Summary
Herpesviruses encode miRNAs that target both virus and host genes; however their role in herpesvirus biology is poorly understood. We previously identified eight miRNAs encoded by OvHV-2; the causative agent of malignant catarrhal fever (MCF) and have now investigated the role of these miRNAs in regulating expression of OvHV-2 genes that play important roles in virus biology. ORF 20 (cell cycle inhibition), ORF 50 (reactivation) and ORF 73 (latency maintenance) each contain predicted targets for several OvHV-2 miRNAs. Co-transfection of miRNA mimics with luciferase reporter constructs containing the predicted targets showed the 5’ UTRs of ORF 20 and ORF 73 contain functional targets for ovhv-miR-2 and ovhv2-miR-8 respectively, and the 3’UTR of ORF 50 contains a functional target for ovhv2-miR-5. Transfection of BJ1035 cells (an OvHV-2 infected bovine T cell line) with the relevant miRNA mimic resulted in a significant decrease in ORF 50 and a smaller but non-significant decrease in ORF 20. However, we were unable to demonstrate a decrease in ORF 73. MCF is a disease of dysregulated lymphocyte proliferation, miRNA inhibition of ORF 20 expression may play a role in this aberrant lymphocyte proliferation. The proteins encoded by ORFs 50 and 73 play opposing roles in latency, it has been hypothesized that miRNA-induced inhibition of virus genes acts to ensure that fluctuations in virus mRNA levels do not result in reactivation in conditions that are unfavourable for viral replication, our data would support this hypothesis.
Introduction
Malignant catarrhal fever (MCF) is a fatal disease of cattle and other ruminants caused by viruses in the genus Macavirus of the subfamily Gammaherpesvirinae (Russell et al., 2009). The disease occurs as a result of infection of susceptible hosts by contact with an asymptomatic carrier species that acts as a virus reservoir. Ovine herpesvirus-2 (OvHV-2) sub-clinically infects most sheep and is the major cause of MCF worldwide (Russell et al., 2009). In both sheep and cattle, OvHV-2 infects CD2+ T lymphocytes (Meier-Trummer et al., 2010; Schock et al., 1998) but only in cattle does virus infection cause dysregulation of lymphoid cell function leading to uncontrolled proliferation, cytotoxicity and MCF disease. The proliferation of infected bovine T cells is dependent on the cytokine interleukin-2 (IL-2) and immortalized T cell lines can be cultured from affected cattle. The infected bovine T cells do not support productive virus replication and have been described as large granular lymphocytes (LGLs) (Reid et al., 1989) in part due to expression of perforin (Nelson et al., 2010); and unlike sheep, infected cattle cannot transmit the virus to other susceptible hosts (Russell et al., 2009). The mechanism by which OvHV-2 induces MCF in cattle is unknown; virus-induced cytopathology is thought not to be involved in lesion development and it has been proposed that tissue damage arises from non-antigen specific, MHC unrestricted cytotoxicity of the virus-infected LGLs (Cook & Splitter, 1988).
MicroRNAs (miRNAs) are short (21-23nt) RNAs that act as post-transcriptional inhibitors of gene expression. Cellular miRNAs expressed in the nucleus are derived from primary transcripts (pri-miRNAs) that are processed by the enzyme Drosha to form a shorter precursor miRNA (pre-miRNA). These pre-miRNAs are exported from the nucleus and once in the cytoplasm are further cleaved by the enzyme Dicer to produce a transient double-stranded precursor; where one strand is designated the miRNA or guide strand and the complementary strand is designated the miRNA* or passenger strand. The miRNA is stably incorporated into the RNA-induced silencing complex and guides it to the target mRNA, which represses translation by a number of mechanisms including mRNA degradation and inhibition of translation. The interaction of miRNAs with target mRNAs is mediated by a seed region, nucleotides 2-7 or 2-8 at the 5’end of the miRNA (Bartel, 2009)Most miRNA targets identified to date are present in the 3’UTR of mRNAs, however some miRNAs have been reported that functionally target the 5’UTR (Grey et al., 2010; Tay et al., 2009).
To date, over 250 virus-encoded miRNAs have been identified, the majority from herpesviruses (Grundhoff & Sullivan, 2011). Herpesvirus-encoded miRNAs have been shown to regulate both cellular and viral gene expression and to influence cell processes including proliferation. In Marek’s disease virus (MDV), the deletion of a single virus-encoded miRNA abrogates virus-induced cellular transformation (Zhao et al., 2011) and in Epstein Barr virus (EBV) a cluster of miRNAs has been implicated in controlling virus-induced B cell proliferation and transformation (Feederle et al., 2011a; Feederle et al., 2011b; Seto et al., 2010). We have previously demonstrated that OvHV-2 encodes at least eight miRNAs (Levy et al., 2012) expressed within the immortalized bovine LGL line, BJ1035; and hypothesize that these play a critical role in MCF pathogenesis. In order to investigate the role of virus-encoded miRNAs in OvHV-2 pathogenesis it is necessary to identify their viral and cellular targets. In this study we investigated the role of OvHV-2 encoded miRNAs in regulating selected virus gene expression. There is no in vitro infection system for studying OvHV-2; the only cells in which virus gene expression can be studied are immortalized LGL lines like BJ1035 (Levy et al., 2012). All cells in these LGL lines are virus genome positive and in the majority OvHV-2 is latent; however, some lytic cycle gene expression occurs in a small proportion of the cells (Rosbottom et al., 2002; Thonur et al., 2006).
Results
Prediction of OvHV-2 encoded miRNA targets in the OvHV-2 genome
Potential miRNA targets within the OvHV-2 genome were initially identified by scanning the entire OvHV-2 genome using BLASTN (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to align the sequences complementary to the ovhv2-miRs in the OvHV-2 genome (GenBank, AY839756) (Hart et al., 2007). Targets were then mapped to the 5’UTR or 3’UTR of OvHV2 genes. Only a small number of OvHV-2 mRNAs have been mapped previously therefore for the majority of genes the 5′UTRs were considered to span the region from the start codon to the predicted TATA box. For 3’UTRs the region from the stop codon to the predicted polyadenylation site was used. This analysis identified potential targets in 33 OvHV-2 genes, representing all classes of virus gene; immediate early, early and late, and both structural and non-structural proteins.
For validation of predicted targets we focussed on genes/proteins predicted to play important roles in virus biology and pathogenesis. We chose to analyse targets present in the 5’ or 3’UTR of three virus genes, ORF 20, ORF 50 and ORF 73. ORF 20 has been shown to induce cell cycle arrest in other herpesviruses (Nascimento et al., 2009), and the other two genes encode proteins that play contrary roles in virus latency. ORF 50 is crucial for virus reactivation from latency and ORF 73 is important for the maintenance of latency (Ackermann, 2006). The positions of the predicted miRNA target sites in ORF 20, ORF 50 and ORF 73 are detailed in Table 1.
Table 1.
| Gene | 5’UTR | 3’ UTR | 5’ UTR targets | 3’ UTR targets |
|---|---|---|---|---|
| ORF 20 | 41641-41538 | ovhv2-miR-2 : 41611-7 | ||
| ORF 50 | 79043-79196 | ovhv2-miR-5 : 79161-7 | ||
| ORF 73 | 121049-120533 | ovhv2-miR-8 : 120824-18 : 120840-34 |
Nucleotide numbers are from (GenBank, AY839756) (Hart et al., 2007).
miRNAs function by targeting expressed mRNAs, ORF 20 and 50 are only predicted open reading frames from the genomic sequence; and the ORF 73 transcript is only partially mapped (Coulter & Reid). In this study we confirmed the existence of these three virus ORFs as transcripts, including the miRNA target sites, using an RT-PCR strategy.
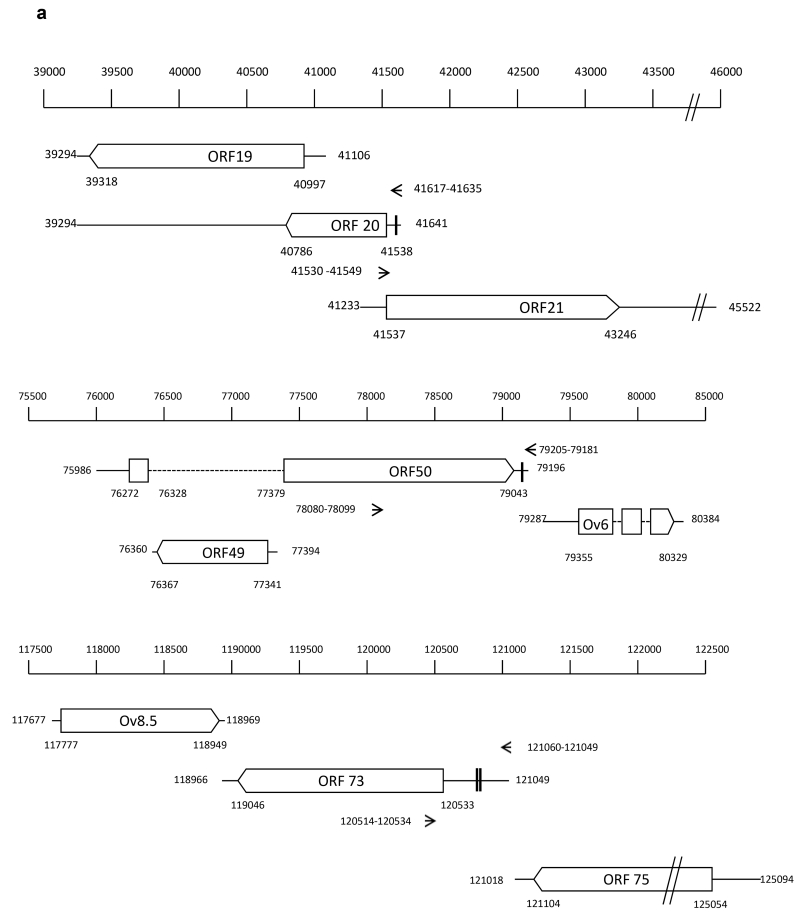
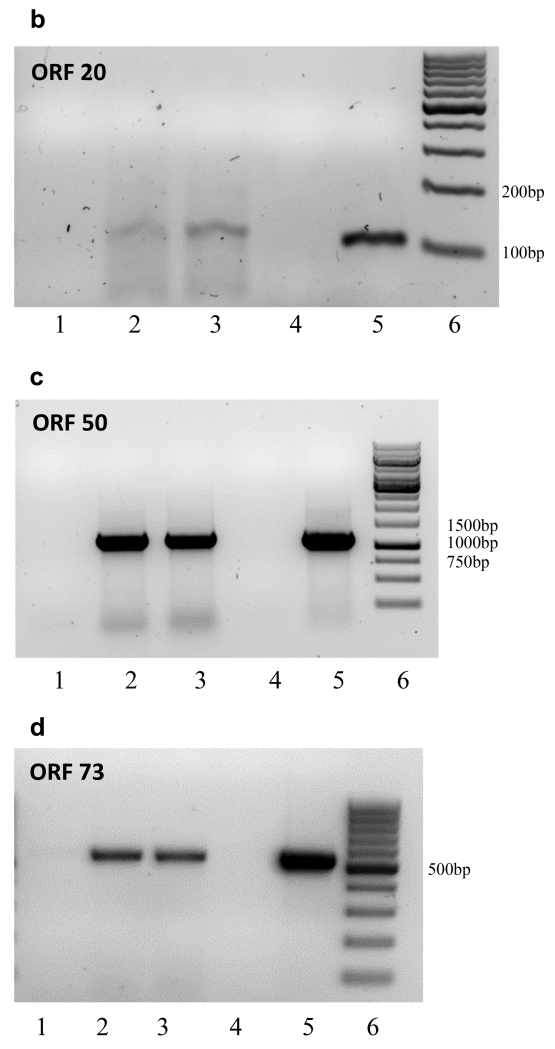
The annotated OvHV-2 genome predicts that ORF 20 overlaps with both ORF 19 and ORF 21 (Fig. 1A). To allow detection of ORF 20 only, cDNA synthesis was primed using a gene-specific primer located 406 bp upstream (42530-41549) of the ORF 19 TATA box (41106). This primer will not prime ORF 21 mRNA as it is the same sense as the ORF 21 transcript. Fig. 1B shows the 118 bp amplicon, the sequence of which was 100% identical to the predicted ORF 20 sequence (39294 – 41641) and includes the target sequence (41617 – 41635) for ovhv-2-miR-2 (Fig. 1B).
Fig. 1.
A. Genomic location of ORF 20 and ORF 50 relative to adjacent genes.
Genes are indicated by open boxes, arrow head represents direction of transcription. Dotted lines represent introns. The nucleotide positions representing the location of the predicted TATA box and poly A site for each gene are indicated. The position of primers used for PCR and cDNA priming (ORF 20) are indicated by arrows and nucleotide position. Predicted miRNA binding sequences are indicated by vertical bars in the respective UTRs.
B. ORF 20 Lane 1, No RT; Lane 2, cDNA primed with 35 pm primer (250 ng); Lane 3, cDNA primed with 10 pm primer (66ng); Lane 4,: No template control; Lane 5, DNA positive control. Lane 6; Marker, Generuler 100bp
C. ORF 50 Lane 1, No RT, Lane 2, cDNA primed with Oligo dT; Lane 3, cDNA primed with random primers; Lane 4, No template control; Lane 5, DNA positive control; Lane 6, Marker, Generuler 1kb
D. ORF73 Lane 1, No RT, Lane 2, cDNA primed with Oligo dT; Lane 3, cDNA primed with random primers; Lane 4, No template control; Lane 5, DNA positive control; Lane 6, Marker, Generuler 100bp
The ORF 50 and Ov6 transcripts are transcribed in the same direction, with the Ov6 TATA box lying 94 bp downstream of the ORF 50 poly A site. ORF 50 is spliced and ORF 49 lies within the ORF 50 intronic sequence transcribed in the opposite direction (Fig. 1A). We were unable to prime cDNA synthesis efficiently using an ORF 50 gene specific primer due to the limited number of nucleotides between the predicted target sequence and the poly A site, we therefore used both oligo dT and random primers to prime cDNA synthesis. PCR primers (78080-78099 and 79205-79181) were designed to generate an 1101bp amplicon, from the middle of the second exon of ORF 50 to the poly A site. The 3’ primer (79025 - 79181) is approximately 1900 bp and 82 bp upstream of ORF 49 and Ov6 TATA boxes respectively; and the 5’ primer (78080 - 78099) is 118 bp upstream of the Ov6 TATA box and 2304 bp upstream of the Ov6 polyA site. Fig. 1C shows the 1101 bp amplicon the sequence of which was 100% identical to the predicted ORF 50 and included the target sequence (79205 - 79181) for ovhv-2-miR-2.
ORF 73 lies downstream of the ORF 75 transcript and is transcribed in the same direction (Fig. 1A). The annotated OvHV-2 genome (GenBank, AY839756) (Hart et al., 2007) states the 5’ terminus of the ORF 73 transcript at nt 121049, 31 bp upstream of the predicted poly A site for ORF 75. We used both oligo dT and random primers to prime cDNA synthesis. PCR primers (120514-120534 and 121060-121041) generates a 536 bp amplicon from within the coding sequence of ORF 73 to the predicted end of ORF 73 mRNA. Fig. 1D shows this 536 bp amplicon, the sequence of which was 100% identical to the predicted ORF 73 and includes the target sequence (120817-120839) for ovhv-2-miR-8.
Inhibition of gene expression
BJ1035 is a mixed population of cells with respect to virus life cycle; the majority are latently infected but a small proportion express early and late virus genes (Rosbottom et al., 2002; Thonur et al., 2006). OvHV-2 miRNAs may be also differentially expressed in the proportion of cells in culture in which the virus is latent compared to those cells where the virus is reactivating making analysis of inhibition of gene expression following introduction of exogenous miRNAs complex. We therefore initially assessed the ability of the ovhv2-miRNAs to interact with their predicted targets using a luciferase expression assay.
We have previously shown that insertion of a predicted 5’UTR downstream of an exogenous promoter, e.g CMV IE, can result in the expression of transcripts in which the start site does not reflect that of the native transcript (Grey et al., 2010). In keeping with our previous studies, when analysing targets in 5’UTRs we cloned a region approximately 1000bp upstream of the AUG in an attempt to allow correct expression from the natural promoter. For all experiments a luciferase expressing vector lacking OvHV-2 sequnces was used to investigate off- target effects: no significant reduction in luciferase expression was seen using any of the ovhv-2-miRs.
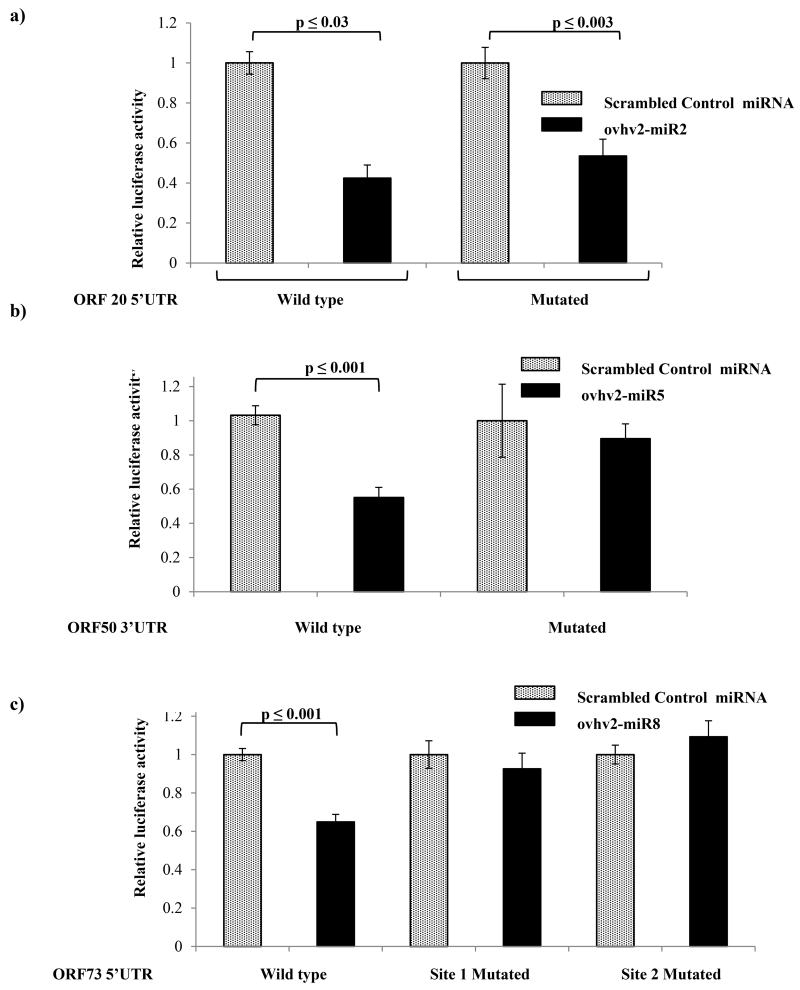
The 5’UTR of ORF 20 contained a predicted target site for ovhv2-miR-2, (Table 1;Fig. 3). BHK-21 cells were co-transfected with reporter constructs and miRNA mimics as described and luciferase expression levels were measured. The combination of the ORF 20 5’UTR reporter and ovhv2-miR-2 resulted in a 57.5 (± 10) % reduction (p ≤ 0.03) in luciferase expression using 100 nM mimics (Fig. 2a) compared to control miRNA. The same degree of inhibition was observed using 50nM mimics (data not shown). Ovhv2-miR-2 inhibited luciferase expression by 47.5(±15) % (p ≤ 0.003) even after the mutation of the predicted target site in the 5’UTR (Fig. 2A), suggesting that inhibition was not due to interaction of ovhv2-miR-2 with the predicted target sequence. The 3’UTR of ORF 50 was predicted to contain a target site for ovhv2-miR-5 (Table 1, Fig. 3) the combination of ORF 50 3’UTR and ovhv2-miR-5 mimic resulted in a 45 (±10) % reduction (p ≤ 0.001) in luciferase expression (Fig. 2B) compared to control miRNA. Mutation of the target site from the 3’UTR abrogated this inhibition.
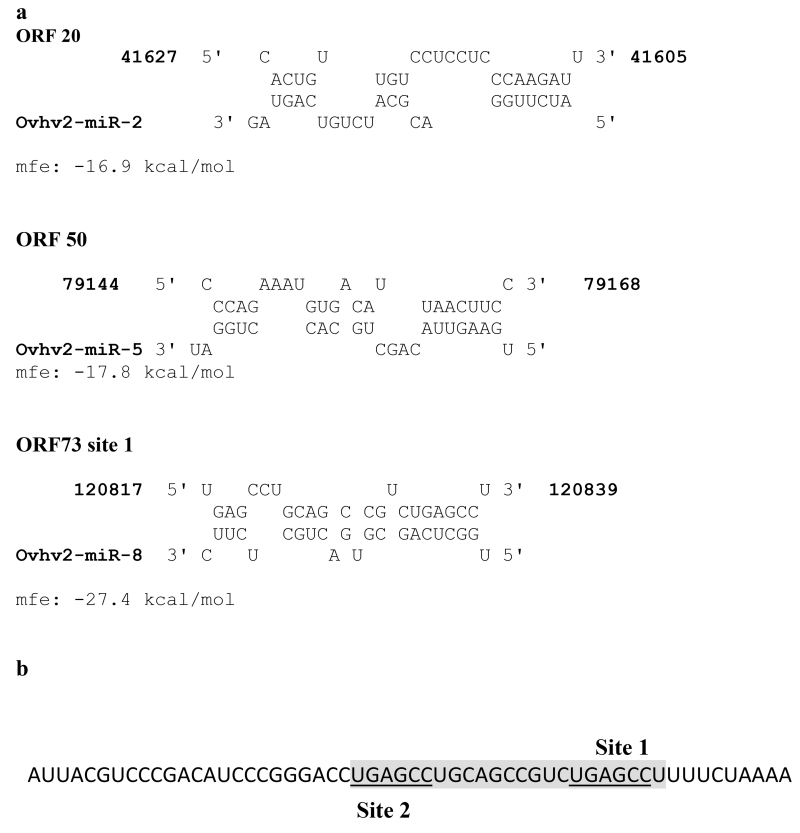
Fig. 3.
A) RNAhybrid analysis of the predicted target sites with the relevant miRNA.
mfe = mean free energy.
B) Sequence of ORF 73 5’UTR surrounding predicted sites 1 and 2 (underlined) the shaded box represents the sequence shown to interact with ovhv2-miR-8 in A.
Fig. 2.
BHK cells (n=6) were co-transfected with: a) PGL-ORF 20, which has the 5’UTR of ORF 20 upstream of firefly luciferase (or with a PGL-ORF 20 in which the predicted ovhv2-miR-2 target site had been mutated), pRL (which expresses renilla luciferase) and either an ovhv2-miR-2 mimic or a scrambled miRNA control: b) psi-ORF 50, which has the 3’UTR of ORF 50 downstream of renilla luciferase (or with a psi-ORF 50 in which the predicted ovhv2-miR-5 target site had been mutated), and either an ovhv2-miR-2 mimic or a scrambled miRNA control: c) PGL-ORF 73, which has the 5’UTR of ORF 73 upstream of firefly luciferase (or with a PGL-ORF 73 in which the predicted ovhv2-miR-8 target sites had been individually mutated), pRL (which expresses renilla luciferase) and either an ovhv2-miR-8 mimic or a scrambled miRNA control 24 h post-transfection firefly luciferase levels were measured, normalised to the renilla luciferase levels and expression in the control and test miRNA samples compared.
The 5’UTR of ORF 73 was predicted to contain two separate sites for ovhv2-miR-8 (Table 1, Fig. 3). The combination of 5’UTR of ORF 73 and ovhv2-miR-8 mimic resulted in a 45(± 8) % decrease (p ≤ 0.001) in luciferase expression (Fig 2C). Mutation of either of the predicted ovhv2-miR-8 target sites abolished the inhibitory effect of ovhv2-miR-8.
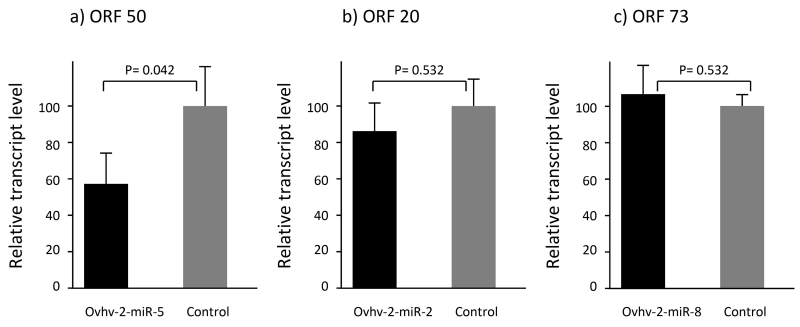
To further investigate inhibition of the expression of these OvHV-2 genes by the ovhv2-miRs we developed RT-qPCR assays for ORF 20, 50 and 73 transcripts. BJ1035 cells were transfected with the relevant ovhv2-miR mimics or control miRNA for 24 or 48h at which point cells were harvested, RNA isolated and qPCR carried out. We were able to demonstrate a 42(±15) % reduction in levels of ORF 50 transcript following transfection with ovhv2-miR-5 (Fig. 4a, P=0.042). Although there appeared to be a 12.8 (±13) % reduction in levels of ORF 20 transcript following transfection with ovhv2-miR-2 (Fig. 4b), this was not significant (P=0.532). Furthermore, we were unable to demonstrate any reduction in levels of ORF 73 transcript following transfection with ovhv2-miR-8 (+ 7.3(±13.9) %) (Fig. 4c, P=0.532).
Fig. 4.
BJ1035 cells (n=3) were transfected with control miRNA mimic or; A) ovhv2-miR-5 mimic, B) ovhv2-miR-2 mimic, D) ovhv2-miR-8 mimic. Twenty four hrhr post infection RNA was extracted and transcript levels of a) ORF 50, b) ORF 20 and c) ORF 73 analysed by RT-qPCR (3 technical replicates per biological replicate). Levels were compared to levels in control transfected cells; error bars represent the standard error of the mean levels as produced by the linear mixed-effect statistical models, taking into account the technical and biological replicates.
Discussion
OvHV-2 induced malignant catarrhal fever in susceptible hosts is a disease of dysregulated lymphocyte proliferation and cell function. However, these susceptible hosts cannot transmit the virus. In contrast, infection of carrier hosts is asymptomatic, but these are infectious. A previous study has shown that this virus expresses at least eight miRNAs (Levy et al., 2012). In this study we investigated the control of expression of three virus genes important for control of cell cycle (ORF 20) and virus latency (ORF 50 and ORF 73) by virus-encoded miRNAs.
The 3’ UTR of ORF 50 was predicted to be targeted by ovhv2-miR-5. This interaction was confirmed by both the luciferase assay and by inhibition of ORF 50 miRNA levels in BJ1035 cells transfected with ovhv2-miR-5. Thus we have demonstrated that expression of ORF 50, whose main role is to drive reactivation from latency, can be inhibited by a viral encoded miRNA.
The 5’UTR of ORF 20 was predicted to contain one site recognised by ovhv2-miR-2. Ovhv2-miR-2 did inhibit luciferase expression; however mutation of the predicted site did not abolish this inhibition. Thus, whilst we have demonstrated that ovhv2-miR-2 inhibits expression of a transcript containing the 5’UTR of ORF 20, we cannot definitively show that this inhibition is a consequence of its interaction with the target site predicted in the original analysis. We also investigated the ability of ovhv2-miR-2 to inhibit ORF 20 expression in BJ1035 cells. We were able to demonstrate a small reduction in ORF 20 mRNA levels in BJ1035 cells transfected with this ovhv2-miR-2; however was not statistically significant.
ORF 73 was predicted to contain two sites recognised by the ovhv2-miR-8 seed sequence located nine bases apart (Fig. 3). ovhv2-miR-8 was able to inhibit gene expression in the luciferase assay and mutation of either of these sites resulted in loss of inhibition. Further analysis of the binding of ovhv2-miR-8 to this region using RNAhybrid showed that site 1 was predicted to bind the miRNA with high efficiency, but RNAhybrid did not identify site 2. Site 2 lies in the region that is likely to interact with the 3’ end of a miRNA interacting at site 1. It is therefore likely that site 1 is functional and that the loss of inhibition seen when site two is mutated is due to loss of non-seed sequence interactions. We were unable to demonstrate any reduction in ORF 73 mRNA levels following transfection of ovhv2-miR-8 into BJ1035 cells.
The inability to demonstrate inhibition of ORF 20 and ORF 73 expression in BJ1035 cells may be due to a number of factors e.g. the inherent variability within BJ1035 cells could result in variation in baseline levels of virus gene expression or the ovhv2-miRs may inhibit translation (the luciferase assay measured protein levels) of ORFs 20 and 73 without significantly affecting mRNA levels. ORF 73 is the main latency associated protein and so will be expressed in the majority of BJ1035 cells. Our transfection efficiency for BJ1035s is approximately 40-45% (data not shown) it is possible that the level of inhibition obtained is not sufficient to be seen under these experimental conditions.
In all three classes of herpesvirus, homologues of ORF 20 (the UL24 family) have been shown to induce cell cycle arrest and inactivate the cyclin B/cdc2 complex (Nascimento et al., 2009). The conservation of the structure and function of these proteins during herpesvirus evolution strongly suggests that they are important in herpesvirus biology (Nascimento et al., 2009 Using ORF 20 deletion mutants of MHV Nascimento et al {Nascimento, 2011 #2962) also demonstrated that ORF 20 is not essential for virus replication, there was a delay in virus clearance from the lungs of animals infected with the mutant virus and the mutants established latency normally. The role, if any, of ORF 20 in MHV pathogenesis is therefore not clear. A recent study has shown that viral cyclins play a key role in control of latency and reactivation of the gammaherpesvirus MHV-68 (Lee et al., 2012). Different mammalian cyclins could substitute for some of the functions of the MHV-68 cyclins with different cyclins mediating persistence or reactivation (Lee et al., 2012). It is possible that changes in expression of ORF 20, mediated in part by ovhv2-miRNAs, could result in a change in the cyclin expression profile in infected cells influencing the balance between productive and latent life cycles. A major part of MCF pathogenesis is uncontrolled proliferation of the infected LGLs, whose aberrant cytotoxic function leads to pathology. Inhibition of ORF 20 expression by ovhv2- miR-2 may down-regulate an ORF 20 mediated block to the cell cycle contributing to the dysregulated lymphocyte proliferation in susceptible hosts. OvHV-2 miRNAs were identified as being expressed in a bovine cell line, their expression pattern in infected ovine cells is unknown, however it is possible that a difference in the regulation of these miRNAs in ovine and bovine cells may result in differences in expression of ORF 20 leading to the observed differences in the outcome of OvHV-2 infection in cattle and sheep.
ORF 50 is a homologue of the replication and transcription activator (RTA) of EBV and Kaposi’s sarcoma herpesvirus (KSHV). In KSHV, the virus encoded miR-K5 and miR-K9 have antagonistic effects on latency. miR-K5 attenuates expression of RTA leading to a reduction in reactivation whilst miR-K9 targets BCLAF-1, a host transcription factor that is associated with reduced viral replication (Ziegelbauer et al., 2009). Using a PARclip approach Gottwein et al. (Gottwein & Cullen, 2010) have reported that ORF 73 of KSHV is targeted by KSHV miR-K10/miR-142- 3p. Deletion of three EBV-encoded miRNAs has been shown to result in a significant increase in expression of virus latent genes (Feederle et al., 2011a; Feederle et al., 2011b; Seto et al., 2010) and Riley et al. (Riley et al., 2012) have shown that EBV-encoded miRNAs target virus-encoded, latency-associated genes and suggested that these miRNAs play a role in control of EBV latency. The OvHV-2 immortalized LGL cell line, BJ1035 is a mixed population of cells with respect to virus life cycle; the majority are latently infected but a small proportion express early and late virus genes(Rosbottom et al., 2002; Thonur et al., 2006). Thus, OvHV-2 miRNAs may be differentially expressed in the proportion of cells in culture in which the virus is latent compared to those cells where the virus is reactivating.
The proteins encoded by ORF 50 and ORF 73 play important, but contrary roles in relation to latency; ORF 50 is critical for virus reactivation and ORF 73 is important in maintenance of viral latency (Ackermann, 2006). Bellare and Ganem (Bellare & Ganem, 2009) proposed that miRNA-induced inhibition of virus genes is not the sole regulator of reactivation/latency but rather that they ensure that fluctuations in virus mRNA levels do not result in reactivation in conditions that are unfavourable for viral replication, i.e. the miRNAs act as a type of rheostat to control the levels of virus gene expression. The apparent contradiction of having miRNAs that act to both inhibit and encourage reactivation expressed in the same cell line can be explained by considering the mixed nature of the BJ1035 line. The differential expression of individual virus miRNAs in individual cells could respond to changes in the cellular environment acting to regulate virus gene expression. The factors which determine the maintenance of, or reactivation from, latency, are complex, by demonstrating miRNA mediated control of both “pro and anti- reactivation genes” our data adds support to the hypothesis that miRNAs represent an additional layer of control exerted by the virus to control the latent state.
Methods
Target identification
Potential miRNA targets within the OvHV-2 genome were identified using BLASTN (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to align the sequences complementary to the ovhv2-miRs in the OvHV-2 genome (GenBank, AY839756) (Hart et al., 2007). Targets were mapped to the 5’UTR or 3’UTR of OvHV2 genes. Only a small number of OvHV-2 mRNAs have been mapped previously therefore, for the majority of genes, the 5′UTRs were considered to span the region from the start codon to the predicted TATA box. For 3’UTRs the region from the stop codon to the predicted polyadenylation site was used. Sites chosen for validation were also analysed by RNAhybrid (Rehmsmeier et al., 2004) allowing no G:U pairing in the seed sequence and a helix constraint of nucleotides 2 to 8.
Cell culture
Baby hamster kidney cells (BHK-21) were cultured in Glasgow’s minimum essential medium (Gibco) supplemented with 10% new born calf serum, 1% v/v Penicillin/Streptomycin, 1% v/v L-glutamine (Sigma) and 10% v/v tryptose phosphate broth. BJ1035 cell were grown in suspension in Iscove’s Modified Dulbecco’s Medium (Invitrogen) (Hart et al; 2007) supplemented with 10% v/v FCS, 1% v/v penicillin/streptomycin and 350 U ml−1 Proleukin (IL-2) (Novartis Pharmaceutical) and cultured at 37°C with 5% CO2.
Cloning of target sites
Sequences spanning the 3’ and 5’ UTRs of the selected genes were amplified by PCR. For 3’UTRs the region from the stop codon to the predicted poly A site was amplified, for analysis of 5’UTRs approximately 1000 bp upstream of the ATG was amplified, 3′UTRs were cloned into the psiCHECK™-2 Vector (Promega) downstream of a renilla luciferase reporter gene (Rluc) and 5′UTRs into the pGL4.10 vector (Promega) upstream of a firefly luciferase reporter gene (Fluc).
Transfection
BHK-21 cells (n=6) were co-transfected with reporter vectors (1.5 μg) with or without the 5’ or 3’UTR of interest and with 50 nM or 100 nM mimic miRNAs (miScript miRNA Mimics, Qiagen) or control miRNA (AllStars Negative Control siRNA, Qiagen) using Lipofectamine 2000 (Invitrogen) (Mimic sequences: ovhv2-miR-2, 5′-AUCUUGGACGCAUCUGUCAGUAG-3′; ovhv2-miR-5, 5′-UGAAGUUACAGCUGCACCUGGAU-3′; ovhv2-miR-8, 5′- UGGCUCAGCGUGACUGCUCUUC-3′). After 24 h, samples were harvested and luciferase levels assessed using the Dual-Luciferase Reporter Assay System (Promega). For psiCheck (3’UTR) based vectors Rluc (target) expression was normalized to Fluc expression from the same plasmid. For pGL4.10 (5’UTR) based vectors, cells were co-transfected with the pRL vector (Promega) to allow normalization of Fluc expression. BJ1035 cells were prepared for transfection by density –gradient centrifugation using lymphoprep (Axis-Shield) and re-suspended at a concentration of 1× 106 cells in a final volume of 100ul of solution V (Lonza). 200 nM of mimic or control miRNA were transfected into the prepared cells using program X-001 of Nucleofector II (Lonza). Transfected cells (n=3) were incubated for 24 hrhr in BJ1035 media prior to RNA extraction.
Site directed mutagenesis
Site directed mutagenesis was performed using the Quick Change Site-Directed Mutagenesis (Stratagene) protocol. Primers for mutagenesis (HPLC purified, Eurofins MWG Operon, Germany) were designed such that each primer was 42-45 nt in length, with a Tm of >78°C. (Table S1). All mutations were confirmed by sequencing.
cDNA synthesis
RNA was isolated from BJ1035 cells using an RNeasy kit (Qiagen). 1 μg RNA was digested with RQ1 DNase (Promega) for 30 min at 37 °C. For analysis of ORF 20, cDNA was primed with 250 ng or 66 ng of specific primer (41530 - CTGAAACATGGCCTCCAACT -41549).For analysis of ORF 50, cDNA was primed using 250 ng oligo dT primer or random primers (Promega; equivalent to 0.5 μg.μg−1 RNA). cDNA was synthesized using AMV reverse transcriptase for 1 hr at 42 °C (oligo dT and specific primer) or 37 °C (random primer).
RT-PCR analysis of transcripts
ORF 20 cDNA was amplified with primer pair TTCATAGTCACTGTTGTCC and CTGAAACATGGCCTCCAACT (nt 41617-41635 and 41530-41549). ORF 50 cDNA was amplified with the primer pair CCCCAACAAGTCAGCATTTT and CACTTTTATTTTAACATCACAAACC (nt 78080-78099 and 79205-79181). ORF 73 cDNA was amplified with the primer pair CCACTTCGTAAAAGCACCATT and GTAATCCTGCCCCAGCTGTA (nt 120514-120534 and 121060-121041). PCR reactions contained 8 pM primers, 120 μM dNTPs, 1 U HotStar Taq plus (Qiagen) in a final reaction volume of 25 μl. Following an initial denaturation of 5 min at 95 °C, 40 cycles (30 s at 95 °C, 30 s at 58 °C and 1 min 15 s at 72 °C) were performed prior to a final extension of 5 min at 72 °C. 100 ng of BJ1035 DNA was used as a positive control for the PCR. Samples were run on a 2% agarose gel and visualized, extracted, cloned into pCR2.1-Topo (Invitrogen) and sequenced.
RT-qPCR
RNA was isolated using an RNeasy kit (Qiagen). 1 μg of RNA was digested with RQ1 Dnase (Promega) for 30 min at 37 °C. cDNA was primed using 250ng Oligo dT primer (Promega; equivalent to 0.5 μg/μg RNA) and synthesized using AMV reverse transcriptase for 1 hr at 42 °C. Semi-quantitative PCR was performed on a Rotorgene Q machine (35 cycles of 15 s 95 °C, 30 s 58 °C and 30 s 72 °C) using SensiFAST™ SYBR Hi-ROX One-Step master mix (Bioline), 2μl of diluted cDNA (1:10) and specific primers for GAPDH (Gossner et al., 2009), ORF 20 (CACTACCCAGCGCTCTTCC (41125-41143); TTGTACCCAACCCCATCAAG (41237-41218); ORF 50 (CCCCAACAAGTCAGCATTTT (78080-78099); TCAGGGGTGACTCCAATG (78267-78250) and ORF 73 (CAG GGCAAAACGTAA AAAGC (119367-119348); GTGTGGAGCGTTAGGATTG (119223-119241) at a final concentration of 8 pM in a final reaction volume of 20 μl. Transcript levels were normalised to GAPDH and the relative expression was calculated using 2−ΔΔCt (Livak & Schmittgen, 2001). Three technical replicates were performed for each biological replicate. Data were plotted as fold change in relation to negative control.
Statistics
For Luciferase assays groups were compared using single factor analysis of variance (ANOVA) test followed by Tukey’s post-hoc test. P values represent results from the post-hoc test. To compare levels of ORF 20, ORF 50 or ORF 73 transcripts after transfection ovhv2-mirs standard linear mixed effect (LME) statistical models were used. Technical replicates were entered as random effects to account for the repeated sampling of the same cell line. Whether the data was test or control was entered as the fixed effect. For ORF 50 and ORF 73 three independent runs were performed, and this run parameter was entered as a potential confounding fixed effect prior to control/test status. Examination of residuals demonstrated a normal distribution. The LME were carried out in R (version 2.15.0 © 2012 The R Foundation for Statistical Computing).
Supplementary Material
Acknowledgements
This project was funded by the Biotechnology and Biological Sciences Research Council (BBSRC) Institute Strategic Programme Grant to The Roslin Institute. AR is in receipt of a postgraduate scholarship from the Pakistan Higher Education Commission.
References
- Ackermann M. Pathogenesis of gammaherpesvirus infections. Veterinary Microbiology. 2006;113:211–222. doi: 10.1016/j.vetmic.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: Target Recognition and Regulatory Functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellare P, Ganem D. Regulation of KSHV Lytic Switch Protein Expression by a Virus-Encoded MicroRNA: An Evolutionary Adaptation that Fine-Tunes Lytic Reactivation. Cell Host & Microbe. 2009;6:570–575. doi: 10.1016/j.chom.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CG, Splitter GA. Lytic function of bovine lymphokine-activated killer cells from a normal and a malignant catarrhal fever virus-infected animal. Vet Immunol Immunopathol. 1988;19:105–118. doi: 10.1016/0165-2427(88)90002-5. [DOI] [PubMed] [Google Scholar]
- Feederle R, Linnstaedt SD, Bannert H, Lips H, Bencun M, Cullen BR, Delecluse H-J. A Viral microRNA Cluster Strongly Potentiates the Transforming Properties of a Human Herpesvirus. Plos Pathogens. 2011a;7 doi: 10.1371/journal.ppat.1001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feederle R, Haar J, Bernhardt K, Linnstaedt SD, Bannert H, Lips H, Cullen BR, Delecluse H-J. The Members of an Epstein-Barr Virus MicroRNA Cluster Cooperate To Transform B Lymphocytes. Journal of Virology. 2011b;85:9801–9810. doi: 10.1128/JVI.05100-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossner AG, Bennet N, Hunter N, Hopkins J. Differential expression of Prnp and Sprn in scrapie infected sheep also reveals Prnp genotype specific differences. Biochemical and Biophysical Research Communications. 2009;378:862–866. doi: 10.1016/j.bbrc.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Gottwein E, Cullen BR. A Human Herpesvirus MicroRNA Inhibits p21 Expression and Attenuates p21-Mediated Cell Cycle Arrest. Journal of Virology. 2010;84:5229–5237. doi: 10.1128/JVI.00202-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey F, Tirabassi R, Meyers H, Wu G, McWeeney S, Hook L, Nelson JA. A Viral microRNA Down-Regulates Multiple Cell Cycle Genes through mRNA 5 ′ UTRs. Plos Pathogens. 2010;6 doi: 10.1371/journal.ppat.1000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundhoff A, Sullivan CS. Virus-encoded microRNAs. Virology. 2011;411:325–343. doi: 10.1016/j.virol.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart J, Ackermann M, Jayawardane G, Russe G, Haig DM, Reid H, Stewart JP. Complete sequence and analysis of the ovine herpesvirus 2 genome. Journal of General Virology. 2007;88:28–39. doi: 10.1099/vir.0.82284-0. [DOI] [PubMed] [Google Scholar]
- Lee KS, Suarez AL, Claypool DJ, Armstrong TK, Buckingham EM, van Dyk LF. Viral Cyclins Mediate Separate Phases of Infection by Integrating Functions of Distinct Mammalian Cyclins. Plos Pathogens. 2012;8 doi: 10.1371/journal.ppat.1002496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy CS, Hopkins J, Russell G, Dalziel RG. Novel virus-encoded microRNA molecules expressed by ovine herpesvirus 2 immortalized bovine T cells. Journal of General Virology. 2012;93:150–154. doi: 10.1099/vir.0.037606-0. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Meier-Trummer CS, Ryf B, Ackermann M. Identification of peripheral blood mononuclear cells targeted by Ovine herpesvirus-2 in sheep. Veterinary Microbiology. 2010;141:199–207. doi: 10.1016/j.vetmic.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Nascimento R, Dias JD, Parkhouse RME. The conserved UL24 family of human alpha, beta and gamma herpesviruses induces cell cycle arrest and inactivation of the cyclinB/cdc2 complex. Archives of Virology. 2009;154:1143–1149. doi: 10.1007/s00705-009-0420-y. [DOI] [PubMed] [Google Scholar]
- Nelson DD, Davis WC, Brown WC, Li H, O’Toole D, Oaks JL. CD8(+)/perforin(+)/WC1(−) gamma delta T cells, not CD8(+) alpha beta T cells, infiltrate vasculitis lesions of American bison (Bison bison) with experimental sheep-associated malignant catarrhal fever. Veterinary Immunology and Immunopathology. 2010;136:284–291. doi: 10.1016/j.vetimm.2010.03.023. [DOI] [PubMed] [Google Scholar]
- Rehmsmeier M, Steffen P, Hochsmann M, Giegerich R. Fast and effective prediction of microRNA/target duplexes. Rna-a Publication of the Rna Society. 2004;10:1507–1517. doi: 10.1261/rna.5248604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid HW, Buxton D, Pow I, Finlayson J. Isolation and characterisation of lymphoblastoid cells from cattle and deer affected with ‘sheep-associated’ malignant catarrhal fever. Res Vet Sci. 1989;47:90–96. [PubMed] [Google Scholar]
- Riley KJ, Rabinowitz GS, Yario TA, Luna JM, Darnell RB, Steitz JA. EBV and human microRNAs co-target oncogenic and apoptotic viral and human genes during latency. Embo Journal. 2012;31:2207–2221. doi: 10.1038/emboj.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosbottom J, Dalziel RG, Reid HW, Stewart JP. Ovine herpesvirus 2 lytic cycle replication and capsid production. Journal of General Virology. 2002;83:2999–3002. doi: 10.1099/0022-1317-83-12-2999. [DOI] [PubMed] [Google Scholar]
- Russell GC, Stewart JP, Haig DM. Malignant catarrhal fever: A review. Veterinary Journal. 2009;179:324–335. doi: 10.1016/j.tvjl.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Schock A, Collins RA, Reid HW. Phenotype, growth regulation and cytokine transcription in Ovine Herpesvirus-2 (OHV-2)-infected bovine T-cell lines. Veterinary Immunology and Immunopathology. 1998;66:67–68. doi: 10.1016/s0165-2427(98)00187-1. [DOI] [PubMed] [Google Scholar]
- Seto E, Moosmann A, Groemminger S, Walz N, Grundhoff A, Hammerschmidt W. Micro RNAs of Epstein-Barr Virus Promote Cell Cycle Progression and Prevent Apoptosis of Primary Human B Cells. PLoS Pathogens. 2010;6 doi: 10.1371/journal.ppat.1001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay Y, Zhang JQ, Thomson AM, Lim B, Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation (vol 445, pg 1124, 2008) Nature. 2009;458:538–538. doi: 10.1038/nature07299. [DOI] [PubMed] [Google Scholar]
- Thonur L, Russell GC, Stewart JP, Haig DM. Differential Transcription of ovine herpesvirus 2 genes in lymphocytes from reservoir and susceptible species. Virus Genes. 2006;2006 doi: 10.1007/s11262-005-5842-3. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Xu H, Yao Y, Smith LP, Kgosana L, Green J, Petherbridge L, Baigent SJ, Nair V. Critical Role of the Virus-Encoded MicroRNA-155 Ortholog in the Induction of Marek’s Disease Lymphomas. Plos Pathogens. 2011;7 doi: 10.1371/journal.ppat.1001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegelbauer JM, Sullivan CS, Ganem D. Tandem array-based expression screens identify host mRNA targets of virus-encoded microRNAs. Nature Genetics. 2009;41:130–134. doi: 10.1038/ng.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.