Abstract
Nigrostriatal dopamine depletion disrupts striatal medium spiny neuron morphology in Parkinson’s disease and modulates striatal synaptic plasticity in animal models of parkinsonism. We demonstrate that long-term nigrostriatal dopamine depletion in the rat induces evolving changes in the phosphorylation of striatal proteins critical for synaptic plasticity. Dopamine depletion increased the phosphorylation of the alpha isoform of calcium–calmodulin-dependent protein kinase II (CaMKIIα) at Thr286, a site associated with enhanced autonomous kinase activity, but did not alter total levels of CaMKIIα or other synaptic proteins. Dopamine depletion decreased CaMKIIα levels in postsynaptic density-enriched fractions without significant changes in other proteins. The activity of protein phosphatase 1 (PP1), a postsynaptic phosphatase that dephosphorylates CaMKII, is regulated by DARPP-32 (dopamine- and cAMP-regulated phosphoprotein of 32 kDa). Dopamine depletion had no effect on DARPP-32 phosphorylation at Thr34, but increased DARPP-32 phosphorylation at Thr75. Levodopa administration reversed the increased phosphorylation of both CaMKIIα and DARPP-32. Normal ageing increased the levels of PP1(γ1 isoform) but decreased levels of the PP1γ1-targeting proteins spinophilin and neurabin. Elevated phosphorylations of CaMKIIα and DARPP-32 were maintained for up to 20 months after dopamine depletion. However, phosphorylation of the CaMKII–PP1 substrate, Ser831 in the glutamate receptor GluR1 subunit, was increased only after sustained (9–20 months) dopamine depletion. Interaction of ageing-related changes in PP1 with the dopamine depletion-induced changes in CaMKIIα may account for enhanced GluR1 phosphorylation only after long-term dopamine depletion. These evolving changes may impact striatal synaptic plasticity, Parkinson’s disease progression and the changing efficacy and side-effects associated with dopamine replacement therapy.
Keywords: ageing, AMPA receptor, CaMKII, DARPP-32, dendrite, postsynaptic density, PP1
Introduction
Parkinson’s disease is a neurodegenerative disorder resulting in striatal dopamine depletion that strikes 1–2% of the population over 60 years of age. Normal ageing induces morphological changes in striatal medium spiny neurons (MSNs) (Ingham et al., 1989), which constitute ≈95% of the total striatal neuron population. However, the loss of nigrostriatal dopamine input results in additional morphological and functional alterations in striatal MSNs. For example, enduring changes in MSN dendrites have been reported in Parkinson’s disease (McNeill et al., 1988; Ingham et al., 1998; Arbuthnott et al., 2000; Meshul et al., 2000; Zaja-Milatovic et al., 2005) and in a rat model of parkinsonism induced by 6-hydroxydopamine (6-OHDA) lesion of the substantia nigra (Ingham et al., 1998; Arbuthnott et al., 2000; Meshul et al., 2000). Moreover, nigrostriatal dopamine depletion in animal models affects striatal long-term depression (Partridge et al., 2000) and long-term potentiation (Centonze et al., 1999; Picconi et al., 2004; Norman et al., 2005).
Both synaptic and morphological plasticity in hippocampal neurons are modulated by intracellular signalling proteins, such as calcium–calmodulin-dependent protein kinase II (CaMKII) and related proteins (Smart & Halpain, 2000; Winder & Sweatt, 2001; Lisman et al., 2002; Colbran & Brown, 2004). The association of CaMKII, protein phosphatase 1 (PP1), spinophilin and neurotransmitter receptors with synapses and postsynaptic densities (PSDs) also is dynamically regulated (Malinow, 2003; Colbran, 2004a; Colbran, 2004b; Griffith, 2004; Schulman, 2004). The morphological and functional effects of striatal dopamine depletion presumably result from changes in the expression and / or function of dendritic cytoskeletal and signalling proteins.
Acute dopamine signalling modulates both N-methyl-d-aspartate (NMDA)- and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors (Svenningsson et al., 2004). Although the effects of chronic dopamine insufficiency associated with Parkinson’s disease are poorly understood, studies in animal models have demonstrated modest changes in levels of some glutamate receptor subunits and of PSD-95 in synaptic membranes (Porter et al., 1994; Oh et al., 1999; Dunah et al., 2000; Betarbet et al., 2004; Picconi et al., 2004), as well as altered phosphorylation of the NMDA receptor NR2B, NR2A and NR1 subunits (Menegoz et al., 1995; Oh et al., 1999; Dunah et al., 2000). However, most of these previous studies analysed whole striatal samples from relatively young animals (3–6 months), typically within 6 weeks of dopamine depletion. Notably, Parkinson’s disease is a progressive disease induced by chronic striatal dopamine depletion, most often in elderly individuals. Normal ageing modifies synaptic plasticity in the striatum and in other brain regions (Ou et al., 1997; Rosenzweig & Barnes, 2003), and also affects intracellular signalling proteins (Norris et al., 1998; Foster et al., 2001; Foster et al., 2003). Thus, the full manifestation of Parkinson’s disease may result from the combined effects of dopamine depletion and normal ageing.
Here, we report novel changes in signalling pathways of the dorsolateral striatum that occur in a graded manner following 6-OHDA lesion of the nigrostriatal pathway. Enduring elevations in the phosphorylation of CaMKII and dopamine- and cAMP-regulated phospho-protein of 32 kDa (DARPP-32) are seen within 3 weeks, whereas phosphorylation of GluR1 increases only after several months of dopamine depletion.
Materials and methods
6-OHDA lesion surgery
Male Sprague-Dawley rats (Harlan; Indianapolis, IN, USA) were housed under a 12 : 12 light : dark cycle with food and water freely available. Experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals (NIH), under the oversight of the Institutional Animal Care and Use Committee. Rats (3 months old) were anaesthetized with ketamine and xylazine, and 6-OHDA HBr (4 µg / µL free base in 0.02% ascorbate) was infused into two sites in the substantia nigra (AP, −5.3; L, 2.3 and 1.0; DV, −8.3; Paxinos & Watson, 1986), at a rate of 0.25 µL / min. Shamlesioned rats received injections of vehicle alone. Age-matched noninjected rats were also studied. At the times indicated after surgery, rats were lightly anaesthetized with isoflurane, decapitated and the brain removed. Data reported here were obtained from five batches of 6–8 rats each that were killed 3 and 6–12 weeks, and 9, 11 and 18–20 months after 6-OHDA lesion surgery. For presentation purposes, we pooled data obtained from animals 3–12 weeks and 9–11 months after surgery. There were no statistically significant differences between batches of animals within each set of pooled data. Moreover, differences observed in the pooled data were observed in both individual groups of animals at each time point.
L-DOPA treatment
Rats 6–12 weeks after 6-OHDA lesion were treated with levodopa (L-DOPA) methyl ester and benserazide (50 and 12.5 mg / kg, i.p.) or benserazide alone every 12 h for 9 days, then were killed (as indicated above) 16 h after the last injection. This L-DOPA treatment paradigm induced a characteristic increase in the contralateral rotation frequency by the 9th day of treatment (data not shown), consistent with previous reports (Schwarting & Huston, 1996). This repeated injection paradigm was designed to mimic the effects of repeated L-DOPA administration to Parkinson’s disease patients.
Dorsolateral striatal tissue homogenates
Punches (1.15 mm ID) of dorsolateral striatum were removed from both hemispheres of 1.0-mm-thick coronal slices at the level of the crossing of the anterior commissure, and flash-frozen on dry ice within 2 min of decapitation. Tissue punches were stored at −80 °C. We confirmed that this procedure minimized postmortem changes in CaMKII phosphorylation (Suzuki et al., 1994; Lengyel et al., 2001).
Whole striatal extracts and subcellular fractionation
Whole extracts were prepared by sonicating striatal tissue punches in 200–300 µL 2% SDS with 10 µg / mL leupeptin and 1 µg / mL pepstatin. PSD-enriched fractions were prepared by homogenizing frozen striatal punches in ice-cold 7 mm Tris–HCl, pH 7.5, containing EDTA, 0.2 mm; EGTA, 0.2 mm; sucrose, 320 mm; benzamidine, 1 mm; aprotinin, 10 µg / mL; leupeptin, 10 µg / mL; pepstatin, 10 µm; microcystin, 1 µm; cypermethrin, 0.5 nm; and NaVO4, 1 mm; using a Kontes tissue homogenizer. After centrifugation (1000 g for 7 min at 4 °C) to separate a pellet fraction (P1) enriched in nuclei, Triton X-100 (1% final) was added to the supernatant fraction (S1). After mixing gently for 30 min at 4 °C, S1 samples were re-centrifuged (100 000 g for 1 h at 4 °C) to separate a supernatant (S2) enriched in cytosolic and detergent-soluble membrane proteins, and a pellet (P2) enriched in cytoskeletal elements including PSDs. Protein concentrations of samples were determined by the method of Lowry (Lowry et al., 1951).
Antibodies
The following primary antibodies were used for immunoblotting: goat anti-CaMKII α / β (McNeill & Colbran, 1995; 1 : 4000), mouse anti-CaMKIIα (ABR, 1 : 4000), rabbit anti-phospho-Thr286-CaMKIIα (Promega, 1 : 2500), rabbit anti-DARPP-32 (Cell Signalling, 1 : 4000), rabbit anti-phospho-Thr34-DARPP-32 (Cell Signalling, 1 : 250), rabbit anti-phospho-Thr75-DARPP-32 (Cell Signalling, 1 : 500), rabbit anti-GluR1 (Upstate, 1 : 4000), rabbit anti-phospho-Ser831-GluR1 (Upstate, 1 : 500), rabbit anti-phospho-Ser845-GluR1 (Upstate, 1 : 2000), rabbit anti-neurabin (1 : 2500) (MacMillan et al., 1999), mouse anti-NR1 (Chemicon, 1 : 3000), rabbit or mouse anti-NR2B (Molecular Probes, 1 : 500), sheep anti-PP1γ1 (1 : 1000; Colbran et al., 2003), mouse anti-PSD-95 (Upstate, 1 : 1000), rabbit anti-spinophilin (1 : 2000; MacMillan et al., 1999) and mouse anti-tyrosine hydroxylase (ImmunoStar, 1 : 1000). Secondary antibodies were from Jackson Immunoresearch (goat anti-rabbit AP, 1 : 1000), Sigma (rabbit anti-mouse AP, 1 : 2000), Promega (goat anti-mouse HRP, 1 : 2000; goat anti-rabbit HRP, 1 : 4000), Vector Laboratories (rabbit anti-goat AP, 1 : 2000) or Alpha-Quest (rabbit anti-goat HRP, 1 : 4000).
Immunoblots
Samples (20–40 µg protein per lane) were fractionated by SDS-PAGE and transferred to nitrocellulose membranes, which were stained with Ponceau-S (Sigma) and then digitally scanned. After blocking, membranes were probed with the indicated primary antibodies: phosphorylation site-specific primary antibodies were incubated overnight at 4 °C, whereas other primary antibodies were incubated for 2 h at room temperature. Membranes were then washed and incubated for 1 h at room temperature with the alkaline phosphatase- or horseradish peroxidase-conjugated secondary antibodies. Alkaline phosphatase-conjugated secondary antibodies were detected with 5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt (BCIP; Pierce) and nitroblue tetrazolium chloride (NTB; Pierce). Horseradish peroxidase-conjugated secondary antibodies were detected with enhanced chemiluminescence (Perkin Elmer) and multiple X-ray film exposures to ensure that signals were within the linear range. In some cases primary and HRP-conjugated secondary antibodies were stripped from the membrane by incubation in stripping buffer (Tris–HCl, 62.5 mm, pH 7.5; SDS, 2%; and 2-mercaptoethanol, 0.8%) for 1 h at 50 °C. Stripping efficiency was confirmed by subsequent incubation with secondary antibody and development. Membranes were then incubated in PBST blocking buffer for 1 h prior to reprobing with different primary antibodies.
Quantification of immunoblots and statistical analyses
Optical densities of total protein loaded in each gel lane (Ponceau S-stained membranes) and specific immunoblotted proteins were measured using NIH Image 1.6 (http://www.rsb.info.nih.gov/nihimage). Immunoblotted protein band densities in each lane were normalized to total protein loaded in the corresponding lane to yield a ‘normalized immunoblot signal’. For analysis of PSD-enriched fractions, the relative total mass of the specific protein in each sample was obtained by correcting the normalized immunoblot signals for the volume of P2 fraction loaded on each lane and the total volume of the corresponding P2 fraction.
Only rats with > 90% depletion of striatal tyrosine hydroxylase (TH) relative to the intact contralateral striatum were included in statistical analyses. Statistical comparisons of normalized immunoblot signals were performed using either one- or two-way anova with Scheffé’s post hoc tests or post hoc t-tests, as indicated. For graphing purposes, the mean normalized immunoblot signal obtained from the intact hemisphere of 6-OHDA-lesioned rats was set at 100% and values from individual samples were expressed relative to this value.
Results
Dopamine depletion increased Thr286 phosphorylation of CaMKIIα
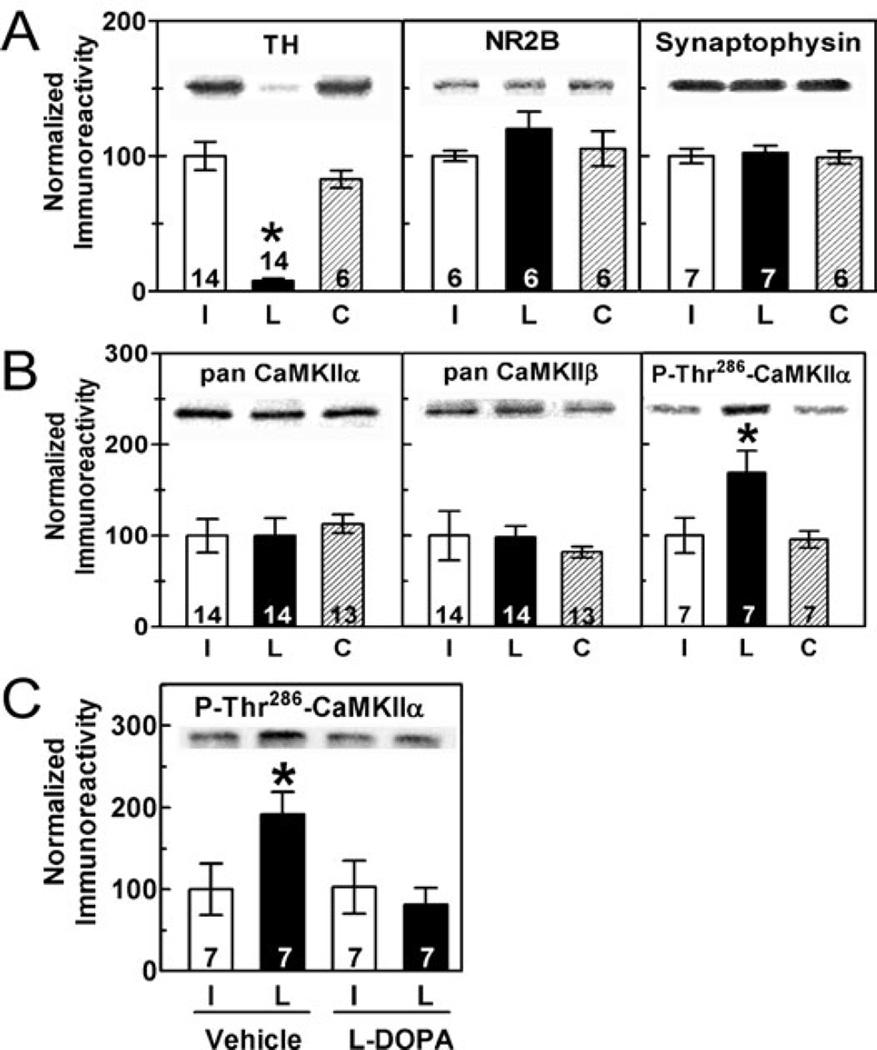
Striatal TH levels were decreased by ≈95% at 3–12 weeks after unilateral 6-OHDA injections into the substantia nigra (Fig. 1A). No changes in total levels of several synaptic proteins were observed, including NMDA receptor NR2B subunit, synaptophysin, CaMKIIα and CaMKIIβ (Fig. 1A and B). In contrast, Thr286 phosphorylation of CaMKIIα was significantly increased by dopamine depletion relative to both the contralateral intact striatum and to control striatum from sham-lesioned or nonlesioned rats (Fig. 1B).
Fig. 1.
Short-term 6-OHDA lesion increased CaMKIIα phosphorylation at Thr286. (A and B) Representative blots and summary graphs quantitating total striatal protein levels or phosphorylation (mean ± SEM) in samples harvested 3–12 weeks postoperatively. The number of rats analysed is indicated within or above each bar. ‘L’ (lesion) and ‘I’ (intact) indicate samples ipsilateral and contralateral to the lesion, respectively. ‘C’ indicates tissue from sham-lesioned rats. Only the decrease in TH (F2,49 = 37.66, P < 0.0001) and the increase of P-Thr286-CaMKIIα (F2,21 = 11.98, P = 0.0003) were significantly altered in dopamine-depleted striatum. (C) The increase in Thr286 phosphorylation of CaMKIIα was reversed by L-DOPA administration (F1,27 = 5.61, P = 0.026).
To test the effects of dopamine replacement on CaMKIIα phosphorylation, 6-OHDA-lesioned rats were treated with either vehicle or L-DOPA for 9 days (see Materials and methods). In rats that received vehicle injections, Thr286 phosphorylation was significantly elevated in the lesioned striatum relative to intact striatum. However, Thr286 phosphorylation in both hemispheres of L-DOPA-injected rats was similar to that in the intact striatum from vehicle-injected rats; i.e. L-DOPA treatment completely reversed the increase in Thr286 phosphorylation (Fig. 1C). However, L-DOPA did not significantly change the total levels of TH, CaMKIIα, CaMKIIβ, PP1γ1 or spinophilin (data not shown).
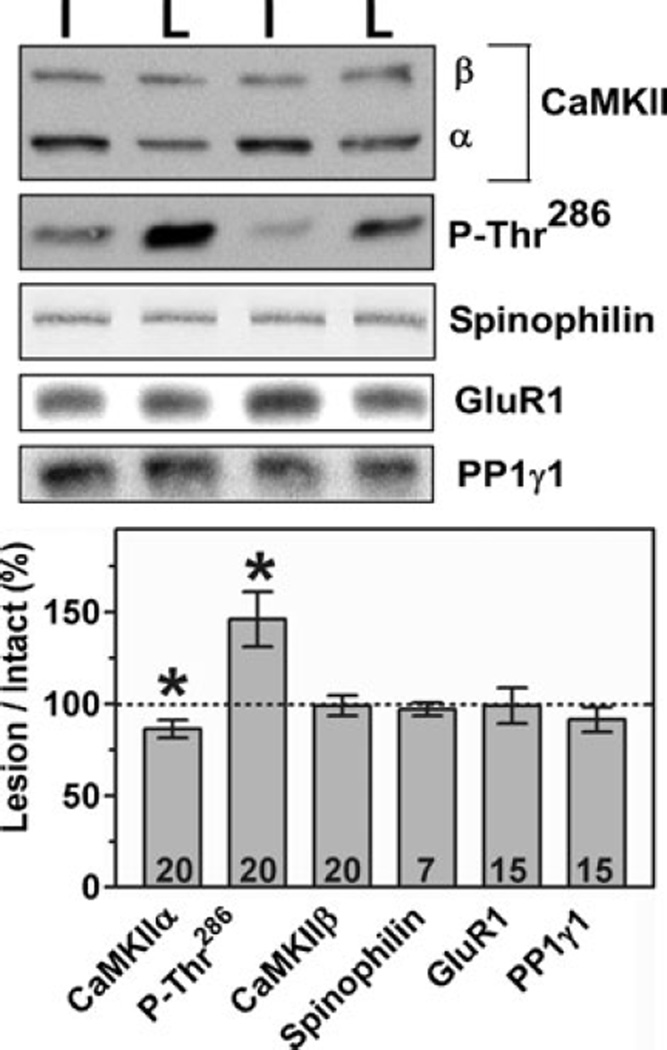
PSD-enriched cytoskeletal fractions from the striatum of 6-OHDA-lesioned rats killed 3 weeks after surgery were also analysed by immunoblotting. As seen in whole striatal extracts, Thr286 phosphorylation of CaMKIIα in the PSD-enriched fractions was significantly elevated. However, total levels of PSD-associated CaMKIIα (but not CaMKIIβ) were slightly but significantly decreased (Fig. 2).
Fig. 2.
6-OHDA lesions changed PSD-associated CaMKIIα. PSD-enriched P2 fractions taken from rats 3 weeks after 6-OHDA lesion surgery were analysed by immunoblotting. The top panel shows representative blots from contralateral (intact) and lesioned hemispheres of two representative animals. The levels in samples from 6-OHDA-lesioned striatum are expressed as a percentage of levels in samples from the contralateral (intact) striatum (mean ± SEM). Total CaMKIIα was significantly decreased (t18 = 2.92, P = 0.009) and Thr286-phosphorylated CaMKIIα was elevated (t19 = 0.33, P = 0.006) following dopamine depletion, as determined by anova followed by post hoc t-test.
Effect of dopamine depletion on striatal phosphatases and PP1 regulatory proteins
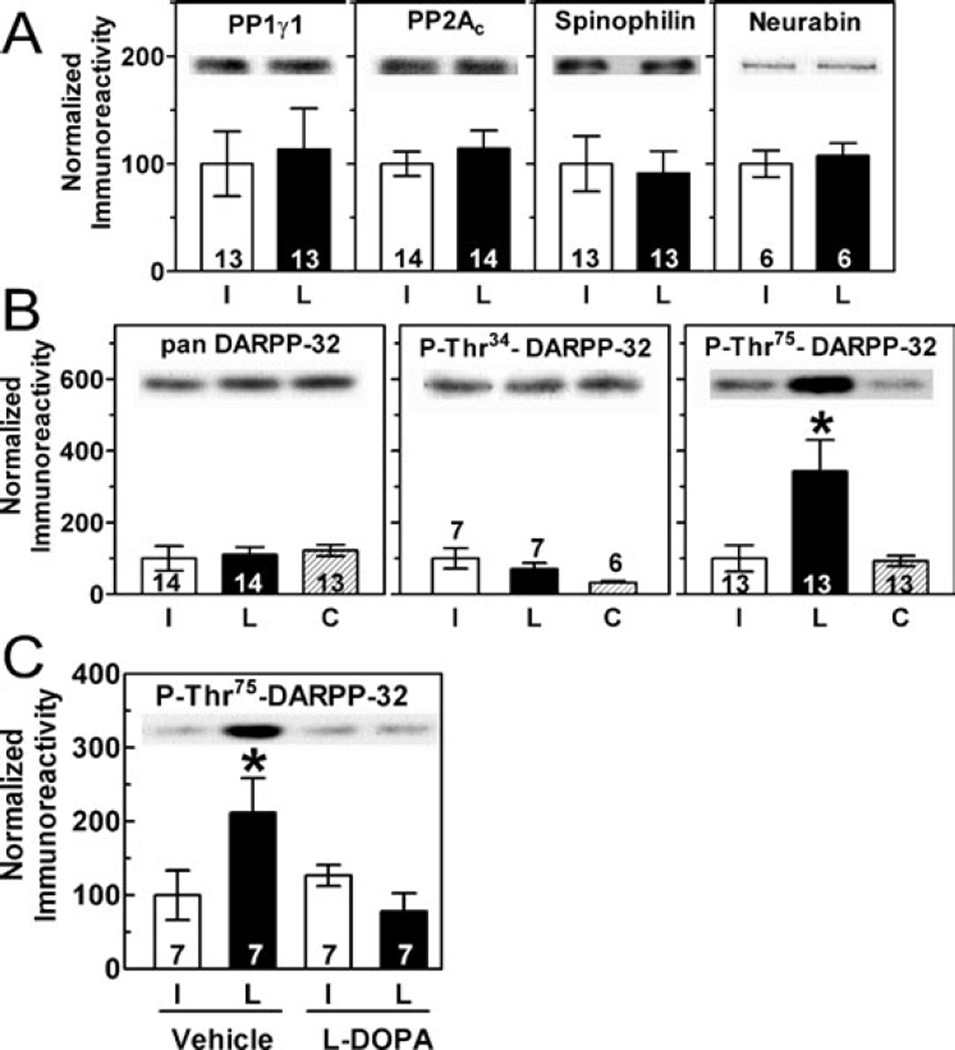
Increased Thr286 phosphorylation of CaMKIIα following dopamine depletion may result from altered regulation of striatal protein phosphatases, major targets of dopamine signalling (Svenningsson et al., 2004). We observed no significant changes in total levels of PP1γ1 or protein phosphatase 2A (PP2A) catalytic subunits, or of the PP1 scaffolding proteins spinophilin and neurabin 3–12 weeks postoperatively (Fig. 3A). Moreover, there were no significant changes in the amounts of PP1γ1 or spinophilin associated with the PSD-enriched cytoskeletal fraction (Fig. 2). Interestingly, while levels of total DARPP-32 remained unchanged, the phosphorylation of DARPP-32 at Thr75 (but not Thr34) was markedly increased in dopamine-depleted striatum (Fig. 3B). The repeated L-DOPA injection paradigm (see Materials and methods) completely reversed the increase in Thr75 phosphorylation (Fig. 3C).
Fig. 3.
Effects of 6-OHDA lesion on striatal protein phosphatases. (A and B) Representative immunoblots and summary graphs quantifying total striatal levels of protein phosphatase catalytic subunits and PP1 targeting and regulatory proteins. Phosphorylation of DARPP-32 at Thr75 was significantly elevated in dopamine-depleted striatal samples (F2,45 = 3.5, P = 0.038). (C) The increase in Thr75 phosphorylation of DARPP-32 was reversed by L-DOPA administration (F1,27 = 22.13, P = 0.0005).
Effects of ageing and striatal dopamine depletion
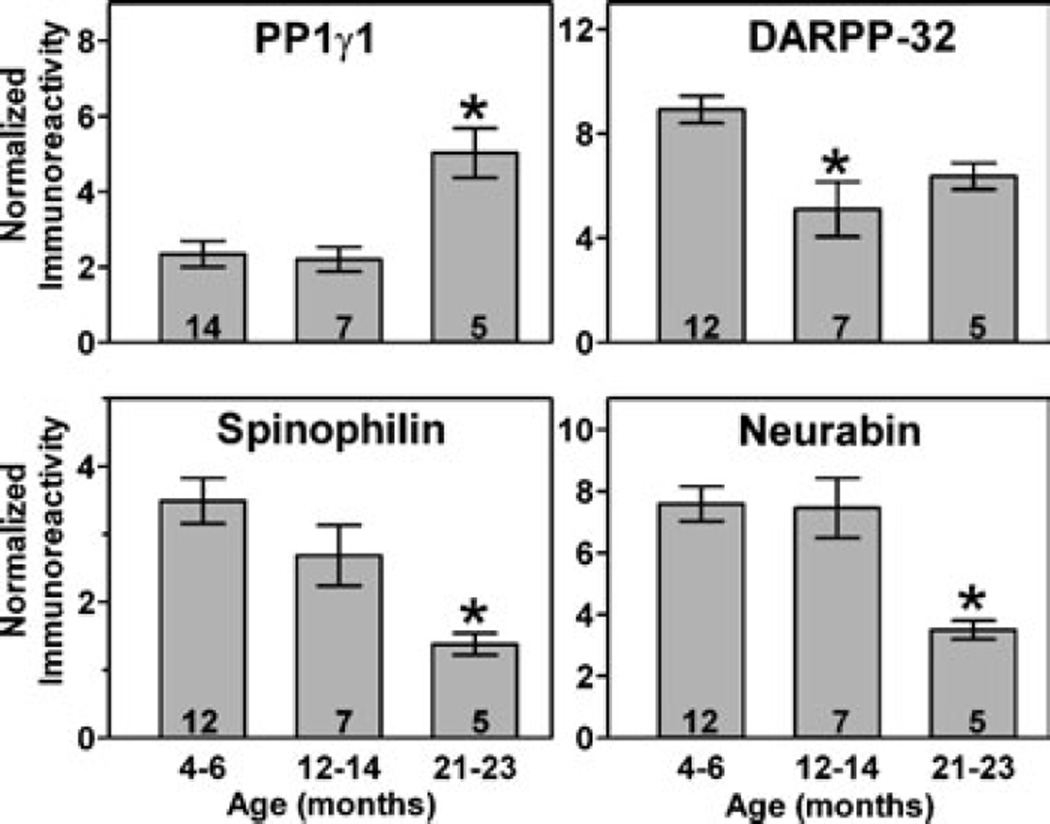
Because Parkinson’s disease is associated with ageing, the effects of ageing on signalling proteins in normal and dopamine-depleted dorsolateral striatum were assessed. There were no significant differences in levels of CaMKIIα, phospho-Thr286-CaMKIIα, phospho-Thr34-DARPP-32, phospho-Thr75-DARPP-32, PP2A, protein phosphatase 2B (PP2B), PSD-95, NMDA receptor subunits or CaMKIIβ in striatum from control rats at 4–6, 12–14 or 21–23 months of age (see Supplementary material, Fig. S1, A, C and D). However, there was a significant increase in PP1γ1 and a significant decrease in spinophilin and neurabin in control rats at 21–23 months of age (Fig. 4). In addition, there was a trend for decreasing levels of DARPP-32 with normal ageing, but this was only statistically significant at 12–14 months of age (Fig. 4).
Fig. 4.
Changes in PP1γ1 and PP1-regulatory proteins during normal ageing. Quantification of protein levels in dorsolateral striatal homogenates from normal rats at 4–6, 12–14 or 21–23 months of age. PP1γ1 was elevated only at 21–23 months (F2,47 = 12.02, P < 0.0001), DARPP-32 was decreased only at 12–14 months (F2,45 = 7.98, P = 0.001), while both spinophilin (F2,45 = 7.77, P = 0.001) and neurabin (F2,43 = 10.46, P = 0.0002) were decreased at 21–23 months.
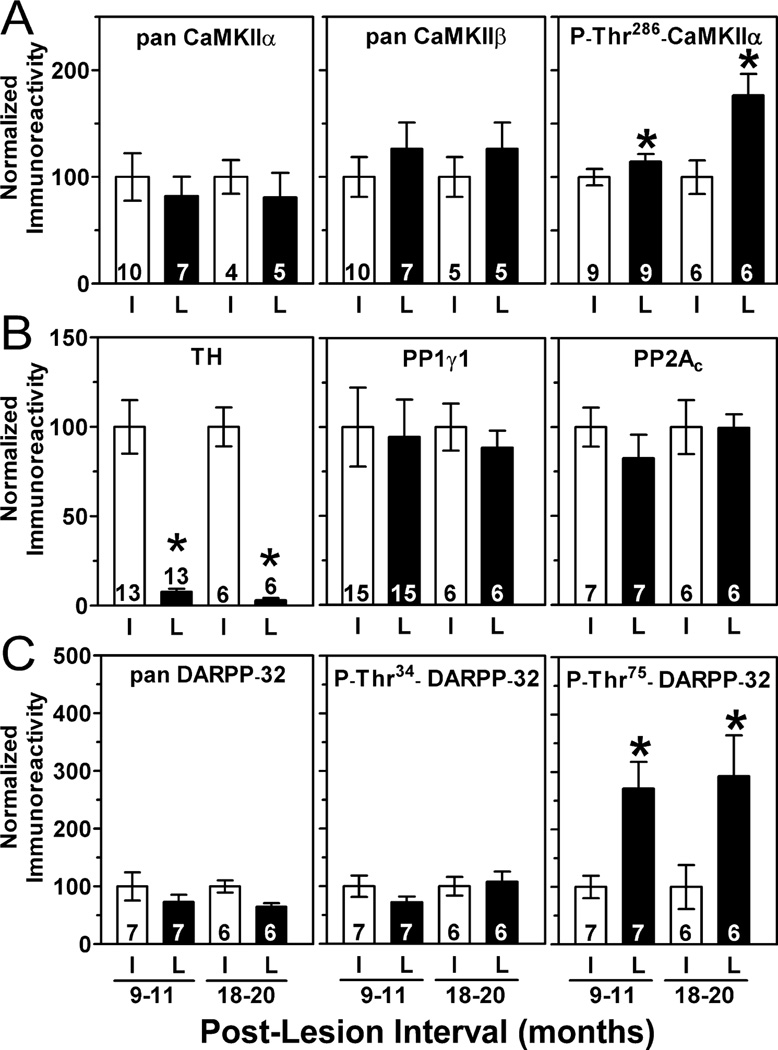
We also analysed dorsolateral striatum from rats killed 9–11 or 18–20 months after 6-OHDA injections (i.e. at 12–14 or 21–23 months of age). Total levels of multiple signalling proteins were unchanged after chronic dopamine depletion (Fig. 5A–C; supplementary Fig. S2). However, the elevated phosphorylation of CaMKIIα at Thr286 and of DARPP-32 at Thr75 seen 3–12 weeks postoperatively was also detected at both later time points (Fig. 5).
Fig. 5.
Effects of chronic dopamine depletion on striatal proteins. (A–C) Long-term dopamine depletion caused an enduring decrease in TH at 9–11 months (t12 = 6.82, P = 0.0001) and at 18–20 months (t5 = 7.99, P = 0.0005). This was paralleled by an enduring increase in phosphorylation of both CaMKIIα at Thr286 at 9–11 months (t11 = 2.28, P = 0.043) and 18–20 months (t5 = 3.50, P = 0.017) and of DARPP-32 at Thr75 at 9–11 months (t6 = 5.03, P = 0.0024) and at 18–20 months (t5 = 3.46, P = 0.018).
Effects of dopamine depletion on AMPA receptor phosphorylation
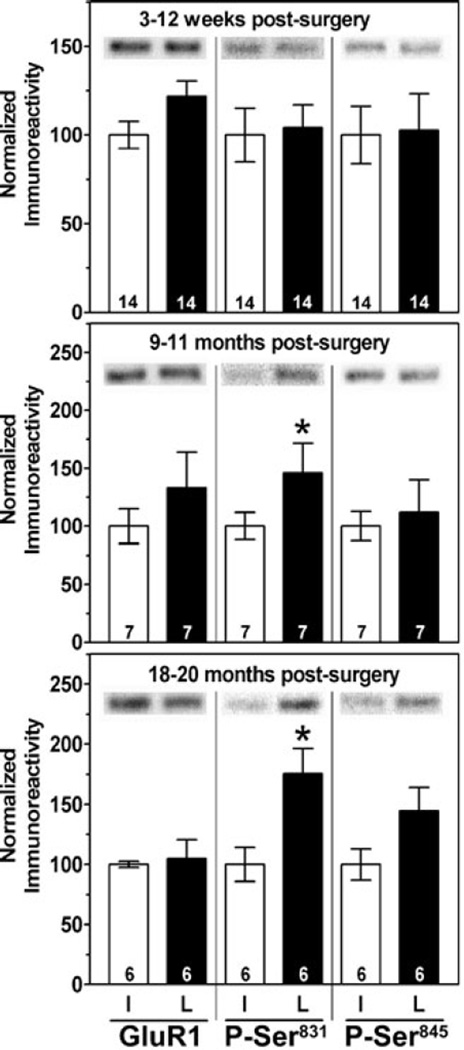
Because ageing and chronic dopamine depletion yield complex changes in the levels and phosphorylation of CaMKIIα, PP1γ1 and PP1 regulatory proteins, we examined a common downstream target of these enzymes, the GluR1 subunit of the AMPA-type glutamate receptor. There were no changes in the levels of GluR1 or of the phosphorylation of GluR1 at Ser831 or Ser845 during normal ageing (4–23 months of age; supplementary Fig. S1, B). Dopamine depletion had no significant effect on the levels of total GluR1 in whole striatal extracts at any time point (Fig. 6), or in PSD-enriched fractions (Fig. 2). Phosphorylation of GluR1 at Ser831 was unaltered 3–12 weeks postsurgery but was significantly elevated in dopamine-depleted striatum at both 9–11 and 18–20 months postoperatively (Fig. 6). In contrast, phosphorylation of GluR1 at Ser845 was not significantly different between the hemispheres at any time point after surgery (Fig. 6).
Fig. 6.
Chronic dopamine depletion selectively increased GluR1 phosphorylation at Ser831. Total GluR1 levels and levels of GluR1 phosphorylated at Ser831 or Ser845 at 3–12 weeks, 9–11 months or 18–20 months following 6-OHDA lesion surgery. There was a significant effect of age (F2,48 = 4.10, P = 0.0322 for Ser831); post hoc tests revealed that Ser831 phosphorylation was increased at both 9–11 months (t6 = 2.495, P = 0.047) and at 18–20 months (t4 = 4.738, P = 0.009) following dopamine depletion. In contrast, there was a trend for increased phosphorylation at Ser845 only 18–20 months following surgery (t5 = 2.490, P = 0.068).
Discussion
Dopamine depletion increases Thr286 phosphorylation of CaMKIIα
Phosphorylation of CaMKIIα at Thr286 was significantly increased within 3 weeks of 6-OHDA lesion surgery in both total homogenates and PSD-enriched subcellular fractions of dorsolateral striatum (Figs 1 and 2). A recent study using whole striatum detected enhanced Thr286 phosphorylation of CaMKIIα in a PSD-enriched fraction but not in the total homogenate (Picconi et al., 2004). Notably, dorsolateral striatum receives the densest nigrostriatal dopamine inputs (Nakano et al., 2000), perhaps suggesting that this region will be most severely affected by 6-OHDA lesion of the substantia nigra. Thus, differences between these studies may reflect the analysis of different parts of the striatum, or the choice of different rat strains.
Thr286 phosphorylation of CaMKIIα confers autonomous (Ca2+–calmodulin-independent) kinase activity (Hudmon & Schulman, 2002; Colbran & Brown, 2004) and stabilizes CaMKII binding to PSD proteins and synapses in hippocampal neurons (Strack et al., 1997; Yamauchi & Yoshimura, 1998; Shen & Meyer, 1999; Colbran, 2004a). Therefore, it was surprising that total CaMKIIα levels in the PSD-enriched fractions isolated from the dorsolateral striatum were decreased (Fig. 2). Significant changes in other PSD-associated protein levels were not detected, although quantitative ultrastructural studies may reveal more subtle changes in subcellular distribution. In addition, it should be noted that total CaMKIIα levels in PSD fractions from whole striatum were unaltered (Picconi et al., 2004). A similar decrease in hippocampal PSD-associated total CaMKIIα in the face of enhanced Thr286 phosphorylation was also recently observed in a mouse model of Angelman’s syndrome, which lacks an E6AP-ubiquitin ligase (Weeber et al., 2003). However, it is important to note that CaMKIIα association with PSDs is regulated by additional mechanisms such as autophosphorylation at Thr305 / 306 (Shen et al., 2000; Elgersma et al., 2002) and a protein kinase C-driven mechanism (Fong et al., 2002), probably involving dynamic interactions of CaMKII with multiple binding partners in the PSD (Colbran, 2004a). Thus, mechanisms of CaMKII targeting to PSDs in striatum and the role of dopamine modulation warrant further investigation.
Mechanisms for increased Thr286 phosphorylation following dopamine depletion
Thr286 autophosphorylation of CaMKII is acutely induced by Ca2+ influx via voltage-gated calcium channels or NMDA receptors or by the release of intracellular Ca2+ stores (Hanson & Schulman, 1992). These effects of Ca2+ mobilization are opposed and reversed by multiple protein phosphatases (Colbran, 2004b). For example, PSD-associated CaMKII is selectively dephosphorylated by PP1, presumably the PP1γ1 isoform that appears to be selectively targeted to dendritic spines by binding to spinophilin and / or neurabin (MacMillan et al., 1999; Strack et al., 1999; Terry-Lorenzo et al., 2002; Carmody et al., 2004; Bordelon et al., 2005). Thus, increased Thr286 phosphorylation following dopamine depletion might be due to increased Ca2+ mobilization resulting from lack of modulation of corticostriatal excitatory inputs to MSNs, or due to inhibition of protein phosphatases acting on CaMKII.
Many acute effects of dopamine in the striatum are mediated by modulation of protein phosphatases, especially via the modulation of PP1 by DARPP-32 and spinophilin (Svenningsson et al., 2004). However, we found no evidence for changes in the total levels of multiple phosphatase catalytic subunits or of PP1 regulatory proteins following dopamine depletion, in either whole dorsolateral striatal extracts or PSD-enriched subcellular fractions. DARPP-32 is phosphorylated at Thr34 by PKA following acute D1-like dopamine receptor activation (presumably decreasing PP1 activity) and dephosphorylated by PP2B in response to activation of D2-like dopamine receptors, as well as the NMDA-, AMPA- and mGluR5-type glutamate receptors (Svenningsson et al., 2004; Nishi et al., 2005). Surprisingly, but consistent with prior reports (Picconi et al., 2003; Chergui et al., 2004), there were no significant changes in Thr34 phosphorylation at any time point following 6-OHDA lesion. Thus, additional cellular mechanisms may normalize DARPP-32 phosphorylation at Thr34 after chronic dopamine depletion.
In contrast to the lack of changes in Thr34 phosphorylation, DARPP-32 phosphorylation at Thr75 was significantly elevated by dopamine depletion. Moreover, L-DOPA injections rescued this increase. Thr75 is phosphorylated by cyclin-dependent kinase 5 (cdk5) and dephosphorylated by PP2A. PP2A activity may be reduced following dopamine depletion due to the loss of a D1-like receptor-activated PKA-dependent activation of PP2A (Nishi et al., 2000), or due to CaMKII-dependent inhibition of PP2A (Fukunaga et al., 2000). It is possible that increased glutamatergic signalling following dopamine depletion also activates cdk5, contributing to the enhanced phosphorylation of DARPP-32 Thr75.
In combination, our studies provide little evidence for alterations in protein phosphatases following dopamine depletion, although the data cannot rule out alterations in specific subtypes. Further studies will be required to determine the contributions of PP2A and cdk5 to the elevated phosphorylation of DARPP-32 following dopamine depletion, and whether these changes play a role in enhancing Thr286 phosphorylation of CaMKII. Alternatively, increased Thr286 phosphorylation of CaMKII following dopamine depletion may be due to stimulation of dendritic Ca2+ signalling, perhaps due to enhanced corticostriatal glutamatergic signalling. Additional studies will be required to resolve these issues.
Consequences of ageing and chronic dopamine depletion
Increased Thr286 phosphorylation of CaMKIIα is evident within 3 weeks of 6-OHDA lesion surgery and is maintained for up to 18 months. However, increased phosphorylation of a well-established CaMKII substrate, Ser831 in the AMPA-type glutamate receptor GluR1 subunit, did not become evident until 9–11 months and was maintained for up to 18 months. Thus, prolonged dopamine depletion can have biochemical consequences beyond those seen in the shorter-term studies that are typically performed.
GluR1 phosphorylation depends not only on CaMKII activity but also on the activity of opposing phosphatases, such as PP1 / PP2A and PP2B (Lee et al., 2000; Snyder et al., 2000; Vinade & Dosemeci, 2000). Total PP1 / PP2A and PP2B activities increase during ageing even though PP2A and PP2B protein levels remain constant (Norris et al., 1998; Foster et al., 2001; Foster et al., 2003; see also supplementary Fig. S1, C). We found that, while total levels of the PP1γ1 isoform increased significantly with ageing, the levels of spinophilin, neurabin and DARPP-32 decreased (Fig. 4). Thus, we hypothesize that in young rats, PSD-targeted PP1γ1 prevents the accumulation of Ser831-phosphorylated GluR1 following dopamine depletion; reduced levels of PSD-associated CaMKIIα may also contribute to the lack of increase in Ser831-phosphorylated GluR1. However, as levels of spinophilin and neurabin decrease with normal ageing, levels of PSD-targeted PP1 may decrease (despite the overall increase in PP1γ1 levels), allowing accumulation of Ser831-phosphorylated GluR1 in response to increased levels of Thr286-phosphorylated CaMKII following dopamine depletion. Further studies are clearly warranted to understand the interplay between changes in signalling pathways induced by dopamine depletion and by normal ageing.
Relationship of observed changes to synaptic plasticity
Dopamine acutely regulates both CaMKII (Gu & Yan, 2004; Picconi et al., 2004) and AMPA receptors (Snyder et al., 2000; Chao et al., 2002), and CaMKII regulates the unitary conductance and trafficking of AMPA-type glutamate receptors (Malinow & Malenka, 2002; Malinow, 2003; Allen, 2004). Moreover, normal synaptic plasticity requires phosphorylation of CaMKII at Thr286 (Lisman et al., 2002; Colbran & Brown, 2004) and of AMPA receptor GluR1 subunits (Lee et al., 2003). Although roles of CaMKII and GluR1 phosphorylation in the striatum are poorly understood, short-term striatal dopamine depletion disrupts multiple forms of striatal synaptic plasticity (Centonze et al., 1999; Partridge et al., 2000; Norman et al., 2005). Recently, CaMKII inhibitors were shown to rescue a defect in striatal synaptic plasticity following short-term dopamine depletion (Picconi et al., 2004). However, our data suggest that long-term dopamine depletion may enhance glutamate receptor-mediated transmission by increasing the levels of Ser831-phosphorylated GluR1. It will be interesting to determine whether prolonged periods of dopamine depletion induce additional changes in striatal synaptic plasticity and whether CaMKII inhibitors are similarly effective after GluR1 phosphorylation has increased.
Relationship of observed changes to morphological alterations in MSNs
Dendritic morphology of cortical and hippocampal neurons is sensitive to changes in expression of many proteins, including CaMKIIα, PSD-95, neurabin and spinophilin (Feng et al., 2000; Oliver et al., 2002; Jourdain et al., 2003; Lee et al., 2004; Li et al., 2004; Tang et al., 2004). Given the morphological changes associated with striatal dopamine depletion in Parkinson’s disease and in animal models of parkinsonism (Ingham et al., 1998; Arbuthnott et al., 2000; Meshul et al., 2000; Zaja-Milatovic et al., 2005), it is somewhat surprising that total striatal levels of all proteins analysed here were unchanged, even after prolonged dopamine depletion. However, our observations confirm and extend previous studies demonstrating a lack of changes in GluR2 / 3, α-actinin-2 and PSD-95 following 6-OHDA lesion (Dunah et al., 2000), although some studies report changes in levels of other glutamate receptor subunits (Porter et al., 1994; Oh et al., 1999; Dunah et al., 2000; Betarbet et al., 2004; Picconi et al., 2004). In contrast to the modest effects of dopamine depletion, decreased spinophilin levels (Fig. 4) may be associated with age-related losses of dendritic spines (Ingham et al., 1989; Ou et al., 1997). Thus, morphological changes which occur following dopamine depletion and during normal ageing are probably caused by different cellular mechanisms.
Relevance to Parkinson’s disease
L-DOPA administration completely reversed increases in the phosphorylation of CaMKII and DARPP-32 at Thr286 and Thr75, respectively. In our studies tissue was harvested 16 h following the final L-DOPA injection and the half-life of L-DOPA is ≈90 min. Thus, little (if any) L-DOPA would be present when animals were killed, consistent with the fact that L-DOPA did not affect phosphorylation of CaMKII or DARPP-32 in the intact (normal) striatum (Figs 1C and 3C). These data suggest that dopamine depletion induces significant sustained alterations in striatal signalling mechanisms that can be reversed by L-DOPA within a few weeks of dopamine depletion. This mechanism may contribute to the sustained therapeutic benefits of L-DOPA administration during initial phases of Parkinson’s disease (Thanvi & Lo, 2004). Additional studies that examine signalling in normal and dopamine-depleted striatum at various times after 6-OHDA lesion surgery may provide useful insights into the evolving responses to dopamine replacement therapy in Parkinson’s disease.
Idiopathic Parkinson’s disease is associated with ageing. It will be important to determine whether changes in phosphorylation of CaMKII, DARPP-32 and GluR1 occur in Parkinson’s disease, although such studies may be complicated by dephosphorylation of these proteins in post mortem human tissue. Nevertheless, to the best of our knowledge, the slow development of increased GluR1 phosphorylation at Ser831 following dopamine depletion represents the first report of unique biochemical effects of long-term (9–20 months) dopamine depletion in rodents. These data may be explained by a novel interaction between ageing and dopamine depletion, indicating that short-term dopamine depletion in animal models of parkinsonism may not fully recapitulate the human disease. Moreover, the evolving responses of signalling proteins following dopamine depletion may play a role in the progression of symptoms during Parkinson’s disease, as well as in the changing efficacy and debilitating side-effects associated with dopamine replacement therapy.
Supplementary Material
Acknowledgments
This work was supported by NIH Program Project Grant NS044282 (R.J.C. and A.Y.D.), RO1 grants NS37508 and MH63232 (R.J.C.), MH45124 and MH57795 (A.Y.D.) and the National Parkinson Foundation Center of Excellence at Vanderbilt University. A.M.B. was supported by an NIH Training Grant (T32-GM08554). We thank Drs M. Ritchie and W. Lambert for advice on statistical analyses, and Dr D. Winder for critical comments.
Abbreviations
- 6-OHDA
6-hydroxydopamine
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- CaMKII
calcium–calmodulin-dependent protein kinase II
- cdk5
cyclin-dependent kinase 5
- DARPP-32
dopamine- and cAMP-regulated phospho-protein of 32 kDa
- L-DOPA
levodopa
- MSN
medium spiny neuron
- NMDA
N-methyl-d-aspartate
- PP1
protein phosphatase 1
- PP2A
protein phosphatase 2A
- PSD
postsynaptic density
- TH
tyrosine hydroxylase
Footnotes
Supplementary material
The following supplementary material may be found on: http://www.blackwellpublishing.com/products/journals/suppmat/EJN4190/EJN4190sm.htm
Fig. S1. No significant changes during normal aging in levels of CaMKIIα, GluR1, PP2AC, PP2B or PSD-95, or phosphorylation of CaMKIIα and DARPP-32.
Fig. S2. No significant changes following chronic dopamine depletion in NR1, PP2B or PSD-95.
References
- Allen PB. Functional plasticity in the organization of signaling complexes in the striatum. Parkinsonism Related Disorders. 2004;10:287–292. doi: 10.1016/j.parkreldis.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Arbuthnott GW, Ingham CA, Wickens JR. Dopamine and synaptic plasticity in the neostriatum. J. Anat. 2000;196:587–596. doi: 10.1046/j.1469-7580.2000.19640587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betarbet R, Poisik O, Sherer TB, Greenamyre JT. Differential expression and ser897 phosphorylation of striatal N-methyl-D-aspartate receptor subunit NR1 in animal models of Parkinson’s disease. Exp. Neurol. 2004;187:76–85. doi: 10.1016/j.expneurol.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Bordelon JR, Smith Y, Nairn AC, Colbran RJ, Greengard P, Muly EC. Differential localization of protein phosphatase-1{alpha}, {beta} and {gamma}1 isoforms in primate prefrontal cortex. Cereb. Cortex. 2005 doi: 10.1093/cercor/bhi070. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody LC, Bauman PA, Bass MA, Mavila N, DePaoli-Roach AA, Colbran RJ. A protein phosphatase-1gamma1 isoform selectivity determinant in dendritic spine-associated neurabin. J. Biol. Chem. 2004;279:21714–21723. doi: 10.1074/jbc.M402261200. [DOI] [PubMed] [Google Scholar]
- Centonze D, Gubellini P, Picconi B, Calabresi P, Giacomini P, Bernardi G. Unilateral dopamine denervation blocks corticostriatal LTP. J. Neurophysiol. 1999;82:3575–3579. doi: 10.1152/jn.1999.82.6.3575. [DOI] [PubMed] [Google Scholar]
- Chao SZ, Lu W, Lee HK, Huganir R, Wolf ME. D1 dopamine receptor stimulation increases GluR1 phosphorylation in postnatal nucleus accumbens cultures. J. Neurochem. 2002;81:984–992. doi: 10.1046/j.1471-4159.2002.00877.x. [DOI] [PubMed] [Google Scholar]
- Chergui K, Svenningson P, Greengard P. Cyclin-dependent kinase 5 regulates dopaminergic and glutamatergic transmission in the striatum. Proc. Natl. Acad. Sci. USA. 2004;101:2191–2196. doi: 10.1073/pnas.0308652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbran RJ. Postsynaptic targeting of calcium / calmodulin-dependent protein kinase II. Biochem. J. 2004a;378:1–16. doi: 10.1042/BJ20031547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbran RJ. Protein phosphatases and calcium / calmodulin-dependent protein kinase II-dependent synaptic plasticity. J. Neurosci. 2004b;24:8404–8409. doi: 10.1523/JNEUROSCI.3602-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbran RJ, Brown AM. Calcium / calmodulin-dependent protein kinase II and synaptic plasticity. Curr. Opin. Neurobiol. 2004;14:318–327. doi: 10.1016/j.conb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Colbran R, Carmody L, Bauman P, Wadzinski B, Bass M. Analysis of specific interactions of native protein phosphatase 1 isoforms with targeting subunits. Meth. Enzymol. 2003;366:156–175. doi: 10.1016/s0076-6879(03)66014-3. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Wang Y, Yasuda RP, Kameyama K, Huganir RL, Wolfe BB, Standaert DG. Alterations in subunit expression, composition, and phosphorylation of striatal N-methyl-d-aspartate glutamate receptors in a rat 6-hydroxydopamine model of Parkinson’s disease. Mol. Pharmacol. 2000;57:342–352. [PubMed] [Google Scholar]
- Elgersma Y, Fedorov NB, Ikonen S, Choi ES, Elgersma M, Carvalho OM, Giese KP, Silva AJ. Inhibitory autophosphorylation of CaMKII controls PSD association, plasticity, and learning. Neuron. 2002;36:493–505. doi: 10.1016/s0896-6273(02)01007-3. [DOI] [PubMed] [Google Scholar]
- Feng J, Yan Z, Ferreira A, Tomizawa K, Liauw JA, Zhuo M, Allen PB, Ouimet CC, Greengard P. Spinophilin regulates the formation and function of dendritic spines. Proc. Natl. Acad. Sci. USA. 2000;97:9287–9292. doi: 10.1073/pnas.97.16.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong DK, Rao A, Crump T, Craig AM. Rapid synaptic remodeling by protein kinase C: reciprocal translocation of NMDA receptors and calcium / calmodulin-dependent kinase II. J. Neurosci. 2002;22:2153–2164. doi: 10.1523/JNEUROSCI.22-06-02153.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Kumar A, Masse J. Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiol. Aging. 2003;24:839–852. doi: 10.1016/s0197-4580(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Masse JR, Norris CM, Kumar A. Calcineurin links Ca2+ dysregulation with brain aging. J. Neurosci. 2001;21:4066–4073. doi: 10.1523/JNEUROSCI.21-11-04066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga K, Muller D, Ohmitsu M, Bako E, DePaoli-Roach AA, Miyamoto E. Decreased protein phosphatase 2A activity in hippocampal long-term potentiation. J. Neurochem. 2000;74:807–817. doi: 10.1046/j.1471-4159.2000.740807.x. [DOI] [PubMed] [Google Scholar]
- Griffith LC. Regulation of calcium / calmodulin-dependent protein kinase II activation by intramolecular and intermolecular interactions. J. Neurosci. 2004;24:8394–8398. doi: 10.1523/JNEUROSCI.3604-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Yan Z. Bidirectional regulation of Ca2+ / calmodulin-dependent protein kinase II activity by dopamine D4 receptors in prefrontal cortex. Mol. Pharmacol. 2004;66:948–955. doi: 10.1124/mol.104.001404. [DOI] [PubMed] [Google Scholar]
- Hanson PI, Schulman H. Neuronal Ca2+ / calmodulin-dependent protein kinases. Annu. Rev. Biochem. 1992;61:559–601. doi: 10.1146/annurev.bi.61.070192.003015. [DOI] [PubMed] [Google Scholar]
- Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+ / calmodulin-dependent protein kinase II. Biochem. J. 2002;364:593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham CA, Hood SH, Arbuthnott GW. Spine density on neostriatal neurones changes with 6-hydroxydopamine lesions and with age. Brain Res. 1989;503:334–338. doi: 10.1016/0006-8993(89)91686-7. [DOI] [PubMed] [Google Scholar]
- Ingham CA, Hood SH, Taggart P, Arbuthnott GW. Plasticity of synapses in the rat neostriatum after unilateral lesion of the nigrostriatal dopaminergic pathway. J. Neurosci. 1998;18:4732–4743. doi: 10.1523/JNEUROSCI.18-12-04732.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Fukunaga K, Muller D. Calcium / calmodulin-dependent protein kinase II contributes to activity-dependent filopodia growth and spine formation. J. Neurosci. 2003;23:10645–10649. doi: 10.1523/JNEUROSCI.23-33-10645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Campomanes CR, Sikat PT, Greenfield AT, Allen PB, McEwen BS. Estrogen induces phosphorylation of cyclic AMP response element binding (pCREB) in primary hippocampal cells in a time-dependent manner. Neuroscience. 2004;124:549–560. doi: 10.1016/j.neuroscience.2003.11.035. [DOI] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, Wenthold RJ, Gallagher M, Huganir RL. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112:631–643. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- Lengyel I, Cammarota M, Brent VA, Rostas JAP. Autonomous activity and autophosphorylation of CaMPK-II in rat hippocampal slices: effects of tissue preparation. J. Neurochem. 2001;76:149–154. doi: 10.1046/j.1471-4159.2001.00058.x. [DOI] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc. Natl. Acad. Sci. USA. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat. Rev. Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MacMillan L, Bass M, Cheng N, Howard E, Tamura M, Strack S, Wadzinski BE, Colbran RJ. Brain actin-associated protein phosphatase 1 holoenzymes containing spinophilin, neurabin, and selected catalytic subunit isoforms. J. Biol. Chem. 1999;274:35845–35854. doi: 10.1074/jbc.274.50.35845. [DOI] [PubMed] [Google Scholar]
- Malinow R. AMPA receptor trafficking and long-term potentiation. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2003;358:707–714. doi: 10.1098/rstb.2002.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- McNeill TH, Brown SA, Rafols JA, Shoulson I. Atrophy of medium spiny I striatal dendrites in advanced Parkinson’s disease. Brain Res. 1988;455:148–152. doi: 10.1016/0006-8993(88)90124-2. [DOI] [PubMed] [Google Scholar]
- McNeill RB, Colbran RJ. Interaction of autophosphorylated Ca2+ / calmodulin-dependent protein kinase II with neuronal cytoskeletal proteins. Characterization of binding to a 190-kDa postsynaptic density protein. J. Biol. Chem. 1995;270:10043–10049. doi: 10.1074/jbc.270.17.10043. [DOI] [PubMed] [Google Scholar]
- Menegoz M, Lau LF, Herve D, Huganir RL, Girault JA. Tyrosine phosphorylation of NMDA receptor in rat striatum: effects of 6-OH-dopamine lesions. Neuroreport. 1995;7:125–128. [PubMed] [Google Scholar]
- Meshul CK, Cogen JP, Cheng H-W, Moore C, Krentz L, McNeill TH. Alterations in rat striatal glutamate synapses following a lesion of the cortico- and / or nigrostriatal pathway. Exp. Neurol. 2000;165:191–206. doi: 10.1006/exnr.2000.7467. [DOI] [PubMed] [Google Scholar]
- Nakano K, Kayahara T, Tsutsumi T, Ushiro H. Neural Circuits and functional organization of the striatum. J. Neurol. 2000;247:V1–V15. doi: 10.1007/pl00007778. [DOI] [PubMed] [Google Scholar]
- Nishi A, Bibb JA, Snyder GL, Higashi H, Nairn AC, Greengard P. Amplification of dopaminergic signaling by a positive feedback loop. Proc. Natl. Acad. Sci. USA. 2000;97:12840–12845. doi: 10.1073/pnas.220410397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, Watanabe Y, Higashi H, Tanaka M, Nairn A, Greengard P. Glutamate regulation of DARPP-32 phosphorylation in neostriatal neurons involves activation of multiple signaling cascades. Proc. Natl. Acad. Sci. USA. 2005;102:1199–1204. doi: 10.1073/pnas.0409138102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman ED, Egli RE, Colbran RJ, Winder DG. A potassium channel blocker-induces a long-lasting enhancement of corticostriatal responses. Neuropharmacology. 2005;48:311–321. doi: 10.1016/j.neuropharm.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Norris CM, Halpain S, Foster TC. Alterations in the balance of protein kinase / phosphatase activities parallel reduced synaptic strength during aging. J. Neurophysiol. 1998;80:1567–1570. doi: 10.1152/jn.1998.80.3.1567. [DOI] [PubMed] [Google Scholar]
- Oh JD, Vaughan CL, Chase TN. Effect of dopamine denervation and dopamine agonist administration on serine phosphorylation of striatal NMDA receptor subunits. Brain Res. 1999;821:433–442. doi: 10.1016/s0006-8993(99)01121-x. [DOI] [PubMed] [Google Scholar]
- Oliver CJ, Terry-Lorenzo RT, Elliott E, Bloomer WAC, Li S, Brautigan DL, Colbran RJ, Shenolikar S. Targeting protein phosphatase 1 (PP1) to the actin cytoskeleton: the neurabin I / PP1 complex regulates cell morphology. Mol. Cell. Biol. 2002;22:4690–4701. doi: 10.1128/MCB.22.13.4690-4701.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou X, Buckwalter G, McNeill TH, Walsh JP. Age-related change in short-term synaptic plasticity intrinsic to excitatory striatal synapses. Synapse. 1997;27:57–68. doi: 10.1002/(SICI)1098-2396(199709)27:1<57::AID-SYN6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Partridge JG, Tang KC, Lovinger DM. Regional and postnatal heterogeneity of activity-dependent long-term changes in synaptic efficacy in the dorsal striatum. J. Neurophysiol. 2000;84:1422–1429. doi: 10.1152/jn.2000.84.3.1422. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Orlando: Academic Press; 1986. [Google Scholar]
- Picconi B, Centzone D, Hakansson K, Bernardi G, Greengard P, Fisone G, Cenci MA, Calabresi P. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat. Neurosci. 2003;6:501–505. doi: 10.1038/nn1040. [DOI] [PubMed] [Google Scholar]
- Picconi B, Gardoni F, Centzone D, Mauceri D, Cenci MA, Bernardi G, Calabresi P, DiLuca M. Abnormal Ca2+–calmodulin-dependent protein kinase II function mediates synaptic and motor deficits in experimental Parkinsonism. J. Neurosci. 2004;24:5283–5291. doi: 10.1523/JNEUROSCI.1224-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RHP, Greene JG, Higgins DS, Greenamyre JT. Polysynaptic regulation of glutamate receptors and mitochondrial enzyme activities in the basal ganglia of rats with unilateral dopamine depletion. J. Neurosci. 1994;14:7192–7199. doi: 10.1523/JNEUROSCI.14-11-07192.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog. Neurobiol. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Schulman H. Activity-dependent regulation of calcium / calmodulin-dependent protein kinase II localization. J. Neurosci. 2004;24:8399–8403. doi: 10.1523/JNEUROSCI.3606-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarting RKW, Huston JP. The unilateral 6-hydroxydopamine lesion model in behavioral brain research. Analysis of functional deficits, recovery, and treatments. Prog. Neurobiol. 1996;50:275–331. doi: 10.1016/s0301-0082(96)00040-8. [DOI] [PubMed] [Google Scholar]
- Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284:162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- Shen K, Teurel MN, Connor JH, Shenolikar S, Meyer T. Molecular memory by reversible translocation of calcium / calmodulin-dependent protein kinase II. Nat. Neurosci. 2000;3:881–886. doi: 10.1038/78783. [DOI] [PubMed] [Google Scholar]
- Smart F, Halpain S. Regulation of dendritic spine stability. Hippocampus. 2000;10:542–554. doi: 10.1002/1098-1063(2000)10:5<542::AID-HIPO4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Allen PB, Fienberg AA, Valle CG, Huganir RL, Nairn AC, Greengard P. Regulation of phosphorylation of the GluR1 AMPA receptor in the neostriatum by dopamine and psychostimulants in vivo. J. Neurosci. 2000;20:4480–4488. doi: 10.1523/JNEUROSCI.20-12-04480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack S, Choi S, Lovinger DM, Colbran RJ. Translocation of autophosphorylated calcium / calmodulin-dependent protein kinase II to the postsynaptic density. J. Biol. Chem. 1997;272:13467–13470. doi: 10.1074/jbc.272.21.13467. [DOI] [PubMed] [Google Scholar]
- Strack S, Kini S, Ebner F, Wadzinski BE, Colbran RJ. Differential cellular and subcellular localization of protein phosphatase 1 isoforms in brain. J. Comp. Neurol. 1999;413:373–384. [PubMed] [Google Scholar]
- Suzuki T, Okimura-Noji K, Tanaka R, Tada T. Rapid translocation of cytosolic Ca2+ / calmodulin-dependent protein kinase II into postsynaptic density after decapitation. J. Neurochem. 1994;63:1529–1537. doi: 10.1046/j.1471-4159.1994.63041529.x. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: An integrator of neurotransmission. Annu. Rev. Pharmacol. Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- Tang Y, Janssen WGM, Hao J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb. Cortex. 2004;14:215–223. doi: 10.1093/cercor/bhg121. [DOI] [PubMed] [Google Scholar]
- Terry-Lorenzo RT, Carmody LC, Voltz JW, Connor JH, Li S, Smith FD, Milgram SL, Colbran RJ, Shenolikar S. The neuronal actin-binding proteins, neurabin I and neurabin II, recruit specific isoforms of protein phosphatase-1 catalytic subunits. J. Biol. Chem. 2002;277:27716–27724. doi: 10.1074/jbc.M203365200. [DOI] [PubMed] [Google Scholar]
- Thanvi BR, Lo TCN. Long term motor complications of levodopa: clinical features, mechanisms, and management strategies. Postgrad. Med. J. 2004;80:452–458. doi: 10.1136/pgmj.2003.013912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinade L, Dosemeci A. Regulation of the phosphorylation state of the AMPA receptor GluR1 subunit in the postsynaptic density. Cell. Mol. Neurobiol. 2000;20:451–463. doi: 10.1023/A:1007019030595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeber EJ, Jiang YH, Elgersma Y, Varga AW, Carrasquillo Y, Brown SE, Christian JM, Mirnikjoo B, Silva A, Beaudet AL, Sweatt JD. Derangements of hippocampal calcium / calmodulin-dependent protein kinase II in a mouse model for Angelman mental retardation syndrome. J. Neurosci. 2003;23:2634–2644. doi: 10.1523/JNEUROSCI.23-07-02634.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder DG, Sweatt JD. Roles of serine / threonine phosphatases in hippocampal synaptic plasticity. Nat. Rev. Neurosci. 2001;2:461–474. doi: 10.1038/35081514. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Yoshimura Y. Phosphorylation-dependent reversible translocation of Ca2+ / calmodulin-dependent protein kinase II to the postsynaptic densities. Life Sci. 1998;62:1617–1621. doi: 10.1016/s0024-3205(98)00117-9. [DOI] [PubMed] [Google Scholar]
- Zaja-Milatovic S, Schantz A, Zhang J, Montine KS, Samii A, Deutch AY, Montine TJ. Dendritic degeneration in neostriatal medium spiny neurons in Parkinson’s Disease. Neurology. 2005;64:545–547. doi: 10.1212/01.WNL.0000150591.33787.A4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.