Abstract
Objectives
To examine changes in patterns of medication utilization in patients with rheumatoid arthritis (RA).
Methods
Data from Tennessee Medicaid (TennCare) databases (1995-2004) were used to identify adults with both a diagnosis of RA and at least one disease modifying anti-rheumatic drug (DMARD) prescription each year. Annual age-specific utilization of DMARDs, glucocorticoids, non-steroidal anti-inflammatory drugs (NSAIDs) and narcotics was measured on the last day of each year to determine the point prevalence of use of these agents.
Results
Records from 23342 patients with treated RA were analyzed. Most patients were female (78%) and white (74%). The median age was 57 years (Interquartile range:48-65). The proportion of patients who had a current DMARD prescription on the index date increased from 62% in 1995 to 71% in 2004 (p<0.001). Methotrexate was the most commonly used DMARD. By the end of 2004, 22% of patients had a current prescription for a biologic, and etanercept represented 51% of all biologic therapies. During the study period, the overall utilization of glucocorticoids decreased from 46% to 38%, whereas NSAID utilization increased from 33% to 38% (p<0.001), and use of narcotics increased from 38% to 55% (p<0.001). A secondary analysis which identified RA patients based on diagnosis codes alone, showed similar patterns, but lower DMARD utilization which increased from 33% to 52% overall and from 0% to 16% for biologics.
Conclusions
The utilization of DMARDs increased in TennCare patients with RA, and by 2004, use of biologics was substantial. Although glucocorticoid utilization decreased, use of both NSAIDs and narcotics increased.
Keywords: rheumatoid arthritis, disease modifying anti-rheumatic drugs, epidemiology
Introduction
The treatment of rheumatoid arthritis (RA) with disease modifying anti-rheumatic drugs (DMARDs) provides control of disease activity, slows joint erosions and improves quality of life. Thus, DMARDs have become the foundation of RA treatment [1].
Novel biologic DMARDs have been licensed for use in RA and data from specialized registries have consistently shown a progressive increase in the utilization of both traditional and biologic DMARDs. However, information on RA treated in the community is limited [2-4]. Moreover, pain control is an important goal in the treatment of RA [1, 5] and although DMARDs can control disease activity, additional pain treatment is often necessary [5]. There is a scarcity of information on changes in the utilization of non-DMARD concurrent medications for RA.
We analyzed the changes in patterns of utilization of DMARDs, glucocorticoids, NSAIDs and narcotics in RA patients enrolled in TennCare, Tennessee's Medicaid Program.
Methods
Study population
TennCare, the managed-care Medicaid agency in Tennessee, provided free access to healthcare insurance to Medicaid-eligible patients and those who lacked other access to healthcare. TennCare covered approximately 21% of the population of Tennessee in 2005, mostly low income persons. The major enrollment categories for the program are children, pregnant women, persons with disabilities and those who require care in nursing facilities. There is also an overrepresentation of females and non-white persons, compared to the Tennessee population [6].
Identification of patients with RA
From 1995 through 2004, we identified all TennCare enrollees aged ≥18 years who had a coded diagnoses of RA (ICD9-CM: 714.0, 714.1, 714.2, 714.3*, 714.4, 714.81) and had at least one DMARD prescription filled. This combination of diagnosis codes and DMARD use had a sensitivity of 83% and a specificity of 85% in identifying RA patients in administrative databases [7].
A previous study suggested DMARD discontinuation was frequent [8], so we estimated current medication utilization using a sequence of annual cross-sectional assessments on a specific index date (December 31 of each year). On that date, cohort members were required to have at least 365 days of continuous enrollment in TennCare during which they had a diagnosis of RA and filled at least one DMARD prescription. Since serious co morbidities may affect medication utilization, we excluded patients with serious medical conditions identified during the baseline year including solid organ transplantation, HIV/AIDS, cancer-except non-melanoma skin cancers, chronic renal disease requiring dialysis, liver failure and respiratory failure.
Study medications
Medication utilization was measured using information from the TennCare pharmacy files. Study medications included synthetic DMARDs (methotrexate, sulfasalazine, hydroxychloroquine, leflunomide, gold salts, penicillamine, cyclosporine and azathioprine), biologics (etanercept, infliximab, adalimumab and anakinra), glucocorticoids (both oral and injectables), NSAIDs (including Cox-2 inhibitors) and narcotic analgesics.
Statistical analysis
We estimated the proportion of RA patients who were using study medications on December 31 each year (point prevalence) and evaluated age-specific changes in medication use over time using cross-sectional time-series analyses that accounted for patients contributing more than one observation. In addition, we analyzed medication utilization among patients who were not receiving DMARDs on the index date. This study was approved by the Institutional Review Board of Vanderbilt University and the Bureau of TennCare.
Sensitivity analysis
Since the application of a specific RA definition in our study enriched our cohorts with RA patients prescribed DMARDs who likely had more severe disease, we performed a secondary analysis using more lenient selection criteria which included patients identified with only one inpatient or two outpatient coded visits for RA (at least 1 month apart).
Results
During the study period we identified 24,250 patients with RA. Approximately 3-4% of patients were excluded because of serious comorbidities in each cohort. The number of patients who met selection criteria increased from 1007 in 1995 to 3466 in 2004 (total number=23342). Overall, the average proportion of female patients was 78%, the median age was 57 years (Interquartile range (IQR):48-65), 74% of patients were white, and 53% lived in urban areas. Nearly 68% of patients were eligible for TennCare based on disability and 1% were nursing home residents.
Medication utilization
The proportion of RA patients receiving at least one study medication (DMARD, NSAID, glucocorticoid or narcotic) on the index date increased from 88% in 1995 to 91% in 2004 (p<0.001). The median number of non-study medications received by patients with RA increased from 2 (IQR:1-4) in 1995 to 5 (IQR:3-8) in 2004.
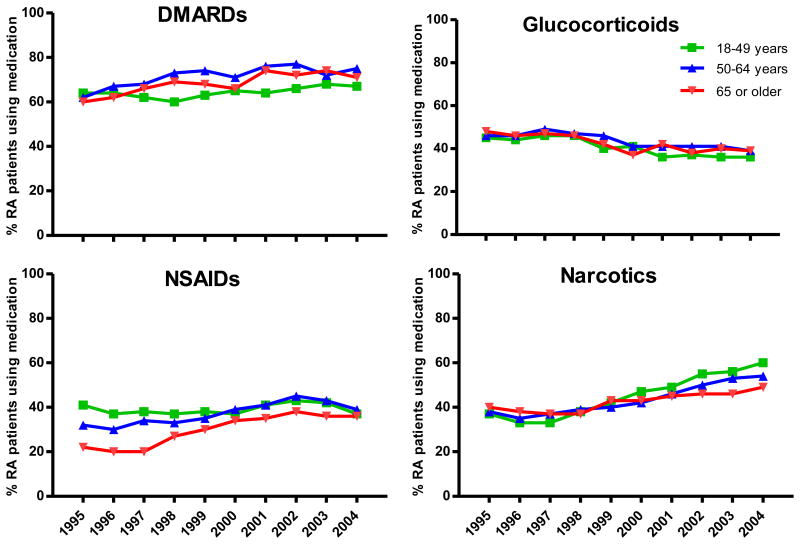
Approximately 62% of patients were receiving DMARDs (alone or in combination with other study medications) in 1995 and this proportion increased to 71% in 2004 (p<0.001). These changes were consistent in all age groups. During the study period, glucocorticoid utilization declined consistently in all age groups from 46% to 38% (p<0.001). The overall utilization of NSAIDs increased (p<0.001) from 33% in 1995 to 43% in 2002, and then declined to 38% in 2004. Patients aged ≥65 years had the largest increase in NSAID utilization. In 1995, 38% of patients were receiving narcotics, whereas in 2004, narcotic utilization increased to 55% (p<0.001) (Figure 1).
Figure 1.
Patterns of medication utilization among TennCare patients diagnosed with RA. 1995 - 2004. Footnote: Data are estimated from TennCare, using December 31st of each calendar year as index date.
DMARD utilization and concurrent medications
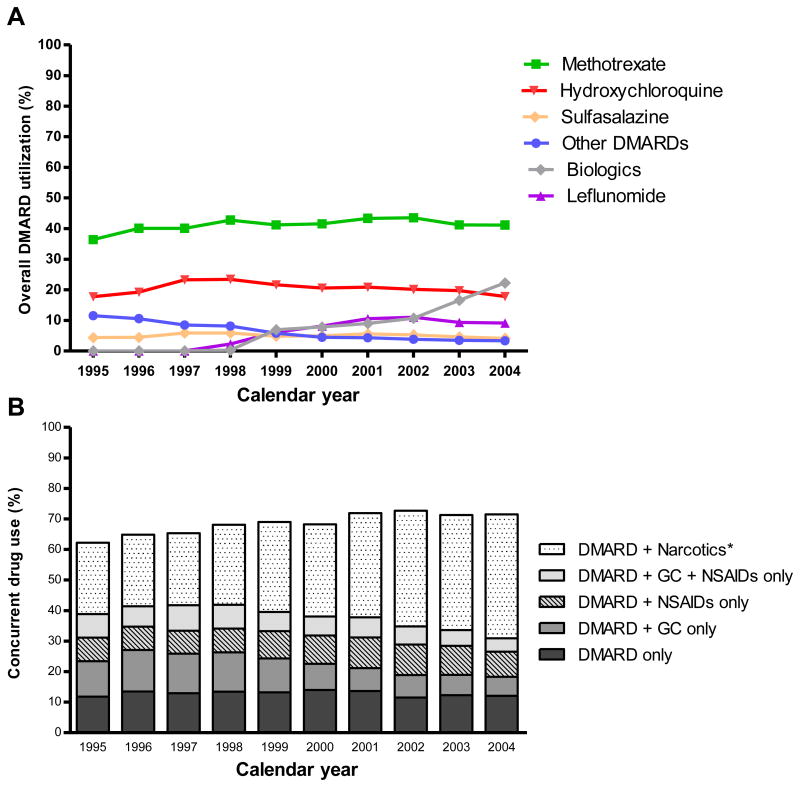
The overall utilization of methotrexate (alone or in combination with other DMARDs) remained stable during the study period (p=0.785); although, there was an increase in the use of methotrexate in combination with other DMARDs (5% to 17%) and a decrease in its use as a single agent (31% to 24%). The overall utilization of hydroxychloroquine decreased (p<0.001), sulfasalazine use remained stable over time (p=0.371) and leflunomide utilization increased (p<0.001). The utilization of other synthetic DMARDs decreased over time (p<0.001 for each) (Figure 2A). The use of biologics (alone or in combination with other DMARDs) started in 1998 and by the end of 2004, 22% of treated patients were receiving biologics (p<0.001). Etanercept accounted for 51% of biologic utilization in 2004.
Figure 2.
Patterns of medication utilization among TennCare patients diagnosed with RA who used DMARDs. 1995 - 2004. A. Overall utilization of DMARDs B. Concurrent non-DMARD medications. Footnote: Data are estimated from TennCare, using December 31st of each calendar year as index date. GC, glucocorticoids. *Used alone or with NSAIDS or glucocorticoids
Approximately 10% of RA patients used DMARDs alone (without concurrent glucocorticoids, NSAIDs or narcotics) and this proportion remained relatively stable over time, whereas use of DMARDs and glucocorticoids (with or without NSAIDs) decreased. The concurrent utilization of DMARDs and narcotics (with or without NSAIDs or glucocorticoids) increased from 23% in 1995 to 41% in 2004 (Figure 2B).
Medication utilization in Non-DMARD users
Although filling one DMARD prescription in the past year was a selection criterion, some RA patients were not receiving DMARDs on the index date. These patients had an overall median age of 56 years (IQR: 46-65). Approximately 79% were female, 71% were white and 51% lived in urban areas. Nearly 66% were enrolled in TennCare based on disability and 1% were nursing home residents. Overall, the proportion of patients without study medications on the index date decreased over time. In addition, the proportion of RA patients receiving glucocorticoids or NSAIDS or both without DMARDs decreased over time; whereas the utilization of narcotics (with or without NSAIDs or glucocorticoids) remained relatively stable (Figure 3, supplementary data).
Sensitivity analysis
The application of less specific selection criteria increased our number of total patients included (from 23342 to 37547). Compared with our primary analysis, there were no material differences in the distribution of demographic data and although the overall and age-stratified changes in patterns of medication use were similar, estimates of medication utilization were consistently lower. Overall DMARD utilization increased from 33% in 1995 to 52% in 2004 (p<0.001). Methotrexate use increased from 19% to 30% (p=0.005), with the increase primarily in combination use with other DMARDs). Overall biologic use increased from 0% in 1998 to 16% in 2004 (p<0.001) whereas glucocorticoid utilization showed a non significant decrease from 36% to 35% (p=0.409). Overall use of NSAIDs and narcotics increased during the study period from 32% to 37% (p<0.001) and from 36% to 56% (p<0.001), respectively.
Discussion
We examined changes in medication utilization in RA patients who were enrolled in TennCare from 1995 to 2004 and had evidence of treatment with a DMARD in the prior year. DMARD utilization increased over time indicating that more RA patients who used DMARDs, remained on therapy, and were current users on the index date. We also documented a substantial uptake of the combination of methotrexate with other DMARDs, and the more recently approved leflunomide and biologic DMARDs. Although glucocorticoid use declined, we observed a modest increase in NSAID use and a large increase in narcotic utilization, especially among RA patients who were concurrently using DMARDs.
Unlike some previous studies of DMARD utilization that identified RA patients relying on diagnosis codes alone, we applied a previously validated algorithm to identify RA patients through administrative data. Since all patients filled a prescription for at least one DMARD in the prior year, our methods overestimate DMARD use in all patients with RA. However, this definition is useful for examining patterns of utilization among those patients judged to be eligible for DMARD therapy and the reported changes were consistently observed when we applied a less specific definition in our sensitivity analysis.
Even though DMARD utilization increased during the study period, a substantial proportion of RA patients were not receiving DMARDs on the index dates. Given the body of evidence supporting the benefits of DMARD therapies, these estimates would suggest suboptimal treatment of RA [3, 4, 9]. However, there are some alternate explanations. The proportion of identified patients who met ACR criteria for RA is unknown. Although our selection criteria likely reduced patient misclassification, any residual misclassification could partially explain this apparent under-treatment [4, 9]. Moreover, the proportion of patients that were in disease remission is unknown. These patients might have discontinued their treatment before the index date. Interestingly, about 10% of patients were not using any study drugs on the index dates. Using data from the Norfolk Arthritis Register, other investigators reported that 18% of patients who initially met ACR criteria for RA were in drug-free disease remission after 3 years [9, 10]. The lack of information on disease duration or disease activity measurements in our databases precluded similar assessments. Furthermore, our study did not assess DMARD doses or prescriber's specialty [3].
Although our findings are consistent with previous studies that showed increasing utilization of DMARDs among all and elderly RA patients [3, 4], some differences should be discussed. In contrast to previous estimates of DMARD utilization that were based on prevalence of use at any time during one year (regardless of continuation on therapy), our study estimated the proportion of patients who were currently using DMARDs on a single day each year (point prevalence). Although our patients had filled a DMARD prescription as part of their inclusion criteria, a sizeable proportion were not using DMARDs on the index date, suggesting that many patients received DMARD therapy intermittently or discontinued treatment altogether. This is consistent with a recent evaluation of the persistence on DMARDs which suggested that discontinuation of these therapies is frequent [8].
We observed increasing trends in narcotic utilization in RA patients. A recent report showed that from 1996 through 2002 the utilization of narcotics increased substantially in the United States, especially among adults aged ≤65 years [11]. By 2005, the State of Tennessee had one of the highest number of narcotic prescriptions per capita in the United States and narcotics were among the most common medications prescribed [12]. Whether the increasing use of narcotics among patients is restricted to TennCare patients is currently unknown. Studies on the patterns of narcotic utilization among RA patients in other populations, and their risks and benefits, would be informative.
Although the precise reasons for DMARD prescribing preferences cannot be assessed in our study, these changes could reflect the incomplete efficacy or relative toxicity profiles of older synthetic DMARDs and the trend towards more aggressive management of inflammation, including combination therapies, leflunomide and biologics. Similarly, the reasons for the changes in the utilization of non-DMARD medications cannot be determined but the observed declines in glucocorticoid utilization could be related to improved disease control resulting from early initiation of DMARD therapy, use of DMARD combination therapies, the introduction of new DMARDs or to concerns about the toxicity of these medications [13]. In the same way, the most recent decline observed in NSAID utilization could be related to the recognition of their gastrointestinal and cardiovascular toxicity.
In accordance with current recommendations for the treatment of RA, the utilization of DMARDs has increased during recent years among TennCare patients with RA. Information on the effectiveness and safety of DMARDs and concurrent medications must inform individual decisions to initiate or continue treatment with these therapies.
Supplementary Material
Figure 3 (Supplementary Data). Patterns of RA-related medication utilization among TennCare patients diagnosed with RA who were not using DMARDs. 1995 - 2004. Footnote: Data are estimated from TennCare, using December 31st of each calendar year as index date. GC, glucocorticoids. *Used alone or with NSAIDS or glucocorticoids
Key Messages.
The utilization of DMARDs has increased during recent years among TennCare patients diagnosed with RA.
The utilization of narcotic analgesics has also increased in this population.
Acknowledgments
We are indebted to the Tennessee Bureau of TennCare of the Department of Finance and Administration, which provided the data.
This project was funded under Contract No. 290200500421 from the Agency for Healthcare Research and Quality, US Department of Health and Human Services as part of the Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) program. The authors of this report are responsible for its content. Statements in the report should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services.
Footnotes
Conflict of Interest Statement: MRG has been a consultant for Merck and received grant funding from Pfizer and Merck. The other authors have no conflicts of interest to disclose.
Contributor Information
Carlos G. Grijalva, Department of Preventive Medicine, Vanderbilt University School of Medicine, Nashville, TN, and the Mid-South
Cecilia P. Chung, Department of Medicine, Vanderbilt University School of Medicine, Nashville, TN, and the Mid-South
C. Michael Stein, Department of Medicine, Vanderbilt University School of Medicine, Nashville, TN, and the Mid-South.
Edward F. Mitchel, Jr., Department of Preventive Medicine, Vanderbilt University School of Medicine, Nashville, TN, and the Mid-South
Marie R. Griffin, Department of Preventive Medicine, Vanderbilt University School of Medicine, Nashville, TN, and the Mid-South, Department of Medicine, Vanderbilt University School of Medicine, Nashville, TN, and the Mid-South, Geriatric Research Education and Clinical Center and Clinical Research Center of Excellence, VA Tennessee Valley Health Care System, Nashville, TN
References
- 1.Guidelines for the management of rheumatoid arthritis: 2002 Update. Arthritis Rheum. 2002;46(2):328–46. doi: 10.1002/art.10148. [DOI] [PubMed] [Google Scholar]
- 2.Soderlin MK, Lindroth Y, Jacobsson LT. Trends in medication and health-related quality of life in a population-based rheumatoid arthritis register in Malmo, Sweden. Rheumatology (Oxford) 2007 Aug;46(8):1355–8. doi: 10.1093/rheumatology/kem143. [DOI] [PubMed] [Google Scholar]
- 3.Edwards CJ, Arden NK, Fisher D, et al. The changing use of disease-modifying anti-rheumatic drugs in individuals with rheumatoid arthritis from the United Kingdom General Practice Research Database. Rheumatology (Oxford) 2005;44(11):1394–8. doi: 10.1093/rheumatology/kei024. [DOI] [PubMed] [Google Scholar]
- 4.Schmajuk G, Schneeweiss S, Katz JN, et al. Treatment of older adult patients diagnosed with rheumatoid arthritis: Improved but not optimal. Arthritis Rheum. 2007 Aug 15;57(6):928–34. doi: 10.1002/art.22890. [DOI] [PubMed] [Google Scholar]
- 5.Solomon DH, Avorn J, Wang PS, et al. Prescription opioid use among older adults with arthritis or low back pain. Arthritis Rheum. 2006 Feb 15;55(1):35–41. doi: 10.1002/art.21697. [DOI] [PubMed] [Google Scholar]
- 6.Bureau of TennCare. TennCare Fiscal Year 2005-2006 Annual report. Vol. 2007. Nashville, TN: p. 30. [Google Scholar]
- 7.Singh JA, Holmgren AR, Noorbaloochi S. Accuracy of Veterans Administration databases for a diagnosis of rheumatoid arthritis. Arthritis Rheum. 2004;51(6):952–7. doi: 10.1002/art.20827. [DOI] [PubMed] [Google Scholar]
- 8.Grijalva CG, Chung CP, Arbogast PG, Stein CM, Mitchel EF, Jr, Griffin MR. Assessment of adherence to and persistence on disease-modifying antirheumatic drugs (DMARDs) in patients with rheumatoid arthritis. Med Care. 2007 Oct;45(10 Supl 2):S66–76. doi: 10.1097/MLR.0b013e318041384c. [DOI] [PubMed] [Google Scholar]
- 9.Wise EM, Isaacs JD. Management of rheumatoid arthritis in primary care--an educational need? Rheumatology (Oxford) 2005 Nov;44(11):1337–8. doi: 10.1093/rheumatology/kei086. [DOI] [PubMed] [Google Scholar]
- 10.Harrison B, Symmons D. Early inflammatory polyarthritis: results from the Norfolk Arthritis Register with a review of the literature. II. Outcome at three years. Rheumatology (Oxford) 2000 Sep;39(9):939–49. doi: 10.1093/rheumatology/39.9.939. [DOI] [PubMed] [Google Scholar]
- 11.Banthin JS, Miller GE. Trends in prescription drug expenditures by Medicaid enrollees. Med Care. 2006 May;44(5 Suppl):I27–35. doi: 10.1097/01.mlr.0000208132.36055.84. [DOI] [PubMed] [Google Scholar]
- 12.BlueCross BlueShield of Tennessee. Inside Tennessee's Medicine Cabinet How Much is Enough? A Blue Report on High Prescription Drug Use in Tennessee and its Consequences. Blue Series. Chattanooga. 2007:1–20. [Google Scholar]
- 13.Moreland LW, O'Dell JR. Glucocorticoids and rheumatoid arthritis: back to the future? Arthritis Rheum. 2002 Oct;46(10):2553–63. doi: 10.1002/art.10567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 3 (Supplementary Data). Patterns of RA-related medication utilization among TennCare patients diagnosed with RA who were not using DMARDs. 1995 - 2004. Footnote: Data are estimated from TennCare, using December 31st of each calendar year as index date. GC, glucocorticoids. *Used alone or with NSAIDS or glucocorticoids