Abstract
Statin treatment has been shown to reduce graft-versus-host disease (GVHD) while preserving graft-versus-tumor (GVT) effect in allogeneic stem cell transplantation (allo-HCT). Herein, we investigated whether lovastatin treatment affects the function of human cytolytic T lymphocytes (CTLs). Upon TCR stimulation, lovastatin significantly inhibited the proliferation of both CD4+ and CD8+ T cells from healthy donors while their intracellular cytokine production including IFN-γ and TNF-α remained the same with a slight decrease of IL-2. Moreover, the specific lysis of target cells by CTL lines derived from patients and normal donors specific for EBV-encoded antigen LMP2 or CMV-encoded antigen pp65 was uncompromised in the presence of lovastatin. In addition, we evaluated the effect of lovastatin on the proliferation and effector function of the CD8+ tumor–infiltrating lymphocytes (TILs) derived from melanoma patients specific for MART-1 antigen. Lovastatin significantly reduced the expansion of antigen-specific TILs upon MART-1 stimulation. However, the effector function of TILs, including the specific lysis of target cells and secretion of cytokine IFN-γ, remained intact with lovastatin treatment. Taken together, these data demonstrated that lovastatin inhibits the proliferation of EBV-, CMV- and MART-1-specific CTLs without affecting cytolytic capacity. The differential effect of lovastatin on the proliferation versus cytoxicity of CTLs might shed some light on elucidating the possible mechanisms of GVHD and GVT effect elicited by alloimmune responses.
Introduction
Allogeneic stem cell transplantation (allo-HCT) is an effective therapy for hematological malignancies1. The limiting factor is graft-versus-host disease (GVHD), a result of alloreactive immune responses elicited by donor T lymphocytes2-4. Meanwhile, the same alloimmune responses are strongly associated with the beneficial graft-versus tumor (GVT) effect5. Novel approaches are being constantly evaluated to minimizing GVHD without compromising the GVT effect6-7.
3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, commonly referred to as statins, are prescribed to reduce plasma cholesterol levels. Recently, several lines of evidence demonstrated that treatment with statins can reduce GVHD in allo-HCT8-10. More important, the GVT effect remained uncompromised in the presence of statins in both a mouse model and human trials8, 10. This potential beneficial effect of statins for tolerance induction while preserving GVT may provide insight into understanding the mechanisms of alloimmune response and using allo-HCT as a platform to deliver immunotherapy1, 11-12.
Statins exert immunosuppressive functions through a variety of mechanisms, including suppression of T cell activation, downregulation of costimulatory molecules and MHC class II on antigen presenting cells (APCs), Th2 polarization, induction of Treg expansion13-15. The immunomodulatory effect of statins on T cell activation is at least partially unrelated to HMG-CoA reductase inhibition and the molecular mechanism has been elucidated16-17. Specifically, lovastatin inhibits the activation of lymphocyte function–associated antigen-1 (LFA-1), which is a costimulatory molecule regulating T cell activation and cytoxicity in the context of the immunological synapse18-19. Lovastatin stabilizes LFA-1 in a low-affinity state allosterically through regulating conformation changes rather than directly interfering with the binding of LFA-1 with its ligands16. Upon T cell activation, LFA-1 changes to the high-affinity state, and the control of LFA-1 activation is critical in inflammatory and immune responses20-24. Therefore, statins can regulate LFA-1 activation by modulating its affinity state and directly affect T cell activation.
We have shown that lovastatin treatment can reduce GVHD mortality and morbidity in a mouse HCT model9. However, pravastatin which has similar potency as lovastatin as the HMG-CoA inhibitor but about 50-fold less potent in blocking LFA-1 activation, failed to protect the mice from developing GVHD, thus indicating that the ability of lovastatin in preventing GVHD involves blocking LFA-1 activation. More importantly, Zeiser et al. demonstrated that preemptive statin treatment provides GVHD protection via Th2 polarization without impacting GVL activity in preclinical studies8. Subsequently, compelling clinical data came from a retrospective study of allo-HCT patients with hematologic malignancies showing that statin treatment of the donor alone or of both donor and recipient significantly decreased the risk of severe GVHD but did not compromise recurrent malignancy and mortality10.
The current data support a hypothesis that GVHD is a systemic Th1 type response including a CD4 response of the Th1 type, generation of CD8 CTLs and an inflammatory cytokine cascade3, 6-7. To elucidate the mechanism of the differential effect of statins on GVHD and GVT, we investigated whether lovastatin treatment has any impact on the function of cytotoxic T cells (CTLs). We found lovastatin inhibited the proliferation of human CTLs upon TCR and antigen-specific stimulation. However, their antigen-specific cytotoxic function against target cells remained intact. Our data demonstrated that statins exert differential immunomodulatory effects on the proliferation and cytoxicity of CTLs, which might partially account for the results seen in both preclinical and clinical settings where statin treatment protects against GVHD while preserving GVT in allo-HCT.
Materials and Methods
T cell proliferation assay
Peripheral blood mononuclear cells (PBMCs) were obtained by sedimentation with Histopaque 1077 (Sigma-Aldrich) using blood from healthy volunteers. All cell preparations were >95% viable by Trypan Blue exclusion. For T cell proliferation assay, PBMCs were stimulated with anti-CD3 mAb (OKT3) at 5 μg/ml. To determine cell proliferation, cells were either pulsed with [3H] thymidine during the last 16 hours of culture and incorporated radioactivity was quantified using a liquid scintillation counter; or cells were labeled with CFSE and cell division was monitored by using the FITC channel in a FACScanto II flow cytometer (BD Biosciences).
Cytokine flow cytometry assay
Intracellular cytokine production was measured using 8-color 10-parameter cytokine flow cytometry as previously described25. Briefly, one hour after simulation of PBMCs with OKT3 (5 μg/ml), Brefeldin A was added to enable accumulation of intracellular cytokines. Following an additional 5 hours of incubation, cells were fixed and permeabilized with Fix & Perm A/B (Caltag, Burlingame, CA) and assessed for the simultaneous expression of surface markers and intracellular cytokines. FACS analyses were performed using mAbs for human CD4, CD8, IL-2, TNF-α and IFN-γ (BD Pharmingen, San Jose, CA). After staining, cells were washed, resuspended in PBS with 1% paraformaldehyde, and analyzed by 5-color, 7-parameter flow cytometry using an LSR-II cytometer (BD, San Jose, CA) and FlowJo software (Treestar, San Carlos, CA). At least 3×105 total events were analyzed with sequential gating of PBMCs in a lymphocyte region (by scatter) and on T cells (by assessing CD4+ or CD8+ staining for intracellular IL-2, IFN-γ, and TNF-α). Gates defining cytokine-positive populations were defined based on the upper limits of fluorescence of unstimulated cells stained with the same antibodies.
EBV- and CMV-CTL lines
According to previously described method, CTL lines from peripheral blood were prepared from patients with EBV-positive Hodgkin's Disease (EBV-specific CTLs) or stem cell donors (CMV-specific CTLs), who were enrolled in clinical trials using antigen-specific T cells for the treatment of EBV-associated Lymphoma (EBV-specific CTLs) or viral infection after transplantation (CMV-specific CTLs) at Baylor College of Medicine with IRB-approved protocols26-27. CTLs specific for the tumor associated EBV antigen Latent Membrane Protein-2 (LMP2), were generated as previously as described27. Briefly, patient dendritic cells were infected with the Ad5f35LMP2 vector at a multiplicity of infection (MOI) of 1000 viral particles per cell. Then cells were restimulated weekly with irradiated autologous lymphoblastoid cell lines (LCLs) transduced with the same Ad5f35LMP2 vector at an MOI of 30,000 viral particles per cell at a responder:stimulator ratio of 4:1. To generate CMVpp65- CTLs as described26, donor PBMCs were stimulated with irradiated autologous monocytes pulsed with pp65 PeptideMix (PepMix). Then cells were restimulated weekly in the presence of IL-2. After a total of three or four stimulations, the CTLs were characterized for immunophenotype and examined for LMP2 or pp65 specificity. For EBV and CMV cytotoxicity assay herein, the cytotoxic specificity of each CTL line was determined using the flow cytometry-based CTL assay measuring the cleavage of caspase-3 in targets cells28. The target cells were labeled with a far-red fluorescent marker dye, DDAO-SE (Invitrogen, Carlsbad, CA), and then incubated with CTLs at different effector:target (E:T) ratios for 3 to 4 hours and fixed, permeabilized, and stained with a PE-conjugated anti-cleaved caspase-3 rabbit mAb (BD Biosciences, San Jose, CA). The stained cells were analyzed within 24 hr using a FACScanto II flow cytometer.
MART-1-specific melanoma tumor-infiltrating lymphocyte (TIL) expansion
TILs for laboratory studies were obtained from patients with HLA-A0201+ (HLA-A2.1+) Stage IV melanoma at M.D. Anderson Cancer Center with IRB-approved protocol29. According to a previously described method, T cells from surgically-removed tumor fragments were initially expanded in TIL culture medium (TIL-CM) containing IL-2, and then harvested and stained for CD8 expression and recognition of the HLA-A2.1
MART-1 peptide tetramer29-30. Human dendritic cells (DCs) were generated from monocytes obtained from HLA-A2.1+ normal donors. The mature DCs were irradiated at 2000 cGy and pulsed with 3 μg/ml of MART-1 peptide (ELAGIGILTV) for 90 minutes. TILs (2 × 106 TILs in 24-well plates) were restimulated by adding 2 × 105 peptide-pulsed mature DCs together with 100 U/ml IL-2.
Measurement of TIL cell division and effector cell activity
TILs from HLA-A2.1+ patients recognizing the HLA-A2.1-restricted MART-1 peptide, as determined by staining with an HLA-A2.1-peptide tetramer, were used in the assays. Cell division was determined by CFSE dilution in TILs. For the CFSE experiments, TILs were washed and resuspended in PBS with 1 μM of CFSE (Molecular Probes-Invitrogen, Carlsbad, CA). Cell division was monitored by using the FITC channel in a FACScanto II flow cytometer (BD Biosciences). Antigen-specific CTL activity was monitored by measuring the cleavage of caspase-3 in targets cells as described28. The target cells were either the HLA-A2.1+ melanoma cell line 624 targets endogenously expressing MART-1 or T2 cells (TAP1/2-deficient HLA-A2.1+ human lymphoma cells) pulsed with the MART-1 peptide. The target cells were first labeled with a far-red fluorescent marker dye, DDAO-SE (Invitrogen, Carlsbad, CA), washed and then pulsed with MART-1 peptide or a control HLA-A2.1-binding HIV rev peptide at 5 μg/ml for 1 hour. The targets were then incubated with TILs at different E:T ratios for 3 to 4 hours. The cells were then fixed, permeabilized, and stained with a PE-conjugated anti-cleaved caspase-3 rabbit mAb (BD Biosciences, San Jose, CA). Staining for intracellular IL-2 and IFN-γ production by TILs coincubated with peptide–pulsed cells for 5 to 6 hours was done by using an intracellular cytokine staining protocol. GolgiStop (BD Biosciences) was added 1 hour into the coincubation time. In each case, stained cells were analyzed in a FACScanto II flow cytometer (BD Biosciences).
Results
Lovastatin modulates human T cell proliferation and cytokine production
We examined whether lovasatin treatment affects human T cell proliferation and cytokine production upon TCR stimulation. First, we compared the effect of lovastatin and pravastatin on human T cell proliferation. As shown in Figure 1A, while pravastatin did not affect the proliferation of PBMC stimulated by OKT3, cell proliferation was significantly reduced in the presence of lovastatin at the 5-10 μM concentration range with slightly reduced proliferation rates at lower concentrations. We further examined the effect of lovastatin at the concentration of 10 μM on the proliferation of both CD4+ and CD8+ T cells using CFSE-labeled cells stimulated with OKT3. As shown in
Figure 1. Effect of lovastatin and pravastatin on T cell proliferation stimulated by OKT3.
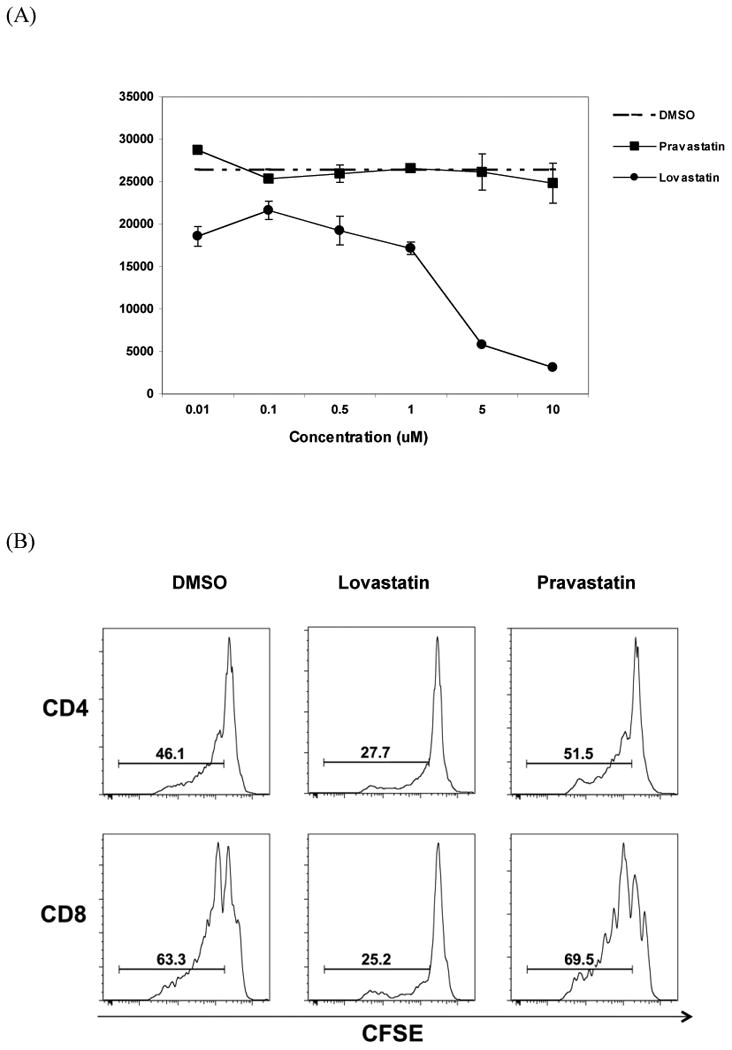
PBMCs were obtained from buffy coats using Ficoll gradient centrifugation and stimulated by OKT3 for 7 days. (A) Proliferation of T cells was determined by the incorporation of [3H] thymidine into the DNA in the presence of various concentrations of lovastatin, pravastatin and vehicle control DMSO. Values represent the mean S.D. of three independent samples run in triplicate. (B) PBMCs labeled with CFSE were stimulated with OKT3 in the presence of 10 μM lovastatin, 10 μM pravastatin, or the vehicle DMSO as indicated. T cell proliferation was assessed by CFSE dye dilution following sequential gating on lymphocytes (by scatter) and CD4+ or CD8+ T cells. A representative data of three independent samples were presented.
Figure 1B, the frequency of dividing cells was significantly reduced in the lovastatin-treated samples compared to DMSO vehicle control (27.7% vs. 46.1% in CD4+ cells; 25.2% vs. 63.3% in CD8+ cells). Pravastatin had no inhibitory effect on T cell proliferation. Thus, lovastatin inhibited the proliferation of CD4+ and CD8+ T cells upon TCR stimulation.
To further investigate the functional consequences of lovastatin on T cells, we used intracellular cytokine staining to measure the production of effector cytokines at the single cell level25. The production of IL-2, TNF-α and IFN-γ was determined simultaneously in five donors to examine whether lovastatin affects T cell polyfunctionality upon OKT3 stimulation31. As shown in Figure 2, the frequency of IL-2 producing cells was slightly reduced in both CD4+ and CD8+ population in the presence of 10 μM lovastatin, with the p value of 0.065 and 0.057 in CD4+ and CD8+ T cells respectively. The frequency of IFN-γ and TNF-α producing cells remained the same with lovastatin treatment in comparison to pravastatin-treated samples and the DMSO control. Taken together, these results demonstrated that lovastatin inhibited T cell proliferation and reduced IL-2 production without affecting IFN-γ and TNF-α production.
Figure 2. Multiple cytokine production in stimulated T cells in the presence of lovastatin.
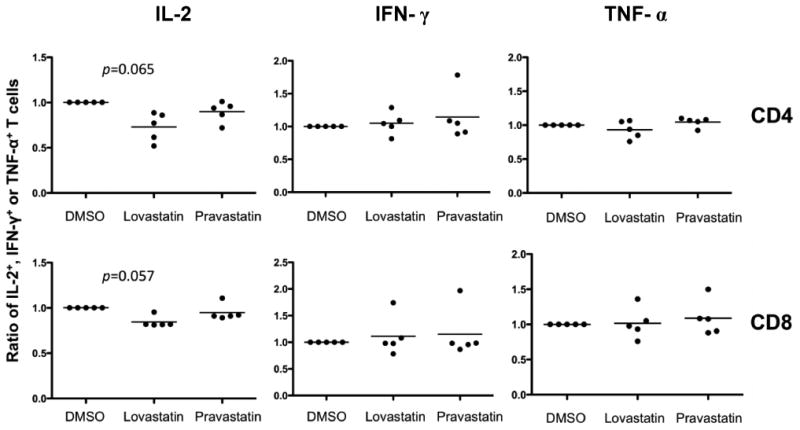
Scatter plots with data from 5 donors for ratio of frequency of IL-2+, IFN-γ+, TNF-α+ in lovastatin- or pravastatin-treated T cells to DMSO control. PBMCs were stimulated with OKT3 in the presence of 10 μM lovastatin, 10 μM pravastatin or the vehicle control DMSO. FACS analyses were performed using mAbs for CD4, CD8, IL-2, TNF-α and IFN-γ. At least 3×105 total events were analyzed and cytokine-positive populations were defined based on the upper limits of fluorescence of unstimulated cells stained with the same antibodies. Median frequency of IL-2+, IFN-γ+, TNF-α+ in stimulated T cells without statin treatment, were 2.9%, 21.1%, 21.1% in CD4+ T cells, and 1.0%, 12.7%, 12.9% in CD8+ T cells. The y axis represents the ratio of IL-2+, IFN-γ+ or TNF-α+ T cells in statin-treated samples normalized to the DMSO control. Statistical analyses were performed using Prism software (GraphPad, San Diego, CA). Intergroup comparisons were performed using Wilcoxon matched pair analysis. All p values were two-tailed and considered significant if less than 0.05 (IL-2 in CD4+ T cells: p=0.065, IL-2 in CD8+ T cells: p=0.057, IFN-γ, TNF-α: p>0.1).
The cytolytic function of EBV- and CMV-CTL lines remains intact in the presence of lovastatin
To determine whether lovastatin affects CTL responses, we assessed the cytolytic capacity of EBV- and CMV- specific CTL lines derived from patients using cytoxicity assay. It has been demonstrated previously that CTLs specific for EBV or CMV, derived from patients and expanded in vitro, produced clinically relevant effects in patients26, 32. We first examined three LMP2 lines generated from individual Hodgkin disease patients specifically targeting EBV-encoded antigen LMP227. These previously characterized CTL lines contained predominantly CD3+/CD8+ T cells and more than 90% of cells were TCR-positive. As shown in Figure 3A, the specific lysis of the autologous LCLs by a representative LMP2-CTL line was measured in the presence of lovastatin, and the killing of target cells at various E:T ratios was not compromised. The specific killing of target cells by three individual CTL lines at E:T ration of 10:1 was summarized in Figure 3A (right), showing that lovastatin treatment did not affect the cytolytic function LMP2-CTLs.
Figure 3. Lovasatin does not affect the specific cytotoxic activity of EBV-specific and CMV-specific CTL lines.
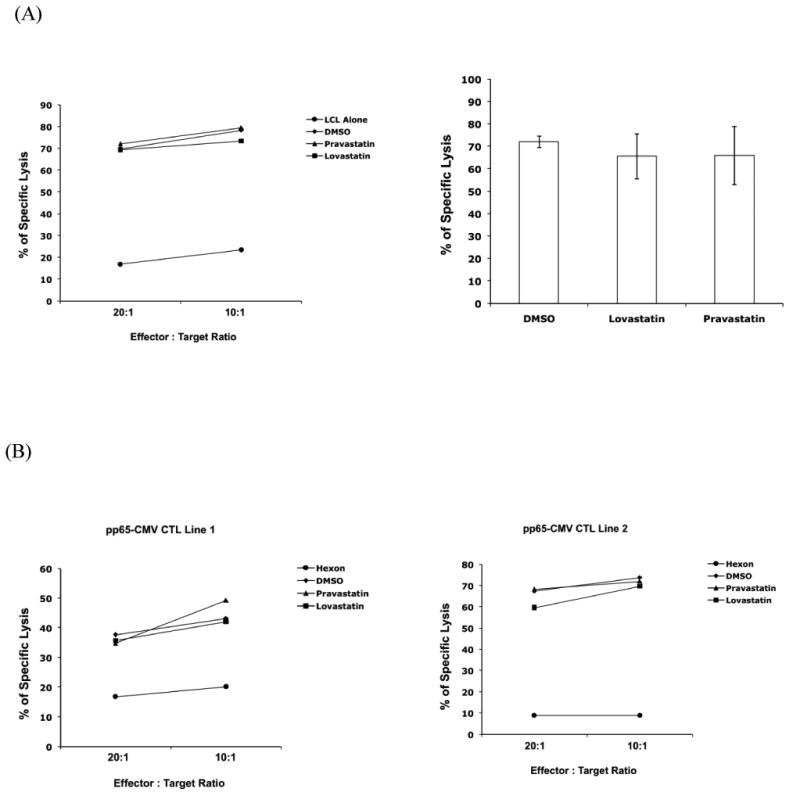
(A) The specific killing activity of the EBV-specific CTLs. Previously characterized LMP2-CTL lines and the corresponding autologous LCLs were recovered in culture27. Autologous LCLs were labeled with DDAO-SE and incubated for 4 hours with corresponding CTL lines at the E/T ratios indicated, in the presence of 10 μM lovastatin, 10 μM pravastatin, or the vehicle DMSO. Killing of autologous LCLs pulsed with LMP2 peptides was measured by the percentage of caspase-3 positive cells. A representative LMP2-CTL line data (left) and means of the percent specific killing from target cells ± SD of the three CTL lines at E:T ratio of 10:1 (right) were presented. (B) The specific killing activity of the CMV-specific CTLs. Two previously characterized pp65-CTL lines were recovered in culture26. The PHA blasts were pulsed with CMVpp65 PepMix (CMVpp65 target) in the presence of 10 μM lovastatin, 10 μM pravastatin, or the vehicle DMSO as indicated, and PHA blasts pulsed with Adv-hexon PepMix (Adv-hexon target) as control. The data were the percentage of caspase positive targets by two individual pp65-CTL lines at E:T of 20:1 and 10:1.
In addition, we examined two individual pp65-CTL lines derived from donors and expanded in vitro specifically targeting CMV-encoded antigen pp6526. Target PHA blasts were pulsed with CMVpp65 PepMix or Adv-hexon PepMix, and the percentage of specific lysis of pp65-pulsed target cells by two pp65-CTL lines was measured at E:T ratio of 20:1 and 10:1 (Figure 3B). We found that the target cell killing was similar in the presence of lovastatin, pravastatin, or DMSO. Thus, these results demonstrated that lovastatin did not impair the cytolytic function of EBV- and CMV-CTL lines.
Lovastatin inhibits the proliferation while preserving the cytolytic activity of MART1-specific melanoma TILs
To further demonstrate the effect of lovastatin on the proliferation and effector function of CTLs, we utilized the well-characterized CD8+ melanoma tumor–infiltrating lymphocytes (TILs)29-30. TILs isolated from HLA-A2.1+ melanoma patients have been shown to expand rapidly in vitro and respond to melanoma antigen restimulation. We examined five TIL cultures that had significant levels of CD8+/MART-1 tetramer+ T cells for their restimulation response to MART-1 peptide-pulsed HLA-A2.1-matched DCs. As shown in Figure 4, there was a significant proliferation of CD8+/MART-1+ cells after peptide stimulation as determined by CFSE dilution. In the presence of lovastatin, there was a significant reduction of proliferating cells (26.5%) in comparison to the DMSO control (70.3%) and pravastatin (69.8%). The same inhibitory effect of lovastatin was also observed in TILs specific for another melanoma peptide antigen gp100 (data not shown).
Figure 4. The proliferation and cytokine production of MART1- specific TILs in the presence of lovastatin.
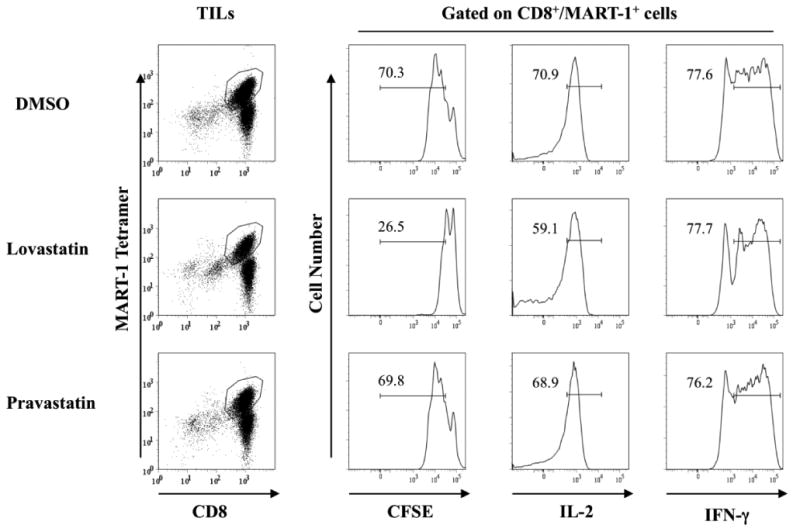
TILs from HLA-A2.1+ patients that recognize the HLA-A2.1-restricted MART-1 epitope were activated with mature dendritic cells pulsed with peptide in the presence of 10 μM lovastatin, 10 μM pravastatin or vehicle control DMSO as indicated. On day four of the stimulation, cells were collected and stained with surface markers to measure cell proliferation and cytokine production. MART-1 tetramer was used to identify antigen specific CD8+ T cells and CFSE was used to track the proliferation of gated cells. For IL-2 and IFN-γ production, TILs were coincubated with peptide–pulsed cells for 5 to 6 hours followed by a standard intracellular cytokine staining protocol. In each case, stained cells were analyzed in a FACScanto II flow cytometer (BD Biosciences). Isotype controls were used for setting the gates to determine the IL-2 and IFN-γ production. Data are representative of three individual TILs.
The cytolytic capacity is critical for the effector function of TILs which including inflammatory cytokine secretion and direct killing by granzyme B and perforin release. We first assessed the IL-2 and IFN-γ production in two MART-1-specific TIL lines derived from melanoma patients. As shown in Figure 4, the majority of CD8+/MART-1+ TILs produced intracellular IL-2 and IFN-γ upon peptide restimulation. There was only a slight decrease in the IL-2 production in lovastatin treated TILs (59.1%) compared to the DMSO control (70.9%) and pravastatin (68.9%). IFN- γ production remained unchanged in the presence of lovastatin. The same trend was also observed in gp100-specific TILs (data not shown).
To investigate whether lovastatin affects the antigen-specific cytolytic ability, TILs derived from three melanoma patients were restimulated with MART-1 peptide-pulsed DCs and tested for CTL activity against target cells. As shown in Figure 5 (top panel), the target cells were the HLA-A2.1+ melanoma cell line 624 targets endogenously expressing MART-1, the specific lysis of target cells by TILs appeared the same in the presence of lovastatin as the DMSO control and pravastatin. The same TIL samples were examined for specific lysis of T2 target cells pulsed with the MART-1 peptide. Although the overall cytolytic capacity for T2 cells was greater than the 624 target cells due to the high levels of pulsed peptide antigen, lovastatin treatment did not compromise the specific killing by TILs (Figure 5, lower panel). These data demonstrated the lovastatin inhibited the proliferation of melanoma TILs following antigenic restimulation, but did not affect antigen-specific effector activity.
Figure 5. Lovasatin does not affect antigen specific cytoxicity of MART1-specific TILs.
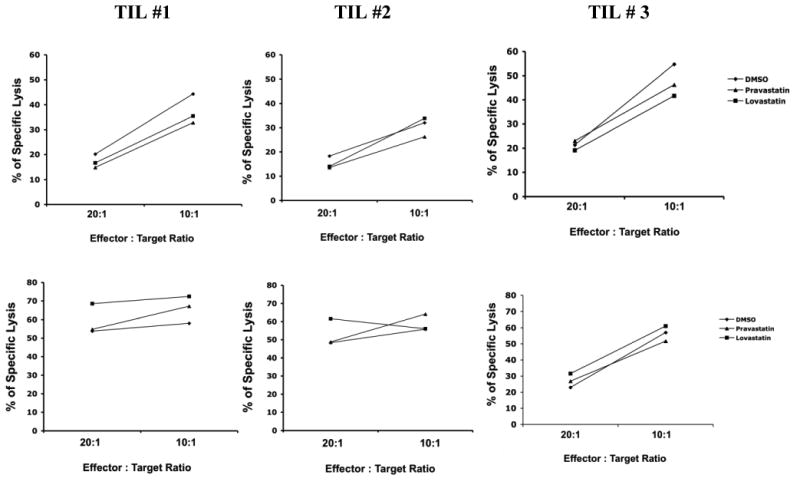
TILs from HLA-A2.1+ patients that recognize MART-1 epitope were used in CTL assays. The target cells were either the HLA-A2.1+ melanoma cell line 624 targets endogenously expressing MART-1 (A) or T2 cells pulsed with the MART-1 peptide (B), in the presence of 10 μM lovastatin, 10 μM pravastatin or vehicle control DMSO as indicated. The control were target cells loaded with HLA-A2.1-binding HIV rev peptide. The targets were then incubated with TILs at different E:T ratios for 3 to 4 hours and stained with an anti-cleaved caspase-3 rabbit mAb. The data were from three different TIL lines.
Discussion
Herein, we reported that while the T cell proliferation was inhibited by lovastatin, specific lysis of target cells by EBV-, CMV- and MART-1-specific CTLs remained uncompromised. In addition to its ability to lower plasma cholesterol levels, clinical studies have indicated that statins have immunosuppressive function14. We and others demonstrated recently that statin treatment reduced GVHD in mouse models8-9. Moreover, the protective effect of statin has been shown in retrospective studies of human GVHD10, 33. In the light of these data, we examined whether lovasatin treatment affects the function of human CTLs including the proliferation, cytokine production and cytotoxicity upon TCR and antigen-specific stimulation.
We found that lovastatin significantly inhibited human T cell proliferation upon OKT3 stimulation only at the concentration 5-10 μM while pravastatin had no inhibitory effect. Pravastatin has similar potency as lovastatin as an HMG-CoA reductase inhibitor but does not sufficiently block LFA-1 activation16. Our results are consistent with findings that lovastatin blocks HMG-CoA reductase at the nanomolar range and LFA-1 inhibition requires higher concentration at the micromolar range14, 16. Taken together, our data indicates that the inhibitory effect of lovastatin on T cell proliferation seen here is probably unrelated to HMG-CoA reductase inhibition. Regardless of the various possible mechanisms proposed for the immunosuppressive function of statins15, lovastatin most likely prevented T cell proliferation upon TCR or antigen-specific stimulation herein by modulating LFA-1 activation.
Collective evidences from both mouse models and clinical data have demonstrated that statin treatment protects GVHD development in allo-HCT. Zeiser et al. showed that atorvastatin treatment in mice through HMG-CoA reductase inhibition, downregulated costimulatory molecules and MHC class II on host APCs, induced Th2 polarization and inhibited expansion of donor CD4+ T cells, which translated to the GVHD reduction8. We demonstrated that lovastatin not pravastatin treatment in mice prevented the migration, homing and proliferation of donor CD4+ and CD8+ T cells, and thus GVHD protection was attributed to LFA-1 inactivation by lovastatin9. Another report showed that while simvastatin treatment downregulated costimulatory molecules and MHC class II on APCs at a lower concentration, the inhibition of CD4+ T cell proliferation required one log higher concentration (5-10 μM)34. Based on these data, multiple mechanisms are probably involved in the role of statins in GVHD protection. Nevertheless, retrospective studies have convincingly confirmed that statin treatment significantly decreased the risk of GVHD in allo-HCT patients with hematological malignancies10, 33.
The beneficial effects of statins for allo-HCT not only rest on GVHD protection but also on preserving the GVT effect8, 10. Although donor T lymphocytes are the primarily mediators for GVHD and GVT, the molecular and cellular basis are still not well understood. Among the main effector cells currently proposed to be responsible for GVHD, including the Th1 type CD4+ T cells, CD8+ CTLs and cytokine cascade, the CD8+ cytotoxic T lymphocytes against allo-antigens are known to contribute to the GVT1. We evaluated the cytolytic function of EBV-, CMV- and MART-1-specific CTLs in the presence of lovastatin. There was a significant inhibition of CTL expansion with a slightly reduction of IL-2 production which probably was partially correlated with the decrease of cell proliferation. However, their specific lysis of target cells and IFN-γ production remained uncompromised. Based on these results, we hypothesize that the effector function of CD8+ CTLs, including direct cytolytic activity and IFN-γ production, might be predominantly responsible for the anti-tumor effect.
Our data showing that the effector cytolytic responses are preserved but the proliferative responses necessary for the expansion of naive T-cells are inhibited in the presence of lovastatin, that provides a potential mechanistic explanation for statins as therapeutic reagents for GVHD associated with allo-HCT without compromising GVT.
Acknowledgments
The work is supported by ACS grant RSG-08-183-01-LIB (Q.M).
Footnotes
The authors have no conflicting financial interests.
Authors Contributions: D.L., Y.L., J.A.H., R.P., T.K.K, J.K., M.C.D, and P.J.H. performed experiments and analyzed data. C.M.B., K.V.K., P.W. and R.E.C. wrote the paper. L.G.R., J.J.M. and Q.M. designed the research and wrote the paper.
References
- 1.Appelbaum FR. Haematopoietic cell transplantation as immunotherapy. Nature. 2001;411:385–389. doi: 10.1038/35077251. [DOI] [PubMed] [Google Scholar]
- 2.Sale GE, Shulman HM, Hackman RC. Pathology of hematopoietic cell transplantation. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas's Hematopoietic Cell Transplantation. Boston, MA: Blackwell Scientific Publications; 2004. pp. 286–299. [Google Scholar]
- 3.Ferrara JLM, Cooke KR, Teshima T. The Pathophysiology of graft-vs.-host disease. In: Ferrara JLM, Cooke KR, Deeg HJ, editors. Graft-vs-Host-Disease. New York, NY: Marcel Dekker; 2005. pp. 1–34. [Google Scholar]
- 4.Korngold R, Friedman TM. Murine models for graft-versus-host disease. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas's Hematopoietic Cell Transplantation. Boston, MA: Blackwell Scientific Publications; 2004. pp. 353–368. [Google Scholar]
- 5.Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555–562. [PubMed] [Google Scholar]
- 6.Socié G, Blazar BR. Acute graft-versus-host disease: from the bench to the bedside. Blood. 2009;114(20):4327–4336. doi: 10.1182/blood-2009-06-204669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7(5):340–352. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 8.Zeiser R, Youssef S, Baker J, et al. Preemptive HMG-CoA reductase inhibition provides graft-versus-host disease protection by Th-2 polarization while sparing graft-versus-leukemia activity. Blood. 2007;110:4588–4598. doi: 10.1182/blood-2007-08-106005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Li D, Jones D, et al. Blocking LFA-1 activation with lovastatin prevents graft-versus-host disease in mouse bone marrow transplantation. Biol Blood Marrow Transplant. 2009;15:1513–1522. doi: 10.1016/j.bbmt.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rotta M, Storer BE, Storb RF, et al. Donor statin treatment protects against severe acute graft-versus-host disease after related allogeneic hematopoietic cell transplantation. Blood. 2010;115:1288–1295. doi: 10.1182/blood-2009-08-240358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 12.Yee C, Greenberg P. Modulating T-cell immunity to tumours: new strategies for monitoring T-cell responses. Nat Rev Cancer. 2002;2(6):409–419. doi: 10.1038/nrc820. [DOI] [PubMed] [Google Scholar]
- 13.Weitz-Schmidt G. Statins as anti-inflammatory agents. Trends Pharmacol Sci. 2002;23:482–486. doi: 10.1016/s0165-6147(02)02077-1. [DOI] [PubMed] [Google Scholar]
- 14.Frenette PS. Locking a leukocyte integrin with statins. N Engl J Med. 2001;345:1419–1421. doi: 10.1056/NEJM200111083451911. [DOI] [PubMed] [Google Scholar]
- 15.Broady R, Levings MK. Graft-versus-host disease: suppression by statins. Nat Med. 2008;14(11):1155–1156. doi: 10.1038/nm1108-1155. [DOI] [PubMed] [Google Scholar]
- 16.Weitz-Schmidt G, Welzenbach K, Brinkmann V, et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7:687–692. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- 17.Gadek TR, Burdick DJ, McDowell RS, et al. Generation of an LFA-1 antagonist by the transfer of the ICAM-1 immunoregulatory epitope to a small molecule. Science. 2002;295:1086–1089. doi: 10.1126/science.295.5557.1086. [DOI] [PubMed] [Google Scholar]
- 18.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 19.Dustin ML. Coordination of T cell activation and migration through formation of the immunological synapse. Ann N Y Acad Sci. 2003;987:51–59. doi: 10.1111/j.1749-6632.2003.tb06032.x. [DOI] [PubMed] [Google Scholar]
- 20.Shimaoka M, Takagi J, Springer TA. Conformational regulation of integrin structure and function. Annu Rev Biophys Biomol Struct. 2002;31:485–516. doi: 10.1146/annurev.biophys.31.101101.140922. [DOI] [PubMed] [Google Scholar]
- 21.Hynes RO. Structural biology. Changing partners. Science. 2003;300:755–756. doi: 10.1126/science.1084854. [DOI] [PubMed] [Google Scholar]
- 22.Ma Q, Shimaoka M, Lu C, Jing H, Carman CV, Springer TA. Activation-induced conformational changes in the I domain region of lymphocyte function-associated antigen 1. J Biol Chem. 2002;277:10638–10641. doi: 10.1074/jbc.M112417200. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Li D, Nurieva R, et al. LFA-1 affinity regulation is necessary for the activation and proliferation of naïve T cells. J Biol Chem. 2009;284:12645–12653. doi: 10.1074/jbc.M807207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li D, Molldrem JJ, Ma Q. LFA-1 regulates CD8+ T cell activation via T cell receptor-mediated and LFA-1-mediated Erk1/2 signal pathways. J Biol Chem. 2009;284(31):21001–21010. doi: 10.1074/jbc.M109.002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim TK, Billard MJ, Wieder ED, McIntyre BW, Komanduri KV. Co-engagement of alpha(4)beta(1) integrin (VLA-4) and CD4 or CD8 is necessary to induce maximal Erk1/2 phosphorylation and cytokine production in human T cells. Hum Immunol. 2010;71(1):23–28. doi: 10.1016/j.humimm.2009.09.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leen AM, Myers GD, Sili U, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12(10):1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 27.Bollard CM, Gottschalk S, Leen AM, et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007;110(8):2838–2845. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He L, Hakimi J, Salha D, Miron I, Dunn P, Radvanyi L. A sensitive flow cytometry-based cytotoxic T-lymphocyte assay through detection of cleaved caspase 3 in target cells. J Immunol Methods. 2005;304(1-2):43–59. doi: 10.1016/j.jim.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Liu S, Hernandez J, Vence L, Hwu P, Radvanyi L. MART-1--specific melanoma tumor-infiltrating lymphocytes maintaining CD28 expression have improved survival and expansion capability following antigenic restimulation in vitro. J Immunol. 2010;184(1):452–465. doi: 10.4049/jimmunol.0901101. [DOI] [PubMed] [Google Scholar]
- 30.Yee C, Thompson JA, Roche P, et al. Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of t cell-mediated vitiligo. J Exp Med. 2000;192(11):1637–1644. doi: 10.1084/jem.192.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Komanduri KV, Donahoe SM, Moretto WJ, et al. Direct measurement of CD4+ and CD8+ T-cell responses to CMV in HIV-1-infected subjects. Virology. 2001;279(2):459–470. doi: 10.1006/viro.2000.0697. [DOI] [PubMed] [Google Scholar]
- 32.Riddell SR, Greenberg PD. T cell therapy of human CMV and EBV infection in immunocompromised hosts. Rev Med Virol. 1997;7(3):181–192. doi: 10.1002/(sici)1099-1654(199709)7:3<181::aid-rmv200>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 33.Hamadani M, Awan FT, Devine SM. The impact of HMG-CoA reductase inhibition on the incidence and severity of graft-versus-host disease in patients with acute leukemia undergoing allogeneic transplantation. Blood. 2008;111(7):3901–3902. doi: 10.1182/blood-2008-01-132050. [DOI] [PubMed] [Google Scholar]
- 34.Shimabukuro-Vornhagen A, Liebig T, von Bergwelt-Baildon M. Statins inhibit human APC function: implications for the treatment of GVHD. Blood. 2008;112(4):1544–1545. doi: 10.1182/blood-2008-04-149609. [DOI] [PubMed] [Google Scholar]


