Abstract
The magnetic resonance imaging–estimated proton density fat fraction (MRI-PDFF) is a novel imaging-based biomarker that allows fat mapping of the entire liver, whereas the magnetic resonance spectroscopy–measured proton density fat fraction (MRS-PDFF) provides a biochemical measure of liver fat in small regions of interest. Cross-sectional studies have shown that MRI-PDFF correlates with MRS-PDFF. The aim of this study was to show the utility of MRI-PDFF in assessing quantitative changes in liver fat through a three-way comparison of MRI-PDFF and MRS-PDFF with the liver histology–determined steatosis grade at two time points in patients with nonalcoholic fatty liver disease (NAFLD). Fifty patients with biopsy-proven NAFLD who participated in a randomized trial underwent a paired evaluation with liver biopsy, MRI-PDFF, and MRS-PDFF at the baseline and 24 weeks. The mean age and body mass index were 47.8 ± 11.7 years and 30.7 ± 6.5 kg/m2, respectively. MRI-PDFF showed a robust correlation with MRS-PDFF both at week 0 and at week 24 (r = 0.98, P < 0.0001 for both). Cross-sectionally, MRI-PDFF and MRS-PDFF increased with increases in the histology-determined steatosis grade both at week 0 and at week 24 (P < 0.05 for all). Longitudinally, patients who had a decrease (≥1%) or increase (≥1%) in MRI-PDFF (confirmed by MRS-PDFF) showed a parallel decrease or increase in their body weight and serum alanine aminotransferase and aspartate aminotransferase levels at week 24 (P < 0.05). This small increase or decrease in liver fat could not be quantified with histology.
Conclusion
In this longitudinal study, MRI-PDFF correlated well with MRS-PDFF and was more sensitive than the histology-determined steatosis grade in quantifying increases or decreases in the liver fat content. Therefore, it could be used to quantify changes in liver fat in future clinical trials.
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of elevated serum aminotransferase levels in the United States.1–3 It has been estimated that approximately 80 million Americans have NAFLD, and the prevalence of this condition is expected to rise with the continuing epidemic of obesity.4,5 Nonalcoholic steatohepatitis (NASH), which is the progressive form of NAFLD, is typically associated with inflammation and cellular injury in addition to steatosis with or without perisinusoidal fibrosis on liver histology.6 Liver biopsy is the gold standard for diagnosing NAFLD and confirming the presence of NASH.7–9 However, liver biopsy is an invasive procedure and is not routinely favored by general practitioners and patients because of potential risks, which include abdominal pain, bleeding, and, very rarely, death.10 It is not practical to subject all patients with NAFLD to a liver biopsy assessment; therefore, noninvasive biomarkers are needed.11–13
Imaging studies are being increasingly used for noninvasive assessments of NAFLD and have shown promise in detecting hepatic steatosis, but they are limited in their assessment of the presence of NASH. Among the imaging modalities, ultrasound and computed tomography are routinely available and are commonly used, but they lack sensitivity and accuracy in quantifying liver fat.14–16 Magnetic resonance (MR)—especially magnetic resonance spectroscopy (MRS)—has emerged as an accurate technique for quantifying liver fat. However, MRS is limited because it measures a small volume of the sampled tissue and is technically difficult to perform, and it is largely used as a research tool with limited clinical availability and application in routine clinical practice.17,18 Conventional magnetic resonance imaging (MRI) techniques are limited by T1 bias, T(2)* decay, and multifrequency signal-interference effects of protons in fat and eddy currents and, therefore, may not be accurate in the quantification of liver fat.18 Advanced MRI techniques eliminate the biases seen with conventional MRI techniques and can provide the magnetic resonance imaging–estimated proton density fat fraction (MRI-PDFF), a novel biomarker that has shown a robust correlation and equivalency with the magnetic resonance spectroscopy–measured proton density fat fraction (MRS-PDFF).19–22 In addition, MRI-PDFF allows fat mapping of the entire liver and can be applied on any clinical MRI platform, whereas MRS measures fat biochemically in small regions of interest (ROIs).
Using a randomized, double-blinded, placebo-controlled clinical trial, we recently showed that MRI-PDFF could detect small amounts of changes in liver fat.23 In the present study, we hypothesized that MRI-PDFF is equivalent to MRS-PDFF in quantifying liver fat cross-sectionally and longitudinally and that the two techniques would correlate with each other over a 24-week time period in patients with biopsy-proven NAFLD. Therefore, we aimed to validate MRI-PDFF by a three-way comparison of the liver MRI-PDFF, MRS-PDFF, and liver histology–determined steatosis grade at two time points (24 weeks apart) and also to assess whether small changes in the liver fat content appreciated by MRI-PDFF have any clinical or biochemical significance.
Patients and Methods
Study Design
This was a secondary longitudinal analysis of a recently conducted randomized, double-blinded, placebo-controlled clinical trial that examined the efficacy of colesevelam versus a placebo in the treatment of NASH. Fifty patients with biopsy-proven NAFLD were randomized to either a placebo or colesevelam and underwent a paired evaluation with liver biopsy, MRI-PDFF, and MRS at the baseline and 24 weeks. The primary results were published previously.24 The patients were enrolled from the San Diego Integrated NAFLD Research Consortium, which is a citywide collaboration designed to study NAFLD that includes the University of California San Diego (UCSD) Medical Center, Sharp Health System, Balboa Naval Medical Center, and Kaiser Permanente Southern California. Patients from these sites with biopsy-proven NASH were referred to the UCSD NAFLD research center. The study was conducted at the Clinical and Translational Research Institute at UCSD. The study protocol received a priori approval by UCSD institutional board review.
Patient Population
Patients were screened at the UCSD NAFLD research clinic. A careful history was taken; a physical examination was performed; and the baseline weight, height, and body mass index (BMI) were taken. The Alcohol Use Disorders Identification Test and the Skinner Lifetime Drinking Questionnaire were used to assess each patient’s alcohol use history. As used by the Nonalcoholic Steatohepatitis Clinical Research Network (NASH-CRN) studies,25,26 this is a widely acceptable approach for assessing alcohol intake in NAFLD patients.
Inclusion and Exclusion Criteria for the Clinical Trial
The inclusion and exclusion criteria were described in detail previously.24 Briefly, the inclusion criteria included the following: (1) the patient was 18 years old or older, (2) liver biopsy was performed within 6 months of enrollment without significant changes in weight between the date of biopsy and the day of enrollment (patients were enrolled only if they had evidence of NASH on biopsy according to the NASH-CRN scoring system27), (3) hepatic steatosis > 5% was present according to MRI-PDFF, and (4) serum alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels were elevated with a cutoff value of 19 U/L for women and 30 U/L for men. The exclusion criteria included (1) evidence of other forms of liver disease (including viral hepatitis autoimmune hepatitis, hemochromatosis, Wilson’s disease, and alpha-1-antitrypsin disease), (2) alcohol intake exceeding 30 g/day in the last 10 years or exceeding 10 g/day in the previous year, (3) a Child-Pugh score > than 7, (4) active substance abuse, (5) severe systemic illness, (6) renal insufficiency (creatinine > 1.5 mg/dL in men and > 1.4 mg/dL in women), (7) human immunodeficiency virus, (8) pregnancy, (9) evidence of hepatocellular carcinoma, (10) contraindications to liver biopsy, and (11) contraindications to MRI.
Patient Population Consideration for This Study
All patients underwent an MRI and MRS assessment. The results of the randomized controlled trial were previously published.24,25 Patients in this substudy were classified as patients who had a longitudinal increase in the fat fraction according to MRI-PDFF (confirmed by MRS-PDFF) or patients who had a decrease in the fat fraction according to MRI-PDFF (confirmed by MRS-PDFF) between the baseline and week 24. A fat fraction change was defined as a ≥1% increase or decrease in MRI-PDFF. We chose a 1% change as the cutoff to explore whether small changes in MRI-PDFF could reflect changes in NAFLD and NASH parameters such as weight and liver enzymes. The coefficient of variation was less than 1%. Therefore, a ≥1% change would be an actual change and would not be biased by diurnal or inter- or intra-reader variability.28
Covariates
The following characteristics were examined. The demographics included age, sex, race, and ethnicity. The anthropometrics included BMI and waist circumference. The laboratory studies included the following: AST, ALT, AST/ALT ratio, gamma-glutamyl transpeptidase (GGT), alkaline phosphatase, albumin, total protein, prothrombin time, total cholesterol, high-density lipoprotein, low-density lipoprotein (LDL) cholesterol, triglycerides, hemoglobin A1c, fasting glucose, serum insulin, and homeostasis model assessment of insulin resistance index.
Histological Assessment
Liver biopsy was performed within 6 months of enrollment. Biopsy samples were scored by an experienced liver pathologist (M.R.P.) who was blinded to the clinical data, treatment allocation, and imaging data. The NASH-CRN histological scoring system was used to score biopsies.27 The nonalcoholic fatty liver disease activity score (NAS) and fibrosis scores were recorded before and after the end of treatment in all patients. The NAS score ranges from 0 to 8 and is the summation of the degree of steatosis (0–3), lobular inflammation (0–3), and hepatocellular ballooning (0–2). Liver fibrosis ranges from 0 to 4, with 0 indicating no fibrosis and 4 indicating cirrhosis. Liver biopsy was performed before randomization and at week 24.
MRI-PDFF and MRS-PDFF Assessment
MRI-PDFF is an optimized MRI-based biomarker that is independent of the scanner’s manufacture, platform, and field strength.29,30 This biomarker is measured with advanced MRI techniques that minimize or correct the confounding factors (T1 bias, T2* bias, and multifrequency interference effects of fat and eddy currents) that corrupt fat fraction estimations with conventional MRI-based techniques.21,31–34 We used a previously described and validated protocol.21 Briefly, the protocol uses a gradient echo sequence with a low flip angle to minimize T1 bias, and it acquires multiple echoes in which fat and water signals are nominally in phase or out of phase with respect to each other. Once data are acquired at each echo of the echo times, they pass to a fitting algorithm that estimates and corrects T2* effects, models the fat signal, and estimates fat and water proton densities; then, the fat content is calculated.
Liver Fat Mapping Protocol and Colocalization of ROIs Before and After 24 Weeks
We used customized software that was developed by the UCSD Liver Imaging Center to create a fat map through the entire liver pixel by pixel. The imaging proton density fat fraction (PDFF) was taken in ROIs (ranging from 300 to 400 mm2) and placed on the PDFF maps; blood vessels, bile ducts, and artifacts were avoided. Three colocalized ROIs were placed in each of the nine liver segments at the baseline and follow-up MR examinations; therefore, we had 27 ROIs in the whole liver at each time point. We used this approach to assess detailed longitudinal fat changes in the entire liver. MRS was used as a reference standard for MRI-PDFF measurements and was taken in a single voxel. Three additional ROIs were placed on the PDFF maps in the same locations as the MRS voxel (one through the superior third of the voxel, one through the middle third of the voxel, and one through the inferior third of the voxel), and these PDFF measurements were averaged. The three ROIs were added to the PDFF maps in the same location as the MRS voxel to assess the accuracy of MRI-PDFF versus MRS-PDFF.
Clinical and Biochemical Significance of MRI-PDFF–Detected Changes in Liver Fat
The variability of MRI-PDFF on repeat scans in the same individual is less than 1%.28 Therefore, we dichotomized the changes in liver fat to greater than 1% or less than 1% to examine the effects of small amounts of changes in liver fat in the setting of a clinical trial.
Reliability and Precision of MRI-PDFF
MRI and MRS were performed by an MR hepatoradiologist (C.S.) who was blinded to the patients’ clinical data and the baseline measurements at week 24. The MR hepatoradiologist has extensive experience with MRI of the liver and has published extensively in this area.29,35 Previous studies have shown excellent precision for MRI-PDFF, even for patients imaged twice on the same day.21,36 In internal quality control analyses, the UCSD Liver Imaging Center, led by the aforementioned MR hepatoradiologist (C.S.), has shown excellent inter-examination precision for MRI-PDFF in each segment (intraclass correlation coefficient ≥0.99, standard deviation < 1%).
Statistical Analysis
The chi-square test was used for comparisons between categorical variables, and a paired t test was used to compare mean differences between continuous variables. Primary and secondary comparisons within groups were calculated with paired t tests, two-tailed, independent-sample t tests, or nonparametric tests as appropriate. Pooled within-group Pearson correlations were used to look at associations across groups. A one-way analysis of variance was used to determine whether there were any significant differences between the means of three or more independent (unrelated) groups. A two-tailed P value < 0.05 was considered statistically significant. Statistical analyses were performed with SPSS 19.
Results
Demographic, Clinical, Biochemical, and Histological Characteristics and Imaging Studies of the Cohort
Fifty patients were randomized to either colesevelam or a placebo in a 1:1 ratio with 25 in each arm. The baseline demographics, laboratory studies, and histology data are shown in Table 1. The average age of the patients in this trial was 47.8 ± 11.7 years. Fifty-four percent of the patients were male, 38% were white, and 28% were Hispanic. The average weight and BMI were 88.3 ± 20.1 kg and 30.7 ± 6.5 kg/m2, respectively. The average serum ALT, AST, and GGT levels were 82.8 ± 58.4, 53.0 ± 39.6, and 78.2 ± 65.4 U/L, respectively (Table 1). The patients underwent liver biopsy, MRI-PDFF, and MRS within 3 months of one another at two time points: the baseline and week 24. MRI-PDFF and MRS were performed on the same day at each time point. The intervals between liver biopsy and MRI were 14.0 ± 56.6 days at the baseline and 20.0 ± 13.7 days at week 24. There was no change in body weight during the interval between liver biopsy and MRI.
Table 1.
Baseline Demographic, Biochemical, and Histological Characteristics of the Subjects
| All Patients (n = 50) | |
|---|---|
| Demographics | |
| Male (%) | 54 |
| Age (years)* | 47.8 ± 11.7 |
| Weight (kg)* | 88.3 ± 20.1 |
| Height (m)* | 1.7 ± 0.1 |
| BMI (kg/m2)* | 30.7 ± 6.5 |
| Ethnic origin (%) | |
| White | 38 |
| Black | 0 |
| Asian | 22 |
| Hispanic | 28 |
| Multiracial | 8 |
| Diabetes | 36 |
| Biochemical profile* | |
| ALT (U/L) | 82.8 ± 58.4 |
| AST (U/L) | 53.0 ± 39.6 |
| AST/ALT ratio | 0.7 ± 0.2 |
| Alkaline phosphatase (U/L) | 78.2 ± 22.5 |
| GGT (U/L) | 78.2 ± 65.4 |
| Total bilirubin (mg/dL) | 0.6 ± 0.4 |
| Direct bilirubin (mg/dL) | 0.1 ± 0.0 |
| Albumin (g/dL) | 4.6 ± 0.3 |
| Glucose (mg/dL) | 108.7 ± 28.8 |
| Insulin (IU/mL) | 27.3 ± 33.9 |
| Hemoglobin A1c (%) | 6.3 ± 0.9 |
| Triglycerides (mg/dL) | 182.2 ± 119.1 |
| Total cholesterol (mg/dL) | 201.3 ± 41.1 |
| LDL (mg/dL) | 119.4 ± 34.6 |
| High-density lipoprotein (mg/dL) | 49.1 ± 15.9 |
| Free fatty acids (mg/dL) | 0.5 ± 0.2 |
| Prothrombin time | 11.4 ± 0.7 |
| Histology* | |
| Steatosis | 2.0 ± 0.7 |
| Lobular inflammation | 1.5 ± 0.7 |
| Ballooning | 1.1 ± 0.7 |
| Fibrosis | 1.2 ± 1.4 |
| NAS | 4.7 ± 1.2 |
All labs were measured while patients were fasting. The NASH-CRN histological scoring system was used for the histological grading and staging of liver biopsy samples.
The data are presented as means and standard deviations.
MRI-PDFF and MRS-PDFF Correlations at Weeks 0 and 24 and Assessment of Liver Fat Changes
MRI-PDFF and MRS-PDFF showed a robust correlation with each other both at the baseline and at the end of the trial at week 24 (r2 = 0.98, P < 0.0001 for both; Fig. 1). We then divided the patients into those who had an increase in the fat fraction and those who had a decrease in the fat fraction on MRI (by 1% or more) between the baseline and week 24. We found that a small increase or decrease in MRI-PDFF correlated well with an increase or decrease in the MRS-measured liver fat fraction (Table 2). Because MRI-PDFF can provide a fat map of the entire liver and thereby enable fat content estimation for each segment separately, we show changes in the liver fat in each of the nine segments of the liver before and after treatment in Table 2, and these small amounts of changes in the liver fat were consistent across all segments of the liver. Furthermore, we performed regression plots of MRI-PDFF measurements and MRS fat fraction measurements at the baseline and week 24 (Supporting Fig. 1). At the baseline, MRI underestimated MRS by 3%. At week 24, MRI underestimated MRS by 1% with a small bias as well. This difference in estimation was small and clinically irrelevant and confirmed the robust correlation between MRI-PDFF and MRS-PDFF.
Fig. 1.
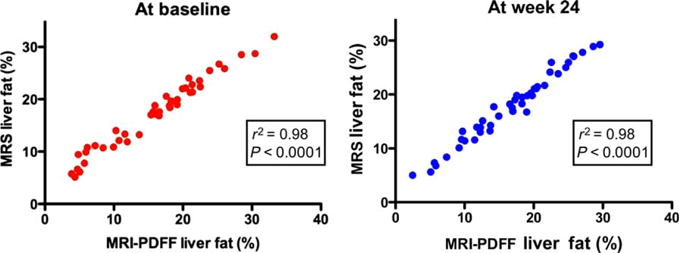
Correlation between MRI-PDFF and MRS at the baseline and week 24. MRI-PDFF showed a strong correlation with the MRS fat fraction at weeks 0 and 24 (r2 = 0.98, P < 0.0001 for both).
Table 2.
Increases or Decreases in Liver Fat Content Were Consistent Across All Segments Between MRS and MRI-PDFF
| Total MRI-PDFF Change Between Enrollment and Week 24 (After Treatment or Placebo)
|
P Value | ||
|---|---|---|---|
| Fat Fraction Decrease ≤ 1% | Fat Fraction Increase ≥ 1% | ||
| Liver segments* | |||
| 1 | −5.2 (5.2) | 4.4 (3.6) | <0.0001 |
| 2 | −6.3 (5.4) | 5.2 (3.4) | <0.0001 |
| 3 | −6.3 (6.1) | 4.8 (3.5) | <0.0001 |
| 4a | −5.6 (5.6) | 4.7 (3.4) | <0.0001 |
| 4b | −5.7 (4.9) | 4.8 (3.8) | <0.0001 |
| 5 | −6.6 (4.8) | 5.1 (3.8) | <0.0001 |
| 6 | −6.3 (4.9) | 5.5 (3.6) | <0.0001 |
| 7 | −6.7 (4.9) | 5.3 (3.8) | <0.0001 |
| 8 | −6.6 (5.1) | 5.1 (3.4) | <0.0001 |
| MRI-PDFF average | −6.6 (4.6) | 5.8 (3.9) | <0.0001 |
| MRS† | −7.6 (5.8) | 4.9 (3.2) | <0.0001 |
| MRI level‡ | |||
| Superior | −6.2 (4.4) | 5.9 (3.4) | <0.0001 |
| Middle | −6.8 (4.6) | 5.3 (3.5) | <0.0001 |
| Inferior | −6.8 (5.0) | 5.5 (3.4) | <0.0001 |
The data are expressed as means differences with standard deviation. An independent sample t test (assuming equal variance) was performed for all continuous variables for comparisons between groups. A paired sample t test was performed for comparisons within groups. Mean differences reflect comparisons between baseline averages minus posttreatment averages.
Longitudinal changes in MRI-PDFFs measured in all nine liver segments were used to calculate average segmental and overall fat fraction changes in patients with increased fat fractions versus patients with decreased fat fractions between weeks 0 and 24. The fat content in each liver segment was calculated via the averaging of three colocalized ROIs. The MRI total average was calculated with 27 ROIs: 3 from each liver segment.
Longitudinal changes in MRS measurements from a 2 × 2 × 2 cm3 cube (voxel) within the liver at week 0 and week 24 in patients with increased fat fractions versus patients with decreased fat fractions were used as reference points.
Internal validation was performed through the colocalization of MRI-based PDFF measurements to the reference MRS voxel. Three ROIs were placed on the PDFF maps in the same locations as the spectroscopic voxel (one through the superior third of the voxel, one through the middle third of the voxel, and one through the inferior third of the voxel).
Correlation of MRI-PDFF and MRS-PDFF With the Histology-Determined Steatosis Grade at the Baseline and Week 24
We then looked at whether MRI-PDFF and MRS-PDFF of the liver would correlate with the liver histology–determined steatosis grade at the baseline and at the end of the trial at week 24. At the baseline, MRI-PDFF increased with an increase in the histology-determined steatosis grade and showed a positive correlation (P < 0.0001). As expected, MRI-PDFF also increased with an increase in the histology-determined steatosis grade at week 24 and showed a positive correlation (P = 0.0027; Fig. 2A). Similarly, MRS-PDFF increased with an increase in the liver histology–determined steatosis grade at the baseline and week 24 (P < 0.0001 and P = 0.0017, respectively; Fig. 2B).
Fig. 2.
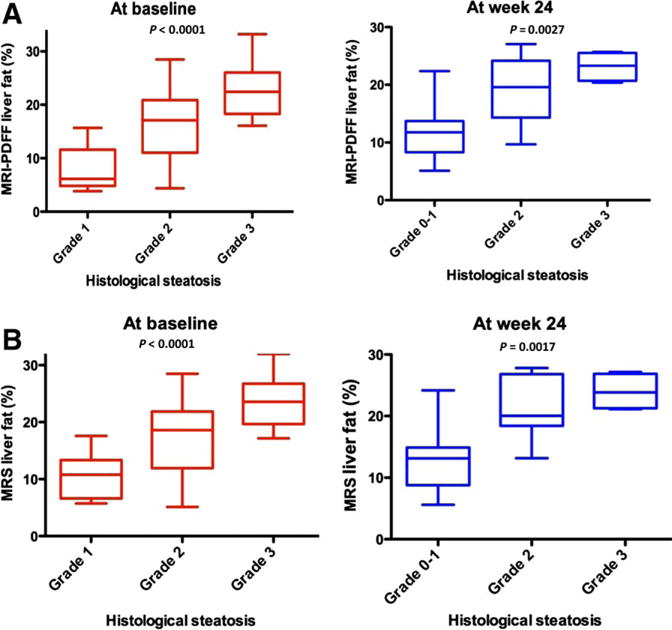
(A) Relationship between MRI-PDFF and the histological steatosis grade. MRI-PDFF increased with an increase in the liver histology–determined steatosis grade at the baseline (P = 0.0001) and at week 24 (P = 0.0027). The comparison was performed with an analysis of variance. (B) Relationship between the MRS fat fraction and the histological steatosis grade. The MRS fat fraction increased with an increase in the liver histology–determined steatosis grade at the baseline (P = 0.0001) and at week 24 (P = 0.0017). The comparison was performed with an analysis of variance.
Association Between Changes in Liver MRI-PDFF and MRS-PDFF and Clinical Parameters
We further investigated whether liver MRI-PDFF would correlate with changes in clinical parameters. We compared this to liver MRS-PDFF changes in liver fat and its relationship with changes in clinical parameters as a reference (Table 3). In this analysis, we divided patients into those who had an increase in MRI-PDFF ≥1% and those who had a decrease in the fat fraction ≥1%.
Table 3.
Comparison of NAFLD Patients Who Had Decrease versus Increase in Their Fat Fraction by MRI-PDFF and MRS Between Week 0 and Week 24
| MRI-PDFF Liver Fat Change Between Enrollment and Week 24 (After Treatment or Placebo)
|
MRS Liver Fat Change Between Enrollment and Week 24 (After Treatment or Placebo)
|
|||||
|---|---|---|---|---|---|---|
| MRI-PDFF Decreased ≥1% (n=16) | Fat Fraction Increased ≥ 1% (n=17) | P Value | Fat Fraction Decreased ≥1% (n=14) | Fat Fraction Increased ≥1% (n=17) | P Value | |
| Body weight changes | ||||||
| Weight (Kg) (CI) | −2.0 (−3.3,−0.6) | 0.3 (−0.9,1.6) | 0.008 | −1.83 (−3.3,−0.3) | 0.4 (−1.2,2.1) | 0.0137 |
| Laboratory changes | ||||||
| ALT (U/L) (CI) | −23.5 (−51.7,4.7) | 31.8 (11.8, 51.8) | 0.0008 | −9.2 (−42.0,13.0) | 32.6 (7.8, 57.5) | 0.0083 |
| AST (U/L) (CI) | −16.8 (−42.8,9.2) | 13.9 (1.4,26.4) | 0.0018 | −3.3 (−11.0,4.3) | 14.6 (−1.5, 30.8) | 0.0131 |
| GGT (U/L) (CI) | −4.2 (−12.7,4.4) | 31.8 (4.3,59.2) | 0.02 | 3.3 (−14.2,20.7) | 36.13 (1.2, 71.1) | 0.088 |
| Histology changes | ||||||
| Steatosis (CI) | −0.8 (−1.2,−0.3) | −0.14 (0.5, −0.4.02) | 0.02 | −0.8 (−1.3,−0.3) | −0.2 (−0.5,0.2) | 0.06 |
| Lobular inflammation (CI) | 0.2 (−0.2,0.6) | −0.08 (−0.5,0.4) | 0.4 | 0.2 (−0.3,0.7) | 0.0 (−0.4,0.4) | 0.5 |
| Ballooning (CI) | 0.0 (−0.5,0.5) | 0.08 (−0.4,0.6) | 0.97 | 0.2 (−0.2,0.7) | 0.3 (−0.3,0.9) | 1 |
| NAS (CI) | −0.6 (−1.7,0.5) | −0.17 (−1.3,1.0) | 0.6 | −0.3 (−1.6,0.9) | 0.1 (−1.1,1.3) | 0.6 |
| Fibrosis (CI) | 0.2 (−0.7,1,1) | −0.08 (−0.5,0.3) | 0.6 | 0 (−1.0,1.0) | 0 (−0.5,0.5) | 0.8 |
Decrease in liver fat of ≥1% by MRI-PDFF is associated with weight loss and decrease in serum ALT, AST, and GGT. Similarly, an increase in liver fat of ≥1% by MRI-PDFF is associated with weight gain and increase in serum ALT, AST, and GGT. Although MRI-PDFF is sensitive in capturing these dynamic changes in weight and serum liver enzymes over a 24-week period, liver biopsy determined steatosis grade (because of its subjective assessment) is not able to show a significant change.
Data are expressed as means with confidence intervals (CI) in parentheses or mean difference.
Independent sample t test assuming equal variance was performed on all continuous variables for comparisons between groups. Mean differences reflect comparison between posttreatment averages minus baseline averages.
Patients who had an increase in liver MRI-PDFF had an increase in their body weight, whereas those who had a decrease in liver MRI-PDFF had a decrease in their body weight (Table 3). Furthermore, patients who had an increase in liver MRI-PDFF showed an increase in their serum ALT, AST, and GGT levels at week 24. On the other hand, patients who had a decrease in liver MRI-PDFF showed a decrease in their serum ALT, AST, and GGT levels at week 24. Furthermore, liver MRI-PDFF correlated with changes in body weight (r2 = 0.49, P = 0.0012), ALT (r2 = 0.55, P = 0.0003), AST (r2 = 0.47, P = 0.003), and GGT (r2 = 0.38, P = 0.01; Fig. 3). The results remained consistent when MRS-measured changes in the liver fat content correlated well with changes in the body weight and liver enzymes (Table 3). Finally, we show changes in serum ALT, body weight, liver MRI-PDFF, and MRS-PDFF between weeks 0 and 24 for two patients who participated in this study (Fig. 4). The patient represented in Fig. 4A had an increase in the fat fraction, and the patient represented in Fig. 4B had a decrease in the fat fraction. The increase in liver fat was associated with parallel increases in the serum ALT level and body weight, and the decrease in liver fat was associated with parallel decreases in the serum ALT level and body weight. The direction of changes in the ALT level, body weight, and fat fraction assessed by MRI-PDFF and MRS-PDFF remained consistent with each other.
Fig. 3.
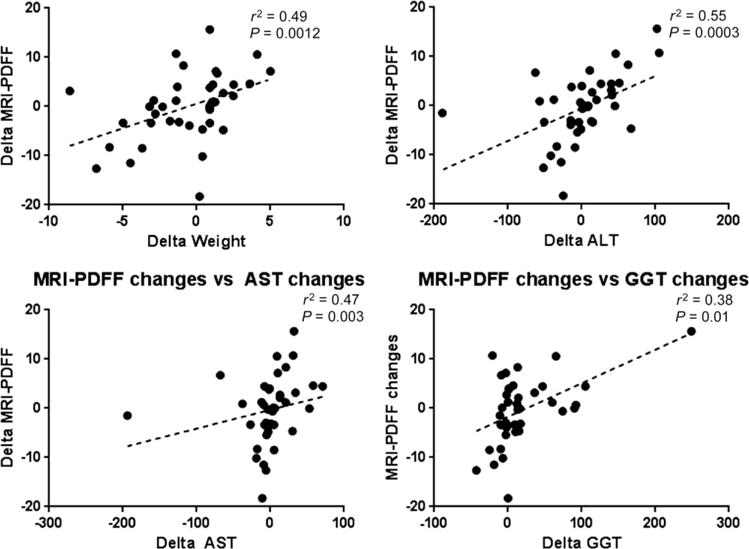
MRI-PDFF changes between weeks 0 and 24 and their association with clinical and laboratory changes in patients with biopsy-proven NAFLD. MRI-PDFF changes correlated with changes in weight (r2 = 0.49, P = 0.0012), ALT (r2 = 0.55, P = 0.0003), AST (r2 = 0.47, P = 0.003), and GGT (r2 = 0.38, P = 0.01).
Fig. 4.
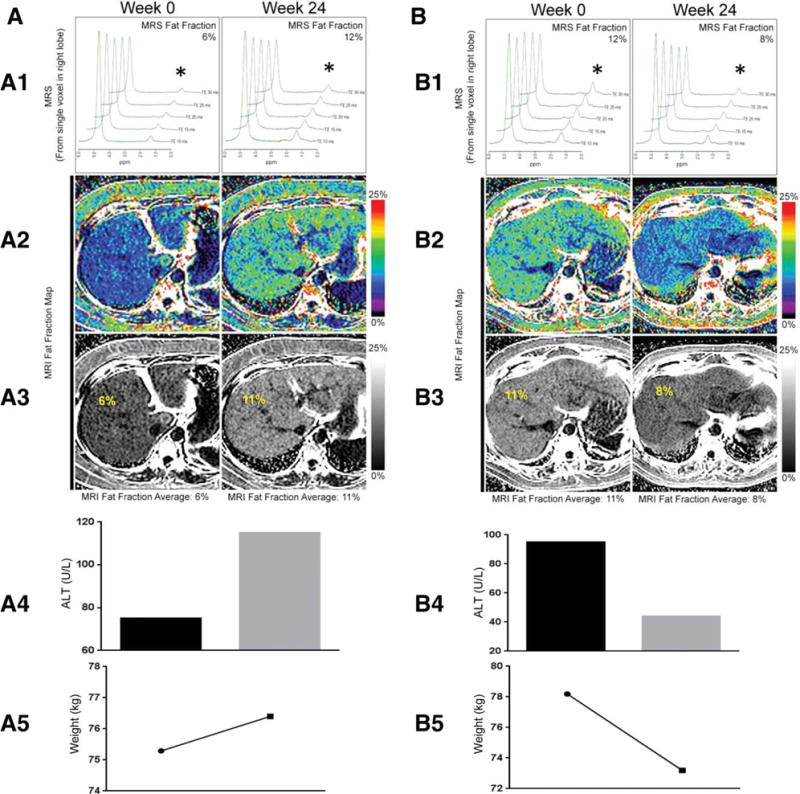
Changes in ALT, weight, MRI-PDFF, and MRS fat fractions between weeks 0 and 24 in two patients: liver fat maps and associated variables for (A) a patient who experienced an increase in MRI-PDFF between weeks 0 and 24 and (B) a patient who experienced a decrease in MRI-PDFF between weeks 0 and 24. (A-1) MRS changes between weeks 0 and 24 showing an increase in the fat fraction from 6% to 12% (asterisks). (A-2,A-3) MRI-PDFF changes between weeks 0 and 24 showing an increase in the fat fraction from 6% to 11%. (A-4) Parallel increase in ALT with an increase in liver fat between weeks 0 and 24 from 75 to 115 U/L. (A-5) Parallel increase in body weight with an increase in liver fat between weeks 0 and 24 from 75.3 to 76.4 kg. (B-1) MRS changes between weeks 0 and 24 showing a decrease in the fat fraction from 12% to 8% (asterisks). (B-2,B-3) MRI-PDFF changes between weeks 0 and 24 showing a decrease in the fat fraction from 11% to 8%. (B-4) Parallel decrease in ALT with a decrease in liver fat between weeks 0 and 24 from 95 to 44 U/L. (B-5) Parallel decrease in body weight with a decrease in liver fat between weeks 0 and 24 from 78.2 to 73.2 kg.
Discussion
In this secondary analysis of a randomized, double-blinded, placebo-controlled clinical trial, we performed three-way comparisons of MRI-PDFF, MRS-PDFF (a quantitative measurement of the liver fat content), and the liver histology–determined steatosis grade (an ordinal scale that allows the assessment of liver fat) at two time points 24 weeks apart. MRS is the only reference standard for measuring liver fat quantity noninvasively, and MRI-PDFF provides a reliable and robust estimate of the liver fat content as shown in previous cross-sectional studies. In this longitudinal study, we have shown that liver MRI-PDFF correlates strongly with the MRS-measured liver fat content both at the baseline and at the end of the trial at week 24 in 50 patients with biopsy-proven NAFLD. In addition, changes in the liver MRI-PDFF and MRS-measured liver fat content reflected changes in clinical/biochemical parameters such as weight and liver enzymes longitudinally at two time points. Moreover, the liver MRI-PDFF and MRS-determined liver fat content correlated with the histology-determined steatosis grade at two time points (weeks 0 and 24). Our data suggest that liver MRI-PDFF is a reliable method for accurately quantifying liver fat and a sensitive method for monitoring changes in the liver fat content in patients with NAFLD in the setting of a clinical trial. The small amounts of changes in the liver fat content that were appreciated by MRI-PDFF but not by liver histology were associated with corresponding changes in body weight and serum ALT, AST, and GGT levels, and this suggests clinical significance for dynamic changes in the liver fat content.
Liver biopsy remains the gold standard for the diagnosis of NAFLD and is used for the assessment of changes in the histology-determined steatosis grade after interventions.9 Because of the subjective assessment of the steatosis grade on histology as well as its sampling variability, it is not able to reliably capture small increases or decreases in liver fat. Noninvasive biomarkers such as imaging studies have been increasingly used for assessments of changes in liver fat in patients with NAFLD. Ultrasound is the most commonly used method because of its availability, low cost, and minimal risk to the patients.14 It measures the fat content of the liver indirectly by assessing the liver texture and echogenicity. However, ultrasound is both machine- and operator-dependent and lacks the ability to accurately quantify the liver fat content. In addition, it is hard to perform on obese patients and lacks sensitivity.14 Computed tomography is more precise than ultrasound, but it has its own disadvantages, including interference due to iron deposition, fibrosis, or edema and, most importantly, the risks associated with exposure to ionizing radiation as well as a sensitivity lower than that of MRI.37,38
MRS remains the only noninvasive reference standard for detecting and quantifying the biochemical fat content in the liver and has been used in several research studies, but it has limited clinical applicability or availability.17,39 MRS measures PDFF biochemically, and MRI estimates PDFF. MRS evaluates the liver fat content in only a single 2 × 2 × 2 cm3 cube (voxel) within the liver. However, this technique relies on factors that lead to estimates of the fat content that are platform- and imaging protocol–dependent.30 An MRI-based assessment of liver fat can provide an image as well as the liver fat content for each segment of the liver. However, conventional MRI-based methods of liver fat assessment are limited because of T1 bias, T(2)* decay, eddy currents, and multifrequency signal-interference effects of protons in fat and, therefore, may not provide an accurate estimation of the liver fat content. MRI-PDFF, a novel biomarker, eliminates the biases seen with conventional MRI techniques and has shown a robust correlation with MRS.19–21,40
MRI-PDFF has improved biases seen with conventional MRI techniques. MRI-PDFF is independent of the field strength, scanner platform, and parameters and correlates highly with hepatic triglyceride.18 In addition, in contrast to MRS, MRI-PDFF can be used with any MRI platform.18 We have shown that MRI-PDFF correlates highly (Pearson r correlation coefficient = 0.98) with MRS. In addition, it has excellent repeatability when the test is repeated on the same day (Pearson r correlation coefficient = 0.99).21 In this study, we have extended our previous finding that MRI-PDFF correlates with MRS-PDFF and histology-determined steatosis grades in a cross-sectional analysis to a longitudinal analysis in which subjects were assessed for changes in their liver fat over a 24-week time period in the setting of a randomized controlled trial.
Strengths and Limitations
We would like to highlight the following strengths of the study. First, the randomized, placebo-controlled study design allowed a systematic assessment of a defined group of patients with biopsy-proven NAFLD and a predefined MRI fat mapping protocol as well as cross-validation of MRI-PDFF by MRS-PDFF for each subject both before and after 24 weeks of treatment. Second, the NASH-CRN histological scoring system was used for characterization of liver histology. Third, the radiologist and pathologists were blinded to histological and MR data, respectively. Fourth, all patients underwent extensive liver fat mapping, and liver fat changes were compared in colocalized ROIs before and after 24 weeks of treatment. To our knowledge, this study illustrates one of the most extensive liver fat phenotypings performed in a clinical trial assessing the efficacy of a drug versus a placebo in the treatment of NASH. This provides a strong rationale for using MRI-PDFF in future clinical trials in NASH. However, we acknowledge the following limitation of this study. This study was not designed to assess the role of MRI-PDFF in screening for NAFLD or detecting NAFLD because we did not have a control group and it would have been unethical to subject normal individuals with normal liver fat content according to MRI-PDFF to a liver biopsy assessment. However, it is highly likely that MRI-PDFF could be used for screening for hepatic steatosis in future studies.
Further Advantages of MRI-PDFF Over MRS-PDFF
MRI-PDFF can be applied to any commercially available platform, whereas MRS-PDFF remains a research tool requiring special coils, is time-consuming, is not routinely available, and will likely not be used in clinical practice because of the complexities of its logistics and the lack of required expertise at most clinical imaging centers. MRS-PDFF requires trained research technologists and is usually analyzed by a physicist using specialized software. In comparison, MRI-PDFF can be determined with routine modern scanners by any MR technologist. MRI-PDFF maps can be generated online within seconds and can be analyzed after minimal training in recognizing segments and avoiding artifacts. Because of the complexity of MRS and the ease of MRI, NASH-CRN, sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases, has decided to include MRI-PDFF but not MRS-PDFF in its active clinical trials.
Emerging Need to Assess Changes in Liver Fat in Lipid-Lowering Trials in Cardiovascular Disease
Newer lipid-lowering therapies are emerging, and some of the novel lipid-lowering agents have been shown to increase the liver fat content. Lomitapide, a microsomal triglyceride transfer protein inhibitor, has been shown to reduce LDL cholesterol in patients with familial hypercholesterolemia, and in doing so, it has been shown to increase the liver fat content.41 Another lipid-lowering agent, mipomersen, an apolipoprotein B synthesis inhibitor, also showed efficacy in lowering LDL cholesterol concentrations in patients with homozygous familial hypercholesterolemia, but a concomitant increase in the liver fat content was noted.42 Therefore, there is an emerging, unmet need for the accurate, noninvasive quantification of changes in the liver fat content, and MRI-PDFF provides a clinically useful tool in such settings.
In conclusion, using a randomized controlled trial, here we show that MRI-PDFF correlates well with MRS-PDFF and is more sensitive than the histology-determined steatosis grade in quantifying longitudinal changes in the liver fat content. MRI-PDFF allows fat mapping of the entire liver and can be determined with any clinical MRI platform, whereas MRS measures fat biochemically in a small ROI and is largely a research tool with limited clinical availability; MRI-PDFF may be used as an imaging biomarker to quantify changes in liver fat in future clinical trials.
Future studies are needed to examine the role of additional MR-based biomarkers beyond liver fat quantification for the assessment of other histological features seen in patients with NASH, including lobular inflammation, ballooning degeneration, and fibrosis. However, we propose that a new standard has emerged for the quantification of liver fat in the setting of clinical trials in NASH.
Supplementary Material
Acknowledgments
Members of the San Diego Integrated NAFLD Research Consortium include Rohit Loomba, M.D., M.H.Sc. (principal investigator), Ottar Lunde, M.D., Robert Gish, M.D., Yuko Kono, M.D., Alexander Kuo, M.D., Heather Patton, M.D., Michel Mendler, M.D., Lisa Richards, N.P., Joanie Salotti, N.P., Archana Bhatt, B.D.S., Brandon D. Ang, and Thu Nguyen, B.S. (University of California San Diego, La Jolla, CA); Michael Bennett, M.D., Tommy Yen, M.D., John Person, M.D., and Cynthia Behling, M.D. (Sharp Health System, San Diego, CA); Lisa Nyberg, M.D., Anders Nyberg, M.D., and Mamie Dong, M.D. (Kaiser Permanente of Southern California, San Diego, CA); and Lt. Cmdr. William Shields, M.D. (Balboa Naval Medical Center, San Diego, CA).
This work was supported by an investigator-initiated study grant to Rohit Loomba by Daiichi Sankyo, Inc. The study was conducted at the Clinical and Translational Research Institute of the University of California San Diego. Rohit Loomba is supported in part by the American Gastroenterological Association Foundation/Sucampo/Association of Specialty Professors Designated Research Award in Geriatric Gastroenterology and by the T. Franklin Williams Scholarship Award; funding was provided by Atlantic Philanthropies, Inc., the John A. Hartford Foundation, the Association of Specialty Professors, the American Gastroenterological Association, and the National Institutes of Health grant K23-DK090303.
The funding agencies did not have any role in the design or conduct of the study; in the collection, management, analysis, or interpretation of the data; or in the preparation, review, or approval of the article.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- GGT
gamma-glutamyl transpeptidase
- LDL
low-density lipoprotein
- MR
magnetic resonance
- MRI
magnetic resonance imaging
- MRI-PDFF
magnetic resonance imaging–estimated proton density fat fraction
- MRS
magnetic resonance spectroscopy
- MRS-PDFF
magnetic resonance spectroscopy–measured proton density fat fraction
- NAFLD
nonalcoholic fatty liver disease
- NAS
nonalcoholic fatty liver disease activity score
- NASH
nonalcoholic steatohepatitis
- NASH-CRN
Nonalcoholic Steatohepatitis Clinical Research Network
- PDFF
proton density fat fraction
- ROI
region of interest
- UCSD
University of California San Diego
Footnotes
Potential conflict of interest: Dr. Sirlin consults for Bayer. Dr. Middleton received grants from Isis, Genzyme, Sanofi, Merck, GE, Siemens, Gilead, Pfizer, Synageva, Biomedical Systems, Bioclinica, Profil, and Takeda.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008;28:339–350. doi: 10.1055/s-0028-1091978. [DOI] [PubMed] [Google Scholar]
- 2.Kim WR, Brown RS, Jr, Terrault NA, El-Serag H. Burden of liver disease in the United States: summary of a workshop. Hepatology. 2002;36:227–242. doi: 10.1053/jhep.2002.34734. [DOI] [PubMed] [Google Scholar]
- 3.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 4.Gupta R, Bhangoo A, Matthews NA, Anhalt H, Matta Y, Lamichhane B, et al. The prevalence of non-alcoholic fatty liver disease and metabolic syndrome in obese children. J Pediatr Endocrinol Metab. 2011;24:907–911. doi: 10.1515/jpem.2011.282. [DOI] [PubMed] [Google Scholar]
- 5.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–725. doi: 10.1053/j.gastro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis. 2012;32:3–13. doi: 10.1055/s-0032-1306421. [DOI] [PubMed] [Google Scholar]
- 7.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 8.Paredes AH, Torres DM, Harrison SA. Nonalcoholic fatty liver disease. Clin Liver Dis. 2012;16:397–419. doi: 10.1016/j.cld.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134:1682–1698. doi: 10.1053/j.gastro.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 10.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 11.Mato JM, Lu SC. Where are we in the search for noninvasive nonalcoholic steatohepatitis biomarkers? Hepatology. 2011;54:1115–1117. doi: 10.1002/hep.24642. [DOI] [PubMed] [Google Scholar]
- 12.Ratziu V, Cadranel JF, Serfaty L, Denis J, Renou C, Delassalle P, et al. A survey of patterns of practice and perception of NAFLD in a large sample of practicing gastroenterologists in France. J Hepatol. 2012;57:376–383. doi: 10.1016/j.jhep.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Corey KE, Lai M, Gelrud LG, Misdraji J, Barlow LL, Zheng H, et al. Non-high-density lipoprotein cholesterol as a biomarker for nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2012;10:651–656. doi: 10.1016/j.cgh.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra P, Younossi ZM. Abdominal ultrasound for diagnosis of nonalcoholic fatty liver disease (NAFLD) Am J Gastroenterol. 2007;102:2716–2717. doi: 10.1111/j.1572-0241.2007.01520.x. [DOI] [PubMed] [Google Scholar]
- 15.Kodama Y, Ng CS, Wu TT, Ayers GD, Curley SA, Abdalla EK, et al. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol. 2007;188:1307–1312. doi: 10.2214/AJR.06.0992. [DOI] [PubMed] [Google Scholar]
- 16.Limanond P, Raman SS, Lassman C, Sayre J, Ghobrial RM, Busuttil RW, et al. Macrovesicular hepatic steatosis in living related liver donors: correlation between CT and histologic findings. Radiology. 2004;230:276–280. doi: 10.1148/radiol.2301021176. [DOI] [PubMed] [Google Scholar]
- 17.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 18.Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011:34. doi: 10.1002/jmri.22580. spcone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yokoo T, Bydder M, Hamilton G, Middleton MS, Gamst AC, Wolfson T, et al. Nonalcoholic fatty liver disease: diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5 T. Radiology. 2009;251:67–76. doi: 10.1148/radiol.2511080666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meisamy S, Hines CD, Hamilton G, Sirlin CB, McKenzie CA, Yu H, et al. Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology. 2011;258:767–775. doi: 10.1148/radiol.10100708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hines CD, Frydrychowicz A, Hamilton G, Tudorascu DL, Vigen KK, Yu H, et al. T(1) independent, T(2) (*) corrected chemical shift based fat-water separation with multi-peak fat spectral modeling is an accurate and precise measure of hepatic steatosis. J Magn Reson Imaging. 2011;33:873–881. doi: 10.1002/jmri.22514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamilton G, Yokoo T, Bydder M, Cruite I, Schroeder ME, Sirlin CB, et al. In vivo characterization of the liver fat 1H MR spectrum. NMR Biomed. 2011;24:784–790. doi: 10.1002/nbm.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Permutt Z, Le TA, Peterson MR, Seki E, Brenner DA, Sirlin C, et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease—MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther. 2012;36:22–29. doi: 10.1111/j.1365-2036.2012.05121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le TA, Chen J, Changchien C, Peterson MR, Kono Y, Patton H, et al. For San Diego Integrated NAFLD Research Consortium (SINC). Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology. 2012;56:922–932. doi: 10.1002/hep.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loomba R, Abraham M, Unalp A, Wilson L, Lavine J, Doo E, et al. For Nonalcoholic Steatohepatitis Clinical Research Network. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012;56:943–951. doi: 10.1002/hep.25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons With Harmful Alcohol Consumption—II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 27.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. For Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 28.Bydder M, Shiehmorteza M, Yokoo T, Sugay S, Middleton MS, Girard O, et al. Assessment of liver fat quantification in the presence of iron. Magn Reson Imaging. 2010;28:767–776. doi: 10.1016/j.mri.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reeder SB, Hu HH, Sirlin CB. Proton density fat-fraction: a standardized MR-based biomarker of tissue fat concentration. J Magn Reson Imaging. 2012;36:1011–1014. doi: 10.1002/jmri.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34:729–749. doi: 10.1002/jmri.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu CY, McKenzie CA, Yu H, Brittain JH, Reeder SB. Fat quantification with IDEAL gradient echo imaging: correction of bias from T(1) and noise. Magn Reson Med. 2007;58:354–364. doi: 10.1002/mrm.21301. [DOI] [PubMed] [Google Scholar]
- 32.Bydder M, Yokoo T, Hamilton G, Middleton MS, Chavez AD, Schwimmer JB, et al. Relaxation effects in the quantification of fat using gradient echo imaging. Magn Reson Imaging. 2008;26:347–359. doi: 10.1016/j.mri.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu H, McKenzie CA, Shimakawa A, Vu AT, Brau AC, Beatty PJ, et al. Multiecho reconstruction for simultaneous water-fat decomposition and T2* estimation. J Magn Reson Imaging. 2007;26:1153–1161. doi: 10.1002/jmri.21090. [DOI] [PubMed] [Google Scholar]
- 34.Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med. 2008;60:1122–1134. doi: 10.1002/mrm.21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang GH, Cruite I, Shiehmorteza M, Wolfson T, Gamst AC, Hamilton G, et al. Reproducibility of MRI-determined proton density fat fraction across two different MR scanner platforms. J Magn Reson Imaging. 2011;34:928–934. doi: 10.1002/jmri.22701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SS, Park SH, Kim HJ, Kim SY, Kim MY, Kim DY, et al. Noninvasive assessment of hepatic steatosis: prospective comparison of the accuracy of imaging examinations. J Hepatol. 2010;52:579–585. doi: 10.1016/j.jhep.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 38.Lee SW, Park SH, Kim KW, Choi EK, Shin YM, Kim PN, et al. Unenhanced CT for assessment of macrovesicular hepatic steatosis in living liver donors: comparison of visual grading with liver attenuation index. Radiology. 2007;244:479–485. doi: 10.1148/radiol.2442061177. [DOI] [PubMed] [Google Scholar]
- 39.Marsman HA, van Werven JR, Nederveen AJ, Ten Kate FJ, Heger M, Stoker J, et al. Noninvasive quantification of hepatic steatosis in rats using 3.0 T 1H-magnetic resonance spectroscopy. J Magn Reson Imaging. 2010;32:148–154. doi: 10.1002/jmri.22064. [DOI] [PubMed] [Google Scholar]
- 40.Yokoo T, Shiehmorteza M, Hamilton G, Wolfson T, Schroeder ME, Middleton MS, et al. Estimation of hepatic proton-density fat fraction by using MR imaging at 3.0 T. Radiology. 2011;258:749–759. doi: 10.1148/radiol.10100659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raal FJ. Lomitapide for homozygous familial hypercholesterolaemia. Lancet. 2013;381:7–8. doi: 10.1016/S0140-6736(12)61845-5. [DOI] [PubMed] [Google Scholar]
- 42.Raal FJ, Santos RD, Blom DJ, Marais AD, Charng MJ, Cromwell WC, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blinded, placebo-controlled trial. Lancet. 2010;375:998–1006. doi: 10.1016/S0140-6736(10)60284-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


