Abstract
Transdifferentiation is the direct conversion from one somatic cell type into another desired somatic cell type. This reprogramming method offers an attractive approach for regenerative medicine. Here, we demonstrate that neonatal fibroblasts can be transdifferentiated into endothelial cells using only four endothelial transcription factors, namely, ETV2, FLI1, GATA2, and KLF4. We observed a significant up-regulation of endothelial genes including KDR, CD31, CD144, and vWF in human neonatal foreskin (BJ) fibroblasts infected with the lentiviral construct encoding the open reading frame of the four transcription factors. We observed morphological changes in BJ fibroblasts from the fibroblastic spindle shape into a more endothelial-like cobblestone structures. Fluorescence-activated cell sorting analysis revealed that ~16% of the infected cells with the lentiviral constructs encoding 4F expressed CD31. The sorted cells were allowed to expand for 2 weeks and these cells were immunostained and found to express endothelial markers CD31. The induced endothelial cells also incorporated fluorescence-labeled acetylated low-density lipoprotein and efficiently formed capillary-like networks when seeded on Matrigel. These results suggested that the induced endothelial cells were functional in vitro. Taken together, we successfully demonstrated the direct conversion of human neonatal fibroblasts into endothelial cells by transduction of lentiviral constructs encoding endothelial lineage-specific transcription factors ETV2, FLI1, GATA2, and KLF4. The directed differentiation of fibroblasts into endothelial cells may have significant utility in diseases characterized by fibrosis and loss of microvasculature.
Keywords: Transdifferentiation, transcription factors, regenerative medicine
Introduction
The endothelium plays a pivotal role in vascular homeostasis and is essential for angiogenesis and tissue perfusion and regeneration. Endothelial cell (EC) therapy may be an attractive approach to promote neovascularization and increase blood flow in ischemic tissues; however, the best source for such cells has not been determined. A variety of cell types have been shown to improve perfusion to ischemic tissues. These include adult stem cells (most usually mesenchymal stromal cells or adipose-derived vascular stromal fraction, or vascular endothelial progenitor cells) or ECs derived from pluripotent cells such as embryonic stem cells (ESCs) or human-induced pluripotent stem cells (hiPSCs).1–6
Transdifferentiation is another potential approach to generating therapeutic cells. In this process, one somatic cell is directly converted into the desired somatic cell type. This reprogramming method is an attractive approach for regenerative medicine as it allows the generation of patient-specific cell types without the risk of teratoma that exists for iPSC-derived cells.7 Recently, several groups have demonstrated that human fibroblasts may be converted into neurons or cardiomyocytes,8–14 by forced expression of lineage determination factors. The set of transcription factors used to induce transdifferentiation were typically determined by screening of combinations of transcriptional factors. This work is consistent with previous observations revealing that cell phenotype is fluid and can be altered by cytoplasmic factors such as transcription factors.15–17
With respect to the therapeutic transdifferentiation of somatic cells into ECs, Ginsberg et al.18 reported the direct reprogramming of amniotic cells into ECs by lentiviral transduction of ETS factors including ETV2, ERG, and FLI1 together with an inhibitor of transforming growth factor (TGF)β. Other authors have reported the conversion of human fibroblasts into ECs by employing pluripotency factors to induce an “intermediate state” followed by the administration of angiogenic factors to induce ECs.19–21 In this article, we provide the first report of direct transdifferentiation of human fibroblasts into ECs using forced expression of lineage-specific endothelial transcription factors.
Materials and methods
Cell culture and induced ECs’ generation
Human primary fibroblast cells, BJ (Foreskin dermal fibroblast; from ATCC, Manassas, VA), were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS), 1 mM l-glutamine (Gibco, Carlsbad, CA), and 1% non-essential amino acids (Gibco) before transduction with lentiviral vectors. Human fibroblasts transduced with lentiviral vectors were maintained on gelatin-coated dishes in DMEM containing 10% FBS overnight. The cells were then treated with differentiation medium, containing 20 ng/mL BMP4, 50 ng/mL vascular endothelial growth factor (VEGF), and 20 ng/mL basic fibroblast growth factor (bFGF) (Peprotech, Rocky Hill, NJ) in DMEM for 3 days. Then the medium was replaced with EC growth medium EGM-2 MV (cc-3162) containing 10 µmol/L SB341542 (TGF-β receptor kinase inhibitor) until day 14 for fluorescence-activated cell sorting (FACS) sorting. The sorted cells were allowed to expand in EGM-2 MV (cc-3162) containing 10 µmol/L SB341542 until 28 days.
Lentivirus production
We used the Thermo Scientific Trans-Lentiviral Packaging System for generating viral constructs carrying selected transcriptional factors. The packaging plasmids and transfer vector encoding the open reading frames of the lineage-specific factors were used to transfect a 293T packaging cell line. Viral supernatants were harvested 48 h after transfection. Equal multiplicity of infection (MOI) of the viruses encoding specific transcriptional factors was used to transduce fibroblasts in the presence of 8 µg/mL polybrene.
Immunofluorescence analysis
The samples were washed once with phosphate-buffered saline (PBS) (Invitrogen, Carlsbad, CA; without Ca2+ and Mg2+) and were fixed with a 4% formaldehyde solution (Electron Microscopy Sciences, Hatfield, PA) containing 0.15% picric acid (Sigma-Aldrich, Carlsbad, CA) in PBS for 20 min, followed by three washes with PBS. Blocking of non-specific binding was achieved by incubating cells with 10% donkey serum (Jackson ImmunoResearch, West Grove, PA). CD31 (R&D Systems, Minneapolis, MN; 1:200, mouse) was diluted in 1% bovine serum albumin (BSA) and incubated overnight at 4°C. After 1 h of washing with 0.1% BSA in PBS, the samples were incubated with Alexa-555–conjugated secondary antibodies (Invitrogen) for 1 h at room temperature and the nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI; Sigma–Aldrich).
Ac-low-density lipoprotein uptake assay
Uptake of Ac-low-density lipoprotein (LDL) was assessed by incubating induced endothelial cells (iECs) and BJ fibroblasts with 5 µg/mL of Alexa Fluor-594–conjugated Ac-LDL (Invitrogen) for 4 h prior to detection by fluorescence microscopy. After incubation, they were washed with 1× PBS before being visualized and photographed using a fluorescence microscope.
Flow cytometry analysis and cell sorting
Cells were washed with PBS and dissociated with Accutase (Innovative Cell Tech, San Diego, CA). After harvesting, the cells were washed twice with ice-cold FACS buffer (Hanks’ Balanced Salt Solution (HBSS) supplemented with 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 2% FBS, and 0.1% sodium azide; Sigma–Aldrich). Undissociated cells were removed by passing the cell suspension through a 70-µm cell strainer (BD Biosciences, San Jose, CA, USA). Cells were incubated with phycoerythrin (PE)-conjugated anti-human CD31 antibody (eBioscience, San Diego, CA, USA) for 20 min at 4°C. An isotype-matched antibody served as a negative control. To purify the iECs, single-cell suspensions were obtained by treatment with Accutase for 20 min at 37°C to dissociate cells. Flow cytometry was then performed using a BD Digital Vantage cell sorter (BD Biosciences). The purified iECs were expanded in EGM-2 medium containing 10 µM SB431542. Analysis was performed using FlowJo software.
RNA extraction and quantitative polymerase chain reaction
Using RNeasy Mini Kit (Qiagen, Valencia, CA), total RNA was extracted and quantitative polymerase chain reaction (PCR) was performed using Taqman gene expression assays (Applied Biosystems, Grand Island, NY). Genes associated with the EC phenotype (including KDR, CD31, CD144, and vWF) and transcription factors (including ETV2, FLI1, GATA2, KLF4, FOXC2, TIE2, CYTL1, and BMX) were analyzed with the data normalized to β-actin housekeeping gene and expressed as relative fold changes using the ΔCt method of analysis.
In vitro vascular-like tube formation angiogenesis assay
The ability of the cells to form tube-like structures was assessed in vitro by seeding 105 cells in 12-well plate coated with Matrigel in the presence of EGM-2 media containing 50 ng/mL VEGF and incubated for 24 h. The formation of tube-like structures was then imaged under bright-field microscopy.
Statistical analyses
Data are presented as means ± standard error of mean (SEM). Comparisons between groups were determined by one-way analysis of variance (ANOVA) with Student’s t-test post hoc test (SPSS Inc., Chicago, IL, USA). The results were considered significant when p-value was less than 0.05.
Results
Overexpression of endothelial transcription factors increases endothelial CD31 expression in human BJ fibroblasts
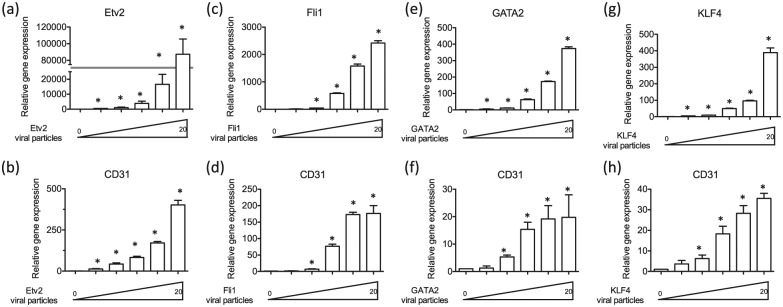
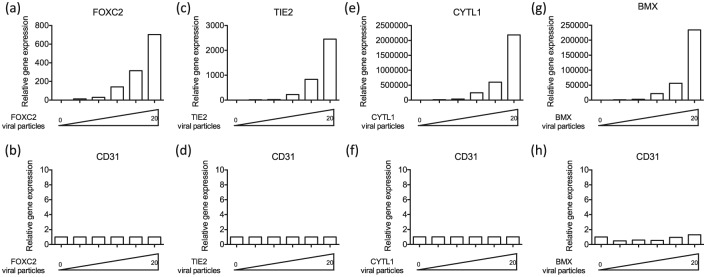
We intended to directly reprogram human fibroblasts into ECs using transcription factors that are known to be critical for EC development and function. Accordingly, based on the literature of EC development and function, we selected the following eight factors: ETV2, FLI1, GATA2, KLF4, FOXC2, TIE2, CYTL1, and BMX. To determine whether these transcription factors were potentially useful in transdifferentiation, we assessed the effect of forced expression of each transcription factor on CD31 expression in the transduced fibroblasts. The CD31 gene encodes an adhesion molecule that is not expressed in fibroblasts, but is expressed in all ECs. Accordingly, we transduced human neonatal BJ fibroblasts with lentiviral constructs containing the open reading frame of each gene with different MOIs (0, 0.5, 1, 5, 10, 20) for 7 days and conducted quantitative real-time-PCR (qRT-PCR). We found that messenger RNA (mRNA) expression of EC gene CD31 was markedly increased in BJ fibroblasts treated with lentiviral constructs encoding ETV2 (Figure 1(a) and (b)), FLI1 (Figure 1(c) and (d)), GATA2 (Figure 1(e) and (f)) and KLF4 (Figure 1(g) and (h)) in an MOI-dependent manner, by comparison to BJ fibroblasts treated with control GFP lentivirus. The potency of the constructs to induce CD31 expression was . On the other hand, there were no changes in mRNA expression of CD31 in BJ fibroblasts treated with lentiviral constructs encoding FOXC2 (Supplemental Figure 1(a) and (b)), TIE2 (Supplemental Figure 1(c) and (d)), CYTL1 (Supplemental Figure 1(e) and (f)), and BMX (Supplemental Figure 1(g) and (h)). These data suggested that ETV2, FLI1, GATA2, and KLF4 had the potential to convert BJ fibroblasts into ECs when acting alone or in combination.
Figure 1.
Overexpression of endothelial transcription factors ETV2, FLI1, GATA2, and KLF4 increases endothelial CD31 expression in human BJ fibroblasts. Expression of the messaged for EC gene CD31 was markedly increased in BJ fibroblasts treated with lentiviral constructs encoding (a, b) ETV2, (c, d) FLI1, (e, f) GATA2, and (g, h) KLF4 in a dose-dependent manner compared to BJ fibroblast treated with control GFP lentivirus. All data are represented as mean ± SEM (n = 3). *p < 0.05 versus control.
“Minus-1” strategy to confirm the importance of endothelial transcription factors
Next, we sought to determine what combination of these selected transcription factors might efficiently induce transdifferentiation of fibroblasts into EC lineage. We compared the effect on CD31 expression of the four transcription factors combined together (4F: ETV2, FLI1, GATA2, and KLF4), versus each three-factor combination. Human BJ fibroblasts were transduced with lentiviral constructs encoding the transcriptional factors with MOI 5. After 7 days, qRT-PCR analysis demonstrated that the 4F combination caused the greatest up-regulation of CD31 expression compared with the control GFP lentivirus-treated group (Figure 2(a)). Of the various three-factor combinations, that not including ETV2 had the least expression of the CD31 gene, indicating that ETV2 was a major contributor to the efficiency of the 4F combination. By comparison, 4F minus FLI1, 4F minus GATA2, or 4F minus KLF4 caused only moderate reductions in CD31 mRNA expression (Figure 2(a)). Furthermore, we also observed a time-dependent effect of 4F to induce endothelial CD31 expression in BJ fibroblasts (Figure 2(b)), which may indicate that other factors contributing to full expression of CD31 may be activated by the 4F combination over time.
Figure 2.
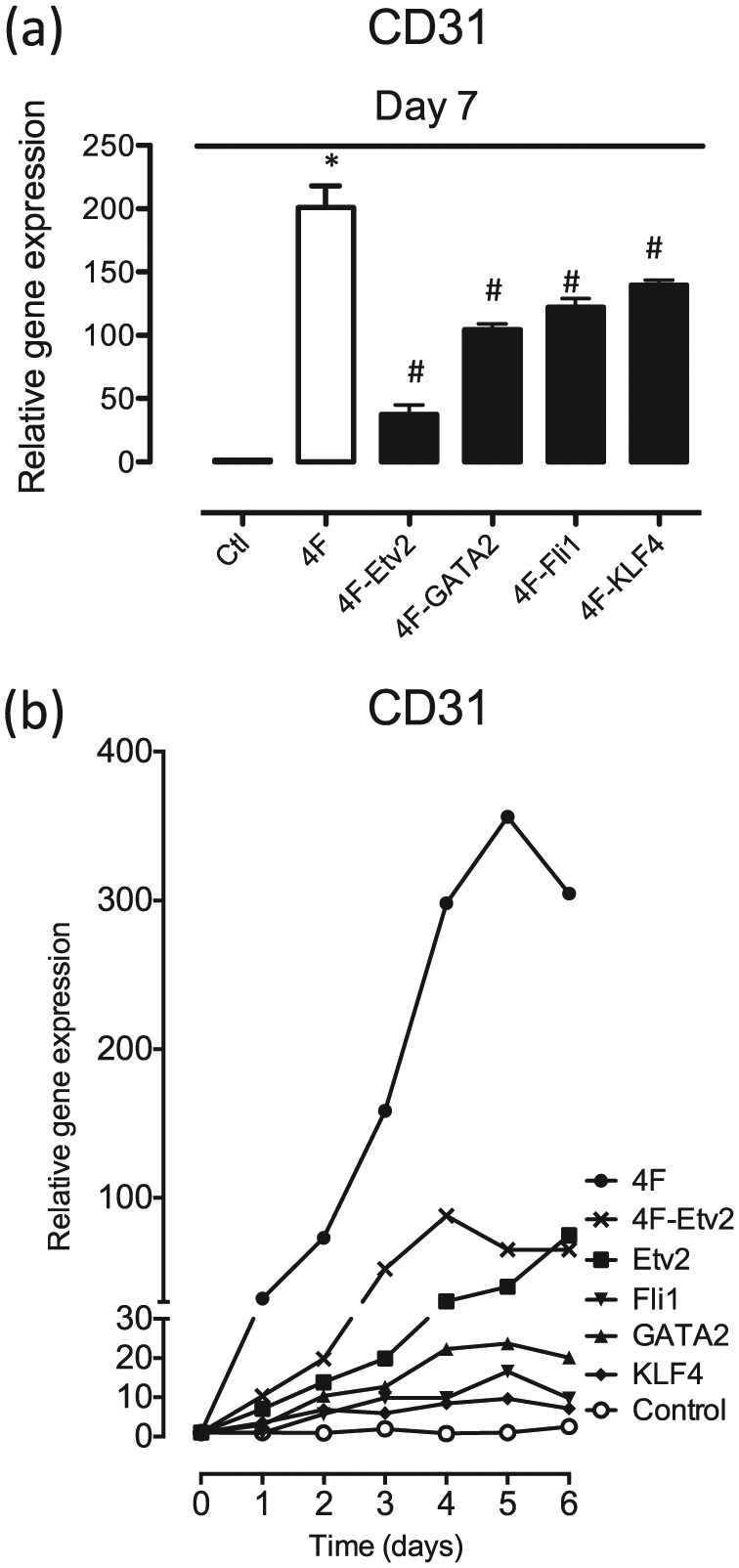
“Minus-1” strategy to confirm the importance of endothelial transcription factors. (a) Analysis of gene expression by qRT-PCR demonstrated that the 4F combination markedly up-regulated CD31 expression compared with the control GFP lentivirus-treated group. Removing ETV2 from the 4F combination caused a substantial reduction in CD31 gene expression, whereas removing any one of the other factors had less effect on CD31 mRNA expression. (b) Time-dependent effect of 4F in inducing endothelial CD31 expression in BJ fibroblasts. All data are represented as mean ± SEM (n = 3). *p < 0.05 versus control. #p < 0.05 versus 4F.
4F increases endothelial genes in a time-dependent manner
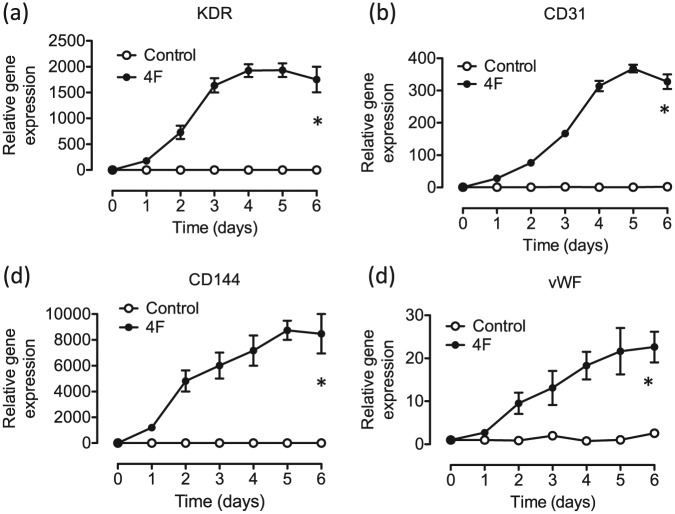
To determine whether 4F could increase other markers of endothelial lineage in BJ fibroblasts, we transduced the cells with lentiviral constructs encoding the 4F and collected the cells daily for the first 6 days and conducted qRT-PCR. We observed a significant up-regulation of endothelial genes including KDR (Figure 3(a)), CD31 (Figure 3(b)), CD144 (Figure 3(c)), and vWF (Figure 3(d)) in BJ fibroblasts infected with the 4F combination compared to the control GFP-virus-infected cells. These results prompted us to investigate whether the 4F could convert human fibroblasts into ECs.
Figure 3.
4F increases endothelial genes in a time-dependent manner. A significant up-regulation of endothelial genes including (a) KDR, (b) CD31, (c) CD144, and (d) vWF in BJ fibroblasts transduced with the 4F combination compared to the control GFP-virus-infected cells. All data are represented as mean ± SEM (n = 3). p < 0.05 versus control.
Direct conversion of human fibroblasts into EC lineage
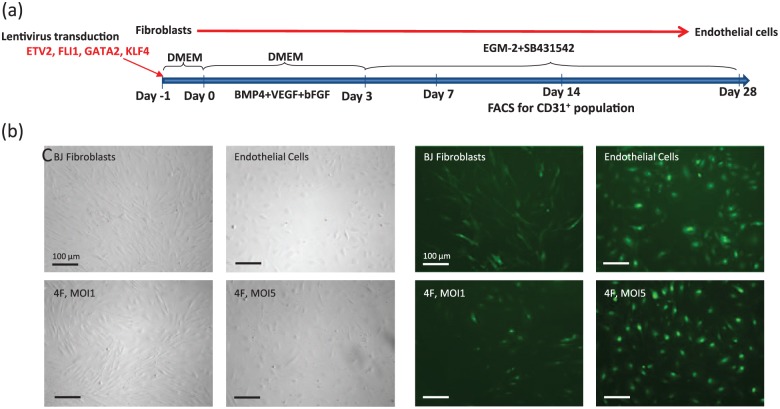
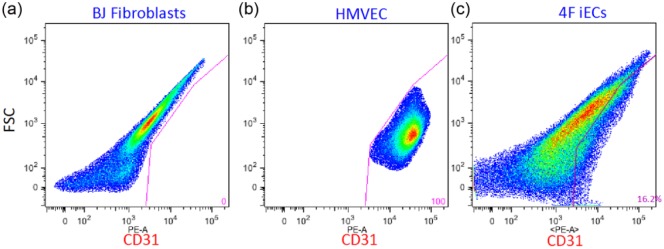
To determine whether we could convert human fibroblasts into ECs using lineage-specific endothelial transcription factors, we transduced neonatal BJ fibroblasts with lentiviruses encoding 4F (ETV2, FLI1, GATA2, and KLF4). After infection of the BJ fibroblasts with lentiviral constructs encoding 4F, we added to the growth medium the following factors: BMP4, VEGF, and bFGF with the intent to facilitate the transition to the EC fate. Three days after the addition of BMP4, VEGF, and bFGF to the culture, the medium was changed to EC growth medium EGM-2 MV (cc-3162) containing 10 µmol/L SB341542. At day 7 after transfection of BJ fibroblasts with 4F (MOI 1 or MOI 5), we observed morphological changes in BJ fibroblasts from the fibroblastic spindle shape into a more endothelial-like cobblestone structures (Figure 4(b) and (c)). On day 14 after the transfection, the cells were collected for FACS sorting (Figure 4(a)). This analysis revealed that ~16% of the cells transduced with the lentiviral constructs encoding 4F (MOI 5) expressed CD31 (Supplemental Figure 2).
Figure 4.
Transdifferentiation by overexpression of 4F generates functional endothelial cells from human fibroblasts. (a) Transdifferentiation of human fibroblasts into endothelial cells. The diagram represents the time course and the sequential addition of the different media. Representative (b) bright-field and (c) FITC images showing the cobblestone structure of induced endothelial cells at day 7 after transduction of BJ fibroblasts with 4F using an MOI 1 or MOI 5.
Functional analysis of the 4F-induced iECs
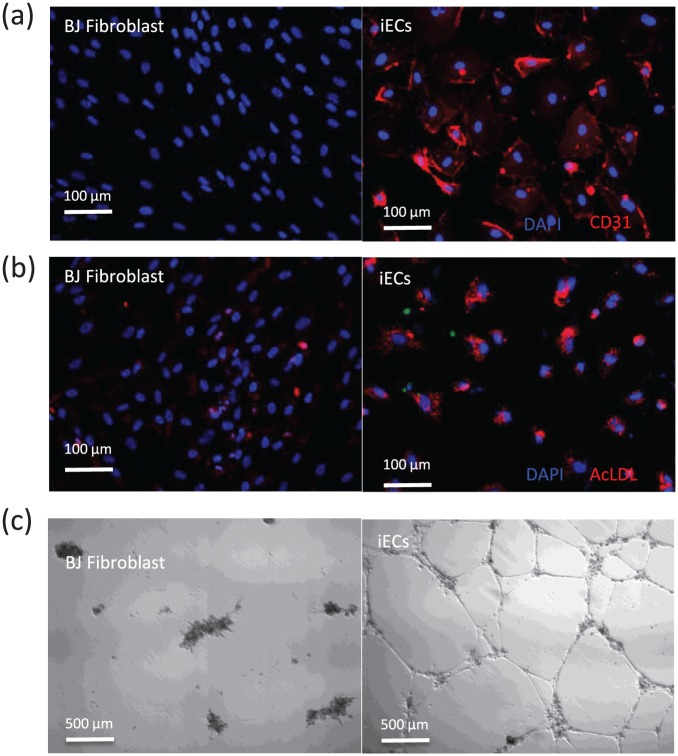
The sorted cells were allowed to expand in EGM-2 MV (cc-3162) containing 10 µmol/L SB341542. After expansion, these cells were immunostained and found to express endothelial markers CD31 (Figure 5(a)). The iECs also took up fluorescently labeled acetylated LDL (Figure 5(b)) and efficiently formed capillary-like networks when seeded on Matrigel (Figure 5(c)). These observations suggest that the iECs are functionally similar to authentic ECs.
Figure 5.
Functional analysis of the 4F-induced iECs: (a) representative images showing CD31 immunofluorescence staining of iECs compared to BJ fibroblasts; (b) microscope image of the incorporation of acetylated LDL by iECs; and (c) the iECs, but not the fibroblasts, form a capillary-like network on Matrigel 16 h after seeding the cells.
Discussion
In this study, we report the direct conversion of human neonatal fibroblasts into ECs by transduction of lentiviral constructs encoding endothelial lineage-specific transcription factors ETV2, FLI1, GATA2, and KLF4. To our knowledge, this is the first report of direct transdifferentiation of human fibroblasts into ECs by forced expression of endothelial transcription factors. Therapeutic transdifferentiation of human fibroblasts into ECs might represent a new approach to enhancing tissue perfusion.
A variety of cell types have been shown to improve perfusion to ischemic tissues. These include adult stem cells (most usually mesenchymal stromal cells or adipose-derived vascular stromal fraction, or vascular endothelial progenitor cells) or ECs derived from pluripotent cells such as ESCs or hiPSCs.1–6 There are obstacles to the therapeutic success of these approaches. The adult stem cells are limited in their replicative capacity.22,23 Furthermore, autologous adult stem cells are often reduced in number or function when they are derived from an ill patient.24 These problems may be overcome with the use of pluripotent stem cells to generate therapeutic cells, as the pluripotent cells may be expanded indefinitely before they are differentiated into the desired cell type.
Indeed, we have previously reported that ECs could be generated from pluripotent stem cells including ESCs25 and induced pluripotent stem cells.2 These ESC-ECs and iPSC-ECs were each able to increase capillary formation and improve blood perfusion when administered to ischemic tissue. Of particular promise is the use of iPSCs, as this strategy avoids the ethical issues of ESCs, and autologous iPSCs may be generated using somatic cells of the patient. However, induction of pluripotency and derivation of the desired cell type requires prolonged tissue culture. In addition, the current methods for differentiation are largely empirical and yield a heterogenous population of cells which need to be sorted for purity of the therapeutic product. Incomplete differentiation and sorting could lead to the inadvertent inclusion of pluripotent cells in the therapeutic product (which could cause teratoma formation) or poorly differentiated cells (which may not function properly in vivo). Finally, concerns have been raised regarding the process of inducing pluripotency, which may inadvertently generate genomic mutations.26 Thus, therapeutic transdifferentiation represents an alternative strategy to the derivation of autologous ECs using iPSCs.
Several groups have successfully demonstrated the direct conversion of human fibroblasts into neurons or cardiomyocytes by forced expression of lineage-specific transcription factors.8–14 With respect to the direct conversion of cells into endothelial lineage, Ginsberg et al.18 reported that amniotic fluid–derived cells could be transdifferentiated into ECs employing lentiviral transduction of ETV2, ERG, and FLI1 together with the inhibition of TGFβ. Interestingly, they reported that this approach did not work for postnatal cells. It is also not clear whether the amniotic fluid–derived cells were in a terminally differentiated state as these cell sources may contain some progenitor cells. More recently, we and other investigators have transdifferentiated fibroblasts into angioblast-like cells or ECs by employing pluripotency factors to induce an intermediate state and adding angiogenic factors to the medium to yield ECs.19–21 Currently, our transdifferentiation protocol (forced expression of four EC transcription factors using a lentiviral construct) converts about 16% of fibroblasts into CD31+ iECs. This yield compares favorably with that of others. For example, Han et al.27 observed a 4% conversion efficiency of murine skin fibroblasts into functional ECs using forced expression of five defined factors (Foxo1, Er71, Klf2, Tal1, and Lmo2). Transdifferentiation of fibroblasts into other somatic cells is also low, for example, the conversion of fibroblasts into induced neurons ranges from 1.8% to 7.7%.9 Reprogramming efficiency in the generation of iPSCs from somatic cells is even lower (about 0.1%).28 It is now known what yield might be required for a therapeutic effect of transdifferentiation of fibroblasts into iECs in vivo, for example, in the setting of ischemia or wound healing. Furthermore, additional insights into the mechanisms of transdifferentiation will likely lead to improved protocols to increase the efficiency of transdifferentiation.
By using a combination of four transcription factors including ETV2, FLI1, GATA2, and KLF4, we have successfully converted human neonatal BJ fibroblasts into functional ECs. These cells express endothelial markers CD31, incorporate acetylated LDL, and can form tubular networks in Matrigel. Generation of functional human ECs could benefit vascular disorders including peripheral arterial diseases. Among the four transcriptional factors that we used to reprogram fibroblast into ECs, ETV2 may probably be the most critical transcription factor in this process. Indeed, removing this factor from the combination substantially reduced CD31 expression in the reprogrammed cells. Previous reports have demonstrated that ETV2 plays an indispensable role in vascular development as confirmed by the lack of vasculature formation in Etv2-deficient mouse embryos.29 It remains largely unknown how ETV2 activates EC genes during the reprogramming processes. Previous studies have reported that ETV2 directly binds to promoters of FLK1, CD31 and CD144.29–31 ETV2 might also regulate epigenetic modifiers that are necessary for epigenetic remodeling during differentiation. For example, ETV2 interacts with the histone demethylase jmjd1a which regulates endothelial gene transcription.32,33 Further investigations are therefore required to delineate the mechanism by which ETV2 induces transdifferentiation of fibroblasts into ECs.
Endothelial-to-mesenchyme transition (EndoMT) contributes to cardiac and renal fibrosis. Lineage tracing studies have confirmed that ECs may transform into mesenchymal cells that generate extracellular matrix and contribute to fibrosis and loss of vascularity. EndoMT is characterized by a loss of endothelial surface antigens (e.g. VE-Cadherin) and functions (e.g. nitric oxide generation) with the adoption of fibroblast morphology and gene expression (such as Twist, Snail, and fibrospondin). Our works suggest that we can reverse this process by overexpressing lentiviral constructs encoding the open reading frames of endothelial transcription factors, and result in mesenchyme-to-endothelial transition, and directly convert fibroblasts into ECs, which might have potential therapeutic implications in reversing fibrotic diseases in humans.
Another advantage of therapeutic transdifferentiation over the use of other cell therapies is that the process of therapeutic transdifferentiation could potentially take place in vivo and in situ. Specifically, rather than injecting adult stem cells or iPSC-derived cells into the target tissue, one could induce fibroblasts in the tissue to become other functional cell types. For example, after a myocardial ischemic injury, one might administer to the patient a cocktail of factors that would transdifferentiate fibroblasts proliferating in the area of injury into vascular cells. In this way, rather than forming an avascular scar, one might be able to generate vascular cells. These vascular cells might self-assemble into a vascular network that would improve perfusion. However, the administration of lentiviral vectors and the insertion of foreign DNA into the patients’ tissue would raise concerns regarding off-target effects and oncogenicity. However, such work in pre-clinical models may provide proof-of-concept for similar approaches that do not use viral vectors. For example, it may be possible to administer mRNA encoding the transcription factors to the injured tissue, which would avoid concerns related to viral vectors. Because mRNA is easily degraded, to enhance its stability and to enhance tissue targeting, it might be provided in a nanoparticle delivery system.
Alternatively, a small molecule approach for therapeutic transdifferentiation may be possible. Previously, we demonstrated that activation of innate immunity promotes epigenetic plasticity, in part, by causing global changes in the expression of epigenetic modifiers that favors DNA accessibility.34 We have further employed this insight to generate iECs using a non-viral approach. Specifically, we stimulated human fibroblasts with an activator of innate immune signaling and added endothelial growth factors (VEGF, BMP4, and bFGF) to induce therapeutic transdifferentiation into ECs.35 However, this transdifferentiation strategy is not efficient in vitro, with yields of iECs of 5%–10% in vitro.
Taken together, we successfully demonstrated for the first time the direct conversion of human neonatal fibroblasts into ECs by transfection of the fibroblasts with lentiviral constructs encoding endothelial transcription factors ETV2, FLI1, GATA2, and KLF4. This study provides proof-of-concept for a novel strategy to treat ischemic syndromes.
Supplementary Material
Supplementary information
Supplemental Figure S1.
No changes in mRNA expression of CD31 in BJ fibroblasts treated with lentiviral constructs encoding (a, b) FOXC2, (c, d) TIE2, (e, f) CYTL1, and (g, h) BMX.
Supplemental Figure S2.
FACS analysis revealed that ~16% of the infected cells with the lentiviral constructs encoding 4F (MOI 5) expressed CD31.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: J.P.C. was supported by National Institutes of Health (U01HL100397, RC2HL103400). W.T.W. was supported by a NIH PCBC Jump Start Award (PCBC_JS_2012/1_02), American Heart Association Postdoctoral Fellowship (POST8830020), and Scientist Development Grant (AHASDG) (13SDG15800004).
References
- 1. Yin L, Ohanyan V, Fen Pung Y, et al. Induction of vascular progenitor cells from endothelial cells stimulates coronary collateral growth. Circ Res 2012; 110: 241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rufaihah AJ, Huang NF, Jame S, et al. Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler Thromb Vasc Biol 2011; 31: e72–e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jujo K, Ii M, Losordo DW. Endothelial progenitor cells in neovascularization of infarcted myocardium. J Mol Cell Cardiol 2008; 45: 530–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cho SW, Moon SH, Lee SH, et al. Improvement of postnatal neovascularization by human embryonic stem cell derived endothelial-like cell transplantation in a mouse model of hindlimb ischemia. Circulation 2007; 116: 2409–2419. [DOI] [PubMed] [Google Scholar]
- 5. Hirata K, Li TS, Nishida M, et al. Autologous bone marrow cell implantation as therapeutic angiogenesis for ischemic hindlimb in diabetic rat model. Am J Physiol Heart Circ Physiol 2003; 284: H66–H70. [DOI] [PubMed] [Google Scholar]
- 6. Kamihata H, Matsubara H, Nishiue T, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation 2001; 104: 1046–1052. [DOI] [PubMed] [Google Scholar]
- 7. Zhang G, Shang B, Yang P, et al. Induced pluripotent stem cell consensus genes: implication for the risk of tumorigenesis and cancers in induced pluripotent stem cell therapy. Stem Cells Dev 2012; 21: 955–964. [DOI] [PubMed] [Google Scholar]
- 8. Zhou Q, Brown J, Kanarek A, et al. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 2008; 455: 627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vierbuchen T, Ostermeier A, Pang ZP, et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010; 463: 1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ieda M, Fu JD, Delgado-Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 2010; 142: 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caiazzo M, Dell’Anno MT, Dvoretskova E, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 2011; 476: 224–227. [DOI] [PubMed] [Google Scholar]
- 12. Son EY, Ichida JK, Wainger BJ, et al. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell 2011; 9: 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoo AS, Sun AX, Li L, et al. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature 2011; 476: 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ambasudhan R, Talantova M, Coleman R, et al. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell 2011; 9: 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blau HM, Chiu CP, Webster C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell 1983; 32: 1171–1180. [DOI] [PubMed] [Google Scholar]
- 16. Yang CS, Rodgers M, Min CK, et al. The autophagy regulator Rubicon is a feedback inhibitor of CARD9-mediated host innate immunity. Cell Host Microbe 2012; 11: 277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blau HM, Pavlath GK, Hardeman EC, et al. Plasticity of the differentiated state. Science 1985; 230: 758–766. [DOI] [PubMed] [Google Scholar]
- 18. Ginsberg M, James D, Ding BS, et al. Efficient direct reprogramming of mature amniotic cells into endothelial cells by ETS factors and TGFbeta suppression. Cell 2012; 151: 559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li J, Huang NF, Zou J, et al. Conversion of human fibroblasts to functional endothelial cells by defined factors. Arterioscler Thromb Vasc Biol 2013; 33: 1366–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Margariti A, Winkler B, Karamariti E, et al. Direct reprogramming of fibroblasts into endothelial cells capable of angiogenesis and reendothelialization in tissue-engineered vessels. P Natl Acad Sci USA 2012; 109: 13793–13798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kurian L, Sancho-Martinez I, Nivet E, et al. Conversion of human fibroblasts to angioblast-like progenitor cells. Nat Methods 2013; 10: 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res 2012; 110: 624–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O’Neill TJ, 4th, Wamhoff BR, Owens GK, et al. Mobilization of bone marrow-derived cells enhances the angiogenic response to hypoxia without transdifferentiation into endothelial cells. Circ Res 2005; 97: 1027–1035. [DOI] [PubMed] [Google Scholar]
- 24. Leeper NJ, Hunter AL, Cooke JP. Stem cell therapy for vascular regeneration: adult, embryonic, and induced pluripotent stem cells. Circulation 2010; 122: 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang NF, Niiyama H, Peter C, et al. Embryonic stem cell-derived endothelial cells engraft into the ischemic hindlimb and restore perfusion. Arterioscler Thromb Vasc Biol 2010; 30: 984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bai Q, Desprat R, Klein B, et al. Embryonic stem cells or induced pluripotent stem cells? A DNA integrity perspective. Curr Gene Ther 2013; 13: 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Han JK, Chang SH, Cho HJ, et al. Direct conversion of adult skin fibroblasts to endothelial cells by defined factors. Circulation 2014; 130: 1168–1178. [DOI] [PubMed] [Google Scholar]
- 28. Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131: 861–872. [DOI] [PubMed] [Google Scholar]
- 29. Lee D, Park C, Lee H, et al. ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell Stem Cell 2008; 2: 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu F, Kang I, Park C, et al. ER71 specifies Flk-1+ hemangiogenic mesoderm by inhibiting cardiac mesoderm and Wnt signaling. Blood 2012; 119: 3295–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Val S, Chi NC, Meadows SM, et al. Combinatorial regulation of endothelial gene expression by ets and forkhead transcription factors. Cell 2008; 135: 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Knebel J, De Haro L, Janknecht R. Repression of transcription by TSGA/Jmjd1a, a novel interaction partner of the ETS protein ER71. J Cell Biochem 2006; 99: 319–329. [DOI] [PubMed] [Google Scholar]
- 33. Loh YH, Zhang W, Chen X, et al. Jmjd1a and Jmjd2c histone H3 Lys 9 demethylases regulate self-renewal in embryonic stem cells. Genes Dev 2007; 21: 2545–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee J, Sayed N, Hunter A, et al. Activation of innate immunity is required for efficient nuclear reprogramming. Cell 2012; 151: 547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sayed N, Wong WT, Ospino F, et al. Transdifferentiation of human fibroblasts to endothelial cells: role of innate immunity. Circulation 2015; 131: 300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.