Abstract
Environmental reward-predictive stimuli provide a major source of motivation for instrumental reward-seeking activity and this has been linked to dopamine signaling in the nucleus accumbens (NAc). This cue-induced incentive motivation can be quite general, not restricted to instrumental actions that earn the same unique reward, and is also typically regulated by one’s current need state, such that cues only motivate actions when this is adaptive. But it is unknown whether cue-evoked dopamine signaling is similarly regulated by need state. Here we used fast-scan cyclic voltammetry to monitor dopamine concentration changes in the NAc core of rats during a Pavlovian-to-instrumental transfer (PIT) task in which the motivating influence of two cues, each signaling a distinct food reward (sucrose or food pellets), over an action earning a third unique food reward (grape-flavored polycose) was assessed in a state of hunger and of satiety. Both cues elicited a robust NAc dopamine response when hungry. The magnitude of the sucrose cue-evoked dopamine response correlated with the PIT effect that was selectively induced by this stimulus. Satiety attenuated these cue-evoked dopamine responses and behavioral responding, even though rats had never experienced the specific food rewards in this state. These data demonstrate that cue-evoked NAc core responses are sensitive to current need state, one critical variable that determines the current adaptive utility of cue-motivated behavior.
Keywords: Pavlovian-to-instrumental transfer, hunger, satiety, mesolimbic dopamine, voltammetry, reward
Environmental reward-predictive stimuli can provide a major source of motivation for reward-seeking behaviors (Dickinson & Balleine 2002). This is exemplified by Pavlovian-to-instrumental transfer (PIT (Lovibond 1983, Estes 1948)), in which a stimulus previously paired with reward can invigorate ongoing appetitive instrumental activity, even though it may never have been associated with the instrumental action. This cue-induced incentive motivation tends to be regulated by one’s current need state; cues only invigorate reward seeking when this is adaptive (Dickinson & Dawson 1987, Balleine 1994, Corbit et al. 2007). But the motivational influence of food or drug cues can become excessive and/or disproportionate with need, and this is thought to contribute to the intense and maladaptive craving and motivation that characterizes addiction (Berridge 2007, Everitt et al. 1999, Milton & Everitt 2012, Ostlund & Balleine 2008, Ludwig et al. 1974) and compulsive overeating (Volkow et al. 2011, Kenny 2011, Watson et al. 2014, Johnson 2013).
Evidence suggests that the nucleus accumbens (NAc) and dopamine signaling therein is vital for cue-induced incentive motivation (Corbit & Balleine 2011, Peciña & Berridge 2013, Wyvell & Berridge 2000, Lex & Hauber 2008). In the NAc core, dopamine phasically responds to unexpected reward cue presentation (Day et al. 2007, McCutcheon et al. 2012, Clark et al. 2013, Cone et al. 2015, Hart et al. 2015, Roitman et al. 2004, Ostlund et al. 2014), especially when that cue acquires motivational value that causes it to become an incentive target (Flagel et al. 2011). Cue-evoked NAc core dopamine signaling also tracks the invigorating influence of a reward-paired cue over an independent reward-seeking action, i.e., PIT (Wassum et al. 2013). Moreover, activation of NAc dopamine D1 (and to a lesser extent D2) receptors is also required for PIT (Lex & Hauber 2008) and NAc dopamine stimulation can enhance PIT (Peciña & Berridge 2013, Wyvell & Berridge 2000).
PIT relies on the mental association formed between the stimulus and the reward it predicts, a so-called stimulus-outcome association (Dickinson & Balleine 2002, Corbit & Balleine 2005). This association allows a Pavlovian cue to generate a detailed, cognitive expectation of its specific predicted reward and thereby bias the selection of available actions towards those that earn that exact same unique reward (Kruse et al. 1983, Colwill & Motzkin 1994, Corbit & Balleine 2005). Cues can also have a more general motivational influence, motivating a relatively broader range of instrumental actions (though typically those earning a categorically similar reward), and exciting general locomotor activity (Bindra 1968), through an association with the more general (i.e., specific identity-independent) motivational features of the paired reward (e.g., nutritive or fluidic properties, not specific taste) (Corbit & Balleine 2005, Balleine 1994). This latter process is particularly sensitive to need state; transient needs influence the specific identity-independent motivational value that allows cues to indiscriminately invigorate appetitive instrumental activity (Corbit et al. 2007, Balleine 1994). Here, we tested the hypothesis that NAc core dopamine signaling tracks this motivational value of a food-predictive stimulus and that cue-evoked dopamine signaling is modulated by need states vital for determining the current adaptive utility of cue-motivated action.
To achieve this we used a novel task in which rats learned that two distinct auditory cues each predicted one of two unique food rewards, prior to being trained in the absence of those cues to lever press to earn a third unique food reward. Then, in a PIT test, NAc core dopamine release was monitored with fast-scan cyclic voltammetry (FSCV) while the influence of each cue over instrumental activity was assessed. PIT was evaluated under conditions of hunger and of satiety to evaluate the sensitivity of behavioral responding and cue-evoked NAc dopamine release to need-based changes in the cue’s motivational value.
MATERIALS AND METHODS
Subjects
Male, Sprague Dawley rats (n=8; 280–320 g upon arrival; Charles River Laboratories, Wilmington, MA) were group housed and handled daily for 5–7 days prior to surgery and training. Except where noted, rats were maintained on a food-restriction schedule whereby they were deprived of food for 18 hrs prior to each day’s training or test session. Rats were provided free access to tap water in the home cage (except where noted) and were fed approximately 3–4 hr after each daily training session. Training and test took place during the dark phase of a 12:12 hr reverse dark:light cycle. All procedures were conducted in accordance with the NIH Guide for the Care and use of Laboratory Animals and approved by the UCLA Institutional Animal Care and Use Committee.
Electrode preparation and calibration
Chronically-implanted, carbon-fiber microelectrodes, which allow stable FSCV dopamine recordings from the same sampling space over months (Clark et al. 2010), were used to make longitudinal, within-subject measures of dopamine concentration changes across multiple tests. Carbon-fiber microelectrodes were prepared as described previously (Clark et al. 2010, Wassum et al. 2013). Electrodes were all pre-calibrated with dopamine (0.25–1 µM in phosphate buffered saline, pH=7.4) in a custom-made flow cell (flow rate 4 ml/min) prior to implantation (average calibration factor = 40.88 nM/nA, SEM=4.22). Preliminary pre- and post-implantation calibrations suggest this value changes <10% due to of chronic implantation for ~70 days.
Surgical procedures
Calibrated microelectrodes were implanted into the NAc core region (see Figure 1A). Rats were anesthetized with isoflurane (5% induction, 1–2% maintenance) and stereotactically implanted with a microelectrode under aseptic conditions (coordinates from Bregma: AP: +1.3 mm; ML: ±1.3; V: −7.0 from dura). A Ag/AgCl reference electrode was placed in the contralateral cortex. Dental cement affixed both electrodes in place and sealed the wound. A nonsteroidal anti-inflammatory agent was administered pre- and post-operatively to minimize pain and discomfort. Following surgery rats were individually housed and allowed to recover for 7 days.
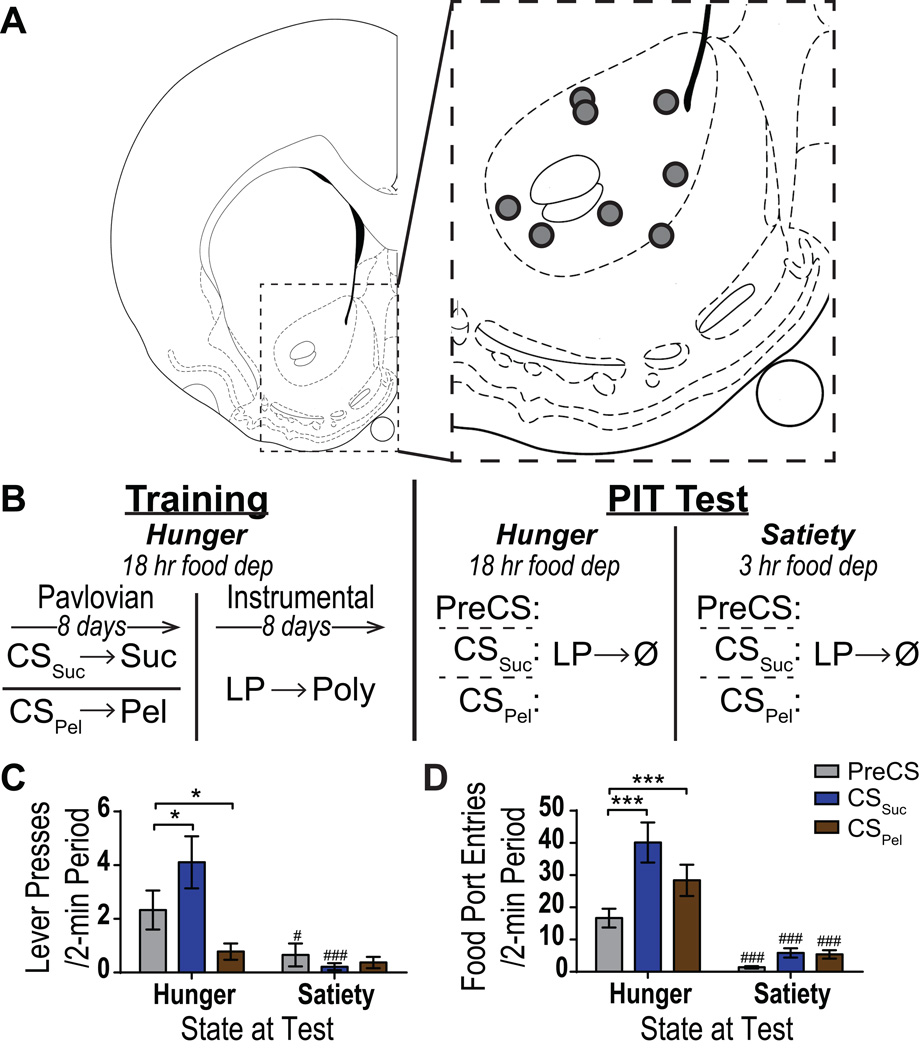
Figure 1. Task design and behavioral results.
A. Schematic representation of recording sites in NAc core. Line drawing of coronal section is reprinted from (Paxinos & Watson 1998), +1.3 mm from bregma. All placements shown in the left hemisphere, but 2 of the 8 recordings were from the right hemisphere. B. Experimental Design. Rats were first given Pavlovian training to associate each of two auditory cues with one of two unique food rewards, sucrose solution (CSSuc) or food pellets (CSPel). In the second phase of training, rats were given instrumental training to learn to lever press (LP) to earn a grape-flavored polycose solution (Poly). Rats were then given a Pavlovian-to-instrumental transfer (PIT) test under a state of either hunger or satiety in to assess the influence of CS presentation on lever pressing. C. The PIT effect. Number of lever presses during each 2-min CS period, averaged across trials compared between the CS-free (PreCS) and CS periods. D. Conditioned food-port approach responding. Number entries into the food-delivery port during each 2-min CS period, averaged across trials compared between the PreCS and CS periods. Error bars indicate ±1 SEM. *p<0.05, ***p<0.001 relative to PreCS control period. #p<0.05, ###p<0.001 relative to same period during hungry test.
Apparatus
All training took place in a set of 8 Med Associates (East Fairfield, VT) operant chambers housed within sound- and light-resistant shells, described previously (Malvaez et al. 2015). Each chamber contained a recessed food-delivery port with a photobeam entry detector and a retractable lever to the left of this port. The chambers were also equipped with syringe pump to deliver solution, as described below, in 0.1 ml increments through a stainless steel tube into a well in the food port and a pellet dispenser to deliver single pellets into the same port. Both a tone and white noise generator were attached to individual speakers on the wall opposite the lever and food-delivery port. A 3-watt, 24-volt house light mounted on the top of the wall opposite the food cup provided illumination. Testing took place in a set of two chambers that were identical to the training chambers with the exception that they were housed within an electrically-isolated faraday cage and were outfitted with an electrical swivel (Crist Instrument Co, Hagerstown, MD) connecting a headstage tether that extended within the operant chamber to the custom-made potentiostat recording unit outside the chamber.
Behavioral training
Each training session was conducted following 18 hrs of food deprivation. Each session began with the illumination of the houselight and insertion of the lever where appropriate, and ended with the retraction of the lever and turning off of the houselight. Experimental design is shown in Figure 1B.
Pavlovian training
Rats were first given Pavlovian training to pair each of two distinct auditory stimuli (tone or white noise, 75 db) with a unique food reward, either sucrose solution (CSSuc) or grain-based food pellets (CSPel; 45 mg, Bio-Serv, Frenchtown, NJ). In preliminary experiments, conducted in a separate group of subjects (n=6), these two food rewards were confirmed to have relatively equal value. Rats were given a consumption choice test in which both the 20% sucrose solution and food pellets were available together for 15 min. On average, rats showed no significant preference for either reward type (average sucrose consumption = 7.62 g, SEM=1.80 v. average grain pellet consumption= 9.10 g, SEM=2.19, t6=0.38, p=0.72; average preference ratio [consumed sucrose g/(consumed sucrose + pellets g)]: 0.47, SEM=0.13).
Each stimulus was conditioned independently, with rats receiving one session each with the tone and white noise each day, separated by 1 hr, for 8 total days. Each session consisted of 8 stimulus presentations (either tone or white noise, 2-min duration), during which either 20% sucrose solution or food pellets, as appropriate, were delivered on a 30-s random-time schedule into the food-delivery port, resulting in an average of 4 stimulus-reward pairings per trial. CSs were separated by a variable inter-trial interval ranging between 2–4 min (3-min mean). The rate at which rats entered the food port was recorded for the 2-min pre-CS period, for the CS-probe period (interval between CS onset and first reward delivery) and for the CS-reward period (interval after first reward delivery to CS offset). Stimulus-reward pairing was counterbalanced across subjects, such that for half the tone served as the CSSuc and the white noise as the CSPel, with the other half receiving the opposite arrangement. The order of Pavlovian conditioning sessions was also counterbalanced across subjects.
Instrumental training
Following Pavlovian training, all rats received 8 total days of instrumental training in which lever pressing was rewarded with delivery of 0.1 ml of grape-flavored 20% polycose solution. Each session lasted until 30 outcomes had been earned, or 40 min elapsed. Rats were given only one instrumental training session per day. Rats received one day each of continuous, random interval (RI)-15 s, and RI-30 s schedules of reinforcement, followed by 5 days of instrumental training on the final RI-60s schedule. The last 2 of these instrumental training sessions were conducted in the voltammetry-equipped operant chamber. For these training sessions, rats were tethered to the voltammetric recording unit for acclimation to the testing environment, but no recordings were made. The CSs were at no point present during instrumental training.
Retraining
Following instrumental training rats were given an additional 2 days of Pavlovian training just as described above, but tethered in the voltammetry-equipped operant chamber. Rats were also given 2 days of instrumental retraining followed by 2 days of Pavlovian retraining in between each of the PIT test sessions described below. No recordings were made during these sessions.
Pavlovian-to-instrumental transfer tests and voltammetry data acquisition
Testing commenced between 30–36 days post-surgery. FSCV was used to measure dopamine concentration changes in the NAc core, as described previously (Wassum et al. 2013), during the PIT tests described below. For each session rats were placed in the operant chamber and tethered to the voltammetric recording unit through an electrical swivel. A custom-made voltammetric potentiostat was used to apply a triangular waveform to the carbon-fiber microelectrode through a head-mounted voltammetric amplifier, as described previously (Clark et al. 2010). The applied potential was held at −0.4 V (vs. the Ag/AgCl reference) and then ramped to +1.3 V and back to −0.4 V at 400 V/s, repeating every 100 ms for a sample rate of 10 Hz. Dopamine is oxidized to dopamine-o-quinone at approximately +0.64 V on this waveform, which is then reduced back to dopamine at −0.2 V. Background-subtraction elucidates these oxidative and reductive peaks providing the dopamine cyclic voltammogram (CV) signature for dopamine detection, described previously (Phillips et al. 2003). Waveform generation and resultant data acquisition were carried out using 2 PCI multi-function data acquisition cards and custom software written in LabVIEW (National Instruments, Austin, TX). After stabilization of the baseline current (~20 min) the behavioral session commenced with the onset of the house light and insertion of the lever.
During each PIT test the lever was continuously available, but pressing was not reinforced. Responding was extinguished for 5 min to establish a low rate of baseline performance, after which each CS was presented 4 times in pseudo random order. No rewards were delivered during CS presentation. Each CS lasted 2 min and CS presentations were separated by a 4-min, fixed inter-CS interval. The 2-min period prior to each CS served as the control, ‘PreCS’ period. Lever pressing and entries into the food-delivery port were monitored throughout the test. Each session was video recorded and locomotor activity was analyzed by video-tracking software (TopScan Clever Sys., Inc., Reston, VA). Rats were tested in a state of hunger (18 hr food deprived/0 hr water deprived), satiety (3 hr food deprived/0 hr water deprived) and thirst (3 hr food deprived/18 hr water deprived). Each rat received two hungry tests and one each of the sated and thirsty tests, with intervening retraining, as described above. Test order was counterbalanced across subjects. Data were averaged across the two hungry tests because neither the behavioral results (no main effect of Hungry test 1 or 2: F1,7=1.15, p=0.32, or Test by CS period interaction: F2,14=0.73, p=0.50 on lever pressing; no main effect of Hungry test: F1,7=0.07, p=0.80, or Test by CS period interaction: F2,14=0.37, p=0.70 on food-port entries), nor cue-evoked dopamine concentration change (no main effect of Hungry test: F1,7=0.09, p=0.77, or Test by CS interaction: F2,14=0.15, p=0.86) differed between these tests.
Rats gained on average 9.97% (SEM=2.22) of their body weight as a result of the satiety treatment, which was significantly more than the negligible weight change when tested hungry (−0.44%, SEM=0.28; t8=4.39, p=0.003). In the state of thirst rats gained on average 5.61% (SEM=1.54) of their body weight, significantly more than the hungry condition (t8=3.58, p=0.009), but less than the sated condition (t8=2.65, p=0.03). In a separate group of subjects (n=7), we found that rats consumed significantly less food when the satiety treatment was accompanied by water deprivation (average food consumption in the satiety condition: 31.43 g/SEM=0.90, satiety + water deprivation: 25.14 g/SEM=0.63; t7=6.49, p=0.0006). The state of thirst was, therefore, confounded by a slight state of hunger beyond that of the satiety condition. Data from this test are presented in Supplemental Figure 1.
Histological verification of recording sites
At the conclusion of each experiment rats were deeply anesthetized with Pentasol (100 mg/kg i.p.) and the recording site was marked by making a small electrolytic lesion at the electrode tip by passing a current (~70 µA) through the carbon fiber microelectrode for 20 s. Rats were then trans-cardially perfused with 0.9% saline followed by 10% formalin in saline. The brains were removed and post-fixed in paraformaldehyde, then cryosectioned into 50 µm slices, mounted onto slides and stained with cresyl violet. Light microscopy was used to examine electrode placement in the NAc core. Histological data are presented in Figure 1A.
Sucrose and grape-flavored polycose discriminability tests
Due to the overlap in features (i.e., carbohydrate-based solution) between the instrumental reward, grape-flavored 20% polycose, and the sucrose solution reward, used to condition the CSSuc during Pavlovian conditioning, in a separate group of subjects (n=6) we conducted two discrimination tests to ensure these two foods were discriminable when both sated and hungry. In the first, we assessed the influence of sensory-specific satiety devaluation of each reward on consumption of the alternate reward. If the rewards are discriminable, then pre-feeding, which should dramatically reduce consumption of the specific pre-fed reward, should not influence consumption of the alternate reward. Consumption following pre-feeding of home chow served as a general satiety control for these experiments. On 3 separate test days rats were given 1-hr access to either home chow, 20% sucrose solution, or grape-flavored 20% polycose. Immediately following this rats were given brief, 15-min individual access to sucrose solution and grape polycose, separated by 15 min, in counterbalanced order. In both cases, pre-feeding on either the sucrose or grape-flavored polycose caused a selective reduction in consumption of that specific reward, relative to pre-feeding on the control home chow food, but did not alter consumption of the alternate reward. See Supplemental Figure 2A.
To ensure sucrose and grape-flavored polycose were discriminable when hungry, in this same separate group of subjects we conducted a second discriminability test, in which either sucrose or grape polycose was devalued by conditioned taste aversion. Taste aversion treatment consisted of 3 days of 30-min access to sucrose (for half the subjects) or grape polycose (for the other half) followed by injection of LiCl (0.15M LiCl, 20 mL/kg, i.p.). Rats were given a consumption test one day prior to the taste aversion treatment, to establish a baseline, and then again on the day after the last taste aversion conditioning session. These tests were conducted 18 hr food deprived and consisted of 15-min individual access to sucrose solution and grape polycose, in counterbalanced order. If the rewards are discriminable, then conditioning an aversion to one should not reduce consumption of the other. The data support the discriminability of the two food rewards; the conditioned taste aversion devaluation resulted in a selective attenuation of consumption of only the devalued food. See Supplemental Figure 2B. Together these data add support the discriminability of sucrose and grape-flavored polycose, both when sated and when hungry, and demonstrate the unique sensory-specific identifying features of these two foods.
Data analysis
Statistical Analysis
Electrochemical data were analyzed using software written in LabVIEW (National Instruments). All data were processed with Microsoft Excel (Redmond, WA) and MATLAB (MathWorks, Inc., Natick, MA). Statistical analyses were conducted with GraphPad Prism (La Jolla, CA) and SPSS (IBM Corp, Chicago, IL). For all hypothesis tests, the α level for significance was set to p<0.05. Data were analyzed with one- and two-way ANOVAs (Geisser-Greenhouse correction), paired t-tests, correlation and regression where appropriate. Bonferoni post-hoc analyses correcting for multiple comparisons were used to clarify all main effects and interactions.
Behavioral Analysis
Lever pressing and entries into the food-delivery port were the primary behavioral output measures. During the PIT tests these measures were counted and averaged across trials for each 2-min period (PreCS, CSSuc, CSPel), with PreCS behavioral output serving as the control for CS-induced changes in behavior. Locomotor activity was also evaluated for each hungry test session with the exception of one test session for two rats, in which tether interference prevented video tracking. For these subjects only the locomotor activity from one hungry test was included. The average locomotor activity during the 1-min periods prior to CS onset served as the baseline for this measure.
Voltammetric Analysis
Electrochemical data were analyzed as described previously (Wassum et al. 2013). Principal component regression (PCR), a chemometric technique that combines principal component analysis with inverse least-squares regression (Heien et al. 2005, Keithley & Wightman 2011), was used to isolate changes in current due to dopamine and pH from the cyclic voltammetric data. We used a standard training set of dopamine, pH and drift CVs as has been described previously (Clark et al. 2010, Wanat et al. 2010, Flagel et al. 2011, Nasrallah et al. 2011, Willuhn et al. 2012, Wassum et al. 2013, Ostlund et al. 2014). This procedure allowed us to distinguish changes in current due to dopamine release from changes due to pH, or to other electroactive substances (Keithley & Wightman 2011, Heien et al. 2005). After this analysis, all data were converted to estimated dopamine concentration via an electrode-specific, in vitro pre-test calibration factor.
To evaluate the dopamine responses to CS presentation we isolated voltammetric data starting 10 s before the onset of the CS and ending with CS offset (120 s). For each trial, voltammetry data were normalized by subtracting the average background current measured during the 1-s baseline period, 10 s prior to CS onset. These data were analyzed in three different ways. First, maximal (i.e., peak) dopamine concentration change during the first 10 s following CS onset provided a quantification of the CS onset-induced dopamine response. Second, the average of the background-subtracted dopamine concentration change during the entire 2-min CS period served as a measure of more prolonged CS-induced dopamine concentration changes. For both of these measures, dopamine concentration change estimates were averaged across trials of the same type for each rat. Third, we identified and quantified transient fluctuations in the dopamine concentration v. time trace, i.e., transient dopamine release events, using Mini Analysis software (Synaptosoft, Decautur, GA), as described previously (Ostlund et al. 2014, Wassum et al. 2013). Increases in concentration that exceeded 2.5× the root mean square noise of concentration v. time trace sampled from the pre-CS period were identified as dopamine transients. Total dopamine transients per trial were counted and averaged across trials of the same type for each rat. Peak amplitude of each transient was calculated as the difference between a peak concentration and the local minimum occurring 0.5–3 s before that peak. Peak amplitudes were averaged across transients for each trial, then averaged across trials of the same type for each rat. Identical analyses were conducted on the PreCS dopamine concentration v. time traces to provide a control comparator.
RESULTS
Pavlovian-to-instrumental transfer task and behavioral performance
This experiment was conducted in three phases. The first two training phases (see Figure 1b-left) were conducted in a state of hunger, 18 hr food deprived, to ensure food rewards were motivationally relevant. In Phase 1, Pavlovian training was used to pair each of two distinct auditory stimuli with delivery of one of two unique, but relatively equally valued, food rewards, either sucrose solution (CSSuc) or food pellets (CSPel). By the end of Pavlovian training all rats entered the food-delivery port significantly more during the CS probe period (at the CS onset before reward delivery) than during the control PreCS period for both the CSSuc (average entry rate increase: 15.61, SEM=2.41; t8 = 6.49, p = 0.0003) and CSPel (average entry rate increase: 7.29, SEM=1.94; t8 =3.75, p = 0.007). In Phase 2, rats were conditioned, in the absence of the Pavlovian cues, to earn a third unique food reward, grape-flavored 20% polycose, by pressing on a lever. At the end of training rats pressed at an average rate of 7.60 presses/min (SEM= 1.42). This training procedure established 2 Pavlovian cues that predicted 2 distinct food rewards and an independent instrumental action that earned a 3rd unique food reward.
In the critical test phase, rats were given a PIT test (Figure 1b- right), in which the lever was available, though pressing was not reinforced, and each CS was presented, also without accompanying reward, in pseudo random order, to assess the motivating influence of each CS over lever-pressing activity. We also assessed CS-induced changes in the rate of entry into the food-delivery port as a measure of Pavlovian conditioned responding and general locomotor activity. FSCV was used to monitor dopamine concentration changes in the NAc core. To assess the influence of need state on both PIT and CS-induced dopamine responses, rats were tested both in a state of hunger (18 hr food deprived), in which all food rewards were motivationally relevant, and in a state of relative satiety (3 hr food deprived), in which the food rewards had much lower motivational value.
As is clear from Figure 1C, in the hungry state presentation of the CSSuc, but not CSPel induced a significant PIT effect (i.e., invigoration of lever pressing). When tested sated, lever pressing was attenuated and neither CS was capable of invigorating lever pressing. There were significant main effects of both CS period (PreCS, CSSuc, CSPel; F2,14=6.29, p=0.01) and of Need state (Hunger, Satiety; F1,7=14.59, p=0.007) on lever pressing, as well as a significant interaction between these factors (F2,14=12.99, p=0.0006). When hungry, the CSSuc significantly elevated lever pressing above PreCS control levels (p<0.05), while, conversely, presentation of the CSPel significantly decreased lever pressing (p<0.0001). When sated, lever pressing during both the PreCS (p<0.05) and CSSuc (p<0.05) periods was significantly lower than that during the hungry test.
Both the CSSuc and CSPel were found to elevate food-port approach responding, and this was attenuated by satiety (Figure 1D). There were significant main effects of both CS period (F2,14=18.37, p=0.0001) and of Need state (F1,7=30.72, p=0.009) on food-port entries, as well as a significant interaction between these factors (F2,14=13.50, p=0.0005). Both the CSSuc (p<0.0001) and CSPel (p<0.001) significantly elevated food-port entries above PreCS control levels when hungry. When sated, food-port entries were significantly lower compared to the hungry test for all CS periods (p<0.0001, in all cases) and neither CS significantly elevated food-port approach (p>0.05, in both cases).
These data show that presentation of the CSSuc induced a robust PIT effect, invigorating the performance of an independently-trained instrumental action that, in training, earned a different food reward. Because sucrose solution and grape-flavored polycose (instrumental outcome) were shown to be discriminable based upon their unique sensory-specific features (e.g., taste; see Supplemental Figure 2), this PIT effect demonstrates the more general motivating influence of the cue over instrumental action. A PIT effect was not observed upon presentation of the CSPel, despite the fact that this cue was an effective CS, as demonstrated by robust conditioned food-port approach responding at the end of training and at test. This behavioral result was replicated in a larger cohort of subjects (Supplemental Figure 3). During both CSs, however, locomotor activity was significantly elevated above baseline levels (distanced traveled: PreCS: 280.5 mm/min SEM=49.62, CSSuc: 390.0 mm/min SEM=61.77, CSPel: 356.0 mm/min SEM=74.60; main effect of CS period: F2,14=7.21, p=0.008, post hoc p<0.05 relative to PreCS, in both cases). Therefore, although PIT was not expressed during presentation of the CSPel, in the state of hunger this cue was equally as capable of eliciting general behavioral excitation as the CSSuc, supporting their roughly equal conditioned motivational value (Bindra 1968).
We took advantage of this situation to examine potentially disparate cue-evoked dopamine responses in a situation in which the general motivational value of the cue was expressed (CSSuc) versus when it was not expressed (CSPel) in instrumental lever-pressing activity. We also evaluated the influence of a downshift in each CSs motivational value, induced by satiety, on CS-evoked dopamine responses. We reasoned that if NAc core dopamine release encodes the motivational value of a CS (and its associated reward), then both the CSSuc and CSPel, which both have similar motivational value via their association with food reward, should induce an elevation in dopamine release that would be attenuated by satiety. If, however, dopamine concentration elevations are merely a secondary consequence of the elevation in instrumental reward-seeking activity brought about by CS presentation, then we would expect dopamine to respond to the CSSuc, but not CSPel. A third possibility, is that cue-evoked dopamine encodes the motivational value of a CS, but, unlike the behavioral expression of PIT (Corbit et al. 2007, Balleine 1994), requires an opportunity to acquire a new value through relevant state experience (i.e., incentive learning (Dickinson & Balleine 1994) or re-tasting the predicted food reward (Dayan & Berridge 2014)). In this case, we would not expect CS-induced dopamine released to be attenuated in the novel sated state, because rats were never provided an opportunity to experience any of the food rewards when sated.
Nucleus accumbens core dopamine signaling during Pavlovian-to-instrumental transfer
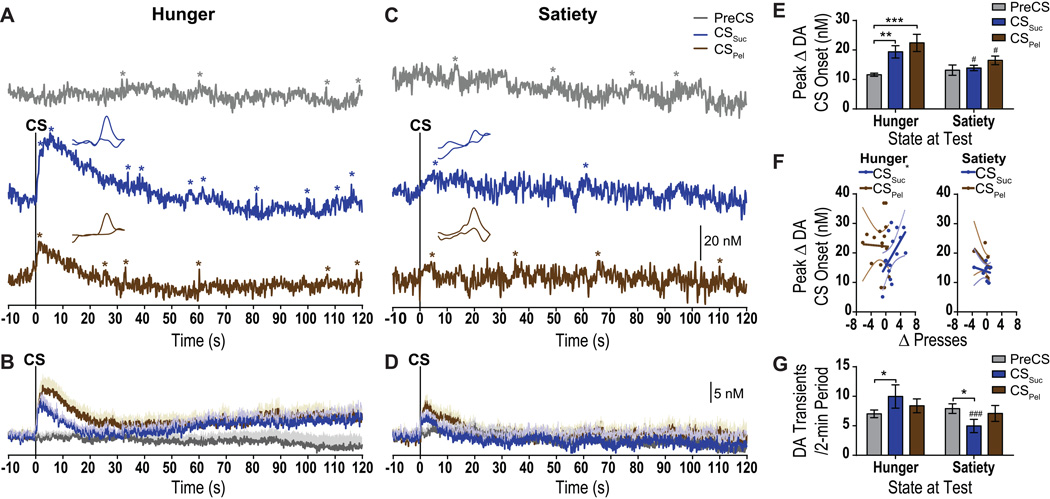
As can be seen in the representative example data in Figure 2A and in the group-averaged data shown in Figure 2B, when rats were tested hungry, the onset of both the CSSuc and CSPel induced a robust elevation in NAc core dopamine concentration. This elevation was markedly attenuated while sated (representative data: Figure 2C; group average: Figure 2D). Indeed, statistical analyses detected a significant main effect of CS period (F2,14=10.58, p=0.002) on peak dopamine concentration change at CS onset, no significant effect of Need state (F1,7=3.30, p=0.11), but a significant interaction between these factors (F2,14=4.09, p=0.04; Figure 2E). Post-hoc analyses (see Figure 2E) further confirmed the observations described above.
Figure 2. Cue-induced dopamine concentration changes in the nucleus accumbens core during Pavlovian-to-instrumental transfer.
A & C. Representative, single-trial dopamine concentration v. time traces during the hungry (A) and sated (C) PIT test from the same rat 10 s before and during the entire 2-min PreCS period (gray) and presentation of the CSSuc (blue) and CSPel (brown). Asterisks mark fluctuations in dopamine that reached threshold for designation as dopamine transients. Scale bar to the lower right represents 20 nM dopamine concentration change. Insets show background-subtracted CVs showing oxidation and reduction peaks that identify the detected electrochemical signal as dopamine, taken from within the first 10 s following CS onset. B. & D. Group-averaged dopamine concentration change during the CSSuc, CSPel, and control PreCS period. Shading reflects +1 between-subject SEM. Scale bar represents 5 nM dopamine concentration change. E. Peak dopamine concentration change in the 10-s period following CS onset. F. Correlation between peak dopamine concentration change at CS onset and the average CS-induced change in lever pressing for the hungry (left) and sated (right) PIT test. Regression line with 95% confidence bands are shown. G. Average number of dopamine transients per 2-min period. Error bars indicate ±1 SEM. *p<0.05, **p<0.01, ***p<0.001 relative to PreCS control period. #p<0.05, ###p<0.001 relative to same period during hungry test.
We also examined the average dopamine concentration change during the entire 2-min duration of the CS presentation and found evidence of only modest elevations in dopamine concentration through each 2-min CS when hungry (see Table 1). We did, however, detect an elevation in the frequency of dopamine release events (i.e., dopamine transients) during CS presentation, especially during the CSSuc (Figure 2G). There was no significant main effect of CS period (F2,14=0.11, p=0.89), or Need state (F1,7=1.07, p=0.33) on the frequency of dopamine transients during each 2-min period, but there was a significant interaction between these factors (F2,14=9.85, p=0.002). Dopamine transient frequency was elevated by the CSSuc (p<0.05), but not significantly by the CSPel (p>0.05) relative to the PreCS period when tested hungry. When tested sated the frequency of dopamine transient was actually lower during the CSSuc relative to the PreCS period (p<0.05) and relative to the CSSuc period during the hungry test (p<0.01). Although the frequency of detected dopamine release events was modified by both CS and need state, neither of these factors influenced the average amplitude of dopamine release events (see Table 2).
Table 1. Average dopamine concentration change during 2-min CS presentation.
Average NAc core dopamine concentration change during the entire 2-min PreCS, CSSuc and CSPel period, relative to the 1-s baseline sampled 10 s prior to CS onset, averaged across trials of the same type, and then across rats (top value). Between-subject SEM is shown below in italics. ANOVA on these data shows no significant main effect of CS period (F2,14=2.47, p=0.12), a marginally insignificant main effect of Need state (F1,7=4.95, p=0.06), with no interaction between these factors (F2,14=1.34, p=0.29).
| Average Δ DA during 2-min Period (nM) | |||
|---|---|---|---|
| PreCS | CSSuc | CSPel | |
| Hunger | −1.33 | 2.57 | 4.37 |
| 1.67 | 1.20 | 1.64 | |
| Satiety | −1.08 | −1.20 | 0.93 |
| 1.73 | 1.97 | 1.07 | |
| Thirst | −2.46 | −0.08 | 1.42 |
| 1.61 | 1.73 | 1.15 | |
Table 2. Average dopamine transient amplitude.
The peak amplitude of each dopamine transient was averaged across all transients within a trial, then averaged across trials and then across rats for the PreCS, CSSuc and CSPel periods (top value). Between-subject SEM is shown below in italics. ANOVA on these data shows neither a significant main effect of CS period (F2,14=0.82, p=0.46), nor of Need state (F1,7=0.69, p=0.43), with no significant between these factors (F2,14=0.96, p=0.41).
| Average DA Transient Amplitude (nM) | |||
|---|---|---|---|
| PreCS | CSSuc | CSPel | |
| Hunger | 15.39 | 15.80 | 14.90 |
| 1.16 | 0.77 | 0.99 | |
| Satiety | 14.66 | 14.89 | 14.71 |
| 1.08 | 1.17 | 1.50 | |
| Thirst | 15.66 | 16.44 | 15.26 |
| 0.93 | 1.31 | 1.30 | |
Interestingly, when tested hungry, rats for which the CSSuc induced a larger elevation in dopamine it also induced greater invigoration of lever-pressing activity. The average amplitude of the CS onset dopamine response positively correlated, between-subjects, with the CS-induced invigoration of lever pressing (i.e., the PIT effect) for the CSSuc (r16=0.53, p=0.04), but not for the CSPel (r16= −0.03, p=0.92; Figure 2F-left). This correlation between CS-evoked dopamine and behavioral responding was selective to PIT; CS-induced dopamine elevation did not significantly correlate with conditioned food-port approach responding (Hungry: CSSuc: r16=0.13, p=0.62; CSPel: r16=0.10, p=0.72; Sated: CSSuc: r8= −0.22, p=0.60; CSPel: r8= −0.41, p=0.31). This correlation was also absent when the PIT effect was abolished by satiety (CSSuc: r8= −0.23, p=0.59; CSPel: r8= −0.51, p=0.19; Figure 2F-right).
DISCUSSION
During appetitive Pavlovian conditioning, a mental association is formed between reward-predictive stimulus and the unconditioned appetitive event (e.g., food item) with which it is associated (Fanselow & Wassum 2015, Berridge 2001, Rescorla & Solomon 1967, Dickinson & Balleine 2002). Subsequently, by recalling the more general (i.e., specific identity-independent) motivational features of the paired reward (e.g., nutritive or fluidic properties, not specific taste), a reward cue can motivate a broad range of instrumental actions (though typically those earning a categorically similar reward) (Corbit & Balleine 2005, Balleine 1994). This process is highly sensitive to need state; a reward cue will only exert this motivational effect if it signals an item that is currently needed (i.e., nutrients when hungry) (Corbit & Balleine 2005, Balleine 1994). Here we found evidence that food cue-evoked dopamine signals in the NAc core are modulated by need state to track the motivational value of a food-predictive stimulus.
Using a novel PIT task, we found evidence for the expression of PIT by a sucrose-predictive cue. The invigoration of instrumental activity induced by the sucrose-predictive cue could be interpreted to reflect primarily the general (rather than specific) form PIT, because the stimulus invigorated responding on an action that, in training, earned a distinct, grape-flavored polycose reward (Dickinson & Balleine 2002, Cartoni et al. 2013, Fanselow & Wassum 2015, Balleine 1994, Dickinson & Balleine 1994). Indeed, sucrose and polycose work through separate taste channels in the rat (Sclafani 1991, Ackroff et al. 1993) and were confirmed here to be discriminable. Interestingly however, in the very same subjects, PIT was not expressed upon presentation of a grain food pellet cue. Rather, the pellet cue actually decreased instrumental responding when rats were hungry. This contrasts to previous demonstrations of general PIT, in which cue- and instrumental-reward associations were mixed across subjects (Corbit & Balleine 2005), or in which only one CS was conditioned per subject (Balleine 1994). The lack of PIT was not due to ineffective Pavlovian conditioning for the pellet cue, because rats readily learned to approach the food-delivery port upon presentation of this CS. It is also unlikely to be due to the CSPel not acquiring motivational value, because it was capable of exciting general locomotor activity. We think this resulted from conditioning the CSs in the same operant chamber. This allowed the chamber context to become predictive of both the sucrose and grain pellet rewards, which may have permitted each cue to not only share an excitatory relationship with its paired reward, but also to acquire conditioned inhibitor properties for at least some general features of the alternate reward. The CSPel could have, therefore, both predicted pellets and the absence of a solution reward and, as a result, inhibited the performance of actions that earned a solution reward in the PIT test. Evidence for the use of such counterfactual associations in PIT has recently been demonstrated (Laurent & Balleine 2015), and both general PIT and non-selective PIT have been demonstrated when a pellet cue is conditioned identically, but in a context that has never been paired with another reward or CS (Wassum et al. 2013, Balleine 1994). Alternatively, it is possible that rats made an online comparison during the test between the food rewards predicted by each CS and elected to respond only during the CS predicting the reward with more similar features to the anticipated instrumental outcome. Because there was no response competition during the test (i.e., rats had only one available instrumental action), this possibility seems less likely, though it remains possible that the PIT induced by the CSSuc relied on the greater overlap in features (i.e., carbohydrate + solution) between the sucrose and the instrumental grape-flavored polycose reward, than between the grain pellets and polycose. Consistent with previous reports (Corbit et al. 2007), the PIT effect observed here to the sucrose-predictive cue was attenuated when the sucrose’s motivational value was decreased by satiety, even though rats never experienced sucrose in this sated state.
In this task, both cues evoked a robust NAc core dopamine response, suggesting this signaling tracked the motivational value of the cues and was not merely a secondary consequence of the elevated instrumental responding induced by the cue. Such dissociation between cue-evoked dopamine release and behavioral output has been demonstrated previously, in that particular case when behavior was guided by consideration of effortful response cost (Hollon et al. 2014). When PIT was expressed, the amplitude of the CS-evoked dopamine response correlated with invigoration of instrumental activity, consistent with previous reports (Wassum et al. 2013, Ostlund et al. 2014) and findings of a similar relationship in the absence of explicit reward-predictive cues (Wassum et al. 2012). Supporting this correlational relationship, NAc core dopamine receptor activation has been shown to be both sufficient to enhance (Wyvell & Berridge 2000, Peciña & Berridge 2013) and necessary for (Lex & Hauber 2008) PIT. Moreover, both the NAc core (Corbit & Balleine 2011) and dopamine receptor activation (Ostlund & Maidment 2012, Wassum et al. 2011, Dickinson et al. 2000) has been suggested to be necessary for the general component of PIT. If NAc core dopamine is driving PIT, then it is somewhat paradoxical that it responded to the presentation of the CSPel, which did not induce a PIT effect. This suggests that downstream mechanisms, perhaps even within the NAc, may, therefore, inhibit or otherwise combine with the influence of the pellet cue-evoked dopamine signal to determine the ultimate impact on behavioral output.
Although both food-predictive cues elicited dopamine release and conditioned food-port approach responding, these variables were not correlated, suggesting that dopamine specifically related to cue-induced invigoration of instrumental activity. This is consistent with findings that reward-paired cues do not robustly elicit NAc dopamine release in rats for which the predominate conditioned response is goal approach (Flagel et al. 2011), and with findings that blockade of NAc core dopamine receptors is without effect on conditioned goal-approach responding (Saunders & Robinson 2012). NAc core dopamine receptor activation is, however, required (Saunders & Robinson 2012) and dopamine release robustly elicited (Flagel et al. 2011) when reward-paired cues are imbued with motivational value that allows a cue-approach (i.e., sign-tracking) conditioned response to predominate. Evidence across multiple behavioral and technical platforms is, therefore, converging to support the interpretation that dopamine release in the NAc core is related to the motivational value of reward-paired cues that allows them to both invigorate ongoing instrumental action and to act themselves as targets for approach (when they are visual and localizable (Cleland & Davey 1983)).
Also supporting with this interpretation when the motivational value of the food cues was diminished by a state of satiety, so too was cue-evoked dopamine response. This finding is seemingly inconsistent with recent evidence that NAc shell, but not NAc core cue-evoked dopamine release is attenuated by within-session satiety (Saddoris et al. 2015), though the amount of food consumed by rats in this study was only a fraction of that consumed during the satiety treatment here. These findings do, however, accord well with those showing that dopamine neuron burst firing (Branch et al. 2013) and food-evoked NAc dopamine release (Cone et al. 2014) are attenuated by satiety and findings that NAc phasic dopamine responses are greater to cues predictive of a nutritive versus non-nutritive palatable reward (McCutcheon et al. 2012, Beeler et al. 2012). Satiety has also been shown to attenuate dopamine efflux measured with microdialysis in food-rewarded tasks (Ahn & Phillips 1999, Ostlund et al. 2011). The activity of the hunger hormone ghrelin and orexin modulates food- and food-cue NAc dopamine responses (Cone et al. 2014, Cone et al. 2015, McCutcheon 2015), providing a potential mechanism for the need-based modulation of cue-evoked dopamine signaling detected here. It is interesting that the cue-evoked dopamine signaling was modified by satiety without the requirement to re-experience (i.e., re-taste) the predicted foods in the novel sated state. This is consistent with findings that dopamine receptor activation is not required for either positive (Wassum et al. 2011, Dickinson et al. 2000) or negative (Lex & Hauber 2010) incentive learning, both of which require such re-tasting.
The data here suggest that cue-evoked NAc core responses are sensitive to current need state, one critical variable that determines the current adaptive utility of cue-motivated behavior. Previous research suggests that cue-evoked dopamine signaling also encodes additional (though likely not all (Gan et al. 2009, Hollon et al. 2014, Wanat et al. 2010)) contributing variables, including expected reward magnitude (Gan et al. 2009, Day et al. 2010). These data combine to suggest that NAc core dopamine signaling is an important substrate for allows cues to motivate behavior, but only when this is adaptive. Because this motivational influence can be disrupted in the addicted state in both non-human, drug-exposure models (LeBlanc et al. 2013, Leblanc et al. 2013, Wyvell & Berridge 2001, Taylor & Jentsch 2001, Glasner et al. 2005, Ostlund et al. 2014) and in human addicts (Garbusow et al. 2014, Martinovic et al. 2014, Hogarth et al. 2014), these results have implications for the understanding and treatment of addiction and other disorders of behavioral control.
Supplementary Material
Acknowledgments
This research was supported by a Hellman Foundation Fellowship, a UCLA Faculty Career Development award and grant DA035443 from NIDA, to KMW. The authors would like to thank Dr. Scott Ng-Evans for his expert hardware and software assistance and Dr. Jesse Cushman and the UCLA Psychology Behavioral testing core for expert technical assistance and resources.
Abbreviations
- PIT
Pavlovian-instrumental transfer
- CS
conditioned stimulus
- CSSuc
sucrose-predictive cue
- CSPel
food pellet-predictive cue
- PreCS
control CS-free period
- FSCV
fast-scan cyclic voltammetry
- NAc
nucleus accumbens core
Footnotes
CONFLICT OF INTEREST STATEMENT:
All authors report no financial interests or other potential conflicts of interest.
REFERENCES
- Ackroff K, Manza L, Sclafani A. The rat's preference for sucrose, polycose and their mixtures. Appetite. 1993;21:69–80. doi: 10.1006/appe.1993.1037. [DOI] [PubMed] [Google Scholar]
- Ahn S, Phillips AG. Dopaminergic correlates of sensory-specific satiety in the medial prefrontal cortex and nucleus accumbens of the rat. J Neurosci. 1999;19:RC29. doi: 10.1523/JNEUROSCI.19-19-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine B. Asymmetrical interactions between thirst and hunger in Pavlovian-instrumental transfer. Q J Exp Psychol B. 1994;47:211–231. [PubMed] [Google Scholar]
- Beeler JA, McCutcheon JE, Cao ZF, Murakami M, Alexander E, Roitman MF, Zhuang X. Taste uncoupled from nutrition fails to sustain the reinforcing properties of food. Eur J Neurosci. 2012;36:2533–2546. doi: 10.1111/j.1460-9568.2012.08167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Reward Learning: reinforcement, incentives and expectations. In: Medin DL, editor. The Psychology of Learning and Motivation. Vol. 40. Academic Press; 2001. pp. 223–278. [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Bindra D. Neuropsychological interpretation of the effects of drive and incentive-motivation on general activity and instrumental behavior. Psychol. Rev. 1968;75:1–22. [Google Scholar]
- Branch SY, Goertz RB, Sharpe AL, Pierce J, Roy S, Ko D, Paladini CA, Beckstead MJ. Food restriction increases glutamate receptor-mediated burst firing of dopamine neurons. J Neurosci. 2013;33:13861–13872. doi: 10.1523/JNEUROSCI.5099-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartoni E, Puglisi-Allegra S, Baldassarre G. The three principles of action: a Pavlovian-instrumental transfer hypothesis. Front Behav Neurosci. 2013;7:153. doi: 10.3389/fnbeh.2013.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JJ, Collins AL, Sanford CA, Phillips PE. Dopamine encoding of Pavlovian incentive stimuli diminishes with extended training. J Neurosci. 2013;33:3526–3532. doi: 10.1523/JNEUROSCI.5119-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JJ, Sandberg SG, Wanat MJ, et al. Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nat Methods. 2010;7:126–129. doi: 10.1038/nmeth.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland GG, Davey GC. Autoshaping in the rat: The effects of localizable visual and auditory signals for food. J Exp Anal Behav. 1983;40:47–56. doi: 10.1901/jeab.1983.40-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwill RM, Motzkin DK. Encoding of the unconditioned stimulus in Pavlovian conditioning. Animal Learning & Behavior. 1994;22:384–394. [Google Scholar]
- Cone JJ, McCutcheon JE, Roitman MF. Ghrelin acts as an interface between physiological state and phasic dopamine signaling. J Neurosci. 2014;34:4905–4913. doi: 10.1523/JNEUROSCI.4404-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone JJ, Roitman JD, Roitman MF. Ghrelin regulates phasic dopamine and nucleus accumbens signaling evoked by food-predictive stimuli. J Neurochem. 2015 doi: 10.1111/jnc.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. J Neurosci. 2005;25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. The general and outcome-specific forms of Pavlovian-instrumental transfer are differentially mediated by the nucleus accumbens core and shell. J Neurosci. 2011;31:11786–11794. doi: 10.1523/JNEUROSCI.2711-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Janak PH, Balleine BW. General and outcome-specific forms of Pavlovian-instrumental transfer: the effect of shifts in motivational state and inactivation of the ventral tegmental area. Eur J Neurosci. 2007;26:3141–3149. doi: 10.1111/j.1460-9568.2007.05934.x. [DOI] [PubMed] [Google Scholar]
- Day JJ, Jones JL, Wightman RM, Carelli RM. Phasic nucleus accumbens dopamine release encodes effort- and delay-related costs. Biol Psychiatry. 2010;68:306–309. doi: 10.1016/j.biopsych.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Dayan P, Berridge KC. Model-based and model-free Pavlovian reward learning: Revaluation, revision, and revelation. Cogn Affect Behav Neurosci. 2014 doi: 10.3758/s13415-014-0277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A, Balleine BW. Motivational control over goal-directed action. Animal Learning and Behavior. 1994;22:1–18. [Google Scholar]
- Dickinson A, Balleine BW. The role of learning in the operation of motivational systems. In: Gallistel CR, editor. Learning, Motivation and Emotion, Volume 3 of Steven's Handbook of Experimental Psychology. Vol. 3. New York: John Wiley & Sons; 2002. pp. 497–533. [Google Scholar]
- Dickinson A, Dawson GR. Pavlovian processes in the motivational control of instrumental performance. The Quarterly Journal of Experimental Psychology Section B: Comparative and Physiological Psychology. 1987;39:201–213. [PubMed] [Google Scholar]
- Dickinson A, Smith J, Mirenowicz J. Dissociation of Pavlovian and instrumental incentive learning under dopamine antagonists. Behav Neurosci. 2000;114:468–483. doi: 10.1037//0735-7044.114.3.468. [DOI] [PubMed] [Google Scholar]
- Estes W. Discriminative conditioning. II. Effects of a Pavlovian conditioned stimulus upon a subsequently established operant response. J. Exp. Psychol. 1948;38:173–177. doi: 10.1037/h0057525. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann N Y Acad Sci. 1999;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Wassum KM. The Origins and Organization of Vertebrate Pavlovian Conditioning. Cold Spring Harb Perspect Biol. 2015 doi: 10.1101/cshperspect.a021717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan JO, Walton ME, Phillips PE. Dissociable cost and benefit encoding of future rewards by mesolimbic dopamine. Nat Neurosci. 2009;13:25–27. doi: 10.1038/nn.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbusow M, Schad DJ, Sommer C, et al. Pavlovian-to-instrumental transfer in alcohol dependence: a pilot study. Neuropsychobiology. 2014;70:111–121. doi: 10.1159/000363507. [DOI] [PubMed] [Google Scholar]
- Glasner SV, Overmier JB, Balleine BW. The role of Pavlovian cues in alcohol seeking in dependent and nondependent rats. J Stud Alcohol. 2005;66:53–61. doi: 10.15288/jsa.2005.66.53. [DOI] [PubMed] [Google Scholar]
- Hart AS, Clark JJ, Phillips PE. Dynamic shaping of dopamine signals during probabilistic Pavlovian conditioning. Neurobiol Learn Mem. 2015;117:84–92. doi: 10.1016/j.nlm.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heien ML, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Wassum KM, Wightman RM. Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc Natl Acad Sci U S A. 2005;102:10023–10028. doi: 10.1073/pnas.0504657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth L, Maynard OM, Munafò MR. Plain cigarette packs do not exert Pavlovian to instrumental transfer of control over tobacco-seeking. Addiction. 2014;110:174–182. doi: 10.1111/add.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollon NG, Arnold MM, Gan JO, Walton ME, Phillips PE. Dopamine-associated cached values are not sufficient as the basis for action selection. Proc Natl Acad Sci U S A. 2014;111:18357–18362. doi: 10.1073/pnas.1419770111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AW. Eating beyond metabolic need: how environmental cues influence feeding behavior. Trends Neurosci. 2013;36:101–109. doi: 10.1016/j.tins.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Keithley RB, Wightman RM. Assessing principal component regression prediction of neurochemicals detected with fast-scan cyclic voltammetry. ACS Chem Neurosci. 2011;2:514–525. doi: 10.1021/cn200035u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011;69:664–679. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse H, Overmier J, Konz W, Rokke E. Pavlovian conditioned stimulus effects upon instrumental choice behavior are reinforcer specific. Learn Motiv. 1983;14:165–181. [Google Scholar]
- Laurent V, Balleine BW. Factual and Counterfactual Action-Outcome Mappings Control Choice between Goal-Directed Actions in Rats. Curr Biol. 2015;25:1074–1079. doi: 10.1016/j.cub.2015.02.044. [DOI] [PubMed] [Google Scholar]
- Leblanc KH, Maidment NT, Ostlund SB. Impact of repeated intravenous cocaine administration on incentive motivation depends on mode of drug delivery. Addict Biol. 2013 doi: 10.1111/adb.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc KH, Maidment NT, Ostlund SB. Repeated cocaine exposure facilitates the expression of incentive motivation and induces habitual control in rats. PLoS One. 2013;8:e61355. doi: 10.1371/journal.pone.0061355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lex A, Hauber W. Dopamine D1 and D2 receptors in the nucleus accumbens core and shell mediate Pavlovian-instrumental transfer. Learn Mem. 2008;15:483–491. doi: 10.1101/lm.978708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lex B, Hauber W. The role of nucleus accumbens dopamine in outcome encoding in instrumental and Pavlovian conditioning. Neurobiol Learn Mem. 2010;93:283–290. doi: 10.1016/j.nlm.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Lovibond PF. Facilitation of instrumental behavior by a Pavlovian appetitive conditioned stimulus. J Exp Psychol Anim Behav Process. 1983;9:225–247. [PubMed] [Google Scholar]
- Ludwig AM, Wikler A, Stark LH. The first drink: psychobiological aspects of craving. Arch Gen Psychiatry. 1974;30:539–547. doi: 10.1001/archpsyc.1974.01760100093015. [DOI] [PubMed] [Google Scholar]
- Malvaez M, Greenfield VY, Wang AS, Yorita AM, Feng L, Linker KE, Monbouquette HG, Wassum KM. Basolateral amygdala rapid glutamate release encodes an outcome-specific representation vital for reward-predictive cues to selectively invigorate reward-seeking actions. Sci Rep. 2015;5:12511. doi: 10.1038/srep12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinovic J, Jones A, Christiansen P, Rose AK, Hogarth L, Field M. Electrophysiological responses to alcohol cues are not associated with Pavlovian-to-instrumental transfer in social drinkers. PLoS One. 2014;9:e94605. doi: 10.1371/journal.pone.0094605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE. The role of dopamine in the pursuit of nutritional value. Physiol Behav. 2015 doi: 10.1016/j.physbeh.2015.05.003. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Beeler JA, Roitman MF. Sucrose-predictive cues evoke greater phasic dopamine release than saccharin-predictive cues. Synapse. 2012;66:346–351. doi: 10.1002/syn.21519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AL, Everitt BJ. The persistence of maladaptive memory: addiction, drug memories and anti-relapse treatments. Neurosci Biobehav Rev. 2012;36:1119–1139. doi: 10.1016/j.neubiorev.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Nasrallah NA, Clark JJ, Collins AL, Akers CA, Phillips PE, Bernstein IL. Risk preference following adolescent alcohol use is associated with corrupted encoding of costs but not rewards by mesolimbic dopamine. Proc Natl Acad Sci U S A. 2011;108:5466–5471. doi: 10.1073/pnas.1017732108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. On habits and addiction: An associative analysis of compulsive drug seeking. Drug Discov Today Dis Models. 2008;5:235–245. doi: 10.1016/j.ddmod.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, LeBlanc KH, Kosheleff AR, Wassum KM, Maidment NT. Phasic mesolimbic dopamine signaling encodes the facilitation of incentive motivation produced by repeated cocaine exposure. Neuropsychopharmacology. 2014;39:2441–2449. doi: 10.1038/npp.2014.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Maidment NT. Dopamine receptor blockade attenuates the general incentive motivational effects of noncontingently delivered rewards and reward-paired cues without affecting their ability to bias action selection. Neuropsychopharmacology. 2012;37:508–519. doi: 10.1038/npp.2011.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Wassum KM, Murphy NP, Balleine BW, Maidment NT. Extracellular Dopamine Levels in Striatal Subregions Track Shifts in Motivation and Response Cost during Instrumental Conditioning. Journal of Neuroscience. 2011;31:200–207. doi: 10.1523/JNEUROSCI.4759-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Peciña S, Berridge KC. Dopamine or opioid stimulation of nucleus accumbens similarly amplify cue-triggered 'wanting' for reward: entire core and medial shell mapped as substrates for PIT enhancement. Eur J Neurosci. 2013 doi: 10.1111/ejn.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PE, Robinson DL, Stuber GD, Carelli RM, Wightman RM. Real-time measurements of phasic changes in extracellular dopamine concentration in freely moving rats by fast-scan cyclic voltammetry. Methods Mol Med. 2003;79:443–464. doi: 10.1385/1-59259-358-5:443. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Solomon RL. Two-process learning theory: Relationships between Pavlovian conditioning and instrumental learning. Psychol Rev. 1967;74:151–182. doi: 10.1037/h0024475. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris MP, Sugam JA, Stuber GD, Witten IB, Deisseroth K, Carelli RM. Mesolimbic dopamine dynamically tracks, and is causally linked to, discrete aspects of value-based decision making. Biol Psychiatry. 2015;77:903–911. doi: 10.1016/j.biopsych.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur J Neurosci. 2012;36:2521–2532. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A. Starch and sugar tastes in rodents: an update. Brain Res Bull. 1991;27:383–386. doi: 10.1016/0361-9230(91)90129-8. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Jentsch JD. Repeated intermittent administration of psychomotor stimulant drugs alters the acquisition of Pavlovian approach behavior in rats: differential effects of cocaine, d-amphetamine and 3,4-methylenedioxymethamphetamine ("Ecstasy") Biol Psychiatry. 2001;50:137–143. doi: 10.1016/s0006-3223(01)01106-4. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat MJ, Kuhnen CM, Phillips PE. Delays conferred by escalating costs modulate dopamine release to rewards but not their predictors. J Neurosci. 2010;30:12020–12027. doi: 10.1523/JNEUROSCI.2691-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Balleine BW, Maidment NT. Differential dependence of Pavlovian incentive motivation and instrumental incentive learning processes on dopamine signaling. Learn Mem. 2011;18:475–483. doi: 10.1101/lm.2229311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Loewinger GC, Maidment NT. Phasic Mesolimbic Dopamine Release Tracks Reward Seeking During Expression of Pavlovian-to-Instrumental Transfer. Biol Psychiatry. 2013;73:747–755. doi: 10.1016/j.biopsych.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Maidment NT. Phasic Mesolimbic Dopamine Signaling Precedes and Predicts Performance of a Self-Initiated Action Sequence Task. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P, Wiers RW, Hommel B, de Wit S. Working for food you don't desire. Cues interfere with goal-directed food-seeking. Appetite. 2014;79:139–148. doi: 10.1016/j.appet.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Burgeno LM, Everitt BJ, Phillips PE. Hierarchical recruitment of phasic dopamine signaling in the striatum during the progression of cocaine use. Proc Natl Acad Sci U S A. 2012;109:20703–20708. doi: 10.1073/pnas.1213460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward "wanting" without enhanced "liking" or response reinforcement. J Neurosci. 2000;20:8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: increased cue-triggered "wanting" for sucrose reward. J Neurosci. 2001;21:7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.