aged ≥15 years | 45.1 (427/947) | 9.1 (44/484) | 36 | 4.96 (3.71–6.63)d | 10.41 (7.89–13.73)m |
| Doherty et al., 2013 | Num: # of men testing during study period Denom: # of men surveyed in post-intervention household survey | 47.3 (229/484) | 32.7 (189/578) | 14.6 | PR: 1.52 (1.19–1.95) | NR |
| Fylkesnes et al., 2013 | Num: # of men testing in year prior to the follow-up survey Denom: # of men surveyed baseline and follow-up | 76.1(N = 255) | 42.2 (N = 261) | 33.9 | 1.8 (1.4–2.3) | NR |
| Low et al., 2013n | Num: # of men reporting ever testing Denom: # of men surveyed in post intervention household survey | 92.7 (580/626) | 53.6 (351/655) | 39.1 | 1.73d | NR |
ANC, antenatal care; CI, confidence interval; HH, household; HTC, HIV testing and counselling; NR, not reported; OR, odds ratio; PR, prevalence ratio; RR, risk ratio.
aPer-protocol analysis: intervention 95.3% (82/86) and control 90.7% (68/75); OR adjusted for male partner's age, occupation and education level.
bOR based on combined indicator of logbook and women's self-report of men's HTC. Adjusted for age, female remunerated activity, partner alcohol consumption, HIV status, whether women reports partner ever tested, ever discussed condom with partner, women suggested HIV-testing to partner.
cAdjusted for women's age, marital status, religion and cohabitation.
dRR calculated using Epi-Info.
eOR for individuals retained at follow-up (HTC at 1 month: 47 versus 28%; HTC before 3-month follow-up: 38 versus 33%). Adjusted for age, race, sex, years of education and baseline testing rates.
fOR calculated using STATA.
gReported OR for men and women.
hData for males provided through personal communication (Corbett, 2013).
iRR for unadjusted mean uptake of voucher versus on-site VCT by men and women.
jRatio of observed/expected proportions, adjusted for age, sex, marital status, education, household contact with TB patient, self-rated health and strata (high–low absenteeism). Adjusted RR for use of voucher versus on-site VCT by men and women 12.5 (8.2–16.8).
kData for males provided through personal communication, 2014. OR of change in ever had HIV-test from pre to postintervention, excluding those who tested preintervention.
lCalculated by assuming that 50% of the target population was male.
mAdjusted for age and sex.
nData available in paper; however, data for men aged at least 15 years provided through personal communication (Low, 2014).
oData for men provided through personal communication; (Brown 2013).
pData for men provided through personal communication; (Wanyenze 2012).
In a Ugandan trial, an invitation requesting partners to attend the next ANC visit showed a similar effect compared with providing partners an information letter [21]. Sixteen percent (n = 82/530) of partners in the intervention arm tested compared with 13% (n = 68/530) in the control arm (RR 1.21, 95% CI 0.90–1.62).
A RCT in Cameroon randomized women who had tested in ANC to couple-oriented post-test counselling or standard counselling [19]. HIV-testing among men was defined as HIV-testing at any site as reported by female partners [intervention 23% (n = 56/239); control 14% (n = 35/245)] and/or logbooks of testing on-site [logbook-only intervention: 15% (n = 35/239); control 6% (n = 14/245)]. The intervention showed a stronger effect when measures were based on logbooks (RR 2.56, 95% CI 1.42–4.64) compared with combining logbook and self-report (RR 1.73, 95% CI 1.18–2.52).
A RCT in Democratic Republic of Congo offered pregnant women invitations for their partner to attend church or bar-based HTC [22]. Partners of women randomized to control were offered invitations for health facility-based HTC. Eighteen percent (n = 166/909) of men randomized to health facility-based HTC were tested compared with 21% (n = 189/906) randomized to a church and 27% (n = 236/891) randomized to bar-based HIV-testing (adjusted OR: church 1.10, 95% CI 0.87–1.39; bar 1.50, 95% CI 1.19–1.89).
Facility-based HIV-testing and counselling through partner notification
In a RCT in Malawi, newly diagnosed HIV patients attending a sexually transmitted infection (STI) clinic were randomized to one of the three modes of partner notification (Table 1) [23]. In the provider referral arm, 44% (n = 23/52) of the male partners were HIV-tested, in the contract referral, 46% (n = 23/50) were tested, whereas in the passive referral arm, 15% (n = 7/48) were tested (RR provider 3.03, 95% CI 1.43–6.42; contract 3.15, 95% CI 1.49–6.66).
Reaching men attending health facilities
Three trials reached men in health facilities. In a South African RCT, patients attending a STI clinic were randomized to risk-reduction counselling or a 20-min HIV information/education session [15]. Of the 228 patients, 66% (n = 151) were men. In the intention-to-treat analyses, 38% (n = 43/114) of the patients in the intervention arm reported HIV-testing 1 month after counselling versus 22% (n = 25/114) in the control arm (RR 1.72; 95% CI 1.13–2.62).
In a CRT of opt-out PITC on tuberculosis (TB) patients HIV-testing in primary healthcare clinics in South Africa, 19% of male TB patients offered PITC tested compared with 8% in ‘opt-in’ clinics (OR 2.40, 95% CI 1.05–5.50; Table 3) [25].
A RCT in Uganda compared HIV-testing among in-patients offered PITC to HIV-testing among patients offered vouchers for HTC 1 week after discharge [24]. All male patients in the PITC arm (n = 109) were tested compared with 65% (n = 62/96) of men who were offered a voucher (RR 1.55, 95% CI 1.34–1.80) [24].
Reaching men in community settings
Eight trials evaluated interventions to reach individuals in community settings. In each trial, men in the intervention had higher levels of ever-testing (Table 3). In a CRT in Zimbabwe, businesses were randomized to on-site HTC or vouchers for off-site HTC. In the intervention sites, 55% of the male employees were HIV-tested. In control sites, 14% of male employees accepted vouchers (adjusted RR for men and women 2.8, 95% CI 1.8–3.8) [31]. Mean reported use of off-site VCT by men and women was 4.3% (adjusted RR: 12.5, 95% CI 8.2–16.8) [31].
In a RCT in Swaziland, 204 students were randomized to an intervention aimed at changing HIV-related knowledge, attitudes and behaviours, or control [30]. Among males aged at least 15 years with complete HIV-testing history data after intervention, 48% (n = 15/31) randomized to intervention reported testing after intervention versus 7% (n = 2/31) in control (RR 7.50, 95% CI 1.87–30.08) [30].
Service utilization data from a CRT of mobile-testing in Tanzania and Zimbabwe reported significantly higher HTC in communities randomized to mobile HTC, compared with HTC in communities randomized to standard VCT [18]. In Tanzania, testing was approximately five times higher in intervention communities [18], with an estimated 44% of men tested compared with 9% the in control communities. In Zimbabwe, testing was approximately 10 times higher in the intervention communities [18], an estimated 53% of men tested compared with 5% in control communities.
A sub-study of a CRT in Uganda compared HIV-testing among households offered home-based HTC with HTC among households offered vouchers for facility-based HTC [26]. The primary trial outcome was mortality among ART patients randomized to home or clinic-based ART delivery [32]. The sub-study targeted household members of the index-ART patient, with household members of patients randomized to home-based ART offered home-based HIV-testing, and household members of patients randomized to facility-based ART offered vouchers for facility-based HTC. Male household members aged at least 15 years in the home-based arm were significantly more likely to test compared with that in the comparison arm (45% versus 9%; RR 4.96, 95% CI 3.71–6.63) [26].
Two CRTs reported the effect of home-based HTC compared with standard of care (Table 1) [27,29]. In South Africa, 47% of men in intervention clusters reported HIV-testing during the study period compared with 33% in control clusters (PR 1.52, 95% CI 1.19–1.95) [27]. In Zambia, 76% of men in intervention clusters reported HIV-testing in the year prior to the follow-up survey compared with 42% in control clusters (RR 1.8, 95% CI 1.4–2.3). In a CRT of home-based HTC in Kenya, 93% (n = 580/626) of men in intervention communities reported ever-testing in a survey conducted 18 months after intervention versus 54% (n = 351/655) in control communities [28].
Discussion
This is the first systematic review of the impact of interventions to increase HIV-testing on men's HIV-testing in sub-Saharan Africa. Previous systematic reviews of HTC explored the contribution of PITC to universal testing of pregnant women [3], determined the effect of home-based HTC compared with facility-based services [33] or the acceptability of home-based HTC [34] or reviewed evidence from community-based HTC studies [50]. Our review suggests that interventions to increase men's testing in sub-Saharan Africa are available, although few interventions targeted men specifically. Increasing men's population levels of HIV-testing provides opportunities to link men to HIV-treatment, prevention and care services. Furthermore, with the evidence that men's HIV-testing is associated with women's uptake and adherence to prevention of mother-to-child transmission (PMTCT) [35,36], men's HIV-testing is crucial to the success of PMTCT interventions and for women's health [9]. Evidence arising from this review may support decision-making by the HTC implementers in countries where men's HIV-testing is low. With four trials conducted in South Africa, evidence arising from these interventions may support HTC implementers operating in this region in developing strategies to target men with HIV-testing services.
This review is subject to limitations. Inclusion was limited to published trials and it may be subject to publication bias, as null-effect interventions may be less likely to be published. As inclusion was not restricted to a specific male population or mode of HTC service delivery, the review includes heterogeneous trials. Combining the evidence to determine overall intervention effects was challenging. We therefore did not perform a meta-analysis or compare results across interventions. To increase comparability, strict study design criteria were applied. However, by including this restriction, we excluded observational studies, which have been included in reviews of home-based HTC and may provide additional evidence in support of our findings [34]. Although heterogeneous, evidence across the trials is consistent, with HIV-testing higher in intervention sites in all trials, highlighting that interventions to increase men's HIV-testing are available.
Trials included in the review may be subject to limitations. The potential for bias in trials that did not report details of how the random sequence was generated or whether allocation was concealed was considered unclear. In a CRT, an HIV-prevention organization working with businesses assisted in selection of businesses to be included in the trial. As such, the selected businesses may have been more responsive to the intervention [31]. Bias may be present in trials of invitations to partners as women may have opened sealed envelopes [22]. Knowledge that partners were invited to ANC [21] or where they would be offered VCT [22] may have deterred women from providing letters to their partners [22]. However, as highlighted by Ditekemena et al. (2011), it is unclear what effect this bias would have on outcomes [22]. Outcome measurement was at risk of bias in some trials in which these were self-reported, as individuals may over-report previous testing due to social desirability bias [27–29].
All trials show large and consistent effects on men's HIV-testing. In trials included in this review, men's attendance to ANC was low at 16–30% consistent with other research [35–37]. Nonetheless, ANC-based strategies have the potential to increase men's HIV-testing. Evidence suggests that couple-HTC increases HIV-positive pregnant women's use of nevirapine for PMTCT and their own health [35,36]. HIV-testing by partners of HIV-positive pregnant women is crucial for PMTCT and women's health [22]. Three-quarters of individuals aged 20–49 years residing in sub-Saharan Africa report cohabiting [38]. With many cohabiting men likely to have a pregnant partner in the course of their relationship, ANC-based HTC has the potential to reach a high proportion of these men [38]. To increase men's ANC-based HIV-testing, promotional campaigns to normalize their ANC attendance may prove effective. Although limited to one trial, the RCT that promoted men's participation had a greater effect than trials with no sensitization directed at men [20]. With little evidence of the impact and cost-effectiveness of promotional campaigns on HTC acceptance in developing countries additional research is warranted.
Couple-HTC is being promoted principally in ANC [36–39]. As few men attend clinics considered ‘female’ environments, couple-HTC should be available in alternate settings including voluntary medical male circumcision (VMMC) clinics and mobile HTC [38,40]. These settings may not only prove more acceptable to men and some women but may encourage couple-HTC by non-pregnant couples. Home-based testing is an additional strategy that may prove more effective at reaching couples. In the South African home-based HTC trial, the prevalence of couple-HTC in the intervention arm was two times higher than in control (RR 2.24, 95% CI 1.49–3.03) [27]. In the Zambian trial, 62% of cohabiting respondents reported being counselled with their partner [29]. Among those tested at home, 70% reported that they received their test result with their partner [29].
Men may be reluctant to access health services due to norms associated with masculinity, including being strong and ‘disease-free’, that may act as a barrier to seeking preventive healthcare services [41]. Men's access to HTC and HIV services may be limited further by fear and perceived lack of confidentiality of services [42–44]. With limited access to healthcare, PITC has reached few men compared with women, despite increased incentives to HIV-test with increasing ART availability [45,46]. Studies have shown that PITC is acceptable [47,48] and diagnoses a high proportion of HIV-positive individuals among those tested at relatively low costs per individual tested [45]. PITC trials included in this review showed a large effect on HIV-testing [25]. In VMMC clinics in Tanzania, 99% of men accepted PITC when offered [49]. However, the majority were young and at low risk of infection [49]. The need for interventions to reach older men therefore remains [49]. Although PITC is unlikely to increase men's population levels of HIV-testing, the strategy can reach a high proportion of men already engaged with health services [50]. PITC is an important mode of service delivery, particularly in generalized epidemics, but PITC alone will not provide men with universal HTC access [50].
Community-based HTC strategies providing services outside of health facilities may prove more acceptable to men than facility-based initiatives [16,36]. Mobile-testing, where services are offered through caravans or tents, in particular, could have a large impact on population levels of HIV-testing by reaching a high number and proportion of men. In the CRT of mobile-HTC included in this review, mobile-testing increased men's HIV-testing by 45% (P < 0.001) across all countries (including Thailand), relative to VCT [51]. Post-intervention survey data from a random sample of men aged 18–32 years showed an increase in HIV-testing in Tanzania from 16% at baseline to 26% in intervention communities, compared with no increase from 6% in control communities [51]. In Zimbabwe, HIV-testing increased from 3 to 25% in the intervention communities compared with 3 to 16% in the control communities [51]. Cost analyses in Kenya show that fully mobile HTC costs approximately $23 per first-time tester compared with $44 at standalone VCT [52]. Although studies show that mobile-testing detects a lower proportion of HIV-positive individuals than facility-based testing, it has the potential to reach a large number of men, particularly those with no history of testing [50,52]. To improve cost-effectiveness and reach, integrated campaigns that deliver a package of interventions, including VMMC and condoms, could be delivered [53,54]. Workplace-HTC reached a relatively high proportion of employed men and is expected to bear little cost to governments [31]. It may be effective at reaching men not at home during home or mobile-HTC campaigns. Nonetheless, even if fully integrated in employment settings, large numbers of men will not have access to workplace-HTC in settings where formal employment is low.
Home-based HTC also had a large effect on HIV-testing. A systematic review of home-based HTC acceptability shows that overall men found at home are as likely as women to be offered testing and to accept an offer of home-based HTC [34]. However, in some studies, the proportion of individuals found at home that were male was low [55]. Home-based HTC may have limited impact on population levels of HIV-testing in areas of high mobility for employment and in the absence of repeat visits to find men who are less likely to be at home. As such, increasing men's HIV-testing at population level through home-based HTC may prove less cost-effective as repeated visits would increase delivery costs [34,56]. Home-based HIV-testing identifies a lower proportion of HIV-positive men among men tested compared with PITC, yet individuals are likely to be identified at earlier stages of infection [34,45]. Home-based testing is an effective strategy that reaches a high proportion of those targeted in addition to increasing couple-HTC [27]. Furthermore, offering home-based HTC may provide an opportunity for counselling and to reach first-time testers. In the Zambian trial, 84% of men accepted counselling services [29]. First-time testing was higher in the intervention arms of the Zambian and South African trial; in Zambia, the prevalence of first-time testing was 68% in the intervention arm versus 29% in the control arm [57]. In South Africa, 46% of all testers in the intervention arm were first-time testers compared with 37% in the control arm [27]. Home-based testing is therefore an important strategy to be delivered alongside other models of HIV-testing [34,50,58].
HIV self-testing is an additional strategy that may prove effective at increasing men's population levels of HIV-testing. A feasibility study conducted in Malawi suggests that home-based self-testing was men's preferred option for future testing [59]. Additional research in developing countries is needed including population-level impact, cost-effectiveness, how to deliver HIV tests, and linkage to HIV prevention and care; yet the model remains a promising alternative to healthcare provider-delivered HIV-testing [59].
Timely diagnosis of HIV infection is crucial to the effectiveness of treatment as prevention initiatives [56]. Strategies that not only encourage first-time testing but also repeat-testing, particularly by high-risk HIV-negative individuals, are required [56]. In the Zambian trial, repeat-testing was higher in intervention communities (94 versus 74%; P < 0.001) [57]. The trial of mobile-testing reports that at the end of the intervention period, approximately 40% of the individuals attending mobile-testing clinics in the three trial countries (including Thailand) were repeating an HIV test within the clinics [18]. Additional research is required to determine the impact of community-based strategies on repeat-testing and whether these modalities encourage repeat-testing by individuals at increased risk of HIV infection [60].
In conclusion, this review provides evidence that interventions to increase HIV-testing are effective at reaching men. To increase men's HIV-testing at a population level, country and time-specific combinations of available strategies are likely to be required [58]. Additional research to determine whether these strategies encourage repeat-testing by high-risk HIV-negative men is required to support decision-making in countries opting for treatment as prevention initiatives.
Acknowledgements
Author contributions: B.H. – conceived the idea, conducted the literature searches, screened the identified titles, appraised the trials and extracted data. B.H. also drafted the first manuscript.
S.T. – screened the identified titles, appraised the trials included in the review and contributed to writing.
J.L. – provided guidance on data presentation and contributed to writing.
H.W. – provided guidance on the conduct of the review and contributed to writing.
J.H. – conceived the idea, provided guidance on the conduct of the review, assessed inclusion eligibility where B.H. and S.T. did not agree and contributed to writing.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (http://www.AIDSonline.com).
References
- 1.UNAIDS. UNAIDS report on the global AIDS epidemic 2013. Washington, DC: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2013. [Google Scholar]
- 2.World Health Organization. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 3.Hensen B, Baggaley R, Wong VJ, Grabbe KL, Shaffer N, Lo YR, et al. Universal voluntary HIV testing in antenatal care settings: a review of the contribution of provider-initiated testing and counselling. Trop Med Int Health 2011; 17:59–70. [DOI] [PubMed] [Google Scholar]
- 4.Baggaley R, Hensen B, Ajose O, Grabbe KL, Wong VJ, Schilsky A, et al. From caution to urgency: the evolution of HIV testing and counselling in Africa. Bull World Health Organ 2012; 90:633–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staveteig S, Wang S, Head SK, Bradley SEK, Nybro E. Demographic patterns of HIV testing uptake in sub-Saharan Africa. DHS Comparative Reports No. 30. Calverton, Maryland, USA: ICF International; 2013. [Google Scholar]
- 6.Lahuerta M, Wu Y, Hoffman S, Elul B, Kulkarni S G, Remien R H, et al. Advanced HIV disease at entry into HIV care and initiation of antiretroviral therapy during 2006–2011: findings from four sub-Saharan African countries. Clin Infect Dis 2014; 58:432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badri M, Lawn SD, Wood R. Short-term risk of AIDS or death in people infected with HIV-1 before antiretroviral therapy in South Africa: a longitudinal study. Lancet 2006; 368:1254–1259. [DOI] [PubMed] [Google Scholar]
- 8.Njeru MK, Blystad A, Shayo EH, Nyamongo IK, Fylkesnes K. Practicing provider-initiated HIV testing in high prevalence settings: consent concerns and missed preventive opportunities. BMC Health Serv Res 2011; 11:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shand T, Thomson-de Boor H, van den Berg W, Peacock D, Pascoe L. The HIV blind spot: men and HIV testing, treatment and care in sub-Saharan Africa. IDS Bull 2014; 1:53–60. [Google Scholar]
- 10.Kenya demographic and health survey 2008–2009. Calverton, Maryland: Kenya National Bureau of Statistics (KNBS) and ICF Macro; 2010. [Google Scholar]
- 11.Lesotho demographic and health survey 2009. Maseru, Lesotho: Ministry of Health and Social Welfare (MOHSW) and ICF Macro; 2010. [Google Scholar]
- 12.Mills EJ, Ford N, Mugyenyi P. Expanding HIV care in Africa: making men matter. Lancet 2009; 374:275–276. [DOI] [PubMed] [Google Scholar]
- 13.Cornell M, McIntyre J, Myer L. Men and antiretroviral therapy in Africa: our blind spot. Trop Med Int Health 2011; 16:828–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [PMC free article] [PubMed] [Google Scholar]
- 15.Simbayi LC, Kalichman SC, Skinner D, Jooste S, Cain D, Cherry C, et al. Theory-based HIV risk reduction counseling for sexually transmitted infection clinic patients in Cape Town, South Africa. Sex Transm Dis 2004; 31:727–733. [DOI] [PubMed] [Google Scholar]
- 16.Fylkesnes K, Siziya S. A randomized trial on acceptability of voluntary HIV counselling and testing. Trop Med Int Health 2004; 9:566–572. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions (version 5.1.0) 2011. [cited 2014] http://handbook.cochrane.org/. [Google Scholar]
- 18.Sweat M, Morin S, Celentano D, Mulawa M, Singh B, Mbwambo J, et al. Community-based intervention to increase HIV testing and case detection in people aged 16–32 years in Tanzania, Zimbabwe, and Thailand (NIMH Project Accept, HPTN 043): a randomised study. Lancet Infect Dis 2011; 11:525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orne-Gliemann J, Balestre E, Tchendjou PT. Increasing HIV testing among male partners. The Prenahtest ANRS 12127 multicountry randomised trial. AIDS 2013; 27:1167–1177. [DOI] [PubMed] [Google Scholar]
- 20.Mohlala BKF, Boily M-C, Gregson S. The forgotten half of the equation: randomized controlled trial of a male invitation to attend couple voluntary counselling and testing. AIDS 2011; 25:1535–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Byamugisha R, Strom AN, Ndeezi G, Karamagi CAS, Tylleskar T, Tumwine JK. Male partner antenatal attendance and HIV testing in eastern Uganda: a randomized facility-based intervention trial. J Int AIDS Soc 2011; 14:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ditekemena J, Matendo R, Koole O, Colebunders R, Kashamuka M, Tshefu A, et al. Male partner voluntary counselling and testing associated with the antenatal services in Kinshasa, Democratic Republic of Congo: a randomized controlled trial. Int J STD AIDS 2011; 22:165–170. [DOI] [PubMed] [Google Scholar]
- 23.Brown L, Miller W, Kamanga G, Nyirenda N, Mmodzi P, Pettifor A, et al. HIV partner notification is effective and feasible in sub-Saharan Africa: opportunities for HIV treatment and prevention. J Acquir Immune Defic Syndr 2011; 56:437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wanyenze RK, Hahn JA, Liechty CA, Ragland K, Ronald A, Mayanja-Kizza H, et al. Linkage to HIV care and survival following inpatient HIV counseling and testing. AIDS Behav 2011; 15:751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pope DS, Deluca AN, Kali P, Hausler H, Sheard C, Hoosain E, et al. A cluster-randomized trial of provider-initiated (opt-out) HIV counseling and testing of tuberculosis patients in South Africa. J Acquir Immune Defic Syndr 2008; 48:190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lugada E, Levin J, Abang B, Mermin J, Mugalanzi E, Namara G, et al. Comparison of home and clinic-based HIV testing among household members of persons taking antiretroviral therapy in Uganda: Results from a randomized trial. J Acquir Immune Defic Syndr 2010; 55:245–252. [DOI] [PubMed] [Google Scholar]
- 27.Doherty T, Tabana H, Jackson D, Naik R, Zembe W, Lombard C, et al. Effect of home based HIV counselling and testing intervention in rural South Africa: cluster randomised trial. BMJ 2013; 346:f3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Low C, Pop-Eleches C, Rono W, Plous E, Kirk A, Ndege S, et al. The effects of home-based HIV counseling and testing on HIV/AIDS stigma among individuals and community leaders in western Kenya: evidence from a cluster-randomized trial (Special Issue: Effects of investing in communities on HIV/AIDS outcomes.). AIDS Care 2013; 25 (s1):S97–S107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fylkesnes K, Sandoy IF, Jurgensen M, Chipimo PJ, Mwangala S, Michelo C. Strong effects of home-based voluntary HIV counselling and testing on acceptance and equity: a cluster randomised trial in Zambia. Soc Sci Med 2013; 86:9–16. [DOI] [PubMed] [Google Scholar]
- 30.Burnett SM, Weaver MR, Mody-Pan PN, Reynolds Thomas LA, Mar CM. Evaluation of an intervention to increase human immunodeficiency virus testing among youth in Manzini, Swaziland: a randomized control trial. J Adolesc Health 2011; 48:507–513. [DOI] [PubMed] [Google Scholar]
- 31.Corbett EL, Dauya E, Matambo R, Cheung YB, Makamure B, Bassett MT, et al. Uptake of workplace HIV counselling and testing: a cluster-randomised trial in Zimbabwe. PLoS Med 2006; 3:e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amuron B, Levin J, Birunghi J, Namara G, Coutinho A, Grosskurth H, et al. Mortality in an antiretroviral therapy programme in Jinja, south-east Uganda: a prospective cohort study. AIDS Res Ther 2011; 8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bateganya M, Abdulwadud OA, Kiene SM. Home-based HIV voluntary counselling and testing (VCT) for improving uptake of HIV testing. Cochrane Database Syst Rev 2010; 1–28.CD006493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabapathy K, van den Bergh R, Fidler S, Hayes R, Ford N. Uptake of home-based voluntary HIV testing in sub-Saharan Africa: a systematic review and meta-analysis. PLoS Med 2012; 9:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Msuya SE, Mbizvo EM, Hussain A, Uriyo J, Sam NE, Stray-Pedersen B. Low male partner participation in antenatal HIV counselling and testing in northern Tanzania: implications for preventive programs. AIDS Care 2008; 20:700–709. [DOI] [PubMed] [Google Scholar]
- 36.Becker S, Mlay R, Schwandt HM, Lyamuya E. Comparing couples’ and individual voluntary counseling and testing for HIV at antenatal clinics in Tanzania: a randomized trial. AIDS Behav 2010; 14:558–566. [DOI] [PubMed] [Google Scholar]
- 37.Musheke M, Bond V, Merten S. Couple experiences of provider-initiated couple HIV testing in an antenatal clinic in Lusaka, Zambia: lessons for policy and practice. BMC Health Serv Res 2013; 13:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization. Guidance on couples HIV testing and counselling including antiretroviral therapy for treatment and prevention in serodiscordant couples. Recommendations for a public health approach. Geneva, Switzerland: World Health Organization; 2012. [PubMed] [Google Scholar]
- 39.Sherr L, Croome N. Involving fathers in prevention of mother to child transmission initiatives: what the evidence suggests. J Int AIDS Soc 2012; 15 (Suppl 2): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aarnio P, Aarnio P, Olsson P, Chimbiri A, Kulmala T. Male involvement in antenatal HIV counseling and testing: exploring men's perceptions in rural Malawi. AIDS Care 2009; 21:1537–1546. [DOI] [PubMed] [Google Scholar]
- 41.Skovdal M, Campbell C, Madanhire C, Mupambireyi Z, Nyamukapa C, Gregson S. Masculinity as a barrier to men's use of HIV services in Zimbabwe. Global Health 2011; 7:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barnabas Njozing N, Edin KE, Hurtig AK. ’When I get better I will do the test’: facilitators and barriers to HIV testing in Northwest Region of Cameroon with implications for TB and HIV/AIDS control programmes. SAHARA J 2010; 7:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leichliter JS, Paz-Bailey G, Friedman AL, Habel MA, Vezi A, Sello M, et al. ’Clinics aren’t meant for men’: sexual healthcare access and seeking behaviours among men in Gauteng province, South Africa. SAHARA J 2011; 8:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greig A, Peacock D, Jewkes R, Msimang S. Gender and AIDS: time to act. AIDS 2008; 2 (Suppl 2):S35–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menzies NA, Abang B, Wanyenze R, Nuwaha F, Mugisha B, Coutinho A, et al. The costs and effectiveness of four HIV counseling and testing strategies in Uganda. AIDS 2009; 23:395–401. [DOI] [PubMed] [Google Scholar]
- 46.Todd J, Wringe A, Floyd S, Zaba B. Antiretroviral therapy in sub-Saharan Africa: evidence about need, uptake and impact from community-based cohort studies. Trop Med Int Health 2012; 17:E1–E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wanyenze RK, Nawavvu C, Namale AS, Mayanja B, Bunnell R, Abang B, et al. Acceptability of routine HIV counselling and testing, and HIV seroprevalence in Ugandan hospitals. Bull World Health Organ 2008; 86:302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wanyenze R. HIV counseling and testing practices at an urban hospital in Kampala, Uganda. AIDS Behav 2006; 10:361–367. [DOI] [PubMed] [Google Scholar]
- 49.Mahler HR, Kileo B, Curran K, Plotkin M, Adamu T, Hellar A, et al. Voluntary medical male circumcision: matching demand and supply with quality and efficiency in a high-volume campaign in Iringa region, Tanzania. PLoS Med 2011; 8: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suthar A, Ford N, Bachanas P, Wong VJ, Rajan JS, Saltzman AK, et al. Towards universal voluntary testing and counseling: a systematic review and meta-analysis of community-based approaches. PLoS Med 2013; 10: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Celentano D. Community-level secondary (behavioral) outcomes of NIMH Project Accept (HPTN 043) presented on behalf of Project Accept Study Team. 7th IAS Conference on HIV Pathogenesis, Treatment and Prevention: 30th June–03 July 2013; Kuala Lumpur, Malaysia 2013. [Google Scholar]
- 52.Grabbe K L, Menzies N, Taegtmeyer M, Emukule G, Angala P, Mwega I, et al. Increasing access to HIV counseling and testing through mobile services in Kenya: strategies, utilization, and cost-effectiveness. J Acquir Immune Defic Syndr 2010; 54:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kahn JG, Muraguri N, Harris B, Lugada E, Clasen T, Grabowsky M, et al. Integrated HIV testing, malaria, and diarrhea prevention campaign in Kenya: modeled health impact and cost-effectiveness. PLoS One 2012; 7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cherutich P, Bunnel R, Mermin J. HIV testing: current practice and future directions. Curr HIV/AIDS Rep 2013; 10:134–141. [DOI] [PubMed] [Google Scholar]
- 55.Sekandi JN, Sempeera H, List J, Mugerwa MA, Asiimwe S, Yin XP, et al. High acceptance of home-based HIV counseling and testing in an urban community setting in Uganda. BMC Public Health 2011; 11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cawley C, Wringe A, Isingo R, Mtenga B, Clark B, Marston M, et al. Low rates of repeat HIV testing despite increased availability of antiretroviral therapy in rural Tanzania: findings from 2003–2010. PLoS One 2013; 8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jurgensen M, Sandoy IF, Michelo C, Fylkesnes K, Mwangala S, Blystad A. The seven Cs of the high acceptability of home-based VCT: results from a mixed methods approach in Zambia. Soc Sci Med 2013; 97:210–219. [DOI] [PubMed] [Google Scholar]
- 58.World Health Organization. Service delivery approaches to HIV testing and counselling (HTC): a strategic HTC policy framework. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 59.Choko AT, Desmond N, Webb EL, Chavula K, Napierala-Mavedzenge S, Gaydos CA, et al. The uptake and accuracy of oral kits for HIV self-testing in high HIV prevalence setting: a cross-sectional feasibility study in Blantyre, Malawi. PLoS Med 2011; 8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barnighausen T, Tanser F, Dabis F, Newell ML. Interventions to improve the performance of HIV health systems for treatment-as-prevention in sub-Saharan Africa: the experimental evidence. Curr Opin HIV AIDS 2012; 7:140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]


