Abstract
Repurposing of existing cancer drugs to overcome their physical limitations, such as insolubility, represents an attractive strategy to achieve enhanced therapeutic efficacy and broaden the range of clinical applications. Such an approach also promises to offer substantial cost savings in drug development efforts. Here we use repurposed FDA-approved topical agent bexarotene (Targretin™), currently in limited use for cutaneous manifestations of T-cell lymphomas, and re-engineer it for use in solid tumor applications by forming self-assembling nanobubbles. Physicochemical characterization studies of the novel prodrug nanobubbles demonstrated their stability, enhanced target cell-internalization capability and highly controlled release profile in response to application of focused ultrasound energy. Using an in vitro model of hepatocellular carcinoma and an in vivo large animal model of liver ablation, we demonstrate the effectiveness of bexarotene prodrug nanobubbles when used in conjunction with catheter-based ultrasound, thereby highlighting the therapeutic promise of this trimodal approach.
Keywords: Liver cancer, thermal ablation, anticancer prodrug nanobubble, drug repurposing
American Cancer Society estimated that in 2015 approximately 1.66 million new cancer cases are expected to be diagnosed in the United States.1 A brand-new drug for treating cancer through a conventional drug discovery pipeline is time consuming and expensive. One solution is to repurpose drugs. In this process, a therapeutic agent that is approved to treat one type of disease or condition is re-examined for treating other diseases. Bexarotene (4-[1-(5,6,7,8-tetrahydro-3,5,5,8,8-pentamethyl2-naphthalenyl)ethenyl] benzoic acid, Targretin®) is a FDA-approved drug, currently in limited use for cutaneous manifestations of T-cell lymphomas (CTCL).2 It is a known orphan nuclear agonist and a member of a subclass of retinoids that selectively activate retinoid X receptors (RXRs).3 These retinoid receptors have biologic activity distinct from that of retinoic acid receptors (RARs). Bexarotene selectively binds and activates retinoid X receptor subtypes (RXRα, RXRβ, RXRγ). RXRs can form heterodimers with various receptor partners such as thyroid receptor, vitamin D receptor, retinoic acid receptors (RARs), and peroxisome proliferator activator receptors (PPARs).4 Once activated, these receptors function as transcription factors that regulate the expression of genes that control cellular differentiation and proliferation4. Despite being clinically approved, the use of this agent is only limited to topical application primarily due to the poor solubility of the agent, dose-limiting toxicities or lack of objective responses. We hypothesize that a re-engineered form of the drug will be more effective for treating primary and secondary hepatocellular carcinoma. Liver is a common site of metastasis from many gastrointestinal and extra gastrointestinal primary cancers, including breast, lung, esophagus, stomach, pancreas, kidney and melanoma.5-8 The recent developments of minimally or non-invasive techniques to treat liver cancer provides quick recovery, less risk of wound infection, and less pain. Cryo-ablation,9,10 radio frequency,11-14 microwave heating,15-17 and high-intensity focused ultrasound (HIFU)18-23 are examples of minimally invasive thermal therapies that are under investigation, with several of these techniques now used in patients.. Ultrasound is a promising noninvasive approach for treating cancer through thermal ablation or hyperthermia. The use of ultrasound to treat tumors has been investigated in prostate,19,20,24 liver,18,22,25-27 kidney,28 brain,29,30 and eye conditions.31,32 However, current systems remain highly dependent on operator skill, and cannot treat many tumors because there is insufficient control of the size and shape of the zone of necrosis, and little/no control over ablator trajectory within tissue and often take long treatment time. Remedying these problems requires advances in end-effector design, robust image guidance, and precise steering of the ablator device to the desired target location. Here we develop catheter-based ultrasound therapeutic device with spatially-tracked 3D ultrasound imaging, and deliver the drug in combination with targeted drug payload using pro-bexarotene nanobubble (PBNB) under therapeutic ultrasound exposure (Figure 1).
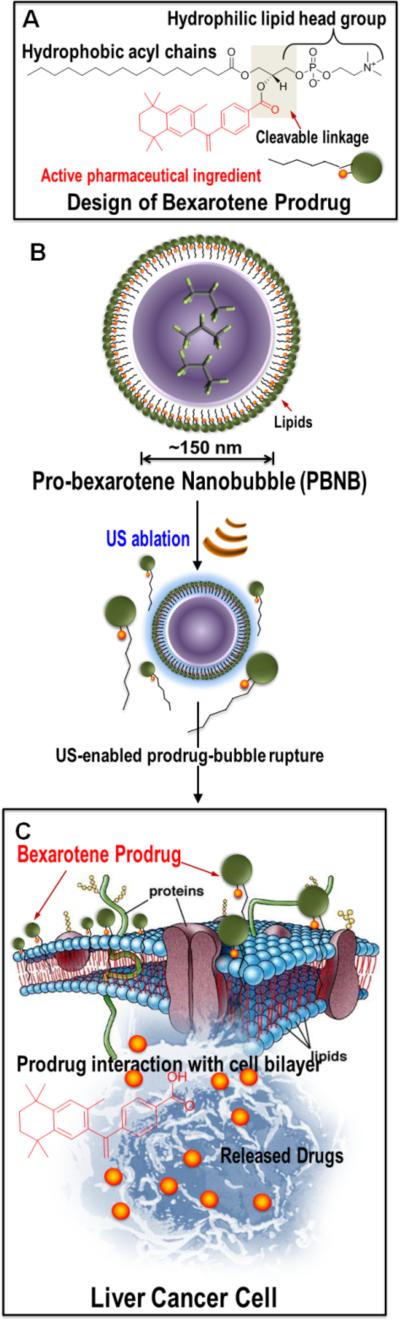
Figure 1.
Overall design of re-purposed therapeutics. (A) Design of bexarotene prodrug and its chemical components; (B) layered arrangement of bexarotene-prodrug after self-assembling into a gas-filled bubble followed by its rupture upon US exposure; (C) prodrug interacts favorably with the cancer cell membrane to get inserted and eventually be cleaved enzymatically releasing the active pharmaceutical ingredient.
Results and Discussion
Synthesis of Pro-bexarotene
We synthesize and characterize the prodrug version of bexarotene (denoted as pro-bexarotene). In this procedure, 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine (16:0 Lyso PC) (24.8 mg, 0.05 mmol), EDC (19.1 mg, 0.1 mmol) and DMAP (catalytic amount) were mixed in anhydrous CHCl3 solution (1 ml) and stirred for 15 mins at ambient temperature. To this mixture we added bexarotene (17.4 mg, 0.05 mmol dissolved in 0.5 ml anhydrous CHCl3) and stirred for 24 h at room temperature. The completion of the reaction was monitored by thin layer chromatography (silica). The resultant organic solution was then washed for several times with water and extracted with excess dichloromethane. Dichloromethane was evaporated under reduced pressure to afford compound 23.1 mg in 56% yield as light yellow solid (Scheme S1). NMR and Mass studies were used to characterize the solid.
1H-NMR (CDCl3, 400MHz): δ 7.93 (d, J = 8.0 Hz, 2H), 7.32 (d, J = 8.0 Hz, 2H), 7.10 (s, 1H), 7.06 (s, 1H), 5.78 (s, 1H), 5.30 (s, 1H), 4.35 (q, J = 4.0 Hz, 2H), 3.89(s, 2H), 3.22 (s, 4H), 2.30 (s, 2H), 1.93 (s, 4H), 1.68 (s, 6H), 1.53 (s, 3H), 1.36 (m, 6H), 1.69 (m, 12H), 1.23 (m, 24H), 0.85 (m, 3H). HRMS m/z: 826.5394 (MH)+, calculated for C48H76NO8P: 826.5387
Susceptibility of pro-bexarotene towards enzyme and pH
We determined whether the pro-bexarotene can be degraded in the presence of enzyme and acidic pH. The prodrug (500 μl) was incubated for 2 hours with 0.278 mg (3.36 × 10−7 Mol) phospholipase A2 or acidic pH (4.1). The samples were continuously agitated on a nutator to prevent settling. The mixture was isolated and analyzed by HRMS to observe signature peaks of cleaved and liberated bexarotene. High resolution mass spectrometry (HRMS) confirmed the release of bexarotene from the prodrug under enzymatic or low pH condition.
Density functional theory (DFT) study
DFT calculations were performed on bexarotene, 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine (lyso-PC), Octafluoropropane (C3F8) and pro-bexarotene. This technique enabled the analysis of electronic distribution, feasibility of forming nano-assemblies and for predicting the pharmacological activities. The pharmacological activities of drugs are affected by the electronic distribution on the drug molecules.33-36 The reactivity of a molecule, for example, intermolecular interaction, is controlled by the frontier molecular orbitals (FMO) such as highest occupied molecular orbitals (HOMO) and the lowest unoccupied molecular orbitals (LUMO). The HOMO energy indicates the region of molecules, which can donate electrons during the complex formation with proteins or other receptor molecules. The LUMO energy region signifies the site of the molecules which can accept electrons from the protein (or other molecules) during interaction.37 In a drug-receptor system, the HOMO state of the drugs generally interact with the LUMO state of the receptor and the LUMO state of the drugs interact with the HOMO state of the receptor.37 The energy gap (HOMO-LUMO gap) is inversely proportional to the activity of the drugs.37 Hence, the drug with the smallest energy bandgap between the HOMO and LUMO is the most active and typically exhibits the highest cytotoxic activity. Similarly, larger energy bandgap leads to greater stabilization and binding with a receptor. The DFT calculation predicts that the Lyso-PC will be least cytotoxic. When comparing, prodrug and drug, the calculation predicts the prodrug to be less cytotoxic (as prodrug ΔE is larger than that of drug, refer Table 1) and more stable than the drug.
Table 1.
Energies of both HOMO and LUMO and their gaps (in eV) calculated for prodrug and its components
| Compound | EHOMO (eV) | ELUMO (eV) | ΔE |
|---|---|---|---|
| Lyso-PC | −4.731 | −1.406 | 3.325 |
| Pro-bexarotene | −4.708 | −1.822 | 2.886 |
| Bexarotene | −6.174 | −3.518 | 2.656 |
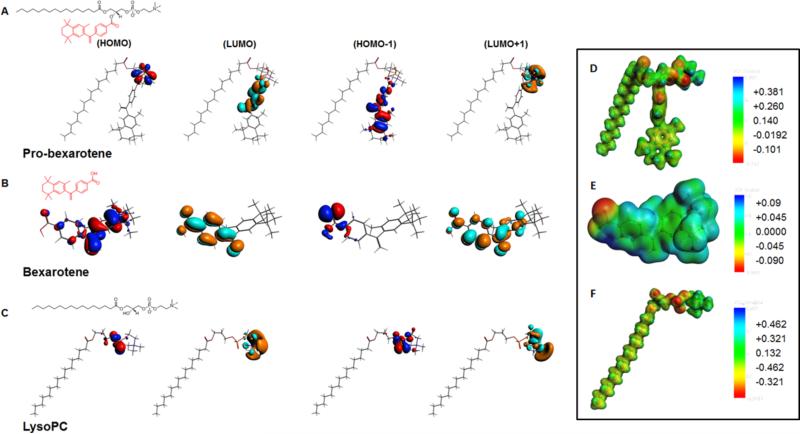
Figure 2 shows the plot of HOMO and LUMO of the prodrug and its components showing the main atomic contribution of the orbitals. The electron rich center is shown in red color, while the electron poor center is shown in blue color in the HOMO plot. The region with high electron density (red color) can be regarded as preferred nucleophilic centers, and regions with low electron density (blue color) are potential electrophilic sites. For Lyso-PC and probexarotene, most of the electron density is concentrated near the phosphodiester bond, making it a hydrophilic end. There is little change in the electron density distribution on Lyso and Probexarotene indicating that the Lyso-PC structural properties remain intact even after the drug insertion. The LUMO of the drug (bexarotene) showed electron deficient centers near the phenyl ring. Therefore, for HOMO-1 energy level, the electron density of pro-bexarotene is pushed towards the phenyl ring (Fig. 2) creating a curvature of the membrane compared to without drug (Lyso-PC). This may suggest the probability of forming nano-assembly is higher for Probexarotene compared to Lyso-PC alone as seen in our experiments. The HOMO molecular orbital of Pro-bexarotene mainly located near the phosphodiester bond, indicating the existence of a possible reactive site. Therefore, electrophilic attacks might take place on these sites. On the other hand, the LUMO of Pro-bexarotene is primarily concentrated on the phenyl ring of the drug end of bexarotene. Therefore, the negatively charged polar residues of the receptor are more favorable on these sites. (Table 1)
Figure 2.
Density functional theory distributions (A) pro-bexarotene; (B) bexarotene; (C) lysoPC and molecular electrostatic potentials (MEPs) of (D) pro-bexarotene; (E) bexarotene and (F) lysoPC.
Further, comparing the dipole moment of pro-bexarotene, bexarotene, and Lyso-PC revealed the compound with higher propensity to form nano-assembly and Interaction with the cell membrane. It is generally understood that the compound with largest dipole moment and larger dipole polarizability will have higher activity38 (Table 2).
Table 2.
Electrical properties of drug and prodrug
| Polarizability | Average polarizability | Dipole moment | Dipole length | |||
|---|---|---|---|---|---|---|
| α xx | α yy | α zz | μ (debye) | (e Bohr) | ||
| Bexarotene | 230.16 | 270.11 | 382.31 | 294.2 | 7.72 | 3.037 |
| Pro-bexarotene | 642.62 | 831.18 | 648.32 | 707.37 | 14.55 | 5.725 |
In order to further understand the activity of pro-bexarotene we plot and compare the molecular electrostatic potentials (MEPs) of pro-bexarotene and its components. (Figure 2D-F) Previously, MEP had been utilized to understand the potency of carbinolamine analogs in the antimalarial drugs,39 LpxC inhibition activity of 2-aryloxazolines, aroylserines and 2-arylthiazolines,40 17b-aminoestrogens's anticoagulant effect,36 and aurora A/B kinase inhibitors activity.37 Comparing the MEP shows that pro-bexarotene compound has an increased negative charge regions (in red) located near phosphodiester bond and Lyso-PC-drug junction. Most of the positive charges (shown in blue) reside on hydrogen atom in the trimethylamine region. This may indicate the site from where the electrons are being pulled away by strongly withdrawing substituents. This may create a hydrophobic end (electron deficient site) and contribute to the directional transport within the lipid bilayer membranes.41 As shown in Table 2, in addition to the geometrical parameters and charge distribution, we calculated the polarizability and dipole moment of the drug and prodrug. These properties are quite useful in describing the quantitative structure property (QSPR) and structure activity relationship (QSAR) of the compound under study.37,42,43 The higher average polarizability and dipole moment of the prodrug compared to the drug signifies the better activity and higher potency of prodrug. The static polarizability of bexarotene is highest along zz direction, whereas for pro-bexarotene, it is highest along the yy direction. The directional dependence of polarizability reveals the possible dipole-dipole interaction between target molecule and the drug/prodrug. This may possibly contribute to the different interaction probability of PBNB on cell surface depending on the symmetry of cell surface ligands.
Preparation and physico-chemical characterizations pro-bexarotene nanobubble (PBNB) and controls
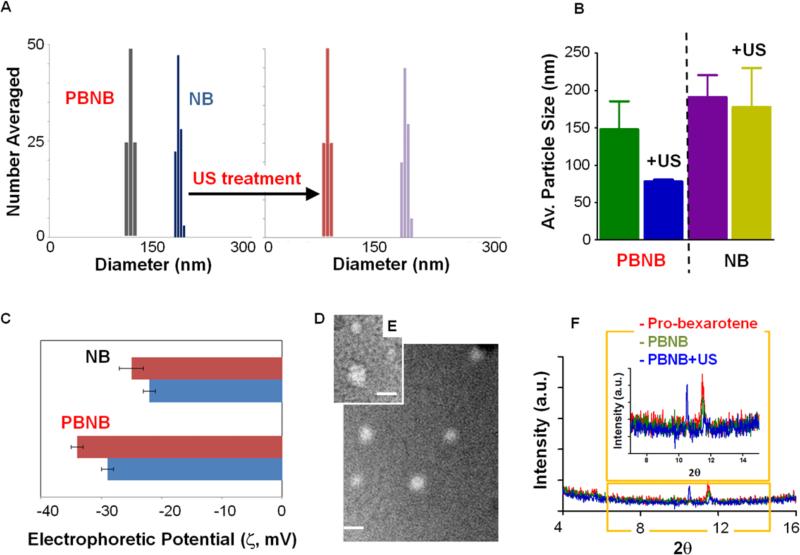
Pro-bexarotene molecules were used to prepare pro-bexarotene-NPs via a membrane hydration method.44 The synthetic system requires45 C3F8 gas purging with simultaneous bath sonication to prepare PBNB from Pro-bexarotene-NPs. Respective control lipid nanobubbles (NB) were also prepared NB without the utilization of pro-bexarotene. As synthesized PBNBs were evaluated for their physico-chemical integrity before and after US exposures. It was found that the size of PBNB (160±20 nm) decreased (80±10 nm) on exposure to US while in the case of NB, size decreased from 200±20 to 170±10 nm, only (Figure 3A, B). The surface potential of PBNB changed from −29±1 to −34±2 on US exposure while for NB changed from −22±1 to −25±1 (Figure 3C). The variation of surface charge potential upon US exposure is possibly due to an effect of re-assembly process of the amphiphilic prodrug. The presence of a higher negative electrophoretic potential also supports the successful lipid coating around these particles.
Figure 3.
Physico-chemical characterization of PBNB and effect of US exposure. (A) Bar diagrams of change in hydrodynamic diameter of PBNBs and NBs after US exposure; (B) Comparison in average particle size variation obtained from three independent experiments; (C) Zeta potential distribution; anhydrous state morphology of (D) PBNB and (E) pro-bexarotene-NPs; (F) XRD patterns of pro-bexarotene-NPs, PBNB and PBNB+US.
X-ray diffraction studies were performed to verify the US exposure mediated disruption of PBNB. Pro-bexarotene-NPs showed ordered-ness with peak at 2θ value of 11.5 and retained even in form of PBNB but US exposure decimated the ordered-ness peak while peak at 2θ value of 10.8 represents the crystallinity peak due to sodium phosphate salt from dulbacco's phosphate buffer (Figure 3F). This demonstrates the loss of pro-bexarotene molecule assembly due to the application of US.
The morphological distribution of PBNB in anhydrous state was studied by transmission electron microscopy (TEM). Negatively stained (0.4% uranyl acetate) pro-bexarotene-NP and PBNB samples were compared for their morphological distributions and variations. The anhydrous state diameter of pro-bexarotene-NP was found to be 70±20 nm while the size of PBNB was 90±10 nm (Figure 3D, E). A slight increase in the particle diameter is the resultant of expansion of outer layer of assembled pro-bexarotene molecules due to a volume expansion by the insertion of gaseous C3F8.
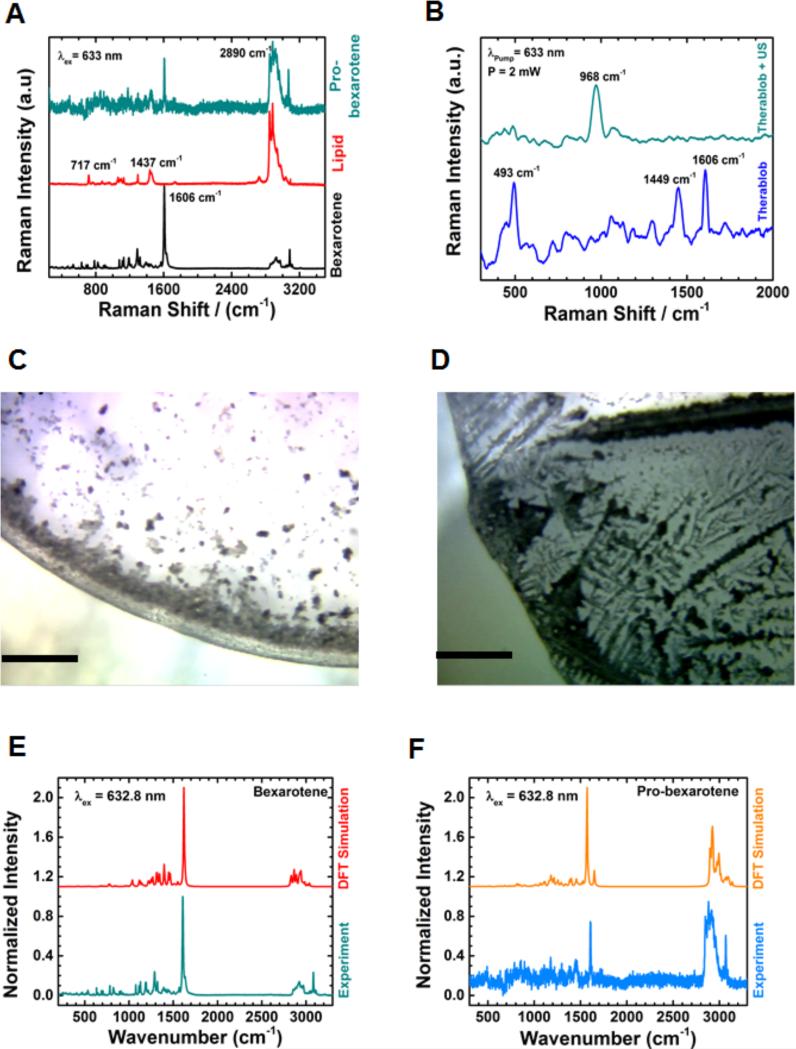
In order to gain insight into the fate of PBNB, pre- and post US exposure, Raman spectroscopic measurements were performed on bexarotene, lipid, pro-bexarotene, PBNB and NB. Figure 4C and 4d show the optical images (5X magnification) of PBNB samples before and after US exposure, respectively. The figures clearly showed a dramatic change in morphology after US exposure revealing a thin spread of lipid molecules (Figure 4D). Findings from Raman experiment further corroborated the XRD result demonstrating the loss of arrangements of probexarotene molecules in PBNBs after US exposure. (Figure 3F) Similar changes were also observed for NBs sample after US exposure (Figure S2A-B).
Figure 4.
Raman scattering patterns of (A) bexarotene, lyso-PC and pro-bexarotene in powdered form; (B) PBNB before and after US exposure; (C) Raman imaging of PBNB before and (d) after US exposure (scale bar 200μm; 5X); and DFT simulated Raman scattering patterns compared with experimental Raman spectra for (E) bexarotene and (F) pro-bexarotene.
Raman spectroscopic analyses of individual components of PBNBs were measured and found to be significantly distinguishable (Figure 4A). Raman peaks from bexarotene (782, 1288, and 1606 cm−1) were non-overlapping with 717, 873, 1062, 1098, 1128, 1295, 1437 and 2890 cm−1 peaks from lipid molecule which were found to be co-existing as 1606, and 2890 cm−1 in case of Pro-bexarotene. The peak at 1606 cm−1 which is assigned to C=C backbone stretching of bexarotene can be found in both bexarotene and Pro-bexarotene. This shows that the preparation of prodrug reserved the crystalline state and chemical properties of bexarotene. The symmetric CH stretching mode at 2890 cm−1 observed for lipid and pro-bexarotene gives evidence that bexarotene is conjugated to the lipid in pro-bexarotene. Figure 4B shows the effect of US on PBNB. A significant change was noticed when PBNB was exposed to US. For example, the peak at 968 cm−1 (CH out of plane vibration of bexarotene) became stronger after US exposure. The peak at 1606 cm−1 (C=C vibration) and 483 cm−1 (CH rocking vibration near P atom of the lipid) were diminished after US exposure to PBNB (Figure 4B).
Presumably, US exposure to PBNBs changes the orientation of lipidic tail and enables the CH out of plane vibrational mode of the drug (bexarotene). The control (NB) did not show any such changes after exposure to US (Figure S2B). Raman studies strongly support the conversion of pro-bexarotenes to PBNBs and assembly disruptive effects of US exposures. Additional DFT studies were performed to further confirm the respective signature peaks for individual components of PBNB and controls (Figure 4E-F).
Molecular dynamic simulations and fate PBNBs after US exposure
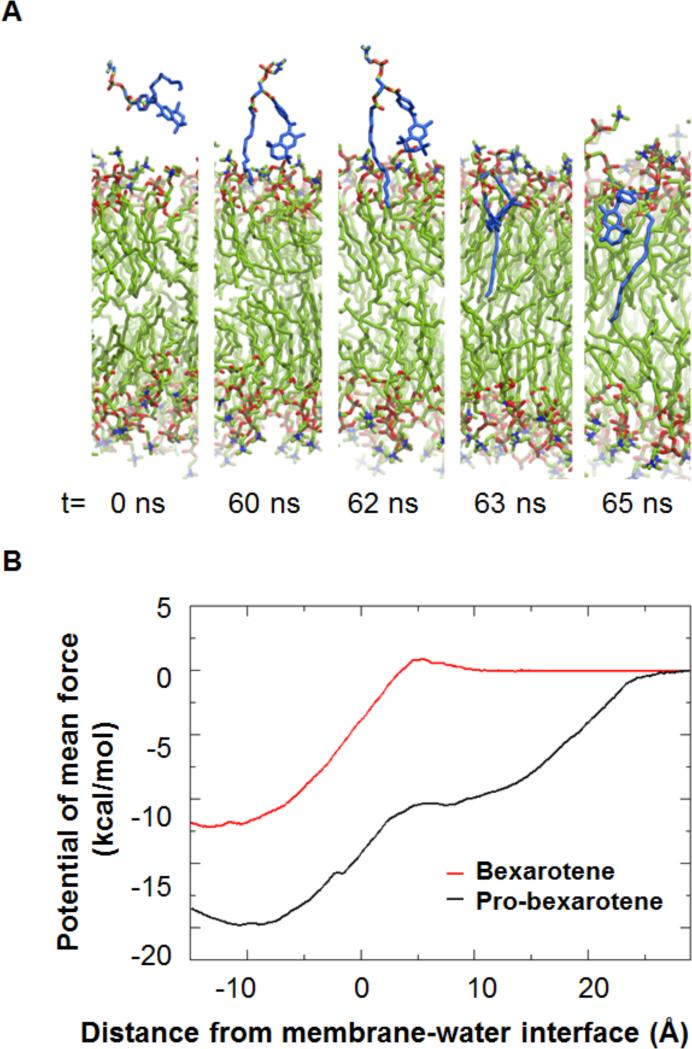
After establishing that PBNB can be prepared stably, molecular dynamic simulations were performed to visualize the interaction of Pro-bexarotene with the cell surface and internalization with the cellular membranes (Figure 5). A very strong cell uptake for the prodrug was observed. We performed molecular dynamics simulation in full atomic detail to model the insertion of the prodrug into a membrane bilayer. Initially, the prodrug molecule was placed 5 Å above the membrane and solvated by water. After ~ 60 ns, the prodrug starts to insert its Lyso PC hydrocarbon tail into the membrane. Within the next 5 ns, the PC hydrocarbon tail goes deeper into the membrane hydrophobic core, bringing along the aromatic group of the prodrug. The PC head group aligns with membrane head group and the whole prodrug molecule behaves similarly as a lipid molecule. After the insertion process, the prodrug stays in the membrane for the rest of the simulation (400 ns). For a dynamic simulated cellular entry process, please see Supporting Information movies derived from NLMD64-65 studies of pro-bexarotene and a lipid bilayer: Mov20).
Figure 5.
Static snaps of molecular dynamic simulations of the insertion process of Probexarotene into a membrane after US exposure to PBNBs (top). Pro-bexarotene inserts more favorably with membrane compared to bexarotene, as shown in terms of free energy (bottom).
Observed from the simulation, the lyso PC hydrocarbon tail interacts favorably with the membrane hydrophobic core, and thus facilitates the prodrug membrane insertion. The bexarotene group, however, prefers regions slightly below the membrane-water interface, i.e., regions close to the lipid glycerol group, similar to aromatic amino acids such as Phenylalanine and Tryptophan46. Based on the potential of mean force calculations, the membrane insertion free energy of the bexarotene group in the prodrug is −19.8±0.8 kcal/mol, which is ~8 kcal/mol more favorable to be delivered into a cell membrane than unbounded bexarotene (insertion free energy as 12.2±0.9 kcal/mol).
In vitro experiments
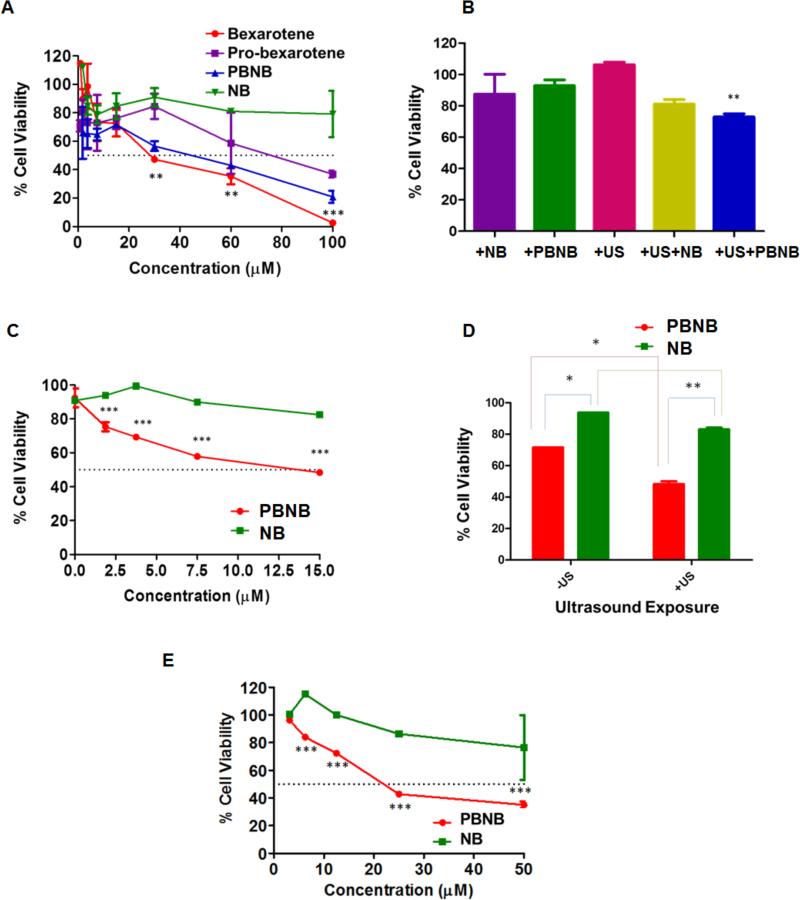
HepG2 cells were treated with bexarotene, pro-bexarotene, PBNB and NB formulations to establish their cytotoxicity in absence of US. IC50 values for bexarotene, pro-bexarotene and PBNB formulations were ~25, 60 and 45 μM, respectively. At 100 μM, the trend of HCC cell growth response remained the same with maximum cell death observed in case of bexarotene (~95%) followed by PBNB (~70%) and pro-bexarotene (~60%), respectively. A two way ANOVA analysis was performed to determine the statistical signficance of the results. Cell viability studies showed statistically significant decrease in case of bexarotene treatment at concentrations of 30, 60 and 100 μM. Similar results were obtained for treatments at 48 and 96h while PBNB improved its efficiency with increased incubation time while Bexarotene allowed more cells to revive their viability at 96h time point (Figure S3). Upon optimization of the US parameters using sub-IC50 concentration of 5 μM, for PBNB formulation, a significant drop in % cell viability was reported (p <0.05; One way ANNOVA with Bonferroni post test) compared to non US exposed cell population (Figure 6B). These effects could be visualized under bright field microscopy for US exposed or unexposed cell populations (Figure S4).
Figure 6.
In vitro MTT assay experiments for establishing HCC growth regression in HepG2 cells after 72h of incubation. (A) MTT assay on HepG2 cells treated with formulations to establish effective concentrations after 72h of treatment; (B) Optimization of US parameters for establishing effective improvements in US mediated enhancements in growth regression of HePG2 cells after 24h of treatments with 5 μM; (C) Effect of therapeutic nanobubble, PBNB on HCC growth regression after optimized US exposure at 72h time point at concentration of 15, 7.5, 3.75, 1.87 and 0.93 μM. A two way ANOVA was performed to be found as biologically significant difference in cell growth inhibition efficiency between PBNB and NB at all used concentrations. (D) Bio-statistical significance of therapeutic nanobubble (PBNB) treatment and US exposure at concentration of 10 μM for 72h in HepG2 cells and (E) Effect of therapeutic nanobubble, PBNB on HCC growth regression after optimized US exposure at 120h time point at concentration of 50, 25, 12.5, 6.25 and 3.125 μM.
Experiments were further performed to optimize concentration of PBNB at optimized US exposure for better growth regression. MTT results show the improvement in IC50 values of PBNBs to ~13 μM (Figure 6C). Biostatistical analysis (Two way ANOVA with Bonferroni post test) of MTT results showed improved cell growth regression at 72h time point with treatment concentration of 15 μM (Figure 6C). It was revealed that the improvement in cell regression ability of PBNB on US exposure by significance of p < 0.005 at concentration of 15, 7.5, 3.75 and 1.87 μM compared to NB treatment at same concentration. Further statistical analysis on comparison of PBNB and NB treatments in absence and presence of US revealed the decrease in cell viability with PBNB compared to NB by p<0.5 while under US exposure, it improved by significance of p<0.05. NB on the other hand did not reduce the % cell viability to any significant level (Figure 6D). It is anticipated that the major role of US exposure is to facilitate the rupture of the prodrug-bubble and assist the internalization of PBNB and NB at the surface of HCC. The US has no role to delegate the functional activity of bexarotene after delivery. Further MTT assay performed on HCC cells for 120h at concentration of 50, 25, 12.5, 6.25 and 3.125 μM revealed reduced cell viability only at higher concentration of 25 and 50 μM (Figure 6E) compared to 72h treatment with 15, 7.5, 3.75 and 1.87 μM (Figure 6C). Lower concentration of 12.5, 6.25 and 3.125 μM did not show any improvement in cell growth regression with an extended incubation period post US exposure. These analyses verify the combinatorial effects of HCC growth regression of PBNBs and US exposure. Cellular assay results were also corroborated with bright field microscopy images (Figure S5-6). PBNBs were found to be highly effective in deterioting the growth density and morphology of HCC cells at 15 μM concentration (Figure S5H). In Fig. 6c, d, the cell viability without US was ~100% for NB (No bexarotene), whereas with PBNB, 6d showed decrease in cell viability to ~65% at 10 μM and US exposure further reduced it to ~45%. Hence, NB exhibited no significant damage to HCC even in presence of lipases.
Mechanistic studies of RXR modulation and probing other biological interactions for PBNB and controls
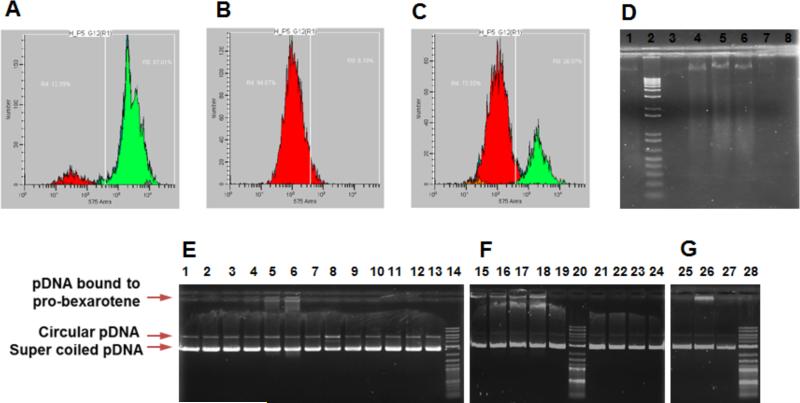
Mechanistic studies on role of US facilitated PBNB treatment in induction of apoptosis were performed in vitro using analysis on sub-G0/G1 cell population in cell cycle study and genomic DNA fragmentation assay. The fate of apoptotic death leads to cell shrinkage, membrane blebbing and fragmentation of genomic DNA into oligonucleosomal subunits. Staining of apoptosized cells with propidium iodide (PI) allows very low PI intercalation in fragmented genomic DNA leading to low fluorescence cell population in cell cycle analysis. Thus apoptotic cell population can be categorized as lowest PI staining population during cell cycle analysis, which can also be identified as sub-G0/G1 cell population. PI staining assay performed on HepG2 cells (1.5 × 105 plated in 12 well plate) after treatment with PBNB for 72h post US exposure. The % apoptotic cell population was quantified as ~95 and 74 % cells for treatments with PBNB + US at concentration of 50 (Figure 7B) μM and 12.5 μM (Figure 7C) represented as red population compared to cells with no significant apoptosis in case of untreated cells (Figure 7A).
Figure 7.
Mechanistic studies on role of US facilitated PBNB treatment in induction of apoptosis and Interaction of bexarotene with duplex plasmid DNA (pDNA). Experiments were performed on HepG2 cells (1.5 × 10 5 plated in 12 well plate) post treated with PBNB for 72h after US exposure. Propidium iodide staining on fixed cell population after US treatment only (A) and treatments with PBNB + US at concentration of (B) 50 μM and (C) 12.5 μM showed around 95 and 74 % cells going through apoptosis (red populations), respectively. (D) A gel laddering experiment performed on extracted genomic DNA from treated cells. It represents electrophoretic mobility of genomic DNA from cells treated with PBNB Lane 1: untreated; Lane 2: 1 Kb ladder; Lane 3: 6.25 μM; Lane 4: 12.5 μM; Lane 5: 25 μM; Lane 6: 50 μM; and treated with NB Lane 7: 25 μM and Lane 8: 50 μM. Cells treated with 12.5, 25 and 50 μM of PBNB produced DNA ladders, implies the cells have gone through the apoptosis. (E-G) Interaction of bexarotene with duplex plasmid DNA (pDNA) and effect of its prodrug and nanobubble form on electrophoretic mobility. Experiment was performed using (E) 500 (F) 250 and (G) 250 ng of pBR322. (E) pDNA was incubated with bexarotene at molar ratio of Lane 1: 1:1; Lane 2: 1:5; Lane 3: 1:10; with pro-bexarotene Lane 4: 1:1; Lane 5: 1:5; lane 6: 1:10; with PBNB Lane 8: 1:1; Lane 9: 1:5; Lane 10: 1:10 and with NB lane 11: 1:1; Lane 12: 1:5; Lane 13: 1:10 while Lane 7: DNA alone and Lane 14: 0.1-1 Kb DNA ladder; (F) pDNA was incubated with probexarotene Lane 15: 1:10; Lane 16: 1:15; lane 17: 1:20; Lane 18: 1:25 and with PBNB Lane 21: 1:10; Lane 22: 1:15; Lane 23: 1:20; Lane 24: 1:25 while Lane 19: DNA alone and Lane 20: 0.1-1 Kb DNA ladder. (G) pDNA was incubated with pro-bexarotene Lane 26: 1:30; with bexarotene Lane 27: 1:30; while Lane 25: DNA alone and Lane 28: 0.1-1 Kb DNA ladder.
Cellular apoptosis ultimately causes the nuclear fragmentation into small, condensed, membrane-bound apoptotic bodies. The genomic DNA results into nucleosome sized pieces of approximately 200 bp and multiples thereof, generating 3′-OH groups at the strand breaks. A gel laddering experiment performed on extracted genomic DNA from treated cells revealed the efficiency of inducing cellular apoptosis in HepG2 cells after treatment with PBNB and other control formulations. It was found that electrophoretic mobility of genomic DNA from cells treated with PBNB 12.5, 25 and 50 μM (Figure 7C; Lane 4, 5 and 6) could show a ladder of increasing intensity while no significant response in NB treated cells at 25 and 50 μM (Figure 7C; Lane 7 and 8) was noticed. Induction of DNA fragmentation further confirmed the role of PBNB for induced apoptosis in HepG2 cells.
Interaction of bexarotene with duplex plasmid DNA and effect of its prodrug and nanobubble form on electrophoretic mobility
Interaction of pDNA with bexarotene (Figure 7E, Lane 1-3), PBNB (Figure 7E, Lane 8-11) and NB (Figure 7E, Lane 12-14) resulted in no change in gel retardation pattern of pDNA. Change in gel retardation was noticed when the concentration reached 1-10 molar ratio with pDNA (Figure 7E). A 1:5 molar ratio (Lane 5) was enough to exhibit considerable interaction and gel retardation for pro-bexarotene showing approx. 20% pDNA binding to pro-bexarotene (Figure 7E). To further clarify the role of nanobubble assembly in loosing pDNA interaction of probexarotene, PBNB-pDNA mixtures were compared with mixtures of pro-bexarotene DNA at 10-25 molar ratios (Figure 7F). An incremental binding pattern was clearly visualized for pro-bexarotene (Lane 15-18), while PBNB-pDNA mixture was found to be non-interacting at all ratios (Lane 21-24). Further increase in molar ratios resulted in distinguishable changes in pDNA interaction pattern of pro-bexarotene (Figure 7G, Lane 26) compared to PBNB (Figure 7G, Lane 25). Overall evaluation of pDNA interaction studies led to the conclusion that pro-bexarotene transformed into a stable nano assembly (PBNB). It also showed that the inability of PBNB and its chemical components to generate DNA fragments via DNA cleaving. This further signifies the induction of apoptosis in DNA fragmentation processes.
RXR agonist assay
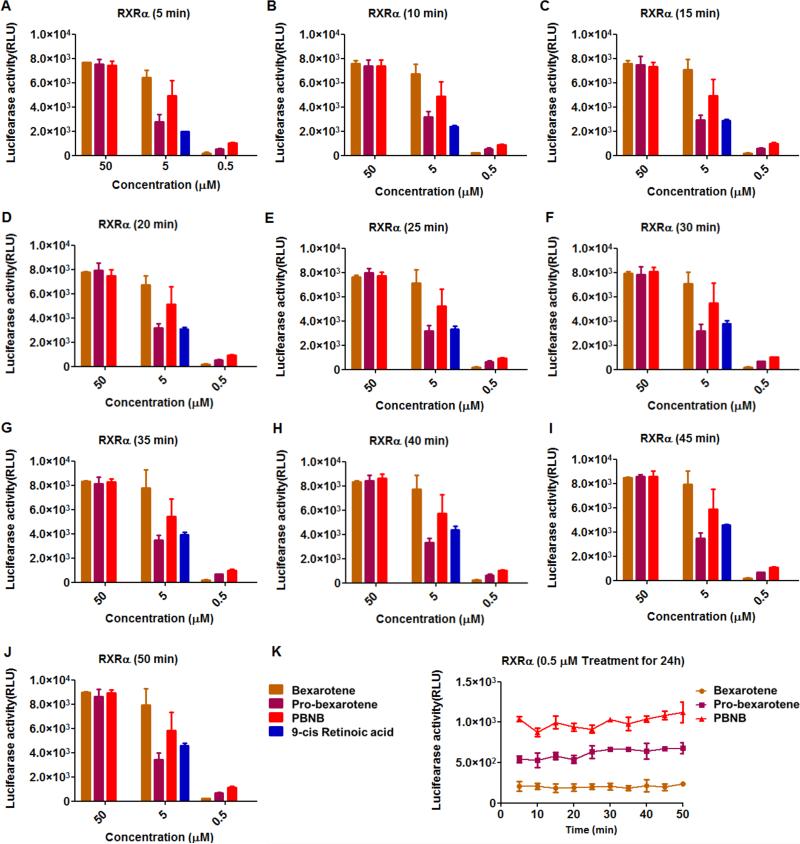
RXRα activity of bexarotene in free (bexarotene), pro-drug (Pro-bexarotene) and prodrug nanobubble (PBNB) form were measured at concentrations of 0.5, 5 and 50 μM. A positive control of 9-cis retinoic acid was used as a positive control at concentration of 5 μM. Luminescence was measured at various time points including (Figure 8A) 5 min; (Figure 8B) 10 min; (Figure 8C) 15 min; (Figure 8D) 20 min; (Figure 8E) 25 min; (Figure 8F) 30 min; (Figure 8G) 35 min; (Figure 8H) 40 min; (Figure 8I) 45 min and (Figure 8J) 50 min. Changes in luciferase activity was proportional to RXRα activity post treatments. Invariably PBNB was found to be a better RXRα agonist compared to bexarotene and pro-bexarotene molecules at a concentration of 0.5 μM. At concentration of 5 μM, bexarotene was better than PBNB and probexarotene in the same order while response at 50 μM was very similar with no significant difference among any of the used formulations. Change in luciferase activity was summarized to see maximum RXRα agonist efficiency in case of PBNB at 0.5 μM followed by Pro-bexarotene and bexarotene at various incubation time points (Figure 8K). Luciferase assay revealed the improvement in RXR activity on conversion of free bexarotene to pro-bexarotene and further to PBNB to improved cell internalization. This was also predicted by molecular dynamic simulations and density functional theory distribution studies.
Figure 8.
RXRα activity of retinoid bexarotene in free (Bexarotene), pro-drug (Pro-bexarotene) and pro-drug bubble (PBNB) form at concentrations of 0.5, 5 and 50 μM. A positive control 9-cis retinoic acid was used at positive control at concentration of 5 μM. After addition of luciferase substrate lumeniscence was measured at various time points including (A) 5 min; (B) 10 min; (C) 15 min; (D) 20 min; (E) 25 min; (F) 30 min; (G) 35 min; (H) 40 min; (L) 45 min and (J) 50 min. (K) Change in luciferase activity with time was summarized only to see maximum RXRα agonist efficiency in case of PBNB at 0.5 followed by Pro-bexarotene and bexarotene.
Ex vivo experiments
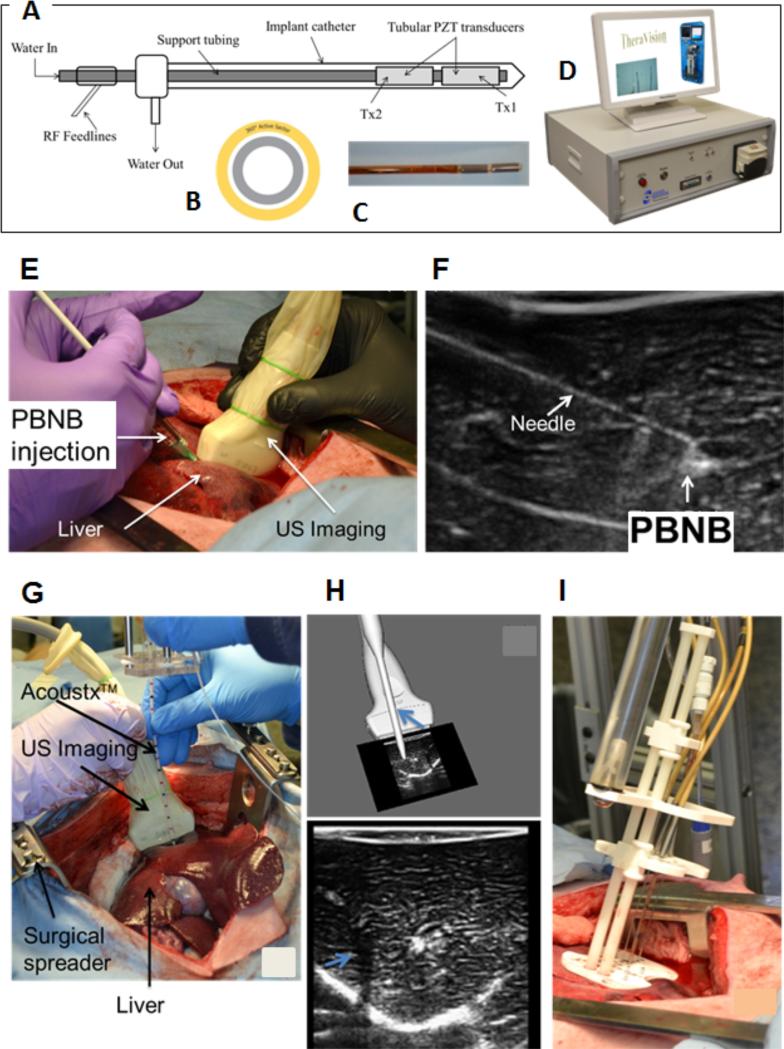
Ex vivo experiments were conducted using freshly excised porcine liver tissue sample. The tissue sample was placed in a thermo-electric enclosure to maintain the tissue temperature to 35-37 °C during the experiment (Figure 9). TheraVision™ (Acoustic MedSystem, IL) ultrasound ablation system and Acoustx™ applicators47 were used to deliver high-intensity ultrasound energy to the target tissue volume (Fig. 9A-D). The PBNB was injected into the tissue using a 21g needle under ultrasound image guidance (Figures 9E, F). The strong acoustic reflection from the needle could be easily identified in the ultrasound B-mode image including the release of the nano-chemotherapeutics at the tip of the needle shown in Figure 9F. Needle thermocouples of Type T (Physitemp, New Jersey, USA) with multiple sensors along the needle length were used for monitoring temperature during the treatment. Each needle was 100±2 mm long and 0.82 mm in diameter with 0.1 °C accuracy. Thermocouples were placed at different distances from the ultrasound applicator and dose was calculated for each thermal sensor.
Figure 9.
(a) Schematic of a catheter-Based ultrasound (CBUS) applicators for percutaneous conformal hyperthermia and thermal ablation to targets in soft tissue, (B) Sectored tubular ultrasound transducer showing 360° sector design for creating ablation pattern during the treatment, (C) magnified view of fabricated interstitial CBUS applicator tip showing the two transducers. Each of these transducers can be controlled individually with respect to the needed dose delivery. (D) Table-top TheraVision™ ablation system, which includes image processing and therapy control algorithms. (E) Injection of PBNB under 3D tracked ultrasound image guidance, and (F) the corresponding ultrasound B-mode showing the needle insertion and PBNB injection site. Gross pathology images for the different configurations from ex-vivo experiments (G) Pro-bexarotene-NPs (RD) + US, (H) NB (RD) + US, (I) Pro-bexarotene-NPs (RD) + US ablation, (J) PBNB (RD) + US ablation, (K) PBNB + US ablation, (L) US ablation only. The white arrows in (G), (H), (I) and (J) indicate the presence of rhodamine (RD) around site of injection.
The template helped in precisely registering the location of each thermocouple with respect to the ultrasound applicator. Acoustic power of 6 Watts per element were delivered to the tissue for the initial 2 minutes and then increased to 7 Watts with total treatment time of 6 minutes for each experiment. The gross pathology images (Figure 9G-L) show red spots indicated by the white arrows in Figure 9 to indicate the diffusion of the rhodamine into the tissue post US ablations. The temperature profiles at various locations are shown in Figure S8. The thermocouple sensor TC-B placed in-between the two applicators shown in Figure S9, shows the highest rise in temperature due to the combined ablation effects contributed from both the applicators. The TC-C placed at 10 mm radially beside Applicator 2 shows a large rise in temperature during the ablation. TC-A exhibited less rapid increase as it was located the greatest distance from both US treatment applicators. Typically necrosis occurs above 43 °C and is time-dependent. Temperature rise above 43 °C was observed in all the cases at 15 mm radially from the applicator. The gross pathology images shown in Figure 9G-L show good ablation pattern created using the dual applicator configurations. Approximately similar lesion size was observed in all the cases. Temperature sensors were inserted into the tissue to monitor temperature profiles and accumulated thermal dose to determine the thermal necrosis zones and also define the safety margin and its reproducibility. Temperature rise of different extent can produce resultant different tissue damage levels including reversible and irreversible damages. The measured temperatures and the accumulated resultant thermal dose estimated from inserted fine needle thermocouple arrays aided in determining the damage level due to the treatment. In other studies, a temperature rise of more than 35 °C was observed during focused ultrasound ablation of uterine fibroids50. Similar temperature rise of more than 30°C above body temperature was observed in RF ablation of liver tumor in a 71 year old patient51. In both of these cases, researchers used magnetic resonance temperature imaging (MRTI) method to monitor temperature profile during ablation of the respective tissue. In another study, researchers used MR-guided RF ablation of liver tumors and observed increase of temperature ranging from 60-100 °C very near the treatment device in the tumor region during ablation52. In microwave treatment of porcine renal cortex experiments, a temperature rise of more than 85 °C at the tip of the antenna was observed53. In this study, similar temperature rises ranging from 15 °C at 15mm to ~40°C at 5mm were observed in in vivo porcine liver, during ablation with Acoustxs™ applicators in the presence of PBNB. Tissue temperature of 70-76 °C was observed at 5 mm radial distance from the axis of the applicators.
In vivo experiments in swine model for optimization of therapy regime
In-vivo experiments were conducted in porcine liver (Figure 10A-C). A custom template was used to insert the applicator and thermocouples as shown in Figure 8C. Real-time 3D tracked ultrasound image guidance was used to insert the Acoustx™ applicator (Figure 9A-B). The targeted liver was exposed to high intensity ultrasound delivered via Acoustx™ applicators for 8 minutes at an acoustic power level of 7 watts. From gross tissue images, the lesion diameter of 2.4±0.1 cm and axial length of 2.0±0.1 cm were observed repeatedly for high intensity ultrasound exposure using consistent energy delivery parameters. The temperature and accumulated thermal dose at various radial distances from the therapy applicator are tabulated in Table 3. The radial distance at which thermal dose of t43 > 240 min was estimated as R = 15 mm, by interpolating the data shown in Table 3. This distance R defines the thermal necrosis boundary and margin of the treatment. Specifically, R was estimated by interpolating thermometry data at various radial distances and angles from the axis of the applicator. Highest temperatures were recorded at the 5 mm radial distance. Temperature of the applicator catheter did not exceed 36°C. Temperatures sufficient to produce thermal dose of at least 240 equivalent minutes were measured for nearly all cases at a radial distance of 15 mm from the applicator center (30 mm diameter treatment zone). Since liver is a highly vascularized organ the drug injected into the target site will also circulate to other organs and hence the prodrug formulation can be delivered either through direct or vascular route. The vascular delivery approach was out of scope for this work, however may be conducted as a separate study in the near future. The thermal damage end-points can be classified into vascular effects, direct damage and functional effects. Histopathological analysis is considered to be the gold standard to understand the tissue effects and damage due to various types of therapy including thermal energy therapy. Cancer cells are durable and resistive enough to insult that they can recover from a harsh environment if not completely destroyed. Histopathological analysis can be used to confirm tissue damage at the cellular level due to thermal therapy. Histopathological and gross appearance of tissue damage were observed at a thermal dose greater than t43>240 minutes in various studies of in vivo tissue including liver, muscle, prostate and kidney from dog, rabbit and pigs as reported by numerous studies.48
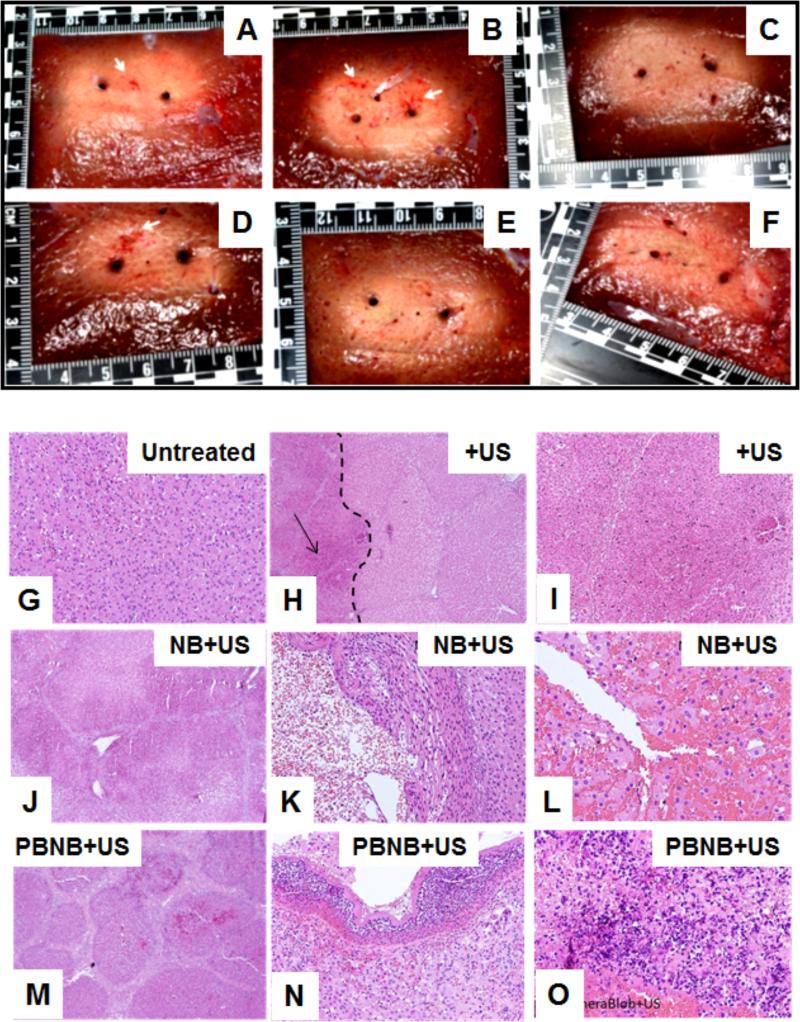
Figure 10.
(A) Insertion of ultrasound Acoustx™ applicators under guidance of combined ultrasound imaging and electromagnetic tracking, (B) ultrasound imaging and applicator position in 2D and 3D plane corresponding to the setup shown in (B) (Arrows indicate the catheter in the 3D view and shadow by the catheter in the B-mode image), and (C) Template with applicator and fine needle thermocouples during treatment; Histopathology images obtained from liver tissues from in vivo experiment of (D) normal liver at 8X magnification, (E) liver ablated with ultrasound at 2X (the dotted line indicates the boundary of the ablated and normal tissue (right) and the arrow points to the ablated region (left)); (F) Magnified ultrasound treated region marked by arrow in (E) at 8X US only, indicates pre-necrotic hepatic damage; (G) Liver tissue treated with ultrasound in presence of nanobubble showing signs of injury, sinusoidal dilation edema of hepatocytes at 4X, with 50% of region showing frank necrosis; (H) necrotic region, thermal damage, and streaming in ~50% of area shown at 20X, and (I) areas of hemorrhage, bubble in cytoplasm (70%) at 40X. Liver tissue treated with combined therapy of high intensity ultrasound and PBNB showing (J) liver bands due to contraction of liver parenchyma in the vicinity of the necrotic area at 4X, (K) liver capsule, inflammation and acute signs of frank necrosis in the liver over entire area shown at 20X, and (L) totally (100%) necrotic area of dead tissue, with inflammation at 40X, (M) PBNB injected liver tissue, (N) PBNB injected normal liver with stress and little hemorrhage at injection site and (O) PBNB injected normal liver with hepatoportal tract and no pathological change.
Table 3.
Mean peak temperature and accumulated thermal dose at various radial distances from the applicator for single applicator insertion configuration
| Tissue | Peak thermometry at different radial distances | |||||||
|---|---|---|---|---|---|---|---|---|
| 5 mm | 10 mm | 15 mm | 20 mm | |||||
| T (°C) | t43 min | T (°C) | t43 min | T (°C) | t43 min | T (°C) | t43 min | |
| Liver | 73.9±2.9 | 5.64×108 | 63.8±3.2 | 1.65×106 | 47.2±0.6 | 154.3 | 44.7±1.1 | 6.1 |
Histopathology images were analyzed to detect tissue damage resulting from the combined therapy (high-intensity ultrasound plus PBNB). Tissues from four different experimental configurations i) untreated, ii) ultrasound (US) only, iii) US and Nanobubble, and (iv) US and PBNB are shown in Figure 10D-L. Specifically, liver tissue was analyzed for histological features. Both normal and thermally damaged tissue was analyzed to differentiate histological changes between them. Histopathology of normal liver shows healthy cell distribution (Figure 10d), while hepatocyte balloon degeneration, liver “congestion” and minor sinusoidal dilatation are evident for high intensity ultrasound treatment (Figure 10E-F). The tissue treated with high intensity ultrasound in presence of nanobubbles exhibits area of necrosis, thermal coagulation, sinusoidal dilation edema of hepatocytes, streaming, hemorrhage and bubbles in cytoplasm (Figure 10G-I). Liver tissue treated with the combined treatment of PBNB and high intensity ultrasound showed bands due to contraction of liver parenchyma in the necrotic region, inflammation sign of stress, dead tissue, thermal coagulation (Figure 10J-L). Cell bursting was also observed. Liver tissue treated with ultrasound in presence of nanobubble (no drug) indicates signs of injury, sinusoidal dilation edema of hepatocytes at 4X, with 50% of region showing frank necrosis and necrotic region, thermal damage, and streaming in ~50% was seen at 20X. At 40X the areas of hemorrhage, bubble in cytoplasm (70%) was seen. Liver tissue treated with combined therapy of high intensity ultrasound and PBNB shows liver bands due to contraction of liver parenchyma in the vicinity of the necrotic area at 4X and liver capsule, inflammation and acute signs of frank necrosis in the liver over entire area at 20X. Totally (100%) necrotic area of dead tissue with inflammation was noticed at 40X. PBNB injected normal liver tissue shows signs of stress and little hemorrhage at injection site along with hepatoportal tract having no pathological change.
In vivo study in transgenic tumor-bearing swine model
A liver cancer animal model that can be used for studying this trimodal ablation therapy was not immediately available. Furthermore, the use of flexible ablation catheter, developed for human use in mind, precluded us from using a rodent-based tumor model. The comparable size of the pig and its resemblance in anatomy, physiology, metabolism, and genetics to humans make it an attractive platform to develop a genetically defined, large animal model of cancer. To this end, in a preliminary experiment, we tested the combination of PBNB and US ablation in a transgenic “oncopig” line encoding Cre recombinase inducible porcine transgenes encoding KRASG12D and TP53R167H.49 These genes are known to represent a commonly mutated oncogene and tumor suppressor in human cancers. Due to the study limitation and unavailability of a tumor model grown in swine liver, an improvised approached was taken. Based on this approach, the liver hepatocyte cells were removed from the oncopig, implanted into the mouse liver and regrew the tumors. The transformed liver tumor cells were used to assess the therapeutic efficacy of PBNB. Liver tissues obtained from the mouse were histologically analyzed for characteristics of HCC.
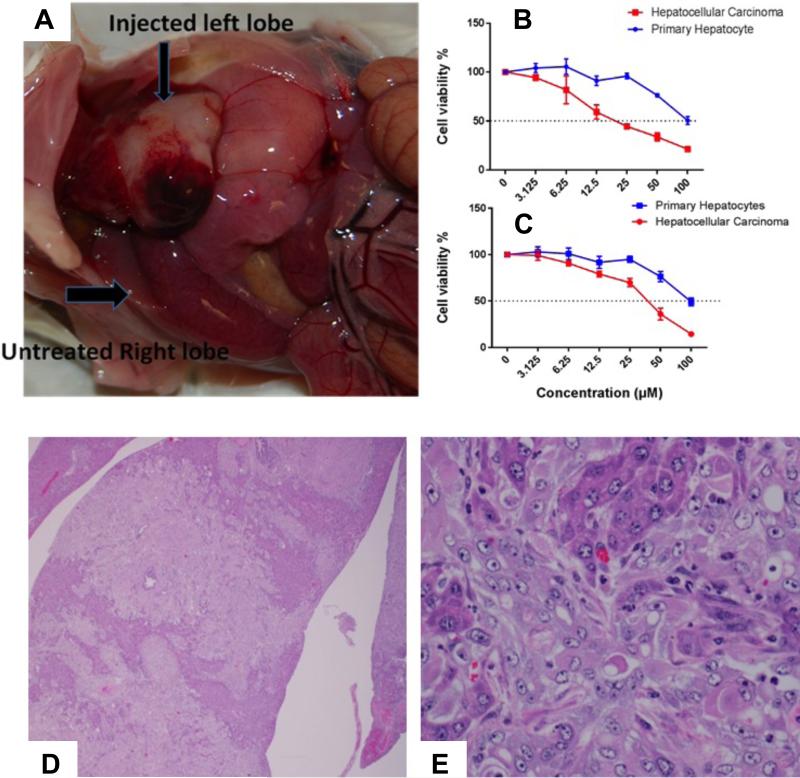
Briefly, hepatocytes were isolated from transgenic onco-pig, containing mutated oncogene Kras (KrasG12D) and p53 (p53R167H) in their genome. KrasG12D and p53R167H mutants were linked by internal ribosome entry sites (IRES) for their simultaneous expression. The cassette was then inserted into a vector following the LoxP-PolyA (STOP)-LoxP sequence. Hepatocytes were isolated from this transgenic oncopig and transfected with Cre plasmid which delegates the PolyA “STOP” sequence and allows transgene expression.49 Histopathology of these cells revealed that they were of hepatocellular carcinoma cells (HCC) nature. The transformed hepatocytes cells were able to successfully generate hepatocellular carcinoma in mouse liver. As shown in the Figure 11A, the injected left lobe of mouse liver with transformed hepatocytes developed tumor, whereas, the untreated right lobe appeared normal. The histopathological analysis on liver tissues excised from mouse liver injected with transformed hepatocellular carcinoma revealed the presence of numerous transplanted cells. (11D-E) These cells were arranged in trabeculae and rare acini supported by a thick fibrovascular stroma. Individual transplanted cells were round to polygonal with moderate amount of light eosinophilic cytoplasm surrounded by indistinct cell border. Nuclei were single to multiple, round to oval with scattered chromatin and single prominent nucleolus. These cells exhibited moderate anisocytosis and anisokaryosis. Mitotic figures were 2 per 10 high power fields. Areas of necrosis and hemorrhages were found to be scattered within these regions. The adjacent hepatocytes exhibited atrophy. Small numbers of neutrophils were also scattered within these regions. Thus the injected liver found to have multiple islands of proliferating viable transplanted cells. These features confirm the morphology of these cells resembled with transplanted transformed hepatocytes and possible nature of a hepatocellular carcinoma. It was found that they were able to produce tumor in mouse liver. The transformed hepatocytes were used for further in vitro assessment of drug activity either as a free small molecule or PBNB. Results indicated a significant cellular regression and improvement in IC50 value of bexarotene in form of prodrug derived PBNB. (Figure 11B-C) Altogether, the results indicated that even though it was not possible to generate a direct tumor implanted in swine liver, we achieved therapeutic efficacy in tumor equivalent in vivo model.
Figure 11.
(A) Left lobe of mouse liver injected with transformed hepatocytes indicating the development of tumor, whereas, the untreated right lobe appeared normal; In vitro assessment of drug activity as a free small molecule bexarotene (B) or PBNB (C) by using MTT assay at 72h; H&E stained sections of mouse liver transplanted with transformed hepatocytes. (D) Dissecting through the liver show sheets of transplanted cells. Bile ducts exhibit hyperplasia (1.2X); (E) transplanted cells arranged in sheets and trabeculae and supported by a thick fibrous stroma. Individual transplanted cells round to polygonal with moderate quantities of light eosinophilic homogenous cytoplasm and surrounded by indistinct cell border. Nuclei appeared single to multiple, round to oval with scattered chromatin and a single prominent central nucleolus. Small numbers of neutrophils look scattered within these regions. Existing trapped hepatocytes exhibit atrophy (3.40X).
Tumor destruction and histopathologic analyses post US exposure
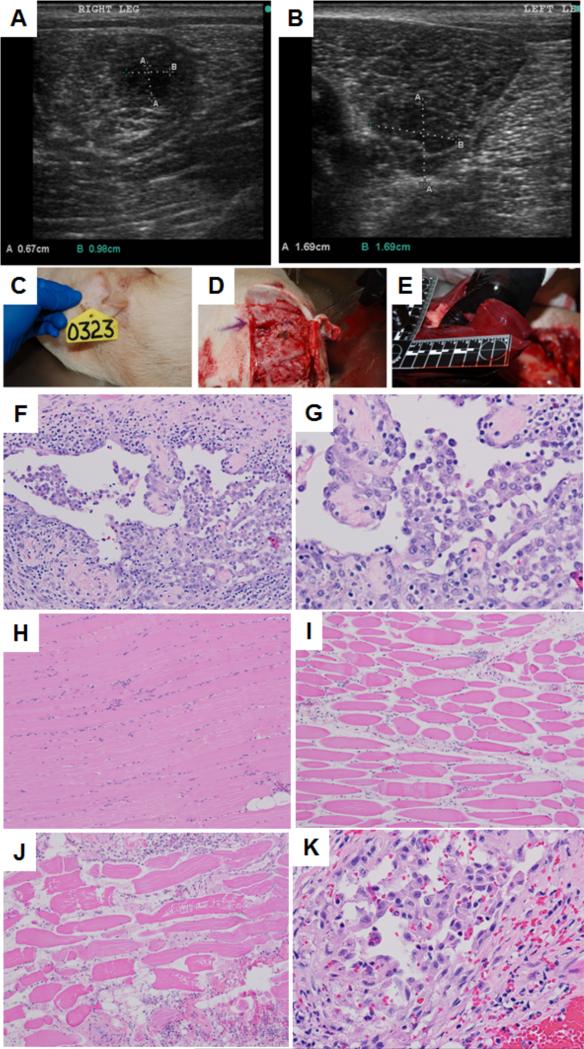
Under an approved protocol by the University of Illinois Institutional Animal Care and Use Committees (IACUC), transgenic oncopig was grown as described before.49 As a makeshift strategy for the assessment of tumor regression in vivo with thermal ablation combined with drug treatment, five week old transgenic piglets were infected with virus at specific sites of right and left thigh. Animal was kept under observation till it grew the tumor of significant size (1.5 cm × 1.5 cm). Tumor growth was followed by US imaging using hand held US imaging machine (Figure 11). Figure 12 indicates the formation of abnormal tissue growth in (A) right and (B) left thigh of a representative transgenic pig. Abnormal tissue growth was regularly followed until being treated with PBNB (1mg/ml, 5 mL total volume) and US.
Figure 12.
Representative US images of abnormal tissue growth in (A) right and (B) left thigh of a transgenic pig. Representative necropsy images: (C) animal identification tag; (B) surgery at site of US exposure; (C) gross pathology of liver tissue; Histopathologic images: (F) neoplastic cells in the tumor from the left thigh showing lining vascular channels and caverns (20X), (G) neoplastic cells in the tumor from the right thigh showing lining vascular channels and caverns (40X), (H) skeletal muscle bundles not treated with thermal ablation (10X), (I) skeletal muscle fibers treated with thermal ablation exhibit sarcoplasmic hyalinization, loss of striation, cell shrinkage and increased endomysial space (10X), (J) skeletal muscle fibers treated with thermal ablation exhibit sarcoplasmic hyalinization, loss of striation, cell shrinkage, sarcoplasmic fragmentation and increased endomysial space (10X), (K) neoplastic cells in the tumor from the right thigh exhibit mild cytoplasmic swelling and vacuolation indicating vacuolar degeneration from the treatment (40X).
Tumor destruction was followed by histopathologic analyses. Figure 12F and G show the presence of neoplastic cells, which are spindeloid to round and contain round nucleus and light eosinophilic cytoplasm surrounded by indistinct cell border. Nuclei are single, round with scattered chromatin and multiple prominent nucleoli. Neoplastic cells stained strongly with anti CD31 antibody confirming the presence of endothelial cells. Therefore, it can be concluded that the tumors are of angiosarcoma type. Scattered within the neoplastic cells are small numbers of lymphocytes and plasma cells. Figure 12H shows the skeletal muscle bundles not treated with thermal ablation. Absence of US treatment is evident here. Figure 12I shows the skeletal muscle fibers treated with thermal ablation and exhibit sarcoplasmic hyalinization, loss of striation, cell shrinkage and increased endomysial space. Figure 12J also confirms the presence of skeletal muscle fibers treated with thermal ablation exhibit sarcoplasmic hyalinization, loss of striation, cell shrinkage, sarcoplasmic fragmentation and increased endomysial space. Lymphocytes and plasma cells are infiltrating endomysial regions. Figure 12K shows the presence of neoplastic cells in the tumor from the right thigh exhibiting mild cytoplasmic swelling and vacuolation. This observation indicates vacuolar degeneration. The abundance of vacuolar degeneration is indicative of the drug treatment.
A detailed necropsy investigation was performed on the treated pig. As evident from our studies, the presence of multifocal areas of atelectasis and hemorrhage within the airways and alveolar spaces in Lung was noticed. There are rare sub-capsular areas of acute hemorrhages observed in liver. Hepatocytes in some regions are mildly swollen and contain granular cytoplasm with a central nucleus. Multiple secondary lymphoid follicles are present in spleen. No significant lesion was observed in gall bladder, thyroid gland and lymph nodes, thymus, adrenal gland and skeletal muscles, salivary gland. No significant blood clot was also observed. Rare medullary interstitial aggregates of lymphocytes, plasma cells and macrophages were observed in kidney, whereas the heart showed the abundance of rare mononuclear aggregates in the myocardium. Small numbers of lymphocytes were found to be present within the lamina propria and are transmigrating through the mucosa. There were rare lymphocytes and plasma cells present within the lamina propria in pyloric stomach. There were rare foci of mineralization present in the tubules admixed with rare lymphocytes and plasma cells in the prostate. Colon appeared to have moderate numbers of lymphocytes and plasma cells admixed with rare eosinophils within the lamina propria. A focal aggregate of mononuclear cells (glial cells) were present within the cortical white matter of brain. Multiple secondary lymphoid follicles were present in the right popliteal lymph node with macrophages containing dark granular pigment (carbon) and hemorrhages (local drainage). Multiple secondary lymphoid follicles were found to be present in the left popliteal lymph node. Macrophages contained green globular pigment (hemosiderin). Moderate numbers of lymphocytes and plasma cells are present in the conjunctiva (Eye). Microscopic Diagnosis showed multifocal mild acute hemorrhages with atelectasis in lung, mild vacuolar degeneration in liver, focal mononuclear cell encephalitis in brain and mild chronic tracheitis in trachea. Focal encephalitis is always considered important with drug treated animals. A cause was not observed in these samples. No parasite or fungus was present in these sections. Hemorrhages in the lungs were secondary to intra-cardiac euthanasia solution. Mild chronic tracheitis and conjunctivitis were likely incidental finding. Mild hepatic vacuolar degeneration was also considered incidental but an association with the drug should be ruled out. Further in depth studies will be necessary to confirm these preliminary findings.
Differential gene expression from swine liver tissue
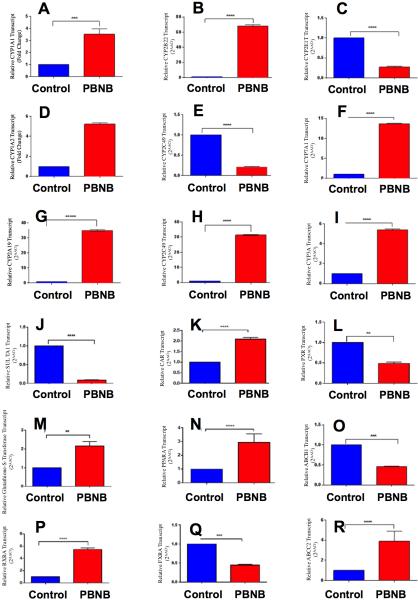
Therapeutic potential of PBNB through RXR inhibition requires regulation and expression of RXR genes. In addition to this, delivery of PBNB should be able to modulate genes related to metabolic pathways involved in phase I and phase II of drug metabolism through liver. Towards this, differential gene expression studies were performed of genes that might be involved in drug metabolism, regulation and transport. Towards this, we identified genes responsive to the treatment of PBNB, including, orphan nuclear receptors (regulators), RXRα, CAR, PPARα, FXR, PXR, genes involved in Phase-I drug metabolism (oxidation reactions) e.g. CYP1A1, CYP1A2, CYP2A19, CYP2B22, CYP2C33, CYP2C49, CYP2E1, CYP3A, CYP7A1, genes involved in Phase-II drug metabolism (conjugation reactions) and glutathione-S-transferase SULTA1, and transporters such as ABCB1 ABCC2. Results indicated that the CYP2E1, CYP2C33, SULTA1, transporters, FXR, PXR and ABCB1 genes were downregulated as expected and other genes revealed 3-70 fold upregulation in various cases. (Figure 13) It clearly confirms the involvement of PBNB through RXR and other gene cascade pathways of swine liver.
Figure 13.
Differential gene expression was studied of genes that might be involved in drug metabolism, regulation and transport in swine liver tissue. Relative changes in PBNB treated animals were analyzed for genes (A) CYP1A1; (B) CYP2B22; (C) CYP2E1; (D) CYP1A2; (E) CYP2C33; (F) CYP3A; (G) CYP2A19; (H) CYP2C49; (I) CYP7A1; (J) SULTA1; (K) CAR; (L) PXR; (M) glutathione-S-Transferase; (N) PPARα; (O) ABCB1; (P) RXRα; (Q) FXR and (R) ABCC2. Results indicated that the genes CYP2E1 (C), CYP2C33 (E), SULTA1 (J), FXR (Q), PXR (L) and ABCB1 (O) were downregulated as expected and other genes revealed 3-70 fold upregulation in various cases.
Conclusions
We successfully demonstrated the integration of cavitation/sonoporation technique with a unique prodrug nanobubbles construct to enhance tissue penetration and ablation by thermal effects of ultrasound. Bexarotene, a known, FDA-cleared anti-cancer agent was repurposed and repackaged into a sensitive nanobubble form for the application with US-mediated ablation therapy. Molecular dynamic simulations, dynamic light scattering, zeta potential, Raman spectroscopy and electron microscopic analysis were used to analyze these particles and response under US exposure. Molecular dynamic simulation studies and free energy data confirmed that PBNB ruptures in response to US directing a favorable cell entry of pro-bexarotene. However, at this stage it was inconclusive whether PBNB interacts with cells via fusion with cell bilayer. An in-depth molecular dynamics simulation study is out of scope and will be reported in the near future. Combined therapy using PBNB with catheter-based high-intensity ultrasound was used to demonstrate the applicability in porcine in vivo liver. Histopathology analysis of tissue treated with combined therapy showed enhanced tissue damage when compared with tissue treated with only high-intensity ultrasound. The presence of nano-chemotherapeutics in prodrug form showed cell bursting due to cavitation effects from bubble bursting. Bubbles in cytoplasm were observed from histopathology analysis in tissue treated with ultrasound in presence of either nanobubble or PBNB. Such results show the significance of the combined therapy where damage occurs from the cellular to the tissue level. This may be due to the fact that the thermal dose and cavitation sensitizes the cells to enhance chemotherapeutic effects.
A liver cancer model was not available in a large animal which would allow us to test our therapeutic regime. An improvised approach was taken where hepatocytes were isolated from transgenic onco-pig, and able to produce tumor in mouse liver as positively confirmed by histopathology of HCC nature. These transformed hepatocytes were used for in vitro assessment of drug activity either as a free small molecule or PBNB, which resulted in a significant cellular regression and improved IC50 by 2 to 2.5 fold in case of PBNB. Since we were dealing with the combination therapeutic approach and just not the drug effect, it was critical to test it in a large animal preclinical model that allows for the use of the flexible ablation catheter. The transgenic tumor pig was developed 49 and the tumor development was monitored by using US imaging and after the administration of drug and ablation therapy, the destruction of the tumor was followed by histopathologic analyses. Detailed analyses showed that while untreated tissues appeared histopathologically normal, in case of ablated animals, sign of sarcoplasmic hyalinization, loss of striation, cell shrinkage and increased endomysial space were predominant. For the drug treated tissue, the vacuolar degeneration was obvious presumably due to the effect from the administration of the drug. Differential gene expression indicated that various genes were responsive to the treatment of PBNB, including, orphan nuclear receptors (regulators), RXRα, CAR, PPARα, FXR, PXR, genes involved in Phase-I and Phase II drug metabolism. It clearly confirms the involvement of PBNB through RXR and other gene cascade pathways of swine liver. In short, these results, together with our in vitro optimization study, ex vivo and in vivo liver model demonstrated the combinatorial treatment approach for this disease, which is normally difficult to intervene and requires complete surgical intervention.
Experimental Section
Materials and Methods
Bexarotene was obtained from Sigma Aldrich, Inc. (St. Louis, MO) while LysoPC was purchased from Avanti Polar Lipids (Birmingham, Al). The hydrodynamic diameter was measured on a Malvern Zetasizer machine equipped with 633 nm laser. Zeta potential measurement was performed on a Malvern Zetasizer instrument, from MRL facility, UIUC. The TEM images were acquired on a JEOL 2100 Cryo TEM machine and imaged by Gatan UltraScan 2k × 2k CCD. The XRD data was collected on instrument Siemens-Bruker D5000 diffractometer and analyzed using software Jade X-ray analysis. The Raman experiments were performed using Renishaw mircoPL /Raman microscope system. A 633 nm HeNe laser (with beam width 1.12 μm) was used to excite the analytes (50X objective, NA= 0.45). The power used for the experiments were 2 mW. All the data were recorded with WiRE3.2 software. All simulations were carried out with NAMD 2.9 (http://www.ks.uiuc.edu/Research/namd/2.9/ug/). TheraVisions™ Ultrasound Ablation system (Acoustic MedSystems, IL) was used to drive the flat square ultrasonic transducer. MTT reduction assay was ended with performing absorption assay on plate reader (Synergy HT, Bio-Tek). Bright field imaging was performed on microscope DMI3000 B, Leica Microsystems, Buffalo Grove, IL.
Nanomedicine approaches have been extensively explored as anti-cancer strategies65 and for diagnostics.66 In this nanomedicinal approach, bexarotene was repurposed as a lipase labile prodrug. Pro-bexarotene-NPs were prepared by membranes formation method. Prepared membranes were hydrated followed by vortexing and bath sonication to prepare Pro-bexarotene-NPs. C3F8 gas was passed through prepared colloidal suspension and air above the suspension generating PBNB. Similarly, the lipid LysoPC was used to generate NBs as control nanobubbles. Average hydrodynamic diameter distributions for PBNB and NB formulations before and after exposure to optimized ultrasonication (PBNB+US and NB+US) were determined using a Malvern Zetasizer nano series–Nano ZS90 at various time points 0-120 h. Zeta potential (ξ) values for the formulations before and after exposure to optimized ultrasonication (PBNB+US and NB+US) were determined with a nano-series Malvern Zetasizer zeta potential analyzer while measurements of ξ were reproducible to within ±5. The morphological investigations on Probexarotene-NPs and PBNBs were performed by transmission electron microscopy using negative staining with Uranyl acetate.
The ordered behavior of Pro-bexarotene-NPs, PBNB and PBNB+US was determined by X-ray diffraction measurement on Siemens-Bruker D5000 diffractometer. Scans were performed for 2θ range of 2 to 50. Raman spectroscopic measurements were performed to reveal unique raman scattering patterns of PBNB and NB before and after US exposure and compared with powdered samples of Pro-bexarotene, Bexarotene and lipid LysoPC. The Raman experiments were performed using Renishaw microPL /Raman microscope system. A 633 nm HeNe laser (with beam width 1.12 μm) was used to excite the analytes (50X objective, NA= 0.45). The power used for the experiments were 2 mW. All the data were recorded with WiRE3.2 software. The fate of PBNBs after US exposure was simulated with NAMD 2.9 (http://www.ks.uiuc.edu/Research/namd/2.9/ug/). All simulations were performed in the NPT ensemble, where pressure was kept constant at 1 atm by the Langevin piston method.
Preparation of PBNBs
Pro-Baxarotene (1 mg/ml) was dissolved in CHCl3/MeOH (4:1) for membrane formation under reduced pressure rotary evaporator at 40 °C for 10 min. Prepared membranes were kept under vacuum for removal of traces of organic solvents. Prepared membranes were first hydrated with hot water (~ 60 °C) for 20 min followed by keeping at 4 °C for > 12h. At the end of hydration process membranes were heated at 60 °C for 10 min followed by 5 min vortexing and 5 min bath sonication. The above cycle was repeated 3 times. Prepared nanoparticles were characterized by DLS and zeta potential measurement. C3F8 gas was passed through prepared soy lecithin nanoparticle colloidal suspension for 3 min. Air above the suspension in preparation-tube was replaced with C3F8 by continuous flow for next 1 min. Prepared nanobubbles were characterized by DLS and zeta potential measurement.
Dynamic light scattering
Average hydrodynamic diameter distributions for PBNB and NB formulations before and after exposure to optimized ultrasonication (PBNB+US and NB+US) were determined using a Malvern Zetasizer nano series–Nano ZS90. Scattered light was collected at a fixed angle of 90°. A photomultiplier aperture of 400 mm was used, and the incident laser power was adjusted to obtain a photon counting rate between 200 and 300 kcps. Only measurements for which the measured and calculated baselines of the intensity autocorrelation function agreed to within +0.1% were used to calculate nanoparticle hydrodynamic diameter values. The measurements for the particles were made at 0 h, 24 h, 48 h, 72 h, 96 h, and 120 hr after synthesis to evaluate the stability. All determinations were made in multiples of five consecutive measurements.
Zeta potential determination
Zeta potential (ξ) values for the PBNB and NB formulations before and after exposure to optimized ultrasonication (PBNB+US and NB+US) were determined with a nano-series Malvern Zetasizer zeta potential analyzer. Measurements were made following dialysis (MWCO 20 kDa dialysis tubing, Spectrum Laboratories, Rancho Dominguez, CA) of nanoparticle suspensions into water. Data were acquired in the phase analysis light scattering (PALS) mode following solution equilibration at 25 °C. Calculation of ξ from the measured nanoparticle electrophoretic mobility (μ) employed the Smoluchowski equation: μ = εξ/η, where ε and η are the dielectric constant and the absolute viscosity of the medium, respectively. Measurements of ξ were reproducible to within ±5 mV of the mean value given by 20 determinations of 10 data accumulations.
Transmission electron microscopy
The Transmission electron microscopy (TEM) was performed on Pro-bexarotene-NPs and PBNBs to evaluate their morphologies. Imaging was performed on samples prepared on copper grids that were coated with a formvar plastic and then coated with carbon for stability followed by negative staining with Uranyl acetate.
X-Ray diffraction studies
The ordered behavior of Pro-bexarotene-NPs, PBNB and PBNB+US was determined by X-ray diffraction measurement. The aqueous aggregates of each formulation were placed on a precleaned glass plate which, upon air drying, afforded a thin film of the formulations on the glass plate. X-ray diffraction (XRD) of an individual cast film was performed using the reflection method with a Siemens-Bruker D5000 diffractometer. The X-ray beam was generated with a Cu anode, and the Cu Kα beam of wavelength 1.5418 Å was used for the experiments. Scans were performed for 2θ range of 2 to 50.
Molecular dynamic simulations
All simulations were carried out with NAMD 2.9 (http://www.ks.uiuc.edu/Research/namd/2.9/ug/).54,55 To ensure statics, 20 independent simulations were performed. Prodrug insertion into the membrane was observed in all the simulations. 15 simulations were terminated once the insertion was observed and 5 of the simulations were performed up to 400 ns to study prodrug-in-membrane stability. Parameters were generated using the CHARMM general force field (CGenFF)56 for bexarotene. CHARMM (c36) force field57 was applied for the Lyso PC and the membrane POPC lipids. A modified TIP3P water model58 in the CHARMM force field was used. Particle-mesh Ewald (PME)59 were used for long-range electrostatic interactions. The r-RESPA multiple time-step integrator60 was applied with time steps of 2 and 4 fs for short-range non-bonded and long-range electrostatic interactions, respectively. The SETTLE algorithm61 maintained water rigid geometry while RATTLE62 constrained the length of covalent hydrogen bonds. Temperature was set to 300 K for all systems by a Langevin thermostat. All simulations were performed in the NPT ensemble, where pressure was kept constant at 1 atm by the Langevin piston method.63
The membrane insertion potential of mean force (PMF) of bexarotene as a part of the prodrug is compared with bexarotene as an independent molecule. Umbrella sampling simulations have been performed to calculate the free energies. Two bexarotene/Pro-bexarotene molecules were placed in the system, one at the membrane center and one 52 Å away from the membrane center (outside of the membrane), and thus two PMFs were obtained from a single calculation. umbrella simulations along the membrane normal at 1 Å interval were performed for 10 ns each, with the umbrella potentials only on the center of mass of the bexaroten group. The resulted distributions of the bexaroten group were obtained and the PMFs were calculated based on the distributions via the weighted histogram analysis method (WHAM).64 The PMFs from the two molecules, one in the membrane and one outside, were then averaged to obtained the final PMF, while their difference gave the error estimation.
Cell culture
HepG2 cells were cultured with Eagle's Minimum Essential Medium (EMEM; Sigma) with 10% fetal bovine serum (FBS), 1x penstrep in T25/T75 culture flasks (Cellstar®; Germany) and were incubated at 37 °C in a 99% humidified atmosphere containing 5% CO2. Cells were regularly passaged by trypsinization with 0.1% trypsin (EDTA 0.02%, dextrose 0.05%, and trypsin 0.1%) in DPBS (pH 7.4). Non-synchronized cells were used for all the experiments.
In vitro treatments
Our preliminary in vitro results were obtained using PBNB with Pro-baxoretene to investigate the synergistic effects of ultrasound and chemotherapy on HepG2 cells. During the ultrasound experiment the cell plates were filled with PBS (pH 7.4), covered by a MicroAmp optical adhesive film (Applied Biosystems, Foster City, CA) to act as an acoustic window and placed inverted in a tank of degassed water. The optical adhesive film also prevented the solution in the wells from mixing with the water bath. The PBS solution had PBNBs and NBs based on the respective control and treatment configuration. A rectangular flat ultrasonic transducer with center frequency of 2.4 MHz was used to expose the cell at the surface of each well for 2 minutes duration at acoustic power of 10 W and pulse rate of 0.5 Hz.
HepG2 (p53 wild type) human HCC tumor cells were obtained from American Type Culture Collection (Rockville, MD) and cultured in RPMI 1640 containing 10% fetal bovine serum (FBS) and human insulin (0.01 mg/mL) at ambient condition of 5% CO2, 99% humidity and temperature of 37°C. HepG2 cells were cultured in ambient conditions prescribed by ATCC and non-synchronized cells were used for all the experiments. Experimental conditions were optimized to achieve maximum cell growth inhibition. Initially growth inhibitory effects of US exposures were investigated in 96 well plates incubating the cells with 60-1.875 μM of Bexarotene, Pro-bexarotene, PBNB and NB at 48, 72 and 96h time points to find out optimum concentration and IC50. Secondly, PBNB and NB formulations were used for optimization of parameters of US exposures to achieve the maximum as described above. Six well plates were plated with cells and treated with PBNB and NB formulations for 2 min while exposed to US.
In another set of experiments, cells were treated with PBNB at various concentrations ranging from 60-7.5 μM for the time period of 2 min while exposed to US with optimal parameter. At the end of the US exposure concentrations were diluted to 15-1.875, respectively and incubated for 72h. In all the experimental conditions, cells were imaged for investigating growth density and morphology variations followed by MTT assay. The percentage cell viability was obtained from plate reader and was calculated using the formula % Viability = {[A630(treated cells)-(background)]/[A630(untreated cells)-background]}x100.
MTT Assay
The % viability of cells treated with Bexarotene, Pro-bexarotene, PBNB and NB in HepG2 cells was investigated by using 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT). NBs were used as negative control for all the experiments. Primarily experiments were performed for evaluating the IC50,s for various used formulations. Experiment was performed in 96 well plates (CellstarR; Germany) growing 8,000 cells per well 24 h before treatments. Experiments were performed for various concentrations of Bexarotene, Pro-bexarotene and PBNB (60, 30, 15, 7.5, 3.75, 1.87, 0.935 μM) present in free or nanoparticle forms while same volume of NB was used as negative controls. Cells were incubated for 48, 72 and 96h before performing the MTT assay. Secondly, PBNB and NB formulations were used for optimization of parameters of US exposures to achieve the maximum. Six well plates were plated with 0.3×106 cells and grew for 24h before the treatments. Cells were treated with PBNB and NB formulations for 2 min while exposed to US. After incubation period, cells were imaged for investigating growth density and morphology variations. Cells were introduced to MTT and trypan blue assays after 24h. In another set of experiments, cells (0.3×106 cells and grew for 24h before the treatments) were treated with PBNB at various concentrations ranging from 15 to 1.875 μM and respective volume of NB. Cells were incubated for further 72h either after exposure to optimized US parameters or as such. Cells were initially incubated with 4x concentration of formulations during US while incubated for 72h at 1x concentrations. After incubation period, cells were imaged for investigating growth density and morphology variations. Cells were further treated with MTT as 200 μl (5 mg/mL) per well and further incubated for 4.5 h. At the end of the incubation entire medium was removed from wells and 2000 μL DMSO was added to dissolve blue colored formazan crystals. Dissolved crystals were transferred to 96 well plates in 20 wells each. The percentage cell viability was obtained from plate reader and was calculated using the formula % Viability = {[A630(treated cells)- (background)]/[A630(untreated cells)- background]}x100.
Mechanistic studies of RXR modulation and other biological interactions for PBNB and controls
HepG2 cells (1.5 × 105) were cultured in 12 well plates and grown till plating density of ~80% was achieved with EMEM containing 10% FBS. Cells were treated with gradually increasing PBNB concentrations i.e., 6.25, 12.5, 25 and 50 μM along with control formulations of NBs. Cells were exposed to therapeutic US as optimized earlier and was allowed to grow for 72h. At the end of incubation period, cells were washed with 200 μL × 3 DPBS to remove traces of serum for better trypsinization followed by collection in 1 mL of 10% FBS containing DMEM. Cells were pelleted by spinning down at 4000 rpm for 5 min at 4 °C. Cells were intermittently vortexed while fixing with 75% ethanol and stored at −20°C for 12 h. Then cells were spun down at 4000 rpm for 5 min at 4 °C and pellets were washed thoroughly with DPBS. Cells were suspended in 100 μ L of DPBS and then treated with RNase (10 μ g) for 12 h at 37 °C. Cells were stained with 2 μg/mL of propidium iodide (PI) for 30 min at RT in dark before performing the flow assisted cell analysis (FACS) and analyzing the percentage apoptotic cell population.
To further probe cellular apoptosis, DNA fragmentation assay was performed on treated cells. Treatments were implemented as described above only with change in their harvesting protocol. At the end of 72h incubation period, cells were harvested in 1 mL of 10% FBS containing culture medium at the end of the incubation period and washed with 1 mL DPBS for two times. Harvested cells were spun at 1000 rpm for 2 min, to obtain cell pellets, which were incubated with 400 μL of lysis buffer (Thermo Scientific) at 65 °C. Lysed cell suspensions were mixed thoroughly with 400 μL of CHCl3 and spun at 12000 rpm at 4 °C for 5 min after intermittent mixing. The water layer was collected and a solution of 100 μL of NaCl with 800 μL of chilled ethanol was added to it before the mixture was maintained at −20 °C overnight. Suspensions were pelleted down at 12000 rpm at 4 °C for 20 min and washed with 75% ethanol. DNA pellets were air dried before dissolving in TE buffer at pH = 7.4. Extracted genomic DNA was run on 2% agarose gel using 1x TAE buffer at 100 V for 30 min.
Interaction of bexarotene with duplex plasmid DNA and effect of its prodrug and nanobubble form on electrophoretic mobility
Propidium iodide staining and DNA laddering assays established the probable induction of apoptosis via US mediated internalization of PBNB. To negate the chemical degradation of genomic DNA by PBNB and its chemical ingredients, gel electrophoresis was performed on plasmid DNA incubated with various formulations. A pBR322 vector DNA (pDNA; New England Biolabs, Ipswich, MA) was used (200 to 500 ng/cocktail) for preparation of various mixtures containing bexarotene, pro-bexarotene, PBNB and NB. The mixtures were allowed to be incubated at rt for 1 h. All the incubated samples were loaded on a 1% agarose gel along with unbound pDNA and DNA ladder (0.1-10 Kb) and ran at 100 V for 30 min. Finally gel was stained in 10 μg/mL ethidium bromide solution in 1× TAE for 5 min, and washed in 1 × TAE solution for 5 min before being imaged (Universal Hood III, Bio-Rad, Hercules, CA).
RXR agonist assay
Experiment was performed using INDIGO's panel of RXR reporter assay (Cayman chemicals, INDIGO Biosciences) which utilizes non-human mammalian cells engineered to express human retinoic acid receptor RXRα. Cells include the luciferase reporter gene functionally linked to a responsive promoter provides quantification of changes in luciferase expression in treated reporter cells. This quantification provides a sensitive surrogate measure of the changes in RXRα activity. Formulations including bexarotene, pro-bexarotene and PBNB were used to verify their RXR agonist abilities at concentration ranging from 50 μM to 0.5 μM along with positive control 9-cis retinoic acid supplied with assay kit at 5 μM. Cells were treated for 24h before measuring the luciferase activity. Experiment was performed using manufacturer's protocol. Luciferase activities were acquired as luminescence using multiwall plate reader at various time points ranging from 5 to 50 min.
Ex-vivo experiments and optimization of catheter-based ultrasonic exposures
Freshly excised porcine liver tissue samples were obtained immediately post-necropsy from the Meat Science Laboratory at the University of Illinois at Urbana-Champaign. The experiment was conducted within 30 minutes from extracting the tissue from the animal. Following treatment, the tissue was dissected for gross pathology and visual inspection. After dissecting, the tissue was submerged into triphenyltetrazolium chloride (TTC) for viability staining. The TTC made the treated region appear in a different color than the normal tissue for easier visual identification of the thermal treatment region.
The catheter based ultrasound (CBUS) interstitial applicator with two element tubular PZT transducers was used in the experiment (Figure S7). The transducers were mounted on a hollow supporting tube. The PZT transducers were 10 mm long with 1.5 mm outer diameter. The applicator was inserted into the tissue for treatment using a 13g implant catheter. Degased water was circulated through the applicator and the catheter for cooling the transducer during ablation. Degased water was used to minimize the presence of bubbles. Transducers with 360° active zones as shown in Figure S7B and C, were used for the experiments.
Using electrical impedance and radiation force balance measurements, the center frequency and efficiency of each transducer was determined. The center frequency of each individual transducer was used to excite the respective transducer to maximize energy output. Continuous wave operation was used to excite the transducers. Typically transducer center frequency ranged from 6.5 – 7.5 MHz with acoustic efficiency of 50 – 60 %. Here the transducer closer to the tip of the catheter was be referred to as T×1 and the second transducer as T×2 as shown in Figure S7.
A multi-channel generator/amplifier/ controller system (TheraVision™, Acoustic MedSystems Inc, Savoy, IL), with independent RF power and frequency control configured to supply high power to the US applicators was used in the experiment (Figure S7D). Real-time ultrasound (US) imaging was acquired using a SonixTouch (Ultrasonix, Canada) clinical ultrasound imaging system, for injecting the nano-medicine into the excised tissue.
Optimization of ultrasonic exposures
In vitro treatments of PBNB and NB formulations before and after exposure to optimized ultrasonication (PBNB+US and NB+US) were performed for optimization of plated cell density, growth span and US exposure power and time followed by concentration and incubation of formulations. Initially, 6 well plates were plated with 3 × 105 cells for 24h before incubating the cells with various concentrations of PBNB and NBs. During the ultrasound experiment the cell plates were filled with PBS (pH 7.4), covered by a MicroAmp optical adhesive film (Applied Biosystems, Foster City, CA) to act as an acoustic window and placed inverted in a tank of degassed water. The optical adhesive film also prevented the solution in the wells from mixing with the water bath.
US set up and optimization
TheraVision™ Ultrasound Ablation system (Acoustic MedSystems, IL) was used to drive the flat square ultrasonic transducer (Fig. S1). The transducer was held positioned such that the acoustic beam is centered at each well in the 12-well plate. A pulse rate of 0.5 Hz was used for all the experiment (Fig. S1B). The transducer was held stationary and the plate was moved to expose each separate well containing cells for the respective configurations in the plate. The size of the transducer was 18 mm diagonally (Acoustic MedSystems, IL), which was slightly smaller than the diameter of each well (22 mm) such that each well is exposed by the collimated acoustic beam. Degased water at 37 °C was used for all the experiments. The RF generator in the TheraVision™ system uses a sophisticated circuit design that minimizes changes in pressure amplitude and frequency settings. Each well was exposed for 2 minutes. During the ultrasound experiment the plates covered by a MicroAmp optical adhesive film (Applied Biosystems, Foster City, CA) to act as an acoustic window and placed inverted in a tank of degassed water (Fig. S1a). The optical adhesive film prevented the solution in the wells from mixing with the water bath and acted as acoustic window. The plate was inverted so that the PBNB would float and be near the cell surface. Typically the four farthest corner wells in a 12-well plate were used for the ultrasound experiment to avoid any ultrasound exposure to the adjacent wells.
High-intensity ultrasound therapy
The Acoustx™ catheter-based ultrasound interstitial applicator with two element tubular PZT transducers was used in the experiment (Fig. S7) for therapeutic ultrasound delivery. Each transducer was 10 mm long with 1.5 mm outer diameter and mounted on a polyamide support structure. The applicator was inserted into a 13g implant catheter, which was inserted into the target liver tissue. Degased water was circulated through the applicator and the catheter for cooling the transducers during ablation. Use of degased water minimized the presence of bubbles. Transducers with 360° angular active zones as shown in Figure 1b and c, were used for the experiments.
The center frequency and efficiency of each transducer were determined using electrical impedance and radiation force balance measurements. The center frequency of each individual transducer was used to excite the respective element to maximize energy output. Continuous wave operation was used for energy delivery. Typically, transducer center frequency ranged from 6.5 – 7.5 MHz with acoustic efficiency of 50 – 60 %. Here the transducer closer to the tip of the catheter was be referred to as T×1 and the second transducer as T×2 as shown in Figure S7.
A multi-channel generator/amplifier/controller system (TheraVision™, Acoustic MedSystems Inc, Savoy, IL), with independent RF power and frequency control configured to supply high power to the US applicators was used in the experiment (Fig. S7D). Real-time ultrasound (US) imaging was acquired using SonixTouch (Ultrasonix, Richmond, BC, Canada) a clinical imaging system, for injecting the nano-medicine into the tissue.
In vivo experimental setup
The PBNB was injected into the liver tissue under ultrasound image guidance (Figure S8). 1 ml of PBNB solution was injected into the in vivo liver tissue. As soon at the needle was extracted from the tissue, the Acoustx™ catheter and fine needle thermocouples were inserted into the target tissue. The ultrasound experiment started within a few minutes from injecting the PBNB nanomedicine. The release of the PBNB solution from the needle tip was clearly visible in the ultrasound B-mode image (Fig. S8b). Ultrasound imaging helped to confirm the injection site of the nanomedicince into the tissue and facilitation of placement of the ultrasound therapy device.
Immediately post injection of PBNB, the Acoustx applicators and fine needle thermocouples were inserted into the tissue using a custom template (Figure S9). Fine needle thermocouple sensor arrays of type T (Physitemp, NJ) were placed at 5 mm, 10 mm, 15 mm and 20 mm distances radially from the center of the applicator. Each sensor needle was 100±2 mm long and 0.8 mm diameter with 0.1 °C accuracy in temperature measurement. Each fine needle thermocouple included multiple sensors along its length at 0 mm, 10 mm and 20 mm from the tip of the needle. The tip of the fine needle thermocouple was placed at a depth axially aligned with the center of the distal transducer along its axis such that at least one sensor is placed within the treatment region for each tubular transducer along the axial length of the applicator. Custom insertion templates were used to insert the Acoustx™ applicators and fine needle thermocouples for thermometry measurement. The custom templates were used to maintain parallel tracks of therapy applicators and fine thermocouple needles during the experiment at specified predetermined radial distances.
The Acoustx™ ultrasound therapy catheter was inserted into the tissue under 3D tracked ultrasound image guidance (Figure S9A). Ultrasound imaging was acquired using a SonixTouch (Ultrasonix, Richmond, BC, Canada) system, which is a FDA approved clinical ultrasound system with an L14-5/38 GPS probe. The SonixGPS system built into SonixTouch system was used for tracking. An in-house software architecture was developed to communicate with the hardware, ultrasound imaging and tracking system. Specifically the ultrasound imaging and tracking was performed using the PLUS software and Open IGT Link interface in conjunction with AMS custom software. For 3D tracked ultrasound targeting, the EM sensor was inserted into the implant catheter to distal tip, which was then inserted to the target tissue guided by combined ultrasound imaging with GPS tracking. After the implant catheter was positioned at the treatment location, the EM sensor was removed from the implant catheter and the ultrasound therapy applicator inserted to the implant catheter tip. A custom template (Figure S9) was used to position the applicator and thermocouples. The tracked imaging screen view of TheraVision system is shown in Fig. S9b, showing the 3D (top) and 2D B-mode (bottom) view of the ultrasound image guidance.
In vivo experiments
In vivo validation tests were performed in porcine liver tissue using the TheraVision™ Ultrasound Ablation System with Acoustx™ interstitial ultrasound applicators (Acoustic MedSystems (AMS), USA). The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC), University of Illinois, Urbana. Champaign, and satisfied all University and National Institutes of Health (NIH) rules for the humane use of laboratory animals. The animal was anesthetized with an intramuscular or intravenous injection of TKX (a combination of 2.5 ml of xylazine (100 mg/ml) and 2.5 ml of ketamine (100mg/ml) added to a Telazol (50 mg tiletamine and 50 mg zolazepam) vial) and administered at a dosage of 2.2 mg/kg of a 50 mg/ml concentration. Following TKX administration, anesthesia was continued by tracheal intubation and maintained at a surgical plane of anesthesia on isoflurane/oxygen (2-4% V/V/2-4 L/min). A ventral midline laparotomy approach was used to expose and access the liver for treatment. A linear array ultrasound imaging probe (L14-5/38, Ultrasonix, Richmond, BC, Canada) was used to image the targeted tissue and locate the desired treatment region. Using ultrasound imaging together with EM tracking system, PBNB nanoparticles were injected using a 23g syringe and then the catheter was inserted into the liver as per the treatment plan. After treatment, the animals were euthanized per the Guide for the Care and Use of Laboratory Animals and followed approved UI IACUC protocols, using sodium pentobarbitol (Fatal plus 10 cc/100lbs IV) and the tissue was harvested for gross and histopathological analysis.
Differential gene expression studies
Differential gene expression was studied of RXR and other genes that might be involved in drug metabolism, regulation and transport by quantitative RT-PCR. mRNA was isolated from porcine liver tissue using RNeasy Mini Kit (Qiagen) as per manufacturer's protocol. Reverse transcription of mRNA was performed from 1μg RNA in the presence of RNase inhibitor, random hexamer primers (50 ng/μl), deoxynucleotides (dNTPs, 10mM), SuperScript III reverse transcriptase (200 U/μl) and reverse transcriptase buffer in a 20 μl final reaction volume using SuperScript III First-Strand Synthesis System for RT-PCR kit (Invitrogen). Reverse transcription was performed at 50°C for 50 min. Relative quantification of the genes involved in phase-I and Phase-II drug metabolism and transport was performed by using Power SYBR green PCR Master Mix (2X) (Applied Biosystems) in Taqman ABI 7900 Real-Time PCR system (Applied Biosystems). The housekeeping gene GAPDH was used as reference gene for normalization of target genes expressed in porcine liver. The specificity of all primer pairs was checked by melting curves of the amplified products.
Statistical Analysis
Statistical significance of differences between control and samples were evaluated using ONE way or TWO-way ANOVA using GraphPad Prizm 5.0 with Bonferroni posttest analysis as applicable. Results were considered statistically significant when the p value was less than 0.05.
Supplementary Material
Acknowledgement
This work was supported by the University of Illinois at Urbana Champaign, USA and was also partially supported by grants to D.P. from Children's Discovery Institute. We acknowledge Paul Neubauer (AMS) for help with the instrumentation set-up. Authors gratefully acknowledge the associate editor Prof. Warren Chan for his suggestion and comments to improve this manuscript.
Footnotes
Author contributions
S.K.M., G.G., M.R.G., M.Y., E.M.W., C.R.B., K.V.T., P.S.R., E.C.B, K.S., A.K.D. and D.P. designed and executed all the materials physico-chemical, DFT simulation, biological and US experiments presented here. Z.W. and K.S. designed and executed molecular dynamics simulation studies. S.K.M., G.G., P.S.R., and D.P. wrote the manuscript. All the authors participated in reviewing and editing it.
Competing financial interests
S.K.M., M.R.G., M.Y., C.R.B., K.V.T., A.D., K.S. declares no conflict of interest. D.P. and E.C.B. have NIH grants. D.P. is founder of two start-up companies, which did not fund this research. P.S.R. has start-up entity, which did not support this work. G.G., E.M.W. are employees of Acoustic Med System (AMS), which supported the high intensity US-portion of the research. The authors declare no competing financial interests. E.C.B is the CEO of AMS.
Supporting Information
Synthesis of pro-bexarotene, Experimental set up for in vitro US studies, Raman spectroscopic details of NB samples, MTT assay experiments, Bright field images of HepG2 cells, Experimental set-up for ex vivo pig liver tissue, Temperature profile of the three thermocouples, Location of the applicators and the flexible. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2013. CA: A Cancer J. Clinic. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Duvic M, Hymes K, Heald P, Breneman D, Martin AG, Myskowski P, Crowley C, Yocum RC. Bexarotene is Effective and Safe for Treatment of Refractory Advanced-stage Cutaneous T-cell Lymphoma: Multinational Phase II-III Trial Results. J. Clin. Oncol. 2001;19:2456–2471. doi: 10.1200/JCO.2001.19.9.2456. [DOI] [PubMed] [Google Scholar]
- 3.Sherman SI, Gopal J, Haugen BR, Chiu AC, Whaley K, Nowlakha P, Duvic M. Central Hypothyroidism Associated with Retinoid X Receptor-selective Ligands. New Engl. J. Med. 1999;340:1075–1079. doi: 10.1056/NEJM199904083401404. [DOI] [PubMed] [Google Scholar]
- 4.Szanto A, Narkar V, Shen Q, Uray IP, Davies PJA, Nagy L. Retinoid X receptors: X-ploring their (Patho)physiological Functions. Cell Death and Diff. 2004;11:S126–S143. doi: 10.1038/sj.cdd.4401533. [DOI] [PubMed] [Google Scholar]
- 5.Hoe AL, Royle GT, Taylor I. Breast Liver Metastases--incidence, Diagnosis and Outcome . J. Royal Soc. Med. 1991;84:714–716. doi: 10.1177/014107689108401207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okano K, Maeba T, Ishimura K, Karasawa Y, Goda F, Wakabayashi H, Usuki H, Maeta H. Hepatic Resection for Metastatic Tumors from Gastric Cancer. Annal. Surg. 2002;235:86–91. doi: 10.1097/00000658-200201000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and Management of Liver Metastases from Colorectal Cancer. Annal. Surg. 2006;244:254–259. doi: 10.1097/01.sla.0000217629.94941.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kagohashi K, Satoh H, Ishikawa H, Ohtsuka M, Sekizawa K. Liver Metastasis at the Time of Initial Diagnosis of Lung Cancer. Med. Oncol. 2003;20:25–28. doi: 10.1385/MO:20:1:25. [DOI] [PubMed] [Google Scholar]
- 9.Shingleton WB, Sewell PE., Jr. Cryoablation of Renal Tumours in Patients with Solitary Kidneys. B. J. U. Internat. 2003;92:237–239. doi: 10.1046/j.1464-410x.2003.04322.x. [DOI] [PubMed] [Google Scholar]
- 10.Bahn DK, Lee F, Badalament R, Kumar A, Greski J, Chernick M. Targeted Cryoablation of the Prostate: 7-year Outcomes in the Primary Treatment of Prostate Cancer. Urol. 2002;60:3–11. doi: 10.1016/s0090-4295(02)01678-3. [DOI] [PubMed] [Google Scholar]
- 11.Curley SA, Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P, Fiore F, Pignata S, Daniele B, et al. Radiofrequency Ablation of Unresectable Primary and Metastatic Hepatic Malignancies: Results in 123 Patients. Annal. Surg. 1999;230:1–8. doi: 10.1097/00000658-199907000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg SN, Solbiati L, Hahn PF, Cosman E, Conrad JE, Fogle R, Gazelle GS. Large-volume Tissue Ablation with Radio Frequency by Using a Clustered, Internally Cooled Electrode Technique: Laboratory and Clinical Experience in Liver Metastases. Radiol. 1998;209:371–379. doi: 10.1148/radiology.209.2.9807561. [DOI] [PubMed] [Google Scholar]
- 13.Gillams AR. The Use of Radiofrequency in Cancer. British J. Cancer. 2005;92:1825–1829. doi: 10.1038/sj.bjc.6602582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Decadt B, Siriwardena AK. Radiofrequency Ablation of Liver Tumours: Systematic Review. Lancet Oncol. 2004;5:550–560. doi: 10.1016/S1470-2045(04)01567-0. [DOI] [PubMed] [Google Scholar]
- 15.Dong BW, Liang P, Yu XL, Zeng XQ, Wang PJ, Su L, Wang XD, Xin H, Li S. Sonographically Guided Microwave Coagulation Treatment of Liver Cancer: An Experimental and Clinical Study. Ajr. Am. J. Roentgenol. 1998;171:449–454. doi: 10.2214/ajr.171.2.9694473. [DOI] [PubMed] [Google Scholar]
- 16.Liang P, Dong B, Yu X, Yu D, Wang Y, Feng L, Xiao Q. Prognostic Factors for Survival in Patients with Hepatocellular Carcinoma After Percutaneous Microwave Ablation. Radiol. 2005;235:299–307. doi: 10.1148/radiol.2351031944. [DOI] [PubMed] [Google Scholar]
- 17.Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave Ablation: Principles and Applications. Radiol. Soc. N. Am. Inc. 2005;25:S69–83. doi: 10.1148/rg.25si055501. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy JE. High-intensity Focused Ultrasound in the Treatment of Solid Tumours. Nat. Rev. Cancer. 2005;5:321–327. doi: 10.1038/nrc1591. [DOI] [PubMed] [Google Scholar]
- 19.Blana A, Walter B, Rogenhofer S, Wieland WF. High-intensity Focused Ultrasound for the Treatment of Localized Prostate Cancer: 5-year Experience. Urol. 2004;63:297–300. doi: 10.1016/j.urology.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Blana A, Rogenhofer S, Ganzer R, Lunz J-C, Schostak M, Wieland WS, Walter B. Eight Years' Experience with High-intensity Focused Ultrasonography for Treatment of Localized Prostate Cancer. Urol. 2008;72:1329–1333. doi: 10.1016/j.urology.2008.06.062. [DOI] [PubMed] [Google Scholar]
- 21.Schlesinger D, Benedict S, Diederich C, Gedroyc W, Klibanov A, Larner J. MRguided Focused Ultrasound Surgery, Present and Future. Med. Phys. 2013;40:080901. doi: 10.1118/1.4811136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang R, Reilly CR, Rescorla FJ. High-intensity Focused Ultrasound in the Treatment of Experimental Liver Cancer. Arch. Surg. 1991;126:1002–1009. doi: 10.1001/archsurg.1991.01410320088012. [DOI] [PubMed] [Google Scholar]
- 23.Yang R, Reilly CR, Rescorla FJ, Faught PR, Sanghvi NT, Fry FJ, Franklin TD, Jr., Lumeng L, Grosfeld JL. Extracorporeal Liver Ablation Using Sonography-Guided High-intensity Focused Ultrasound. Investig. Radiol. 1992;27:796–803. doi: 10.1097/00004424-199210000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Madersbacher S, Marberger M. What is the Newest Technology in Treating Prostate Cancer? Transrectal High-intensity Focused Ultrasound. In: Mydlo JH, Godec CJ, editors. Academic Press; Oxford: 2003. pp. 529–534. [Google Scholar]
- 25.Ter Haar G, Rivens I, Chen L, Riddler S. High Intensity Focused Ultrasound for the Treatment of Rat Tumours. Phys. Med. Biol. 1991;36:1495–1501. doi: 10.1088/0031-9155/36/11/009. [DOI] [PubMed] [Google Scholar]
- 26.Prat F, Centarti M, Sibille A, Fadil FAE, Henry L, Chapelon JY, Cathignol DD. Extracorporeal High-intensity Focused Ultrasound for VX2 Liver Tumors in the Rabbit. Hepatol. 1995;21:832–836. [PubMed] [Google Scholar]
- 27.Prat F, Lafon C, de Lima DM, Theilliere Y, Fritsch J, Pelletier G, Buffet C, Cathignol D. Endoscopic Treatment of Cholangiocarcinoma and Carcinoma of the Duodenal Papilla by Intraductal High-intensity US: Results of a Pilot Study. Gastrointest. Endosc. 2002;56:909–915. doi: 10.1067/mge.2002.129872. [DOI] [PubMed] [Google Scholar]
- 28.Klingler C, Margreiter M, Marberger M. New Ablative Treatments for Small Renal masses: HIFU Ablation. Arch. Espanol. de Urol. 2013;66:79–89. [PubMed] [Google Scholar]
- 29.Hynynen K, Jolesz FA. Demonstration of Potential Noninvasive Ultrasound Brain Therapy Through an Intact Skull. Ultrasound Med. Biol. 1998;24:275–283. doi: 10.1016/s0301-5629(97)00269-x. [DOI] [PubMed] [Google Scholar]
- 30.Jeanmonod D, Werner B, Morel A, Michels L, Zadicario E, Schiff G, Martin E. Transcranial Magnetic Resonance Imaging-guided Focused Ultrasound: Noninvasive Central Lateral Thalamotomy for Chronic Neuropathic Pain. Neurosurg. Focus. 2012;32:E1. doi: 10.3171/2011.10.FOCUS11248. [DOI] [PubMed] [Google Scholar]
- 31.Aptel F, Charrel T, Lafon C, Romano F, Chapelon J-Y, Blumen-Ohana E, Nordmann J-P, Denis P. Miniaturized High-intensity Focused Ultrasound Device in Patients with Glaucoma: A Clinical Pilot Study. Investig. Ophthalmol. Vis. Sci. 2011;52:8747–8753. doi: 10.1167/iovs.11-8137. [DOI] [PubMed] [Google Scholar]
- 32.Charrel T, Aptel F, Birer A, Chavrier F, Romano F, Chapelon JY, Denis P, Lafon C. Development of a Miniaturized HIFU Device for Glaucoma Treatment with Conformal Coagulation of the Ciliary Bodies. Ultrasound Med. Biol. 2011;37:742–754. doi: 10.1016/j.ultrasmedbio.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 33.Matysiak J. Evaluation of Electronic, Lipophilic and Membrane Affinity Effects on Antiproliferative Activity of 5-substituted-2-(2,4-dihydroxyphenyl)-1,3,4-thiadiazoles Against Various Human Cancer Cells. Eur. J. Med. Chem. 2007;42:940–947. doi: 10.1016/j.ejmech.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 34.Schon U, Antel J, Bruckner R, Messinger J, Franke R, Gruska A. Synthesis, Pharmacological Characterization, and Quantitative Structure-activity Relationship Analyses of 3,7,9,9-tetraalkylbispidines: Derivatives with Specific Bradycardic Activity. J. Med. Chem. 1998;41:318–331. doi: 10.1021/jm970120q. [DOI] [PubMed] [Google Scholar]
- 35.Banavath HN, Sharma OP, Kumar MS, Baskaran R. Identification of Novel Tyrosine Kinase Inhibitors for Drug Resistant T315I Mutant BCR-ABL: A Virtual Screening and Molecular Dynamics Simulations Study. Sci. Rep. 2014;4:6948. doi: 10.1038/srep06948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raya A, Barrientos-Salcedo C, Rubio-Póo C, Soriano-Correa C. Electronic Structure Evaluation Through Quantum Chemical Descriptors of 17β-aminoestrogens with an Anticoagulant Effect. Eur. J. Med. Chem. 2011;46:2463–2468. doi: 10.1016/j.ejmech.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 37.Zheng Y, Zheng M, Ling X, Liu Y, Xue Y, An L, Gu N, Ji M. Design, Synthesis, Quantum Chemical Studies and Biological Activity Evaluation of Pyrazole– benzimidazole Derivatives as Potent Aurora A/B Kinase Inhibitors. Bioorg. Med. Chem. Lett. 2013;23:3523–3530. doi: 10.1016/j.bmcl.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 38.Magoulas GE, Bariamis SE, Athanassopoulos CM, Haskopoulos A, Dedes PG, Krokidis MG, Karamanos NK, Kletsas D, Papaioannou D, Maroulis G. Syntheses, Antiproliferative Activity and Theoretical Characterization of Acitretin-type Retinoids with Changes in the Lipophilic Part. Eur. J. Med. Chem. 2011;46:721–737. doi: 10.1016/j.ejmech.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Bhattacharjee AK, Karle JM. Functional Correlation of Molecular Electronic Properties with Potency of Synthetic Carbinolamines Antimalarial Agents. Bioorg. Med. Chem. 1998;6:1927–1933. doi: 10.1016/s0968-0896(98)00146-1. [DOI] [PubMed] [Google Scholar]
- 40.Kadam RU, Chavan A, Roy N. Pharmacophoric Features of Pseudomonas aeruginosa Deacetylase LpxC inhibitors: An Electronic and Structural Analysis. Bioorg. Med. Chem. Lett. 2007;17:861–868. doi: 10.1016/j.bmcl.2006.11.069. [DOI] [PubMed] [Google Scholar]
- 41.Jentzsch AV, Hennig A, Mareda J, Matile S. Synthetic Ion Transporters that Work with Anion-π Interactions, Halogen Bonds, and Anion-macrodipole Interactions. Acc. Chem. Res. 2013;46:2791–2800. doi: 10.1021/ar400014r. [DOI] [PubMed] [Google Scholar]
- 42.Hansch C, Steinmetz WE, Leo AJ, Mekapati SB, Kurup A, Hoekman D. On the Role of Polarizability in Chemical-biological Interactions. J. Chem. Inf. Comput. Sci. 2003;43:120–125. doi: 10.1021/ci020378b. [DOI] [PubMed] [Google Scholar]
- 43.Karelson M, Lobanov VS. Quantum-chemical Descriptors in QSAR/QSPR Studies. Chem. Rev. 1996;96:1027–1043. doi: 10.1021/cr950202r. [DOI] [PubMed] [Google Scholar]
- 44.Misra SK, Naz S, Kondaiah P, Bhattacharya S. A Cationic Cholesterol Based Nanocarrier for the Delivery of p53-EGFP-C3 Plasmid to Cancer Cells. Biomaterials. 2013;35:1334–1346. doi: 10.1016/j.biomaterials.2013.10.062. [DOI] [PubMed] [Google Scholar]
- 45.Yin T, Wang P, Zheng R, Zheng B, Cheng D, Zhang X, Shuai X. Nanobubbles for Enhanced Ultrasound Imaging of Tumors. Int. J. Nanomed. 2012;7:895–904. doi: 10.2147/IJN.S28830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacCallum JL, Bennett WF, Tieleman DP. Distribution of Amino Acids in a Lipid Bilayer from Computer Simulations. Biophys. J. 2008;94:3393–3404. doi: 10.1529/biophysj.107.112805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghoshal G, Heffter T, Williams E, Bromfield C, Salgaonkar V, Rund L, Ehrhardt JM, Diederich CJ, Burdette EC. In situ Treatment of Liver Using Catheter Based Therapeutic Ultrasound with Combined Imaging and GPS Tracking. 2013:SPIE:BIOS 85840T. [Google Scholar]
- 48.Yarmolenko PS, Moon EJ, Landon C, Manzoor A, Hochman DW, Viglianti BL, Dewhirst MW. Thresholds for Thermal Damage to Normal Tissues: An Update. Int. J. Hyperth. 2011;27:320–343. doi: 10.3109/02656736.2010.534527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schook LB, Collares TV, Hu W, Liang Y, Rodrigues FM, Rund LA, Schachtschneider KM, Seixas FK, Singh K, Wells KD, et al. A Genetic Porcine Model of Cancer. PLoS One. 2015;10:e0128864. doi: 10.1371/journal.pone.0128864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rieke V, Pauly KB. M R Thermometry. J. Mag. Reson. Imag. 2008;27:376–390. doi: 10.1002/jmri.21265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lepetit-Coiffé M, Laumonier H, Seror O, Quesson B, Sesay M-B, Moonen CTW, Grenier N, Trillaud H. Real-time Monitoring of Radiofrequency Ablation of Liver Tumors Using Thermal-dose Calculation by MR Temperature Imaging: Initial Results in Nine Patients, Including Follow-up. Eur. Radiol. 2010;20:193–201. doi: 10.1007/s00330-009-1532-1. [DOI] [PubMed] [Google Scholar]
- 52.Rempp H, Hoffmann R, Roland J, Pereira P, Schick F, Claussen C, Clasen S, Tübingen DE, Erlangen DE, Heilbronn DE. MR Temperature Mapping at the Radiofrequency Ablation of Liver Tumors: Correlation Between Temperature Data and the Ablation Zone. 2013 [Google Scholar]
- 53.He X, McGee S, Coad JE, Schmidlin F, Iaizzo PA, Swanlund DJ, Kluge S, Rudie E, Bischof JC. Investigation of the Thermal and Tissue Injury Behaviour in Microwave Thermal Therapy Using a Porcine Kidney Model. Int. J. Hyperthermia. 2004;20:567–593. doi: 10.1080/0265673042000209770. [DOI] [PubMed] [Google Scholar]
- 54.Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kalé L, Schulten K. Scalable Molecular Dynamics with NAMD. J. Comput. Chem. 2005;26:1781–180. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feller SE, Zhang YH, Pastor RW, Brooks BR. Constant Pressure Molecular Dynamics Simulation: the Langevin Piston Method. J. Chem. Phys. 1995;103:4613–4621. [Google Scholar]
- 56.Vanommeslaeghe K, Hatcher E, Acharya C, Kundu S, Zhong S, Shim J, Darian E, Guvench O, Lopes P, Vorobyov I, et al. CHARMM General Force Field: A Force Field for Drug-like Molecules Compatible with the CHARMM all-atom Additive Biological Force Fields. J. Comput. Chem. 2010;31:671–690. doi: 10.1002/jcc.21367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klauda JB, Venable RM, Freites JA, O'Connor JW, Tobias DJ, Mondragon- Ramirez C, Vorobyov I, MacKerell AD, Jr., Pastor RW. Update of the CHARMM all-atom Additive Force Field for Lipids: Validation on Six Lipid Types. J. Phys. Chem. B. 2010;114:7830–7843. doi: 10.1021/jp101759q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.MacKerell Jr. A. D., Bashford D, Bellott M, Dunbrack RL, Jr., Evanseck JD, Field MJ, Fischer S, Gao J, Guo H, Ha S, et al. All-atom Empirical Potential for Molecular Modeling and Dynamics Studies of Proteins. J. Phys. Chem. B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- 59.Darden T, York D, Pedersen LG. Particle Mesh Ewald: An N-log(N) Method for Ewald Sums in Large systems. J. Chem. Phys. 1993;98:10089–10092. [Google Scholar]
- 60.Tuckerman M, Berne BJ, Martyna GJ. Reversible Multiple Time Scale Molecular Dynamics. J. Chem. Phys. 1992;97:1990–2001. [Google Scholar]
- 61.Miyamoto S, Kollman PA. Settle: An Analytical Version of the SHAKE and RATTLE Algorithm for Rigid Water Models. J. Comput. Chem. 1992;13:952–962. [Google Scholar]
- 62.Andersen HC. Rattle: A “Velocity” Version of the Shake Algorithm for Molecular Dynamics Calculations. J. Chem. Phys. 1983;52:24–34. [Google Scholar]
- 63.Feller SE, Zhang Y, Pastor RW, Brooks BR. Constant Pressure Molecular Dynamics Simulation: the Langevin Piston Method. J. Chem. Phys. 1995;103:4613–4621. [Google Scholar]
- 64.Grossfield Alan. WHAM: the Weighted Histogram Analysis Method. Version 2.0.9. http://membrane.urmc.rochester.edu/content/wham.
- 65.Kim BYS, Rutka JT, Chan WCW. Nanomedicine. N. Engl. J. Med. 2010;363:2434–2444. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- 66.Hauck TS, Giri S, Gao Y, Chan WCW. Nanotechnology Diagnostics for Infectious Diseases Prevalent in Developing Countries. Adv. Drug Del. Rev. 2010;62:438–448. doi: 10.1016/j.addr.2009.11.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.