Abstract
Plant hormones play pivotal roles in growth, development and stress responses. Although it is essential to our understanding of hormone signalling, how plants maintain a steady state level of hormone receptors is poorly understood. We show that mutation of the Arabidopsis thaliana co-chaperone SGT1b impairs responses to the plant hormones jasmonate, auxin and gibberellic acid, but not brassinolide and abscisic acid, and that SGT1b and its homologue SGT1a are involved in maintaining the steady state levels of the F-box proteins COI1 and TIR1, receptors for jasmonate and auxin, respectively. The association of SGT1b with COI1 is direct and is independent of the Arabidopsis SKP1 protein, ASK1. We further show that COI1 is a client protein of SGT1b–HSP70–HSP90 chaperone complexes and that the complexes function in hormone signalling by stabilizing the COI1 protein. This study extends the SGT1b–HSP90 client protein list and broadens the functional scope of SGT1b–HSP70–HSP90 chaperone complexes.
Plants utilize two major categories of proteins for hormone perception. Two-component kinases and receptor-like kinases function as receptors for cytokinins, ethylene and brassinosteroids, whereas E3 ubiquitin ligases serve as receptors for auxin, jasmonates and gibberellins1,2.
Jasmonates (JA) play key roles in modulating defence responses and in regulating growth and development. JA can activate defence responses to fungal pathogens and insects3, suppress microbe-associated molecular pattern (MAMP)-triggered innate immunity4 or promote plant growth and development5. JA bioactive forms include methyl jasmonate (MeJA) and jasmonoyl-isoleucine (JA-Ile). Coronatine (COR), secreted by many strains of the bacterial phytopathogen Pseudomonas syringae, is a potent JA-Ile structural mimic6. JA-Ile and COR facilitate the interaction between the JA receptor COI1 and jasmonate-ZIM domain proteins (JAZs)6. COI1 is a 592-amino acid F-box protein and an integral part of the Skp1–Cullin–F-box SCFCOI1 E3 ubiquitin ligase complex. JAZ proteins are negative regulators of JA-responsive transcription factors. JA–COR-mediated binding of COI1 to JAZ proteins leads to polyubiquitination and degradation of JAZ proteins via the 26S proteasome7,8, thus releasing JA-responsive transcription factors such as MYC26.
SGT1 (suppressor of G2 allele of skp1), first identified in yeast9, is a conserved, essential protein that has been shown to function as a co-factor of heat shock protein 90 (HSP90) in both plants10–12 and mammals13,14. SGT1 has an N-terminal tetratricopeptide repeat (TPR) domain, a middle CHORD-Sgt1 (CS) domain that is homologous to the Hsp20/crystalline domain of the human p23 co-chaperone proteins, and a C-terminal Sgt1-specific (SGS) domain15. HSP proteins are highly conserved molecular chaperones that are involved in the assembly, stabilization and maturation of critical signalling proteins and complexes. The substrates of HSP chaperones, termed ‘client’ proteins, include hormone receptors, E3 ligases, kinases, transcription factors and Nod-Like immune receptors16. In plants, SGT1 associates through its CS domain with the N-terminal ATPase domain of HSP90 and another co-factor, RAR1 (Required for Mla12 Resistance)17,18, respectively. SGT1 and RAR1 assist HSP90 in stabilizing plant disease resistance (R) proteins10,17,19,20.
Here we describe the Arabidopsis hsm1 mutant, which harbours a mutation in the SGT1b gene. A combination of genetic, biochemical and pharmacological evidence demonstrates that the JA–COR receptor COI1 is a client protein of SGT1b–HSP70–HSP90 chaper-one complexes and that SGT1b–HSP70–HSP90 chaperones play a key role in JA–COR signalling. The auxin receptor TIR1 is also a client protein of SGT1b, and SGT1b is required for auxin as well as gibberellic acid signalling, suggesting that SGT1b plays an important role in stabilizing the E3 ubiquitin ligase receptors in at least three important hormone signalling pathways.
Results
Identification and characterization of the hsm1 mutant
To identify genes involved in jasmonate signalling, we isolated Arabidopsis thaliana mutants insensitive to COR-mediated suppression of fig22-elicited expression of a CYP71A12::GUS transgene in the root elongation zone4 (Fig. 1a). Flg22 is a synthetic 22 amino acid peptide corresponding to a conserved epitope of eubacterial flagellin that activates an Arabidopsis immune response via FLS221.
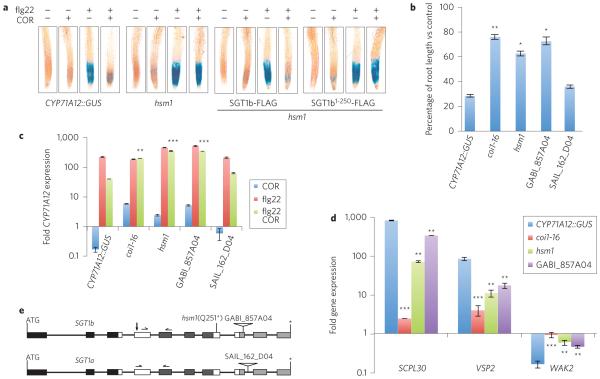
Figure 1. The hsm1 mutant is allelic to the SGT1b gene and is defective in JA–COR responses.
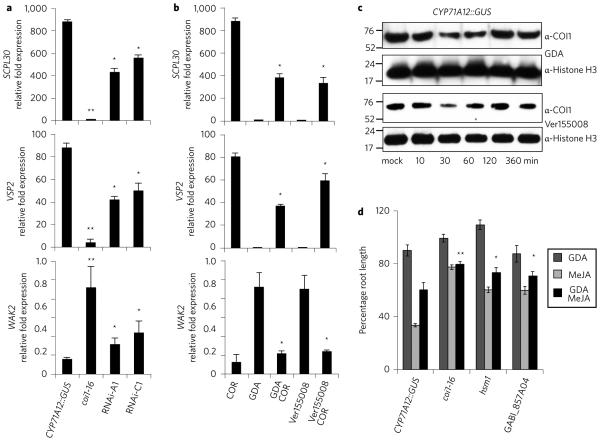
a, GUS staining in the CYP71A12::GUS reporter line, the hsm1 mutant, and the hsm1 mutants expressing SGT1b-FLAG and SGT1b1–250-FLAG transgenes. b, MeJA-mediated root growth inhibition. Seedlings were grown vertically on MS agar plates supplemented with 20 μM MeJA and root lengths were measured 7 days after sowing. Data are represented as mean ± s.e.m. of a minimum of 15 seedlings. The two-tailed Student’s t-test was used to compare means between the CYP7A12::GUS reporter line and the mutants. *P < 0.05, **P < 0.01. c, Transcript levels of the CYP71A12 gene in roots were measured 6 h after treatment with COR (0.5 μM), fig22 (100 nM), or both. Data are represented as mean ± s.e.m. of a minimum of 30 roots. **P < 0.01, ***P < 0.001. d, Transcript levels of three JA–COR-responsive genes, SCLP30 (At4g15100), VSP2 (At5g24770) and WAK2 (At1g21270), in roots 6 h after treatment with 0.5 μM COR. **P < 0.01, ***P < 0.001. All experiments were repeated at least twice with similar results. e, Schematic diagrams of SGT1b and SGT1a genes and T-DNA mutants. Boxes represent exons and horizontal lines represent introns. Filled boxes represent the TPR (black), CS (dark grey) and SGS (light grey) domains, while open boxes represent the flexible loop regions. Horizontal arrows mark the positions of primers used in measuring the transcript levels. Vertical arrow denotes the peptide epitope used for generating the SGT1b-specific antibody.
By screening for mutants retaining strong GUS activity during COR and fig22 co-treatment, we isolated 27 loss-of-function hsm (hormone-mediated suppression of MAMP-triggered immunity) mutants as described in Methods. Genetic analyses showed that 9 of these 27 mutants are allelic to COI1 and that one is allelic to MYC2. One of the remaining 17 mutants, hsm1, was insensitive to MeJA-triggered root growth inhibition, similar to the coi1-16 mutant (Fig. 1b), whereas the other 16 hsm mutants were either hypersensitive or as sensitive as the parental transgenic plant (Supplementary Fig. 1). We selected hsm1 for further analysis, reasoning that because hsm1 phenotypically mimics coi1-16, it might be required for maintaining steady state levels of COI.
In contrast to ‘wild-type’ CYP71A12::GUS roots, hsm1 roots showed strong GUS reporter activity (Fig. 1a) and high levels of CYP71A12 transcript (Fig. 1c) following co-treatment with COR + fig22. In addition, COR-triggered upregulation of the JA-responsive genes SCPL30 and VSP2 and downregulation of the JA-responsive gene WAK2 were substantially diminished in the hsm1 mutant (Fig. 1d).
hsm1 contains a mutant allele of SGT1b
We used a genome resequencing-assisted approach22,23 to map and identify the HSM1 gene. Computational analyses described in Methods showed that the SGT1b gene (At4g11260) was the most likely candidate corresponding to hsm1. In hsm1, a ‘C’ to ‘T’ nucleotide change introduces a premature stop codon at position Q251 (Fig. 1e).
We confirmed allelism between hsm1 and SGT1b by transforming hsm1 with a FLAG epitope-tagged wild-type SGT1b gene (SGT1b-FLAG) and an SGT1b1–250-FLAG construct (mimicking the truncated SGT1b protein in hsm1). Expression of the 35S: SGT1b-FLAG transgene, but not the 35S:SGT1b1–250-FLAG transgene, complemented the hsm1 mutant (Fig. 1a). A corresponding T-DNA insertion mutant, GABI_857A04, was also insensitive to COR-mediated suppression of fig22-triggered upregulation of CYP71A12 (Fig. 1c), COR-mediated upregulation of SCPL30 and VSP2 (Fig. 1d) and COR-mediated downregulation of WAK2 (Fig. 1d). MeJA-mediated root growth inhibition was also significantly suppressed in GABI_857A04 (Fig. 1b).
The Arabidopsis genome encodes two SGT1 proteins, SGT1a and SGT1b, which share 77% amino acid identity. In contrast to loss-of-function SGT1b mutants, however, an SGT1a T-DNA insertion mutant, SAIL_162_D04 (Fig. 1e), was phenotypically indistinguishable from the parental reporter line (Fig. 1b,c).
sgt1b mutants have low levels of SGT1b protein
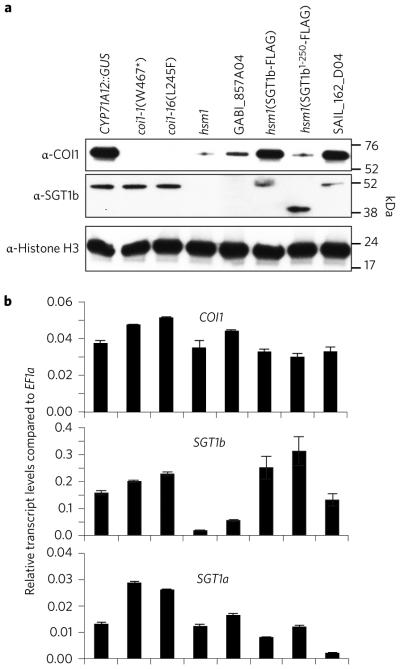
Immunoblot analysis showed that SGT1b protein could be readily detected in CYP71A12::GUS plants but was below the detection limit in the hsm1 and GABI_857A04 mutants (Fig. 2a). SGT1b protein was also readily detected in coi1-1 and coi1-16 mutants, in the sgt1a mutant SAIL_162_D04, and in the transgenic plants described above in which the SGT1b-FLAG construct was expressed from the 35S promoter in the hsm1 mutant background (Fig. 2a). Interestingly, hsm1(SGT1b1–250-FLAG) transgenic plants also accumulated a high level of truncated SGT1b1–250-FLAG protein, although the truncated protein does not appear to be active (see Fig. 1a). Quantitative RT-PCR analysis showed that the transcript levels of SGT1b, but not SGT1a, were greatly reduced in the hsm1 and GABI_857A04 mutants (Fig. 2b), suggesting that SGT1b plays a role in SGT1b transcription.
Figure 2. SGT1b and SGT1a are involved in maintaining COI1 stability.
a, Immunoblot assay of COI1 and SGT1b proteins in roots of 10-day-old Arabidopsis seedlings. b, Transcript levels of COI1, SGT1b, and SGT1a in the parental line and mutants.
sgt1b mutants contain low levels of COI1 protein
Due to the fact that SGT1b is a known co-factor of heat shock protein chaperones, we reasoned that COI1 may be a client protein of SGT1b–HSP complexes. Indeed, significantly lower levels of COI1 protein were detected in hsm1 and GABI_857A04 (Fig. 2a). Stable transgenic expression of SGT1b-FLAG, but not SGT1b1–250-FLAG, in the hsm1 mutant background restored COI1 protein levels (Fig. 2a). Consistent with the lack of a discernible JA phenotype in sgt1a RT-PCR analysis showed that COI1 transcript levels were roughly the same in the parental reporter line, coi1 mutants, the sgt1a mutant and sgt1b mutants (Fig. 2b). Collectively, these results suggest that SGT1b is required for maintaining COI1 stability, but not for COI1 transcription.
sgt1b mutants contain low levels of COI1 protein
Due to the fact that SGT1b is a known co-factor of heat shock protein chaperones, we reasoned that COI1 may be a client protein of SGT1b-HSP complexes. Indeed, significantly lower levels of COI1 protein were detected in hsm1 and GABI_857A04 (Fig. 2a). Stable transgenic expression of SGT1b-FLAG, but not SGT1b1-250-FLAG, in the hsm1 mutant background restored COI1 protein levels (Fig. 2a). Consistent with the lack of a discernible JA phenotype in sgt1a mutants (Fig. 1b,c), COI1 protein accumulated to wild-type levels in the sgt1a SAIL_162_D04 mutant (Fig. 2a). Quantitative RT-PCR analysis showed that COI1 transcript levels were roughly the same in the parental reporter line, coi1 mutants, the sgt1a mutant and sgt1b mutants (Fig. 2b). Collectively, these results suggest that SGT1b is required for maintaining COI1 stability, but not for COI1 transcription.
SGT1a is also involved in stabilizing COI1
The residual levels of COI1 protein detected in hsm1 and GABI_857A04 plants (Fig. 2a) may be due to the SGT1a protein, a close homologue of SGT1b. However, the embryonic lethality of an sgt1b sgt1a double mutant24 makes it difficult to test this hypothesis directly. We therefore examined COI1 protein levels after transiently knocking down both SGT1b and SGT1a in Arabidopsis mesophyll protoplasts using a pair of artificial microRNAs (amiRNAs) (Supplementary Fig. 2a). Unexpectedly, when we transfected a COI-HA construct in which COI1-HA is expressed from the native COI1 promoter, we found approximately equal levels of COI1-HA protein in CYP71A12::GUS and hsm1 protoplasts (Supplementary Fig. 2b). However, co-transfection of protoplasts with the COI1-HA construct plus the SGT1-amiRNAs significantly reduced COI1-HA levels (Supplementary Fig. 2b), suggesting that in mesophyll protoplasts, both SGT1a and SGT1b play significant roles in stabilizing COI1 protein.
An alternative explanation to the reduction of COI1 protein levels observed in sgt1b mutants (Fig. 2a and Supplementary Fig. 2b) is reduced translation of COI1 mRNA. However, this seems unlikely because COI1 levels dropped in protoplasts in which protein synthesis was blocked with cycloheximide and in which SGT1a and SGT1b were knocked down with amiRNAs (Supplementary Fig. 2b), suggesting that in the absence of the SGT1 proteins, the COI1 protein synthesized before the cycloheximide treatment is unstable.
COI1 associates with SGT1b, HSP70 and HSP90 in planta
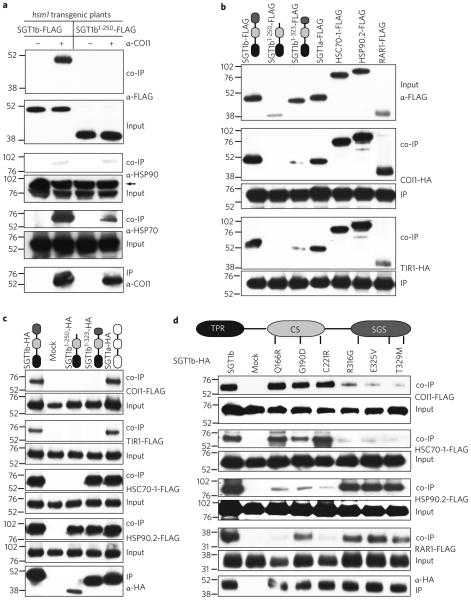
If COI1 is a client of SGT1b–HSP chaperone complexes, we reasoned that COI1 should co-immunoprecipitate (co-IP) with SGT1b. We used the stable transgenic plants described above expressing SGT1b-FLAG or SGT1b1–250-FLAG in the hsm1 mutant background for co-IP experiments. We readily detected SGT1b-FLAG protein following immunoprecipitation using anti-COI1 antibody of lysates from the roots of SGT1b-FLAG transgenic plants (Fig. 3a), demonstrating in planta association of COI1 with SGT1b. We also found that both SGT1b-FLAG and SGT1a-FLAG co-immunoprecipitated with COI1-HA in protoplast lysates (Fig. 3b).
Figure 3. COI1 associates with SGT1b–HSP70–HSP90 complexes.
a, Co-immunoprecipitation of SGT1b-FLAG, HSP70 and HSP90 using anti-COI1 antibody in roots of SGT1b-FLAG and SGT1b1–250-FLAG transgenic plants in the hsm1 background. Arrow indicates the input HSP90 proteins. b, Co-immunoprecipitation of SGT1b-FLAG, SGT1b1–250-FLAG, SGT1b1–323-FLAG, SGT1a-FLAG, HSC70-1-FLAG, HSP90.2-FLAG, RAR1-FLAG by COI1-HA or TIR1-HA in mesophyll protoplasts. The SGT1b1–250-FLAG construct mimics the premature truncated SGT1b protein in the hsm1 mutant and the SGT1b1–323-FLAG construct mimics the premature truncated SGT1b protein in the eta3 mutant. All experiments were repeated twice with similar results. c, Co-immunoprecipitation of COI1-FLAG, TIR1-FLAG, HSC70-1-FLAG and HSP90.2-FLAG by SGT1b-HA, SGT1b1–250-HA, SGT1b1–323-HA or SGT1a-HA in mesophyll protoplasts. d, Co-immunoprecipitation of COI1-FLAG, HSC70-1-FLAG, HSP90.2-FLAG and RAR1-FLAG by SGT1b-HA variants in protoplast lysates. Numbers on the sides of the blots are protein markers in kDa.
In contrast to full-length SGT1b-FLAG, SGT1b1–250-FLAG was not co-immunoprecipitated with COI1 in root extracts, even though the truncated SGT1b-FLAG protein accumulated to a comparable level as SGT1b-FLAG (Fig. 3a; see Input by α-FLAG) and a significant amount of COI1 was immunoprecipitated (Fig. 3a, see IP by α-COI1). SGT1b1–250-FLAG, as well as a second truncated SGT1b protein (SGT1b1–323-FLAG) that contains a 36 amino truncation25, also failed to be co-immunoprecipitated with COI1-HA in mesophyll protoplasts (Fig. 3b, co-IP). Similarly, in co-IP assays in protoplast lysates in which the HA and FLAG tags were swapped between COI1 and SGT1b, COI1-FLAG was not co-immunoprecipitated by SGT1b1–250-HA or SGT1b1–323-HA (Fig. 3c). The premature stop codon in SGT1b in hsm1 lies in the middle of a flexible hinge region connecting the middle CS domain and the C-terminal SGS domain of SGT1b protein. Therefore the entire SGS domain is absent in truncated SGT1b1–250 and is incomplete in SGT1b1–323, suggesting that the SGT1b SGS domain is required for COI1 binding.
Because SGT1b associates with COI1 (Fig. 3a–c) and because previous work has shown that SGT1b associates with HSP9010,17,19,20, we reasoned that HSP90 might also associate with COI1. However, COI1 associated to a much lesser extent with HSP90(s) than with COI1 in root extracts and the low level of co-immunoprecipitated HSP90(s) was independent of SGT1b (Fig. 3a). This may be due to the dynamic and transient association of HSP90 with its client proteins, as previously reported16, rather than a lack of COI1-HSP90 association. In support of the latter conclusion, when COI1-HA and one of the four cytosolic HSP90s, HSP90.2-FLAG, were transiently overexpressed in mesophyll protoplast lysates, a strong association between COI1-HA and HSP90.2-FLAG was observed (Fig. 3b).
To identify additional proteins that associate with SGT1b, we carried out mass spectrometric analyses of proteins co-immunopre-cipitated with SGT1b-FLAG (and SGT1b1–250-FLAG as a control) in mesophyll protoplasts. In addition to the expected HSP90 family proteins, we identified a number of HSP70 family proteins as the most abundant interactors (Supplementary Table 1), consistent with a previous study in which SGT1b co-immunoprecipitated with HSP70 proteins in Arabidopsis24. No HSP70 proteins were co-immunoprecipitated by SGT1b1–250-FLAG (Supplementary Table 1). We confirmed that SGT1b but not SGT1b1–250 interacts with HSP70s by showing co-IP of one of the five cytosolic HSP70s, HSC70-1-FLAG and SGT1b-HA in Arabidopsis proto-plasts (Fig. 3c).
The finding that SGT1b associates with HSP70 proteins prompted us to test whether COI1 associates with HSP70. Indeed, we observed a strong in planta association of COI and HSP70s in SGT1b-FLAG transgenic roots and a weaker association in SGT1b1–250-FLAG transgenic roots (Fig. 3a). The association between COI1-HA and HSC70-1-FLAG was confirmed via co-IP in protoplast lysates (Fig. 3b). Taken together, these results show that COI1 associates with and is a client protein of SGT1b– HSP70–HSP90 complexes.
SGT1b domains involved in SGT1b–COI1–HSP complex formation
To gain insight into the specific domains of SGT1b involved in the formation of SGT1b–COI1–HSP complexes, we examined COI1–SGT1b and SGT1b–HSP interactions in protoplasts using mutant SGT1b proteins containing single amino acid changes in various domains. We tested three SNPs in the middle CS domain and three SNPs in the C-terminal SGS domain of SGT1b that were reported previously to abolish SGT1b–HSP90 association17. The amino acid substitutions in the CS domain, Q166R, G190D and C221R, had no effect on SGT1b–HA and COI1–FLAG association, whereas the mutations in the SGS domain, R316G, E325V and T329M, significantly diminished the association (Fig. 3d). The SGS domain is also required for association with HSC70-1-FLAG (Fig. 3d), consistent with our findings (Fig. 3c) and a previous report24. In contrast, the CS domain is required for association with HSP90 and RAR1 (Fig. 3d), which is also consistent with a previous report17.
SGT1b is involved in auxin and gibberellic acid, but not brassinosteroid and abscisic acid signalling
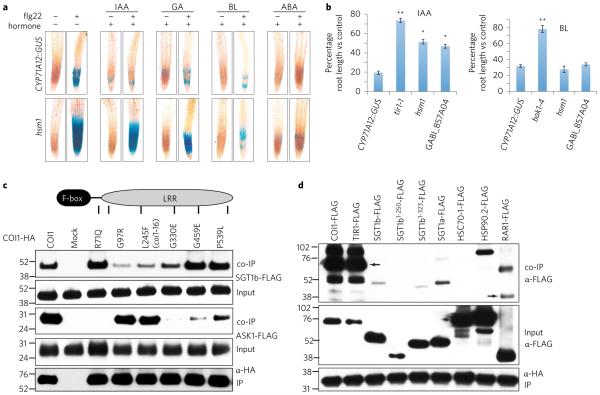
Previous studies have shown that similarly to JA–COR (Fig. 1), other plant hormones, including auxin and brassinolide, can block MAMP-mediated signalling26–28. Indeed, we found that auxin (indole-3-acetic acid, IAA), gibberellic acid (GA), brassinolide (BL) and abscisic acid (ABA) to various extents block fig22-mediated activation of CYP71A12::GUS in Arabidopsis roots (Fig. 4a).
Figure 4. SGT1b is involved in responses to hormones that utilize F-box proteins as receptors but functions independently of ASK1.
a, GUS histochemical staining in the root elongation zone of the CYP71A12::GUS reporter line and the hsm1 mutant following treatments with fig22 (100 nM) and IAA (10 μM), GA (50 μM), BL (5 μM) and ABA (5 μM). b, Hormone-mediated root growth inhibition in roots. Seedlings were grown vertically on MS agar plates supplemented with 0.5 μM IAA or 1 μM BL and root lengths were measured 7 days after sowing. Data are represented as mean ± s.e.m. of a minimum of 15 seedlings. The two-tailed Student’s t-test was used to compare means between the CYP7A12::GUS reporter line and mutants. *P < 0.05, **P < 0.01. c, Co-immunoprecipitation of SGT1b-FLAG and ASK1-FLAG by COI1-HA variants in protoplast lysates. d, Co-immunoprecipitation of SGT1b-FLAG variants and HSP-FLAGs by ASK1-HA. Arrows mark the position of COI1-FLAG, TIR1-FLAG and RAR1-FLAG. Numbers on the sides of the blots are protein markers in kDa. All experiments were repeated twice with similar results.
Consistent with previous work that suggested a role for SGT1b in auxin signalling25, we found that hsm1 retained strong GUS reporter activity upon IAA + fig22 co-treatment (Fig. 4a), and that hsm1 and GABI_857A04 are impaired in an IAA-mediated root growth inhibition response (Fig. 4b) and in IAA-mediated suppression of fig22-triggered CYP71A12 expression (Supplementary Fig. 3). Auxin signalling is mediated by an F-box protein, TIR1, which is evolutionarily related to and functionally analogous to COI129. TIR1 is required for the IAA-mediated suppression of fig22-elicited activation of CYP71A12 (Supplementary Fig. 3). We reasoned that TIR1 may also be a client protein of SGT1b–HSP90–HSP70 complexes. Indeed, SGT1b-FLAG, SGT1a-FLAG, HSC70-1-FLAG and HSP90.2-FLAG were co-immunoprecipitated by TIR1-HA in mesophyll protoplast lysates (Fig. 3b). In addition, knocking down SGT1b and SGT1a levels reduced TIR1-HA levels in protoplasts (Supplementary Fig. 4).
Importantly, hsm1 mutant roots also retained strong CYP71A12::GUS reporter activity following co-treatment of fig22 + GA, but not co-treatment of fig22 with BL or ABA (Fig. 4a). Like JA–COR and IAA, but in contrast to BL and ABA, GA also relies on an Arabidopsis F-box protein, SLY1, for perception30. These data suggest that SGT1b is broadly involved in responses to hormones that utilize F-box proteins as receptors. However, SGT1b does not appear to be involved in brassinolide signalling. BL exhibited an inhibitory effect on root growth in both wild-type and sgt1b mutant seedlings but did not inhibit root growth in a bak1-4 mutant, which is known to be impaired in BL signalling (Fig. 4b). Similarly, BL had a modest but significant effect in blocking fig22-mediated activation of CYP71A12 in both wild-type and sgt1b mutants, but not in a bak1-4 mutant (Supplementary Fig. 3), consistent with the data in Fig. 4a.
Association of SGT1b and COI1 is independent of the Arabidopsis SKP1 protein, ASK1
The fact that SGT1b is involved in stabilizing two F-box hormone receptors, COI1 and TIR1, and possibly SLY1, raises the possibility that SGT1b might associate with these F-box proteins indirectly by interacting with ASK1, the SKP1 protein in Arabidopsis. To examine this, we used a set of loss-of-function coi1 alleles to examine their association with SGT1b and ASK1. The COI1 variants examined included coi1-16 (L245F) as well as five coi1 alleles identified in the genetic screen that yielded hsm1. Although the coi1-16 mutant accumulates a very low level of mutant COI1 protein in planta (Fig. 2a), the corresponding COI1L245F-HA construct accumulated to relatively high levels in mesophyll protoplast lysates, as did the five other COI1 SNP variants (Fig. 4c, IP). SGT1b-FLAG associated with COI1R71Q-HA, COI1G459E-HA and COI1P539L-HA similarly as with wild-type COI1-HA but associated weakly with COI1G97R-HA, COI1L245F-HA and COI1G330E-HA (Fig. 4c), suggesting that the N-terminal half of the LRR domain of the COI1 protein is required for association with SGT1b-FLAG. ASK1-FLAG associated with COI1G97R-HA and COI1L245F-HA similarly as with wild-type COI1-HA but weakly with COI1G330E-HA, COI1G459E-HA and COI1P539L-HA (Fig. 4d).
Interestingly, although COI1R71Q-HA associated strongly with SGT1b-FLAG, there was no detectable association between COI1R71Q-HA and ASK1-FLAG, suggesting that COI1-SGT1b association is direct. Moreover, ASK1-HA only weakly associated with SGT1b-FLAG, SGT1b1–323-FLAG and SGT1a-FLAG (Fig. 4d), suggesting that ASK1-SGT1b association is mediated indirectly by COI1-like F-box proteins or HSP90.
HSP70 and HSP90 play a role in JA–COR responses
Having established that COI1 associates with SGT1b–HSP70–HSP90, we studied the biological relevance of HSPs in JA–COR responses using two independent RNAi knockdown lines, RNAi-A1 and RNAi-C1, in which cytosolic HSP90 genes are non-specifically targeted and HSP90 transcript levels are significantly reduced31. Both RNAi-A1 and RNAi-C1 accumulated modestly lower levels (approximately two-fold, but statistically significant) of SCPL30 and VSP2 transcripts and slightly higher WAK2 transcript upon COR treatment in roots compared to the parental reporter line (Fig. 5a).
Figure 5. HSP70 and HSP90 are required for JA–COR responses.
a, Transcript levels of SCPL30, VSP2 and WAK2 in roots of HSP90 RNAi-A1 and RNAi-C1 lines 6 h after treatment with 0.5 μM COR. Data are represented as mean ± s.e.m. of a minimum of 30 roots. *P < 0.05, **P < 0.01. b, Transcript levels of SCPL30, VSP2 and WAK2 in CYP71A12::GUS roots after a 30 min pretreatment with 20 μM GDA followed by treatment with 0.5 μM COR. Roots were harvested 6 h after COR treatment. Data are represented as mean ± s.e.m. of a minimum of 30 roots. *P < 0.05. c, Immunoblot analyses of COI1 protein levels in roots of the CYP71A12::GUS reporter line after treatment with 25 μM GDA or 100 μM Ver55008. Roots were harvested at the times indicated. Numbers on the sides of the blots are protein markers in kDa. d, MeJA-mediated root growth inhibition in roots. Seedlings were grown vertically on MS agar plates supplied with 20 μM MeJA, 25 μM GDA or 50 μM Ver155008 and root lengths were measured 7 days after sowing. Data are represented as mean ± s.e.m. of a minimum of 15 seedlings. *P < 0.05, **P < 0.01. All experiments were repeated twice with similar results.
We also inactivated HSP90 and HSP70 activity with specific inhibitors, including geldanamycin (GDA), a specific inhibitor of HSP90s. Pretreatment with GDA for 30 min, compared to simultaneous treatment or to 2-hour or overnight pretreatments with GDA, had an optimal effect on reducing SCPL30 and VSP2 and modestly increasing WAK2 transcript levels in roots (Fig. 5b, Supplementary Fig. 5). Similar results were obtained with pretreatment by Ver155008, an inhibitor of HSP70 activity (Fig. 5b).
Immunoblotting revealed that COI1 protein levels noticeably decreased 30 and 60 min after GDA and Ver155008 treatments before returning to normal levels after 2 h (Fig. 5c). This correlates well with the time window of GDA pretreatment effects on SCPL30 and WAK2 expression (Supplementary Fig. 5), suggesting that HSP proteins function in JA–COR responses by directly affecting COI1 protein levels. In a root growth inhibition assay, GDA treatment mitigated MeJA-mediated inhibition of root growth in the CYP71A12::GUS reporter line (Fig. 5d). The mitigation also occurred in hsm1 and GABI_857A04 mutants, but to a lesser extent (Fig. 5d).
Discussion
SGT1b is required for JA, IAA and GA responses in plants
Previous genetic studies suggested that SGT1b is required for jasmonate and auxin hormone responses in plants24,25,32, although the underlying molecular mechanisms were not addressed. In this study, we combined biochemical and genetic approaches to demonstrate that COI1 stability is compromised in sgt1b mutants, that COI1 associates with SGT1b in vivo, and that the diminution of JA–COR responses in sgt1b mutants directly correlates with reduced COI1 stability. We also show that TIR1, like COI1, associates with SGT1b (Fig. 3b,c) and that SGT1b stabilizes TIR1 in protoplast lysates (Supplementary Fig. 4). In addition, we made the unexpected discovery that IAA and GA, similarly to JA–COR, block fig22-mediated signalling in an SGT1b-dependent manner. In contrast, SGT1b does not appear to be involved in brassinosteroid and ABA signalling. Collectively, these results suggest that SGT1b plays a broad and essential role in plant hormone signalling pathways that involve F-box proteins and E3 ubiquitin ligases such as COI1 and TIR1 as components of hormone receptor complexes.
hsm1 and GABI_857A04 do not resemble coi1 null alleles
Although our data show that SGT1b is required to maintain COI1 protein stability, hsm1 and GABI_857A04, have less severe phenotypes than coi1 null alleles such as coi1-1 and coi1-16, which are completely defective in JA–COR responses33–35. The absence of stable COI1 protein in coi1-1 and coi1-16 (Fig. 2a) is consistent with their null phenotypes. In contrast to coi1-1 and coi1-16 mutants, hsm1 and GABI_857A04 are indistinguishable from wild-type plants with respect to fertility, trichome development and anthocyanin accumulation (data not shown), similar to leaky coi1 mutants36, which is most likely attributable to residual levels of COI1 protein (Fig. 2a). Because SGT1a can stabilize COI1 and TIR1 proteins in protoplasts similarly to SGT1b (Supplementary Fig. 4), it is likely that SGT1a is responsible for maintaining residual COI1 levels in sgt1b mutants. Notably, because sgt1b mutants have residual levels of COI1 activity, they can be used to identify branches of the JA signalling network that require higher or lower levels of COI1 protein. In other words, JA–COR-mediated suppression of fig22 responses and root growth inhibition apparently require high levels of COI1 protein because they are impaired in sgt1b mutants, whereas JA– COR-mediated fertility, trichome development and anthocyanin accumulation only require very low levels of COI1 activity and are normal in sgt1b mutants.
HSP chaperones are required for JA–COR responses
A notable function of HSP chaperones in plants is the stabilization of R proteins. Several R proteins can be co-immunoprecipitated with HSP90, including the Arabidopsis RPM110, the tobacco N12 and the barley MLA1 and MLA6 proteins37. Recent studies have also shown that the Arabidopsis F-box proteins Zeitlupe and FKF1, involved in flowering time regulation11,38, and the Arabidopsis transcription factor BES1, involved in BR signalling39, are also HSP90 clients. Here we show that both HSP70 and HSP90 play important roles in JA–COR responses. Knockdown of HSP90 levels via RNAi reduced the transcript levels of the JA–COR upregulated genes SCPL30 and VPS2 (Fig. 5a). Pretreatment with the HSP90 inhibitor GDA or the HSP70 inhibitor Ver155008 significantly attenuated COR-triggered gene induction and root growth inhibition, respectively (Fig. 5b,d).
These biochemical and pharmacological studies suggest that COI1 is a client protein of SGT1b–HSP70–HSP90 chaperone complexes. Given the facts that the auxin and JA receptors TIR1 and COI1, respectively, function analogously to F-box proteins, that SGT1b is required for auxin signalling (Fig. 4a,b)25, and that TIR1 also associates with SGT1b–HSP70–HSP90 complexes (Fig. 3b), it is likely that HSP70 and HSP90 also play important roles in stabilizing TIR1 as they do in stabilizing COI1. More generally, it appears that SGT1b may be more specific than HSP90 with respect to its clients. That is, HSP90 functions broadly in many hormone pathways, including the BR pathway39, whereas SGT1b only appears to be required for the hormone pathways that utilize F-box proteins as receptors.
Methods
Plant materials, growth conditions and chemical treatments
Arabidopsis plants included in this study are in the Columbia (Col-0) background. For most experiments, seedlings were grown in 6-well or 12-well plates in 1X liquid MS medium (pH 5.7) supplemented with 0.5% sucrose and 0.5 g l−1 MES hydrate. Seedlings were grown in a growth chamber at 22 °C under 16 h of light with a light intensity of 100 μE. The medium was exchanged on day 8 and experiments were performed on day 10 by directly adding 100 nM fig22, 0.5 μM COR, 0.5 μM IAA, 1 μM BL, 25 μM GDA or 50 μM Ver15508 as indicated. To separate roots from the shoots, sterilized seeds were sown onto pre-cut, autoclaved polypropylene mesh (9275T8, McMaster-Carr Inc.). For root growth inhibition assays, sterilized seeds were sown onto 1X MS medium solidified with 8 g l−1 phytoagar containing 0.5% sucrose, 20 μM MeJA, 0.5 μM IAA, 1 μM BL, 25 μM GDA or 50 μM Ver15508 as indicated. Seedlings were grown by orienting the plates vertically in a growth chamber. The GABI_857A04 line was ordered from the GABI-Kat seed stock centre and the SAIL_162_D04 line was ordered from the Arabidopsis Biological Resources Center and confirmed by genotyping according to the Center’s instructions.
Genome resequencing-assisted mapping and cloning of HSM1
The HSM1 (SGT1b) gene was cloned following a genome resequencing–assisted mapping and cloning procedure for EMS mutants22,23. Briefly, hsm1 was outcrossed to Ler to yield an F1 hybrid, followed by self-fertilization to produce F2 segregants. A total of 89 F2 segregants (66 homozygous and 23 heterozygous for the GUS reporter gene) were selected on the basis of retained GUS reporter activity in roots following COR and fig22 co-treatment. Genomic DNA from these 89 segregants was extracted, pooled and subjected to library preparation for Illumina-based next-generation sequencing23. Paired-end sequencing was carried out in one lane of an Illumina Hi-seq machine. In total, 52,236,571 reads were obtained and aligned to the Col-0 reference genome. By implementing a suite of biocomputational programmes23, an enrichment of Col-0 SNPs, that is corresponding to the enrichment of the hsm1 mutation, was observed in the bottom arm of chromosome 4 spanning a region of 6.8 to 6.9 million base pairs in AGI coordinates, thereby delimiting the causal hsm1 mutation. Further sequence analyses revealed a single candidate gene with a ‘C’ to ‘T’ nucleotide change in the coding region of the SGT1b gene and this nucleotide change was confirmed by Sanger sequencing.
Co-immunoprecipitation and protein blot analyses
For co-IP analysis using root tissue, about 200–300 mg of roots were ground in liquid N2 into a fine powder and resuspended in 500 μl IP buffer containing 50 mM HEPES-KOH, pH 7.4, 10 mM EDTA, 25 mM sucrose, 5% glycerol supplied with 2 mM DTT, 100 nM PMSF and 1 tablet of EDTA-free protease inhibitor cocktail (Roche) per 10 ml buffer. The lysate was briefly cleared by centrifugation in a microfuge at maximum speed at 4 °C for 10 min. The supernatants were pre-cleared by incubating with 30 μl IgG agarose beads (Sigma-Aldrich) at 4 °C for 2 h followed by incubation with 30 μl Protein A magnetic beads (New England Biolabs) at 4 °C for 2 h. Pre-cleared lysates were incubated with 25 μl of anti-COI1 antibody (AgriSera) at 4 °C for 4 h followed by three washes with IP buffer containing 50 mM HEPES-KOH, pH 7.4, 10 mM EDTA and 100 mM NaCl. The beads were finally resuspended in 50 μl 2X SDS sample buffer.
Co-IP with protoplast lysates was performed as described previously40
Briefly, harvested protoplasts were resuspended in 0.5 ml IP lysis buffer (25 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 10% glycerol, 0.5% Triton X-100 supplemented with 1 tablet of EDTA-free protease inhibitor cocktail (Roche) per 10 ml buffer). The cells were lysed by vigorous vortexing for 40 s followed by centrifugation in a microfuge at maximum speed for 10 min at 4 °C. The supernatant was incubated with 10 μl magnetic anti-HA beads (Thermo Fisher Scientific) for 3 h at 4 °C followed by three washes with IP lysis buffer. The beads were finally resuspended in 50 μl 2X SDS sample buffer.
Immunoblot assays were performed following standard procedures. Samples were separated on NuPAGE 4–12% Bis-Tris protein gels (Invitrogen), transferred onto PVDF membrane (Fisher Scientific), and blocked in TBS-T buffer with 5% non-fat dry milk at room temperature for 1–2 h. Blots were incubated with primary antibody in TBS-T buffer with 5% non-fat dry milk at 4 °C overnight. The antibodies included in this study are α-SGT1b (this study), α-COI1 and α-HSP70 (AgriSera), α-HSP90 (Santa Cruz), α-FLAG (Sigma-Aldrich), α-HA (Roche) and α-HistoneH3 (Abcam).
Protoplast preparation and transfection
Preparation of mesophyll protoplasts has been described previously40. 50 μg of each HA- and FLAG-tagged plasmid or empty vector was used to co-transfect 0.5 ml protoplast lysates. Protoplast cells were harvested 12 h after transfection for co-IP assays. For measuring COI1-HA and TIR1-HA levels after knocking down SGT1b and SGT1a in protoplasts, 24 μg plasmid DNA corresponding to each of the two SGT1-specific amiRNAs (see Supplementary Table 2 for sequences), 1 μg COI1-HA or TIR1-HA, or SGT1b-HA or SGT1a-HA plasmid, and 1 μg GFP-HA plasmid were used to co-transfect 0.5 ml mesophyll protoplasts, and protoplasts were harvested 24 h after transfection. For CHX treatment in protoplasts, 50 μg plasmid DNA corresponding to each of the two amiRNAs or 100 μg COI1-HA was used to transfect 1 ml of mesophyll protoplasts. After 12 h, 10 μM CHX was added to the transfected protoplasts and the protoplasts were harvested after 1 h.
Supplementary Material
Acknowledgements
We thank D. Bartenstein, C. Mankiw and M. Cerulli for help in EMS mutant screening, C. Queitsch for providing the HSP90 RNAi-A1 and RNAi-C1 constructs, M. Borowsky for computational analyses of the Illumina sequencing reads and mapping of the hsm1 mutant, C. Haney and D. McEwan for discussion and critical reading, and J. Li, O. Liu, Y. Niu, L. Li and J. Sheen for plasmids, constructs and technical advice. This work was supported by NSF grants MCB-0519898 and IOS-0929226 and NIH grants GM48707 and P30 DK040561 awarded to F.M.A.
Footnotes
Author contributions
X-C. Z., Y. M. and F. M. A. designed the study; X-C. Z., Y. M., Z. C. and J. B. performed experiments; X-C. Z. and F. M. A. wrote the manuscript.
Additional information
Supplementary information is available online. Reprints and permissions information is available online at www.nature.com/reprints. Correspondence and requests for materials should be addressed to F.M.A.
Competing interests
The authors declare no competing financial interests.
References
- 1.Chow B, McCourt P. Plant hormone receptors: perception is everything. Genes Dev. 2006;20:1998–2008. doi: 10.1101/gad.1432806. [DOI] [PubMed] [Google Scholar]
- 2.Spartz AK, Gray WM. Plant hormone receptors: new perceptions. Genes Dev. 2008;22:2139–2148. doi: 10.1101/gad.1693208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bari R, Jones JD. Role of plant hormones in plant defence responses. Plant Molec. Biol. 2009;69:473–488. doi: 10.1007/s11103-008-9435-0. [DOI] [PubMed] [Google Scholar]
- 4.Millet YA, et al. Innate immune responses activated in Arabidopsis roots by microbe-associated molecular patterns. Plant Cell. 2010;22:973–990. doi: 10.1105/tpc.109.069658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acosta IF, Farmer EE. Jasmonates. Arabidopsis Book. 2010;8:e0129. doi: 10.1199/tab.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheard LB, et al. Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature. 2010;468:400–405. doi: 10.1038/nature09430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chini A, et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature. 2007;448:666–671. doi: 10.1038/nature06006. [DOI] [PubMed] [Google Scholar]
- 8.Thines B, et al. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature. 2007;448:661–665. doi: 10.1038/nature05960. [DOI] [PubMed] [Google Scholar]
- 9.Kitagawa K, Skowyra D, Elledge SJ, Harper JW, Hieter P. SGT1 encodes an essential component of the yeast kinetochore assembly pathway and a novel subunit of the SCF ubiquitin ligase complex. Mol. Cell. 1999;4:21–33. doi: 10.1016/s1097-2765(00)80184-7. [DOI] [PubMed] [Google Scholar]
- 10.Hubert DA, et al. Cytosolic HSP90 associates with and modulates the Arabidopsis RPM1 disease resistance protein. EMBO J. 2003;22:5679–5689. doi: 10.1093/emboj/cdg547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim TS, et al. HSP90 functions in the circadian clock through stabilization of the client F-box protein ZEITLUPE. Proc. Natl Acad. Sci. USA. 2011;108:16843–16848. doi: 10.1073/pnas.1110406108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Burch-Smith T, Schiff M, Feng S, Dinesh-Kumar SP. Molecular chaperone Hsp90 associates with resistance protein N and its signaling proteins SGT1 and Rar1 to modulate an innate immune response in plants. J. Biol. Chem. 2004;279:2101–2108. doi: 10.1074/jbc.M310029200. [DOI] [PubMed] [Google Scholar]
- 13.Davies AE, Kaplan KB. Hsp90-Sgt1 and Skp1 target human Mis12 complexes to ensure efficient formation of kinetochore-microtubule binding sites. J. Cell. Biol. 2010;189:261–274. doi: 10.1083/jcb.200910036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lingelbach LB, Kaplan KB. The interaction between Sgt1p and Skp1p is regulated by HSP90 chaperones and is required for proper CBF3 assembly. Mol. Cell. Biol. 2004;24:8938–8950. doi: 10.1128/MCB.24.20.8938-8950.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubacq C, Guerois R, Courbeyrette R, Kitagawa K, Mann C. Sgt1p contributes to cyclic AMP pathway activity and physically interacts with the adenylyl cyclase Cyr1p/Cdc35p in budding yeast. Eukaryot. Cell. 2002;1:568–582. doi: 10.1128/EC.1.4.568-582.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taipale M, et al. Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell. 2012;150:987–1001. doi: 10.1016/j.cell.2012.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boter M, et al. Structural and functional analysis of SGT1 reveals that its interaction with HSP90 is required for the accumulation of Rx, an R protein involved in plant immunity. Plant Cell. 2007;19:3791–3804. doi: 10.1105/tpc.107.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M, et al. Structural and functional coupling of Hsp90- and Sgt1-centred multi-protein complexes. EMBO J. 2008;27:2789–2798. doi: 10.1038/emboj.2008.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin MJ, et al. Regulatory role of SGT1 in early R gene-mediated plant defenses. Science. 2002;295:2077–2080. doi: 10.1126/science.1067747. [DOI] [PubMed] [Google Scholar]
- 20.Azevedo C, et al. Role of SGT1 in resistance protein accumulation in plant immunity. EMBO J. 2006;25:2007–2016. doi: 10.1038/sj.emboj.7601084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez-Gomez L, Boller T. FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol. Cell. 2000;5:1003–1011. doi: 10.1016/s1097-2765(00)80265-8. [DOI] [PubMed] [Google Scholar]
- 22.Cuperus JT, et al. Identification of MIR390a precursor processing-defective mutants in Arabidopsis by direct genome sequencing. Proc. Natl Acad. Sci. USA. 2010;107:466–471. doi: 10.1073/pnas.0913203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X-C, Millet YA, Ausubel FM, Borowsky M. Next-gen sequencing-based mapping and identification of ethyl methanesulfonate-induced mutations in Arabidopsis thaliana. Curr. Protoc. Mol. Biol. 2014;108:7.18.1–7.18.16. doi: 10.1002/0471142727.mb0718s108. [DOI] [PubMed] [Google Scholar]
- 24.Noel LD, et al. Interaction between SGT1 and cytosolic/nuclear HSC70 chaperones regulates Arabidopsis immune responses. Plant Cell. 2007;19:4061–4076. doi: 10.1105/tpc.107.051896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray WM, Muskett PR, Chuang HW, Parker JE. Arabidopsis SGT1b is required for SCF(TIR1)-mediated auxin response. Plant Cell. 2003;15:1310–1319. doi: 10.1105/tpc.010884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albrecht C, et al. Brassinosteroids inhibit pathogen-associated molecular pattern-triggered immune signaling independent of the receptor kinase BAK1. Proc. Natl Acad. Sci. USA. 2012;109:303–308. doi: 10.1073/pnas.1109921108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Oirdi M, et al. Botrytis cinerea manipulates the antagonistic effects between immune pathways to promote disease development in tomato. Plant Cell. 2011;23:2405–2421. doi: 10.1105/tpc.111.083394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savatin DV, Ferrari S, Sicilia F, De Lorenzo G. Oligogalacturonide-auxin antagonism does not require posttranscriptional gene silencing or stabilization of auxin response repressors in Arabidopsis. Plant Physiol. 2011;157:1163–1174. doi: 10.1104/pp.111.184663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435:441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- 30.Fu X, et al. The Arabidopsis mutant sleepy1gar2-1 protein promotes plant growth by increasing the affinity of the SCFSLY1 E3 ubiquitin ligase for DELLA protein substrates. Plant Cell. 2004;16:1406–1418. doi: 10.1105/tpc.021386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sangster TA, et al. Phenotypic diversity and altered environmental plasticity in Arabidopsis thaliana with reduced Hsp90 levels. PLoS ONE. 2007;2:e648. doi: 10.1371/journal.pone.0000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uppalapati SR, et al. SGT1 contributes to coronatine signaling and Pseudomonas syringae pv. tomato disease symptom development in tomato and Arabidopsis. New Phytol. 2011;189:83–93. doi: 10.1111/j.1469-8137.2010.03470.x. [DOI] [PubMed] [Google Scholar]
- 33.Feys B, Benedetti CE, Penfold CN, Turner JG. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell. 1994;6:751–759. doi: 10.1105/tpc.6.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noir S, et al. Jasmonate controls leaf growth by repressing cell proliferation and the onset of endoreduplication while maintaining a potential stand-by mode. Plant Physiol. 2013;161:1930–1951. doi: 10.1104/pp.113.214908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG. COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science. 1998;280:1091–1094. doi: 10.1126/science.280.5366.1091. [DOI] [PubMed] [Google Scholar]
- 36.He Y, Chung EH, Hubert DA, Tornero P, Dangl JL. Specific missense alleles of the Arabidopsis jasmonic acid co-receptor COI1 regulate innate immune receptor accumulation and function. PLoS Genet. 2012;8:e1003018. doi: 10.1371/journal.pgen.1003018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bieri S, et al. RAR1 positively controls steady state levels of barley MLA resistance proteins and enables sufficient MLA6 accumulation for effective resistance. Plant Cell. 2004;16:3480–3495. doi: 10.1105/tpc.104.026682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song YH, et al. Distinct roles of FKF1, Gigantea, and Zeitlupe proteins in the regulation of Constans stability in Arabidopsis photoperiodic flowering. Proc. Natl Acad. Sci. USA. 2014;111:17672–17677. doi: 10.1073/pnas.1415375111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lachowiec J, et al. The protein chaperone HSP90 can facilitate the divergence of gene duplicates. Genetics. 2013;193:1269–1277. doi: 10.1534/genetics.112.148098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li JF, Bush J, Xiong Y, Li L, McCormack M. Large-scale protein-protein interaction analysis in Arabidopsis mesophyll protoplasts by split firefly luciferase complementation. PLoS ONE. 2011;6:e27364. doi: 10.1371/journal.pone.0027364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.