In addition to regulating sleep/wake cycles, the circadian rhythm is a critical regulator of many behavioral and physiological functions including locomotive activity, alertness, body temperature, heart rate, endocrine function, digestion and immunity. It is well established that like many other physiological processes, blood pressure (BP) exhibits a diurnal rhythm and this rhythmicity has clinical significance. Hypertensive patients with an abnormality in the circadian rhythm of BP called “non-dipping” hypertension (a blunted BP decline at nighttime) exhibit an increased incidence of cardiovascular mortality.1 Similarly, nocturnal hypertension as often observed in sleep apnea and shift work, has substantial impact on cardiovascular morbidity.2 The morning surge of BP (after the dip) has been correlated with higher incidence of stroke, myocardial infarction and sudden cardiac death than other times of the day.3
The circadian rhythm is controlled by a central “pacemaker” located in the Suprachiasmatic Nucleus (SCN). Mammalian circadian rhythms generally demonstrate a day/night pattern and are entrained by light because the SCN receives ganglionic input from retinal photosensitive cells that perceive light/dark cycles. Individual tissues also exhibit rhythmicity both as a result of synchronization by neural and humoral signals originating in the SCN and because the machinery controlling circadian rhythms are expressed ubiquitously. Loss of this central control leads to erratic oscillation of behavioral, endocrine and BP rhythms.
The cellular machinery maintaining the circadian clock is composed of a series of transcription factors generically called clock genes. The expression of clock genes in the SCN, driven by either exogenous cues or intrinsic rhythmicity, maintains the overall circadian rhythm.4 The role of clock genes in BP regulation has been the subject of several excellent reviews.5,6 The primary transcription factors making up the signaling pathway are heterodimers between Bmal1-Clock or Bmal1-Npas2. These heterodimers are required for expression of the Period and Cryptochrome genes. Per (Per1, Per2 and Per3) and Cry (Cry1 and Cry2) isoforms then feedback and inhibit expression of their activators, Bmal, Clock and Npas. This activation/inhibition is oscillatory or rhythmic. Clock genes are expressed in peripheral cells, have been shown to regulate endothelial function and expression of renal tubular transporters, among other functions, and may act tissue-specifically.7,8 For example, deficiency of Bmal1 in vascular smooth muscle deranges the circadian rhythm of BP and reduces BP without affecting SCN-controlled locomotor activity.9 In humans, the circadian pattern of Per2 expression was reported to be altered in peripheral blood from healthcare shift workers suggesting that alterations in clock genes in blood samples may be used as a marker of circadian disruption.10
Although we know that loss of clock gene function can cause abnormalities in BP regulation, the interaction between these pathways and intrinsic regulators of BP such as the renin-angiotensin system (RAS) remains poorly understood. At least one study suggests that hypertensive chronic kidney disease patients who take at least one antihypertensive medication at bedtime (a RAS blocker in 75% of patients) exhibited lower sleep-time BP, decreased incidence of a non-dipper BP profile, better control of ambulatory BP, and a lower risk for adverse cardiovascular events or death approximately 5 years after followup.11 In the current issue of Hypertension, Rudic’s laboratory report on studies that may provide further insight into the relationship between angiotensin-II (Ang-II) and circadian dysfunction.12 They used mice deficient in one or more isoforms of the Period genes, and found that circadian dysfunction, Ang-II, and low salt may synergize to cause non-dipping hypertension via alterations in the RAS pathway.
Wildtype (WT) and Period 2-deficient mice (Per2-KO) mice demonstrated similar circadian rhythms in their BP and locomotor activity under normal light/dark conditions, that is 12 hour continuous cycles of light followed by dark. Thus under normal light entrained conditions, Per2-deficiency has no effect on the level of BP or its rhythmicity. Exposure to constant darkness (DD) however impaired the rhythm of physical activity and blunted the expected temporal phase-shift in BP rhythm in Per2-KO mice. Chronic Ang-II infusion caused the typical robust hypertensive response in WT and Per2-KO mice in LD conditions, and normal rhythmicity of BP was preserved in Per2-KO mice. Whereas day/night variations of BP in mice acclimatized to constant darkness (DD) were preserved in WT, which have functional Per2, the Per2-KO mice exhibited an impaired BP rhythm. Recalling that mice are nocturnally active, the selective elevation in daytime BP in Per2-KO mice connotes non-dipping hypertension. Per2-KO mice also exhibited a higher degree of Ang-II-induced aortic medial hypertrophy compared to WT mice suggesting a progression toward end-organ damage. These changes were confirmed in mice deficient in two Period isoforms (Per2, 3-KO) and in mice deficient in all three Period isoforms (Per-TKO). Therefore the impairment of circadian rhythm exacerbates hypertension and vascular remodeling induced by exogenous Ang-II.
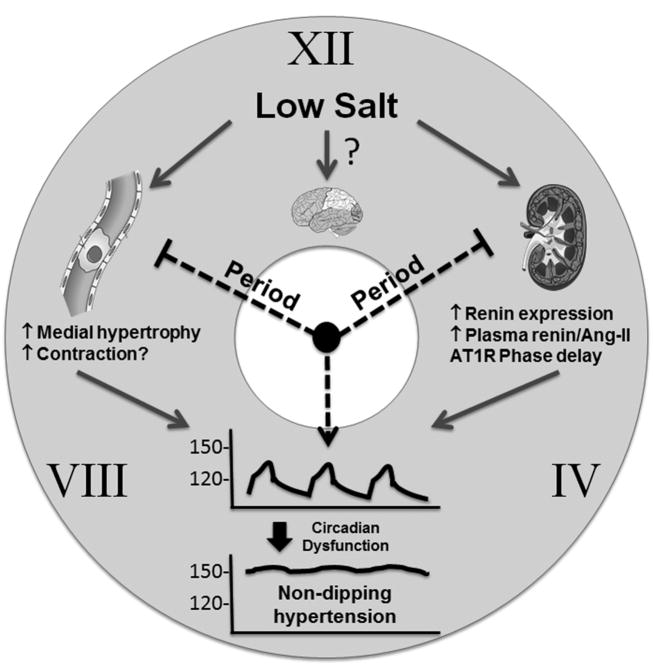
The authors next asked if increased endogenous RAS activity also causes non-dipping hypertension in circadian dysfunction. To do this, they fed WT and Per-TKO (lacking all 3 isoforms of Per) mice a low salt diet (0.01-0.02%), a potent stimulus of the endogenous RAS. Supporting the activation of the RAS was increased renal renin mRNA, aortic renin protein, and circulating renin, increases that were greater in Per-TKO than WT mice. WT mice receiving low salt diet exhibited a 10-mmHg reduction in BP with normal day/night variations. In contrast, low salt treated Per-TKO mice exhibited a non-dipping BP phenotype characterized by much higher BP in daytime than WT mice, and a severe blunting of the circadian rhythm of BP. Accordingly, Per-TKO mice also exhibited increased medial hypertrophy. Notably, this phenotype was evident under normal 12 hour LD conditions. Thus the phenotype in Per-TKO mice treated with low salt in LD conditions mirrored the phenotype caused by Ang-II infusion under DD conditions. This suggests that deficiency of Period genes is sufficient under low salt diet conditions to cause circadian impairment even when normal LD conditions are preserved. Interestingly, the same phenotype emerged in WT mice chronically fed a low salt diet that were induced to have abnormal circadian rhythms due to a shortened light/dark cycle (4 hours light/dark for 31-43 days). Thus, a low salt diet in mice when combined with altered circadian rhythms causes abnormal BP regulation even if the Period genes are intact. This is interesting in light of data reporting that there is a blunted nocturnal fall in BP (a non-dipping phenotype) in patients with salt-sensitive essential hypertension.13
Mechanistically, the authors showed that the phenotypes were reversed by Losartan suggesting they are AT1 receptor-dependent. Moreover, because the BP reduction in response to Losartan was much greater in Per-TKO than WT mice suggests a contribution of the RAS to low salt-induced non-dipping hypertension. Circadian dysfunction in low salt Per-TKO mice also triggered a phase delay of AT1 receptor expression in the aorta and kidney, resulting in higher daytime expression. However, there was no change in the level of AT1 receptor expression in the SCN suggesting, but not proving that the phenotype is not due to disruption of AT1 receptor signaling in the central clock. Whether other aspects of central clock function are altered was not addressed.
These data therefore imply that circadian dysfunction caused either by loss of clock gene function or induced by altering light/dark cycles may be a risk factor for altered BP rhythms and hypertension under conditions of a low salt diet, or if fully extrapolated, under conditions which activate the RAS. This study opens the door to some interesting questions. Are expression of RAS genes regulated by clock genes such as Per, or is this a response to other physiological perturbations caused by the deficiency of Per or altered circadian rhythmicity in the presence of low salt? Are these effects mediated by alterations in the central clock or by one or more peripherally acting circadian pathways? What are the mechanisms causing hypertension under circadian disruption, are they central, renal, vascular, or a combination? Certainly, the study strongly suggests that RAS activation may interact with circadian pathways in unanticipated ways, and implies, that if the same pathways are operant in humans, patients with circadian abnormalities might be particularly sensitive to RAS activation and perhaps RAS blockade. No doubt, this study may add fuel to the debate on the benefits/risks on cardiovascular health of a low salt diet in people with hypertension or those with circadian abnormalities such as those with sleep disorders or shift work.
Figure. The Circadian Clock of BP.
A low salt diet in combination with a loss of circadian function, induced either by deficiency in Period genes, or by changes in light entrainment, alters BP rhythmicity and causes non-dipping hypertension. These effects may be mediated by activation of RAS gene expression and activity in kidney and blood vessels.
Acknowledgments
Sources of Funding: This work was supported through research grants from the NIH (HL084207, HL048058, HL125603, and HL062984). The authors also gratefully acknowledge the generous research support of the Roy J. Carver Trust.
Footnotes
Conflict of Interest/Disclosure: None
References
- 1.Timio M, Venanzi S, Lolli S, Lippi G, Verdura C, Monarca C, Guerrini E. “Non-dipper” hypertensive patients and progressive renal insufficiency: a 3-year longitudinal study. Clin Nephrol. 1995;43:382–387. [PubMed] [Google Scholar]
- 2.Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Guerrieri M, Gatteschi C, Zampi I, Santucci A, Santucci C, Reboldi G. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793–801. doi: 10.1161/01.hyp.24.6.793. [DOI] [PubMed] [Google Scholar]
- 3.Elliott WJ. Circadian variation in the timing of stroke onset: a meta-analysis. Stroke. 1998;29:992–996. doi: 10.1161/01.str.29.5.992. [DOI] [PubMed] [Google Scholar]
- 4.Zylka MJ, Shearman LP, Weaver DR, Reppert SM. Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron. 1998;20:1103–1110. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]
- 5.Rudic RD, Fulton DJ. Pressed for time: the circadian clock and hypertension. J Appl Physiol (1985) 2009;107:1328–1338. doi: 10.1152/japplphysiol.00661.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richards J, Diaz AN, Gumz ML. Clock genes in hypertension: novel insights from rodent models. Blood Press Monit. 2014;19:249–254. doi: 10.1097/MBP.0000000000000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gumz ML, Stow LR, Lynch IJ, Greenlee MM, Rudin A, Cain BD, Weaver DR, Wingo CS. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Invest. 2009;119:2423–2434. doi: 10.1172/JCI36908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viswambharan H, Carvas JM, Antic V, Marecic A, Jud C, Zaugg CE, Ming XF, Montani JP, Albrecht U, Yang Z. Mutation of the circadian clock gene Per2 alters vascular endothelial function. Circulation. 2007;115:2188–2195. doi: 10.1161/CIRCULATIONAHA.106.653303. [DOI] [PubMed] [Google Scholar]
- 9.Xie Z, Su W, Liu S, Zhao G, Esser K, Schroder EA, Lefta M, Stauss HM, Guo Z, Gong MC. Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J Clin Invest. 2015;125:324–336. doi: 10.1172/JCI76881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang MZ, Ohman-Strickland P, Kelly-McNeil K, Kipen H, Crabtree BF, Lew JP, Zarbl H. Sleep interruption associated with house staff work schedules alters circadian gene expression. Sleep Med. 2015;16:1388–1394. doi: 10.1016/j.sleep.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Bedtime dosing of antihypertensive medications reduces cardiovascular risk in CKD. J Am Soc Nephrol. 2011;22:2313–2321. doi: 10.1681/ASN.2011040361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pati P, Fulton DJR, Bagi Z, Chen F, Wang Y, Kitchens J, Cassis LA, Stepp DW, Rudic RD. A low salt diet and circadian dysfunction synergize to induce angiotensin II-dependent hypertension in mice. Hypertension. 2016;XXX:XXX–XXX. doi: 10.1161/HYPERTENSIONAHA.115.06194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uzu T, Kazembe FS, Ishikawa K, Nakamura S, Inenaga T, Kimura G. High sodium sensitivity implicates nocturnal hypertension in essential hypertension. Hypertension. 1996;28:139–142. doi: 10.1161/01.hyp.28.1.139. [DOI] [PubMed] [Google Scholar]