Abstract
Among 249 patients with teratoma-associated encephalitis, 211 had N-methyl-D-aspartate receptor antibodies and 38 were negative for these antibodies. Whereas antibody-positive patients rarely developed prominent brainstem–cerebellar symptoms, 22 (58%) antibody-negative patients developed a brainstem–cerebellar syndrome, which in 45% occurred with opsoclonus. The median age of these patients was 28.5 years (range = 12–41), 91% were women, and 74% had full recovery after therapy and tumor resection. These findings uncover a novel phenotype of paraneoplastic opsoclonus that until recently was likely considered idiopathic or postinfectious. The triad of young age (teenager to young adult), systemic teratoma, and high response to treatment characterize this novel brain-stem–cerebellar syndrome.
The discovery of anti–N-methyl-D-aspartate receptor (NMDAR) encephalitis in 20071 has brought attention to a relationship between systemic teratomas and autoimmune encephalitis. Since 2007, we have studied 249 patients with teratoma-associated encephalitis; most of these patients had antibodies against the NR1 subunit of the NMDAR, but 38 were NMDAR antibody negative. When these 38 patients were compared with those with NMDAR antibodies, a novel brainstem–cerebellar syndrome that frequently associates with opsoclonus emerged. The current study describes the clinical differences between NMDAR antibody-positive and antibody-negative patients with systemic teratoma, and focuses on the novel brainstem–cerebellar syndrome and the subgroup of patients with opsoclonus.
Patients and Methods
From January 2007 until September 2012, serum and CSF of 249 patients with teratoma-associated encephalitis were studied at the Department of Neurology, Hospital of the University of Pennsylvania and at the Neurology Service, Hospital Clinic, August Pi i Sunyer Biomedical Research Institute, University of Barcelona. The presence of a systemic teratoma was confirmed pathologically in 234 patients and radiologically in 15. Information was obtained by the authors or provided by referring physicians at symptom onset and at regular intervals during the course of the disease using a comprehensive questionnaire that includes all symptoms shown in the Figure.2 Sera and cerebrospinal fluid (CSF) were examined for antibodies to NMDA, α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid, γ-aminobutyric acid (B), and mGluR5 receptors, LGI1, Caspr2, onconeuronal proteins (Hu, CRMP5, Ma1–2, amphiphysin), and GAD65, using reported techniques including brain immunohistochemistry, immunoblot, and cell-based assays.3–5 Patients without NMDAR antibodies were further studied for antibodies to dipeptidyl-peptidase-like protein-6 (DPPX), α1-glycine receptor, D2 subunit of the dopamine receptor, and unknown cell-surface antigens using reported techniques.3–5
Outcome was assessed with the modified Rankin scale (mRS),6 grading it as full recovery (mRS = 0), substantial improvement (mRS = 1–2), partial improvement (mRS > 2 after having had at least 1 point of improvement), and no improvement. Three patients without NMDAR antibodies have been previously reported.7–9 Studies were approved by the internal review boards of the University of Pennsylvania and University of Barcelona.
Statistical Analysis
Comparative analyses between patients with and without NMDAR antibodies were performed with SPSS version 20 (IBM, Armonk, NY), using the Fisher exact test for contingency tables and Mann–Whitney U tests for continuous variables.
Results
Two hundred eleven patients were found to have NMDAR antibodies, and 38 were negative for these antibodies. Compared with antibody-positive patients, the 38 patients without NMDAR antibodies showed no differences with respect to gender and age of symptom onset (NMDAR antibody-negative patients: 92% female, median age = 28 years [interquartile range (IQR) = 20–32, range = 12–55] vs antibody-positive patients: 99% female, median age = 25 years [IQR = 19–30, range = 7–65], p = 0.05 and p = 0.11, respectively). However, significant differences were identified with respect to symptom presentation and repertoire of symptoms during the first month of the disease (see Fig 1). Whereas 18 (47%) patients without NMDAR antibodies initially presented with brainstem–cerebellar dysfunction, this presentation did not occur in any of the patients with NMDAR antibodies (p < 0.0005). In contrast, whereas 144 of 211 (68%) patients with NMDAR antibodies presented with psychosis and behavioral abnormalities, this presentation occurred only in 4 of 38 (11%) patients without these antibodies (p < 0.0005).
FIGURE 1.
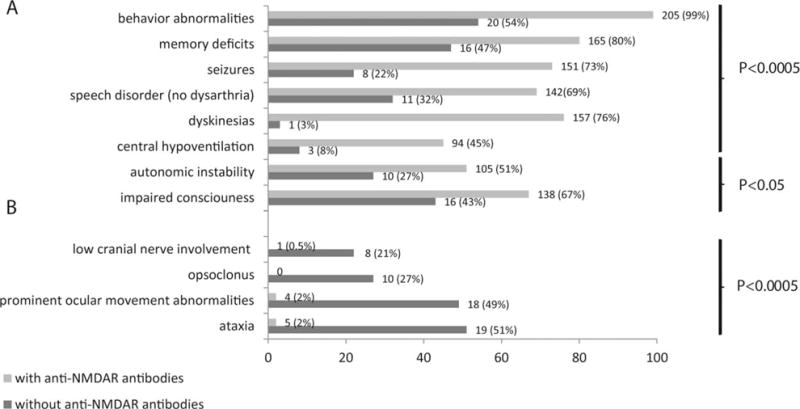
Comparison of symptoms of patients with teratoma-associated encephalitis and N-methyl-D-aspartate receptor (NMDAR) antibodies with those without NMDAR antibodies. (A) Patients without NMDAR antibodies (indicated in dark gray) less frequently developed symptoms considered characteristic of anti-NMDAR encephalitis (behavioral abnormalities, memory deficits, seizures, dyskinesias, speech disorder, and central hypoventilation, all p < 0.0005, and impaired level of consciousness and autonomic dysfunction, p < 0.05). (B) In addition, patients without NMDAR antibodies more frequently developed brain-stem–cerebellar symptoms and opsoclonus, which are rare in anti-NMDAR encephalitis (all p < 0.0005). From 1 patient without NMDAR antibodies and 4 with antibodies, detailed clinical information was not available, and these patients were excluded from analysis; in 3 additional patients without NMDAR antibodies, information for memory deficits and speech disorder was not available.
The Figure shows that during the first month of the disease, 76% of the patients with NMDAR antibodies developed dyskinesias, often involving the face and mouth, whereas only 1 (3%) patient without these antibodies developed dyskinesias, without affecting the face and mouth (p < 0.0005); similar differences were seen for most symptoms typical of anti-NMDAR encephalitis. In contrast, 22 of 38 (58%) patients without NMDAR antibodies developed brainstem–cerebellar symptoms during the first month of the disease, 10 (45%) of them with opsoclonus, whereas these symptoms rarely occurred in patients with NMDAR antibodies. The identification of a predominant brainstem–cerebellar syndrome led us to focus on this disorder and the subgroup of patients with opsoclonus, both described below (the other 16 patients are shown in the Supplementary Table).
Brainstem–Cerebellar Syndrome
The median age of the 22 patients with brainstem–cerebellar symptoms was 28.5 years (IQR = 22–32, range = 12–41). Twenty (91%) were female, all with ovarian teratoma; 2 male patients had testicular teratoma. Main symptoms included ataxia in 86%, opsoclonus–myoclonus in 45% (described below), dysarthria in 36%, decreased level of consciousness in 32%, diplopia or ophthalmoparesis in 18%, and seizures in 18%. Other symptoms are listed in the Table 1.
TABLE 1.
Clinical Features in Patients with Brainstem-Cerebellar Syndrome and Systemic Teratoma without N-Methyl-D-Aspartate Receptor Antibodies
| Opsoclonus | Patient No. |
Age, yr/Sex |
Main Symptoms |
Neurologic Symptoms before Tumor Diagnosis |
Brain MRI |
CSF | Treatment | Response to Treatment |
Immunological Studies with Cultures of Neurons |
|---|---|---|---|---|---|---|---|---|---|
| With | 1 | 20/F | Opsoclonus–myoclonus, limb ataxia, dysarthria, meningeal signs, drowsiness, tonic seizures, autonomic instability (ileus, urinary retention) | Yes | Meningeal enhancement | 182 WBC/μ1 (87% L), 99mg/dl protein; repeat study: 326 WBC/μl, 159mg/dl protein | Tumor removal, steroids | Complete, related to immunotherapy | Negative |
| 2 | 15/F | Opsoclonus–myoclonus, ataxia, drowsiness, vomiting, blurred vision | Yes | Normal | 37 WBC/μl 64mg/dl protein | Tumor removal, steroids, IVIg | Complete, related to tumor removal | Negative | |
| 3 | 26/F | Opsoclonus–myoclonus, ataxia, dysarthria, aphasia, 3 days after tumor removal | No | Normal | 72 WBC/μl (69% L), 49mg/dl protein. OB positive | Steroids, IVIg, plasma exchange (3×), rituximab (3 cycles); 1 cycle of bleomycin, etoposide, and carboplatin; 3 cycles of etoposide and cisplatin | Partial response to steroids, IVIg, plasma exchange; complete recovery after chemotherapy and rituximab | Negative | |
| 4 | 31/F | Opsoclonus–myoclonus, ataxia, tinnitus | Yes | Normal | Mild pleocytosis with increased lymphocytes and protein concentration | Tumor removal (bilateral), steroids, IVIg, plasma exchange, chlorambucil | Complete, related to immunotherapy and tumor removal; relapsed 7 years later with mild ataxia and memory deficits | Reactivity of serum with cell surface of neurons | |
| 5 | 22/F | Opsoclonus–myoclonus, ataxia, abnormal behavior, impaired consciousness; severe bradycardia requiring sinus pacemaker | Yes | Normal | 10 WBC/μl, 57mg/dl protein. OB positive | Tumor removal, steroids | Partial, related to immuno therapy; complete after tumor removal | Negative | |
| 6 | 24/F | Opsoclonus without myoclonus, truncal ataxia, vertigo, abdominal pain, generalized weakness, hyporeflexia | Yes | Normala | <5 WBC/μl, <45mg/dl protein | Tumor removal, steroids, IVIg | Complete, related to immuno therapy | Negative | |
| 7 | 30/F | Opsoclonus without myoclonus, dizziness, meningeal signs, seizures, abnormal behavior (not psychotic), weakness, hyporeflexia, central hypoventilation | Yes | 1st normal; repeat study: brainstem edema and meningeal enhancement | 134 WBC/μl (88% L), 88 mg/dl protein; repeat study: 414 WBC/μl (97% L), 110mg/dl protein | Steroids, IVIg, plasma exchange | Complete, related to immunotherapy; remained with motor weakness 3 months after disease onset | Negative | |
| 8 | 29/F | Opsoclonus–myoclonus, sense of unsteadiness and body “shakiness” (26th week of pregnancy) | No | Not done | <5 WBC/μl, <45mg/dl protein, OB positive | Tumor removal, steroids | Complete, related to immunotherapy | Negative | |
| 9 | 28/F | Opsoclonus–myoclonus, dysarthria, ataxia, behavioral disinhibition, hypersexuality, hyperphagia, cognitive decline | No | Normal | 12 WBC/μl <45mg/dl protein | Tumor removal, steroids, IVIg, plasma exchange, azathioprine | Partial, related to immunotherapy; improved dysarthria and opsoclonus; ataxia still improving at last follow-up (15 months) | Reactivity of serum with cell surface of neurons | |
| 10 | 32/F | Opsoclonus–myoclonus, dysarthria, diplopia, ataxia | Yes | Normal | 30 WBC/μl, <45mg/dl protein | Tumor removal, steroids, IVIg, rituximab (4 doses) | Partial, related to immunotherapy and tumor removal; mild ataxia and dysarthria at last follow-up (13 months) | Negative | |
| Without | 11 | 19/F | Right hand tremor, ataxia, bilateral dysdiadochokinesia, dysmetria | Yes | Normal | 124 WBC/μl, <45mg/dl protein | Tumor removal, steroids | Complete, related to immunotherapy and tumor removal | Negative |
| 12 | 32/F | Subacute tremor, unsteady gait | Yes | Normal | <5 WBC/μl, <45mg/dl protein | Tumor removal | Complete, related to tumor removal | Negative | |
| 13 | 31/F | Subacute onset of vomiting, nystagmus, ataxic gait, dysarthria, myoclonus; all symptoms resolved after removal of ovarian teratoma, but the patient developed abnormal behavior, memory deficit, labile affect, and optic neuritis; recurrence of symptoms and oculomotor paresis 4 months later | Yes | 1st normal; at clinical relapse 7 months later: abnormality at the level of oculomotor nuclei | 16 WBC/μl <45mg/dl protein, OB negative | Tumor removal, steroids, IVIg | Partial with tumor removal, complete with immunotherapy; relapse 8 months later (4 months after recovery) | Negative | |
| 14 | 23/M | Cerebellar ataxia | Yes | NA | NA | NA | NA | Negative | |
| 15 | 33/F | Severe cerebellar ataxia | Yes | NA | NA | Tumor removal | NA | Negative | |
| 16 | 15/F | Left side ataxia, dysarthria, paresthesias, dysdiadochokinesia with left hand (onset 1.5 months after tumor removal) | No | Normal brain MRI and PET scan | <5 WBC/μl <45mg/dl protein |
Not treated | Symptoms stable with no improvement 4 months after presentation | Reactivity of CSF with cell surface of neurons | |
| 17 | 33/F | 6-week episode of severe cerebellar ataxia that resolved without any specific treatment; relapse 2 years later: ataxia and memory problems; ovarian teratoma found | Yes | Normal | <5 WBC/μl, <45mg/dl protein | Not treated | Complete without treatment, but relapsed 2 years later | Negative | |
| 18 | 36/F | Subacute ataxia, memory problems, confabulation, bilateral intention tremor, bilateral gaze directed nystagmus | Yes | Diffuse bilateral atrophy, enlarged ventricles (history of alcohol abuse) | <5 WBC/μl, <45mg/dl protein, OB negative | NA | NA | Negative | |
| 19 | 28/F | Suspected viral meningoencephalitis (drowsiness, fever, headache), followed by seizures, brainstem symptoms, ataxia, cognitive and behavioral abnormalities | Yes | 1st normal; 1 year later: diffuse brain atrophy (predominant in cerebellum) | 66 WBC/μl, 126mg/dl protein | Tumor removal | No response; dependent for activities of daily living (dressing, feeding, ambulation) due to cognitive deficits and tetraparesis | Negative | |
| 20 | 12/M | Left side ataxia, bilateral tremor, weakness, short-term memory loss; status epilepticus after testis teratoma removal | Yes | FLAIR hyperintensities in limbic region; right cortical atrophy | 13 WBC/μl, <45mg/dl protein. OB positive | Tumor removal, steroids, IVIg | Partial, ataxia and coordination problems 4 months after onset | Negative | |
| 21 | 41/F | Subacute diplopia, ophthalmoplegia, impaired consciousness; left ovarian teratoma discovered at workup of encephalitis (history of a right ovarian teratoma removed 10 years earlier) | Yes | Normal | 25 WBC/μl, 85mg/dl protein | IVIg, plasma exchange | Complete, related to immunotherapy | Reactivity of serum with cell surface of neurons | |
| 22 | 31/F | Myoclonus of lips, diplopia, confusion, catatonia, orthostatic hypotension | Yes | Increased FLAIR signal in medial temporal lobes | 106 WBC/μl (98% L), 63.2mg/dl protein | Tumor removal, steroids | Complete, related to tumor removal and immunotherapy | Negative |
Normal brain MRI, but decreased degree of tracer accumulation in the brainstem and bilateral cerebral hemispheres on single photon emission computed tomography.
CSF = cerebrospinal fluid; F = female; FLAIR = fluid-attenuated inversion recovery; IVIg = intravenous immunoglobulin; L = lymphocyte; M = male; MRI = magnetic resonance imaging; NA = not available; OB = oligoclonal bands; PET = positron emission tomography; WBC = white blood cell count.
Neurological symptoms developed before tumor diagnosis in 18 patients (82%; median = 1 month, IQR = 0.9–2 months, range = 3 days to 24 months) and after tumor diagnosis in 4 (10 days and 1.5, 2, and 3.5 months, respectively). Two of these 4 patients had the tumor removed 3 days and 1.5 months before developing encephalitis, respectively. All patients had mature teratomas, except 1 who had an immature ovarian teratoma. Serum of 3 patients (2 with opsoclonus) and CSF of another patient showed weak immunolabeling of cultures of rat neurons (data not shown); no antibodies were identified in the other patients.
Treatment and follow-up information was available for 19 (86%) patients, including all patients with opsoclonus (described below). Fifteen (79%) received immunotherapy, 13 of them with tumor resection; 2 had tumor resection without immunotherapy, and 2 were not treated (1 had tumor removal before developing encephalitis). With a median follow-up of 15 months (range = 3–84), 14 patients (74%) had full recovery, 3 (16%) had partial improvement, and 2 had no improvement (1 of them was not treated). Two patients with complete recovery and 1 with partial recovery relapsed 2 years, 7 years, and 8 months after disease onset, respectively.
Opsoclonus–Myoclonus Syndrome
Ten women (median age = 27 years, IQR = 22–30, range = 15–32) with brainstem–cerebellar syndrome developed opsoclonus; accompanying symptoms are listed in the Table 1. Four had prodromal fever or viral-like symptoms, and another one was 26 weeks pregnant. Symptoms developed before the tumor diagnosis in 7 (median = 1 month, IQR = 0.1–1.5 months, range = 3 days to 2 months) and after tumor diagnosis in 3 (10 days, 2 months, and 3.5 months, respectively). One of these 3 patients had undergone tumor resection 3 days before developing opsoclonus; the other 2 patients had not had tumor treatment.
At symptom onset, 7 patients had CSF lymphocytic pleocytosis (median = 37 white blood cells/μl, range = 10–182), 6 had increased protein concentration (median 5= 64/dl, range 49–100), and 3 of 3 had oligoclonal bands. Brain magnetic resonance imaging and electroencephalographic studies were abnormal in 2 of 9 and 3 of 5 patients (see Table 1).
All patients were treated with methylprednisolone: 3 alone, 3 combined with intravenous immunoglobulin (IVIg), and 4 with IVIg and plasma exchange. Two patients received rituximab after failing initial immunotherapy, and 1 received azathioprine (see Table 1). Nine patients had resection of the teratoma; pathological studies showed mature teratoma in 8, including 1 with bilateral teratomas, and immature teratoma in 1. Chemotherapy was used in 2 patients (see Table 1). Valproic acid, clonazepam, levetiracetam, or phenobarbital did not control the opsoclonus–myoclonus (data not shown).
The median time of follow-up was 19.5 months (IQR = 6–39, range = 3–84). Eight patients had full recovery, and 2 had mild residual dysarthria and ataxia at 13- and 15-month follow-up, respectively. Six of the 8 patients with full recovery became asymptomatic within the first 3 months of treatment, and the other 2 patients within 6 and 12 months, respectively.
Discussion
This study shows that patients with systemic teratoma can develop several forms of encephalitis without NMDAR antibodies, among which a syndrome that associates with brainstem–cerebellar symptoms stands out. Almost 50% of patients with this syndrome developed opsoclonus in association with the triad of young age (teenager to young adult), presence of an ovarian teratoma, and high response to treatment. The subacute presentation of symptoms, frequent CSF pleocytosis, and response to immunotherapy coupled with the detection of antibodies to neuronal cell-surface antigens in some patients suggest an immune-mediated pathogenesis.
All patients with opsoclonus were young women (aged 15–32 years), considered too young for carcinoma-associated opsoclonus, which usually occurs in patients >50 years old,10 and too old for neuroblastoma-associated opsoclonus, which usually affects children <5 years old.11 It is likely that this type of opsoclonus has been previously considered idiopathic or postinfectious and that the presence of a teratoma was missed or not felt to be related.
Compared with patients with anti-NMDAR encephalitis, those without these antibodies were less likely to initially present with psychosis and behavioral change. Although there was overlap of some symptoms, such as limbic dysfunction and psychiatric manifestations, the frequency of other symptoms, such as dyskinesias, rarely occurred in patients without NMDAR antibodies. In contrast, patients with anti-NMDAR encephalitis did not initially present with brainstem–cerebellar dysfunction or opsoclonus. Of note, ataxia can be a presentation of anti-NMDAR encephalitis in children2,12; this is not reflected here, because young children usually do not have teratomas.
This study has several practical implications. Any teenager or young adult, especially if female, who develops subacute brainstem–cerebellar symptoms or opsoclonus–myoclonus suspected to be immune-mediated (because of the rapid onset of symptoms and/or CSF pleocytosis) should be investigated for a teratoma in the ovary (or testes for male patients). Detection of a teratoma should prompt its removal along with the use of immunotherapy (most patients described here received steroids, IVIg, and/or plasma exchange). A limitation of this study is that it is retrospective; future studies will establish the frequency of these disorders and may identify patients with higher levels of cell-surface antibodies that could lead to the characterization of the antigens.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the NIH (RO1NS077851 [NINDS], J.D.; RO1MH094741 [NIMH], J.D.); National Cancer Institute (RO1CA89054, J.D.); Fundació la Marató de TV3 (J.D.); and Fondo de Investigaciones Sanitarias, Madrid, Spain (PI11/01780, J.D.; PI12/00611, F.G.); a fellowship from the Dutch Cancer Society (KWF2009–4451, M.J.T.); and a McKnight Neuroscience of Brain Disorders award (J.D.). T.A. received a personal grant from the Instituto Carlos III (FI12/00366).
We thank Dr I. Kawachi, Dr N. Singhal, Dr F. Cendes, Dr J. Gàllego Pérez-Larraya, Dr Y. Hayashi, Dr E. Deegan, J. Honnorat, and V. Rogemond for providing clinical information; other physicians and family members of patients who provided clinical information; Dr M. R. Rosenfeld for critical review of the manuscript; and E. Aguilar for anti-NMDAR antibody testing.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Potential Conflicts of Interest
J.D.: grants/grants pending, Euroimmun, NIH; patents, royalties, Athena Diagnostics Euroimmun; editorial board, Up-To-Date.
References
- 1.Dalmau J, Tuzun E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ances BM, Vitaliani R, Taylor RA, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128:1764–1777. doi: 10.1093/brain/awh526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai M, Hughes EG, Peng X, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol. 2009;65:424–434. doi: 10.1002/ana.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 7.Lou E, Hensley ML, Lassman AB, Aghajanian C. Paraneoplastic opsoclonus-myoclonus syndrome secondary to immature ovarian teratoma. Gynecol Oncol. 2010;117:382–384. doi: 10.1016/j.ygyno.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Fitzpatrick AS, Gray OM, McConville J, McDonnell GV. Opsoclonus-myoclonus syndrome associated with benign ovarian teratoma. Neurology. 2008;70:1292–1293. doi: 10.1212/01.wnl.0000308947.70045.7a. [DOI] [PubMed] [Google Scholar]
- 9.Kawachi I, Saji E, Toyoshima Y, et al. Treatment responsive opsoclonus-ataxia associated with ovarian teratoma. J Neurol Neurosurg Psychiatry. 2010;81:581–582. doi: 10.1136/jnnp.2009.177261. [DOI] [PubMed] [Google Scholar]
- 10.Bataller L, Rosenfeld MR, Graus F, et al. Autoantigen diversity in the opsoclonus-myoclonus syndrome. Ann Neurol. 2003;53:347–353. doi: 10.1002/ana.10462. [DOI] [PubMed] [Google Scholar]
- 11.Tate ED, Allison TJ, Pranzatelli MR, Verhulst SJ. Neuroepidemiologic trends in 105 US cases of pediatric opsoclonus-myoclonus syndrome. J Pediatr Oncol Nurs. 2005;22:8–19. doi: 10.1177/1043454204272560. [DOI] [PubMed] [Google Scholar]
- 12.Armangue T, Titulaer MJ, Málaga I, et al. Pediatric anti-N-methyl-D-aspartate receptor encephalitis-clinical analysis and novel findings in a series of 20 patients. J Pediatr. 2013;162:850.e2–856.e2. doi: 10.1016/j.jpeds.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


