Abstract
Poly(ethylene glycol) (PEG)-based hydrogels are extensively used in a variety of biomedical applications due to ease of synthesis and tissue-like properties. Recently, there have been varied reports regarding PEG hydrogel’s degradation kinetics and in vivo host response. In particular, these studies suggest that the surrounding tissue environment could play a critical role in defining the inflammatory response and degradation kinetics of PEG implants. In the present study we demonstrated a potential mechanism of PEG hydrogel degradation, and in addition we show potential evidence of the role of the surrounding tissue environment on producing variable inflammatory responses.
Keywords: Poly(ethylene glycol), hydrogel, degradation, inflammatory response, reactive oxygen species (ROS), neutrophils, macrophages
Biomaterials play important roles in modern tissue engineering and regenerative medicine strategies. In particular, polymeric scaffolds including synthetic polymers, have been engineered to create a 3D environment for cells and to mimic the physical properties of native tissue (Seliktar, 2005). Among synthetic polymers, poly(ethylene glycol) (PEG) has been explored and is frequently used in applications ranging from drug delivery devices to tissue engineering scaffolds (Lin and Anseth, 2009). In particular PEG has been frequently applied as a 3D cell culture system and vehicle due to its ease of use and cytocompatibility including its resistance to protein adsorption (Kim et al., 2010). All materials, including PEG, elicit a foreign body response (FBR) when implanted in vivo (Anderson et al., 2008). The FBR leads to nonspecific protein adsorption, followed by inflammatory cells that secrete cytokines, chemotactic factors, and reactive oxygen species (ROS), all of which could play a role in scaffold degradation (Anderson et al., 2008). Consequently, there are varied reports describing the degradation kinetics of PEG-based materials (Lynn et al., 2010, Hillel et al., 2011, Wang et al., 2007). Our lab has observed an increased inflammatory response from PEG-HA implants placed in the human abdomen in comparison to subcutaneous dorsal implants in rats (Hillel et al., 2011). Potential reasons for the variation inflammatory response could be species-specific differences (rat versus human), mismatch in mechanical properties of the PEG-based material compared to native tissue environment, location of implant, and/or mechanical disruption/agitation of the abdominal implants. However, it was noted that the greatest inflammatory response to the PEG-containing implants was found in proximity to adipose tissue. Recent reports suggest that the local environment of adipose tissue might play a role in inciting or augmenting an inflammatory response, potentially through the effects of adipokines (Mathis and Shoelson, 2011, Hillel et al., 2011, Lee et al., 2009). Consequently, the ROS generation from this inflammatory response may impact the behavior of the PEG-based hydrogels including degradation kinetics. In this work, we investigate a potential degradation mechanism based on ROS, a byproduct of inflammatory cells and local tissue environment.
Traditionally, it is believed that poly(ethylene glycol) diacrylate (PEGDA) hydrogels degrade due to ester hydrolysis at the sterically protected acrylate end groups, which is the cause of a slow degradation rate (Metters et al., 2000). We utilized the Fenton reaction (Wardman and Candeias, 1996) to completely degrade PEGDA hydrogels within 4 days. The Fenton reagent used in our studies consisted of 50% hydrogen peroxide and 6.2 mM FeCl3. Hydrogel solution was prepared by mixing 10% (w/v) PEGDA (3.4 kDa Sunbio) in sterile phosphate-buffered saline (PBS). The photoinitiator, Igracune 2959 (Ciba Specialty Chemicals, Tarrytown, NY), was added to the PEGDA solution and mixed thoroughly to make a final concentration of 0.05% (w/v). One hundred microliters of the solution was loaded into cylindrical molds (6 mm diameter) and polymerized with UV light (3 mW/cm2) for 5 minutes. Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS) of the completely degraded hydrogel did not show any molecular weights of 3400 Da (which is the initial molecular weight of the PEGDA macromer used for polymerization) (Figure 1A). Instead we observed molecular weights below 2000 Da and consistent mass differences of 44 Da, thereby suggesting degradation of the PEG backbone (44 Da) rather than hydrolysis of the ester groups.
Figure 1.
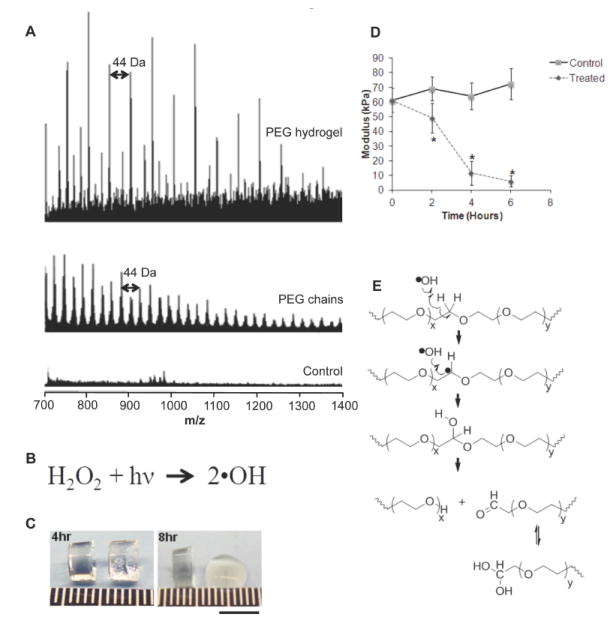
(A) MALDI-TOF MS of a completely degraded PEGDA hydrogel via Fenton reaction. Note the consistent mass differences of 44 Da depicting degradation of PEG backbone. PEGDA used was 3400 Da. (B) UV radiation dissociates hydrogen peroxide into hydroxyl radicals. (C) Control (left) and treated hydrogels (right) at 4 and 8 hrs. Scale bar: 5mm. (D) Young’s modulus of control and treated hydrogels (n=5). Data were analyzed with an unpaired, two-tailed Student’s t test. Statistical significance was determined and represented with asterisks: *p < 0.05. (E) Mechanism of hydroxyl radicals degrading the backbone of PEG into PEG-alcohol and PEG-aldehydes.
The Fenton reaction consists of various forms of ROS similar to those secreted by inflammatory cells in vivo. We sought to investigate two common types of ROS, hydrogen peroxide and hydroxyl radicals. We utilized UV radiation to dissociate hydrogen peroxide into hydroxyl radicals (Figure 1B). Our control group consisted of PEGDA hydrogels in 30% hydrogen peroxide and our treatment group was PEGDA hydrogels in 30% hydrogen peroxide but exposed to UV light. After 8 hours, the treated hydrogels were visibly degraded (Figure 1C). The Young’s modulus of the ROS-treated hydrogels decreased significantly, suggesting breakdown of the hydrogel network, compared to controls hydrogels, which showed no change (Figure 1D). These results demonstrate that hydroxyl radicals can play a role in PEG degradation (Figure 1E), which correlates with our previous work describing hydroxyl radicals modifying the backbone of PEG (Reid et al., 2010).
As mentioned above, there are multiple factors that may impact PEG behavior and response in vivo, in particularly the location of implants. Our lab reported an increased inflammatory response in PEG-HA implants placed in proximity to adipose tissue (Hillel et al., 2011). A recent report demonstrates the degradation kinetics of collagen gels vary significantly in a location specific manner (Artzi et al., 2011). Therefore, we decided to evaluate the early inflammatory response to PEG hydrogels within different tissue environments in the rat including the subcutaneous space, intraperitoneal, and the posterolateral aspect of the thigh which contains a depot of fat (Cinti et al., 2006). All procedures were performed according to the Institutional Animal Care and Use Committee at Johns Hopkins University School of Medicine. Six week old male Sprague-Dawley rats (Charles River, Wilmington, MA) weighing approximately 200 g were used in these studies. In all cases, surgical access was made through a 1 cm skin incision placed approximately 2 cm from the implantation site. This approach was used to minimize inflammation and trauma near the construct. For the subcutaneous implants, constructs were placed in bluntly dissected surgical pockets on the dorsolateral surface at the level of the scapula. Intraperitoneal placement was within the abdominal cavity between the left lateral and caudate lobes of the liver. Access to the abdominal cavity was through a 1cm midline incision below the xiphoid process. Implants placed within adipose tissue were achieved through bluntly dissected pockets within the belly of the biceps femoris located on the posterolateral aspect of the thigh. The intramuscular pocket was closed with running 5-0 Vicryl (Ethicon, Somerville, NJ). In all cases the skin incision was closed with running 4-0 Vicryl. For each location, one 100 microliter construct was polymerized ex vivo and rinsed multiple times in sterile PBS over 2 days to remove any unreacted reactants and unincorporated polymer, and was evaluated in at least 3 different animals. After 7 days the implants were explanted with surrounding tissue and fixed in 4% paraformaldehyde for 24 hours, and later dehydrated, embedded in paraffin, and sectioned using a microtome (~7 μm thick). Tissue sections were stained with hematoxylin and eosin and analyzed by a pathologist (Figure 2).
Figure 2.
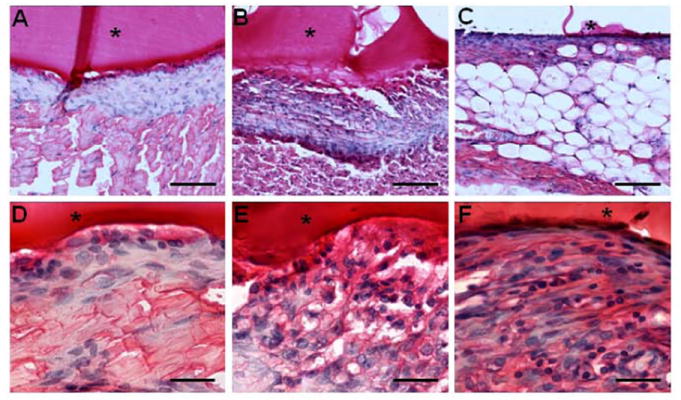
The in vivo host response to PEGDA hydrogels when implanted in the (A,D) dorsal subcutaneous space, (B,D) abdominal cavity, and (C,F) postlateral muscle of Sprague-Dawley rats at 7 days. Tissue was stained with hematoxylin and eosin. (A-C) The sections were imaged with a 20x objective. Scale bar: 100 μm. (D-F) The sections were imaged with a 63x objective. Scale bar: 50 μm. The location of the implanted material is denoted by asterisk.
Histopathological analysis found that subcutaneous implants showed the least inflammatory response and did show evidence of macrophages. PEG hydrogel implanted within the abdominal cavity between the left lateral and caudate lobes of the liver showed a higher inflammatory response compared to subcutaneous implants, and were primarily composed of more macrophages and a few neutrophils. However, implants within adipose tissue elicited the highest inflammatory response with neutrophils and macrophages within the capsule. This supports the notion that the local environment, in particularly those such as adipose tissue, may play a role inciting or augmenting an inflammatory response (Mathis and Shoelson, 2011, Hillel et al., 2011, Lee et al., 2009). Consequently, the ROS generation from this inflammatory response can affect the biomaterial eventually causing degradation by mechanisms defined here via hydroxyl radicals elicited by macrophages and neutrophils (Smith, 1994, Tauber and Babior, 1977). In conclusion, we demonstrated a proposed mechanism of PEG hydrogel degradation, and provided evidence of the role of the local tissue environment on biomaterial response.
Acknowledgments
The authors acknowledge the financial support from NIH/NIDCR (3R01DE016887-03S1).
References
- Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artzi N, Olivia N, Puron C, Shitreet S, Artzi S, Ramos AB, Groothuis A, Sahagian G, Edelman ER. In vivo and in vitro tracking of erosion in biodegradable materials using non-invasive fluorescence imaging. Nature Mater. 2011;21:704–709. doi: 10.1038/nmat3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti S, Mantovani G, Anker SD, Inui A, Morley JE, Fanelli FR, Scevola D, Schuster MW, Yeh S-S. Cachexia and Wasting: A Modern Approach. Springer; Milan: 2006. Functional Anatomy of the ‘Adipose Organ’. [Google Scholar]
- Hillel AT, Unterman S, Nahas Z, Reid B, Coburn JM, Axelman J, Chae JJ, Guo Q, Trow R, Thomas A, Hou Z, Lichtsteiner S, Sutton D, Matheson C, Walker P, David N, Mori S, Taube JM, Elisseeff JH. Photoactivated composite biomaterial for soft tissue restoration in rodents and in humans. Sci Transl Med. 2011;3:93ra67. doi: 10.1126/scitranslmed.3002331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Dadsetan M, Ameenuddin S, Windebank AJ, Yaszemski MJ, Lu L. In vivo biodegradation and biocompatibility of PEG/sebacic acid-based hydrogels using a cage implant system. J Biomed Mater Res Part A. 2010;95A:191–197. doi: 10.1002/jbm.a.32810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Lee YJ, Choi H, Ko EH, Kim J-W. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J Biol Chem. 2009;284:10601–10609. doi: 10.1074/jbc.M808742200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-C, Anseth K. PEG Hydrogels for the controlled release of biomolecules in regenerative medicine. Pharmaceut Res. 2009;26:631–643. doi: 10.1007/s11095-008-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn AD, Kyriakides TR, Bryant SJ. Characterization of the in vitro macrophage response and in vivo host response to poly(ethylene glycol)-based hydrogels. J Biomed Mater Res Part A. 2010;93A:941–953. doi: 10.1002/jbm.a.32595. [DOI] [PubMed] [Google Scholar]
- Mathis D, Shoelson SE. Immunometabolism: an emerging frontier. Nat Rev Immunol. 2011;11:81–83. doi: 10.1038/nri2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metters AT, Anseth KS, Bowman CN. Fundamental studies of a novel, biodegradable PEG-b-PLA hydrogel. Polymer. 2000;41:3993–4004. [Google Scholar]
- Reid B, Tzeng S, Warren A, Kozielski K, Elisseeff J. Development of a PEG derivative containing hydrolytically degradable hemiacetals. Macromolecules. 2010;43:9588–9590. doi: 10.1021/ma1020648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seliktar D. Extracellular stimulation in tissue engineering. Ann NY Acad Sci. 2005;1047:386–394. doi: 10.1196/annals.1341.034. [DOI] [PubMed] [Google Scholar]
- Smith J. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol. 1994;56:672–686. doi: 10.1002/jlb.56.6.672. [DOI] [PubMed] [Google Scholar]
- Tauber AI, Babior BM. Evidence for hydroxyl radical production by human neutrophils. J Clin Invest. 1977;60:374–379. doi: 10.1172/JCI108786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D-A, Varghese S, Sharma B, Strehin I, Fermanian S, Gorham J, Fairbrother DH, Cascio B, Elisseeff JH. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nat Mater. 2007;6:385–392. doi: 10.1038/nmat1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardman P, Candeias LP. Fenton chemistry: An introduction. Radiat Res. 1996;145:523–531. [PubMed] [Google Scholar]


