Abstract
Combining beta-blockers with exposure therapy has been advocated to reduce fear, yet experimental studies combining beta-blockers with memory reactivation have had contradictory results. We explored how beta-blockade might affect the course of safety learning and the subsequent return of fear in a double-blind placebo-controlled functional magnetic resonance imaging study in humans (N=46). A single dose of propranolol before extinction learning caused a loss of conditioned fear responses, and prevented the subsequent return of fear and decreased explicit memory for the fearful events in the absence of drug. Fear-related neural responses were persistently attenuated in the dorsal medial prefrontal cortex (dmPFC), increased in the hippocampus 24 h later, and correlated with individual behavioral indices of fear. Prediction error-related responses in the ventral striatum persisted during beta-blockade. We suggest that this pattern of results is most consistent with a model where beta-blockade can prevent the return of fear by (i) reducing retrieval of fear memory, via the dmPFC and (ii) increasing contextual safety learning, via the hippocampus. Our findings suggest that retrieval of fear memory and contextual safety learning form potential mnemonic target mechanisms to optimize exposure-based therapy with beta-blockers.
Introduction
The primary treatment for trauma- and stressor-related disorders is exposure therapy, which is based on the principle of fear extinction. Although such extinction-based therapies can be initially effective, 19–62% of patients experience return of fear following treatment (Vervliet et al, 2013). This risk of relapse, that is, fear recovery, highlights the need for effective treatments that persist. Here we aimed to investigate the effects of beta-blockade on extinction and the subsequent return of fear in humans.
Reports that reactivating fear memories in the presence of beta-blockers can prevent the return of fear have advocated combining psychotherapy with beta-blockers to improve outcome (Debiec and Ledoux, 2004; Kindt et al, 2009; Kroes et al, 2010; Muravieva and Alberini, 2010; Schwabe et al, 2012). Beta-blockers can reduce retrieval of fear memories (Kroes et al, 2010; Muravieva and Alberini, 2010), and have been suggested to impair reconsolidation (Debiec and Ledoux, 2004; Kindt et al, 2009; Schwabe et al, 2012), both associated with a reduction of fear responses. Research on reconsolidation proposes that upon reactivation, stable memories can become flexible again and susceptible to strengthening or weakening and require restorage to be maintained (Nader et al, 2000; Sara, 2000). Critically, memory needs to be reactivated by a brief single reminder for the original memory to become flexible and undergo reconsolidation (Eisenberg et al, 2003). In contrast, extinction-based psychotherapy typically involves reactivating fear memory by repeated and prolonged exposure to a fear-evoking stimulus in the absence of aversive consequences resulting in reduction of fear responses (Vervliet et al, 2013). Unlike reconsolidation, extinction does not alter the original memory but forms a novel coexisting safety memory that competes with the expression of fear (Myers and Davis, 2007; Quirk and Mueller, 2008). The influence of beta-blockade on extinction learning is unclear, but several studies report that beta-blockade during extinction impairs the consolidation of the novel safety memory resulting in a subsequent increase in fear (Bos et al, 2012; Cain et al, 2004; Mueller et al, 2008; Ouyang and Thomas, 2005). In sum, fear memory reactivation first involves retrieval, and, dependent on reactivation conditions, reconsolidation or extinction can subsequently become the dominant memory process (Eisenberg et al, 2003). We hypothesize that beta-blockade will affect the dominant memory mechanism and can thus either decrease or increase the return of fear, and hence influence therapeutic outcome positively or negatively. Combining beta-blockers with exposure therapy is therefore precarious until we better understand and can experimentally manipulate the mnemonic mechanisms that should be targeted to prevent fear recovery.
We also aimed to investigate the effect of beta-blockade on different memory systems. Based on previous research, we hypothesized that beta-blockade might alternatively attenuate autonomic fear responses (Kindt et al, 2009), reduce explicit emotional memory (Kroes et al, 2010) and/or the subjective feeling for fearful events (Schwabe et al, 2013). Although merely attenuating autonomic fear responses could be considered clinically optimal, intact explicit knowledge may increase the likelihood of fear recovery (Phelps et al, 2001; Raio et al, 2012) and could thus also be a necessary target of effective treatment strategies.
Finally, we aimed to investigate the influence of beta-blockade on the neural mechanisms underlying fear memory and their relationship to behavior. Within the domain of fear conditioning and extinction, distinct brain regions including the amygdala, midbrain, dorsomedial prefrontal cortex (dmPFC), ventromedial prefrontal cortex (vmPFC), hippocampus, and insula have been attributed with specific functions and can collectively be described as a fear and safety neurocircuitry (Milad et al, 2007; Myers and Davis, 2007; Quirk and Mueller, 2008). We hypothesized that beta-blockade may influence this fear and safety neurocircuitry as it is under noradrenergic control (Hermans et al, 2011). Furthermore, extinction learning has been proposed to critically depend on mismatch between expectancy and outcome, that is, prediction error signals (Rescorla and Wagner, 1972). The ventral striatum reflects a neural signature of prediction errors (O'Doherty et al, 2003) that may drive learning in the fear and safety neurocircuitry (Schiller et al, 2008). Ventral striatal prediction error-related responses have been found to be affected by dopamine manipulation (Pessiglione et al, 2006). Considering the close relationship between the two catecholamines dopamine and noradrenaline and their pharmacological manipulation (Dayan and Finberg, 2003; Fang and Yu, 1995; Smith and Greene, 2012), we hypothesized that beta-blockade might alter this neural signature of prediction errors and potentially affect learning. Further, it has been suggested that alteration of reactivated fear memory by beta-blockade depends on a prediction error signal (Sevenster et al, 2013). We therefore hypothesized that individuals' neural signatures of prediction error responses might be correlated with the absence of fear recovery.
In the current study, we tested whether beta-blockade during extinction learning one day after acquisition would result in either a subsequent increase or decrease in the expression of conditioned fear as measured one day later, in the absence of drug. We designed our study such that we could probe effects of beta-blockade on neural activity in the fear and safety neurocircuitry and its relationship to autonomic, explicit, and subjective measures of fear; an approach that could potentially provide insight into memory and neural mechanisms underlying a potential therapeutic effect.
Materials and methods
For a full description of the Materials and Methods see the Supplementary Information. Briefly, 54 healthy young human participants were initially included in the study. In a double-blind design, participants were pseudorandomly assigned to one of the drug groups so that for each of four consecutive participants two would receive a beta-blocker (40 mg propranolol HGl) and two placebo (microcrystalline cellulose). Five participants were excluded on day 1 as they displayed no conditioned skin conductance responses (SCRs), three participants could not complete the study because of scanner problems and one participant in the propranolol group did not complete the reinstatement and re-extinction task because of scanner problems. The placebo group comprised 24 participants (11 males, 13 females) and the propranolol group 22 participants (8 males, 14 females). All participants gave written informed consent. The study was approved by the institutional ethics committee (CMO Regio, Arnhem-Nijmegen, The Netherlands; CMO2010/257).
Over three consecutive days participants were differentially cue-conditioned to a stimulus signaling threat (CS+) of transcutaneous electrical shock (US) and a cue signaling safety (CS−) in a specific context on day 1 (Supplementary Figure S1a). On day 2 participants received a single dose of propranolol or placebo and underwent an extinction paradigm. On day 3, in the absence of drug, the possible return of fear was first tested as spontaneous recovery during a recall task. Next, a stronger test of fear recovery was used where first the general level of arousal was increased by four unsignaled shocks, and next fear reinstatement was assessed during a re-extinction task. The context of extinction, recall, and re-extinction differed from conditioning (ABBB design) to better match treatment settings, and to maximize the chance of detecting hippocampal responses (Kalisch et al, 2006; Marschner et al, 2008; Milad et al, 2007). We measured the influence of beta-blocker administration on day 2 on (a) learned fear as indexed by SCRs (Bach et al, 2011) on days 2 and 3, (b) explicit memory and subjective experience of the fearful events tested at the end of day 3, (c) neural functioning using blood oxygenation level-dependent functional magnetic resonance imaging (BOLD-fMRI) on days 2 and 3, (d) the relationship between behavior (SCR and explicit memory) and neural responses, and (e) a neural signature of reinforcement learning.
Results
We first determined that no incidental between-group differences existed at baseline and that propranolol was active on Day 2 only. There were no significant differences with respect to age, trait anxiety, heart rate, and blood pressure on day 1. The single dose of propranolol affected blood pressure on day 2, but not day 3, replicating previous reports (Kindt et al, 2009; Kroes et al, 2010) (Supplementary Figure S1c and Supplementary Results).
Beta-Adrenergic Blockade Results in a Loss of Fear, and Prevents Return of Fear
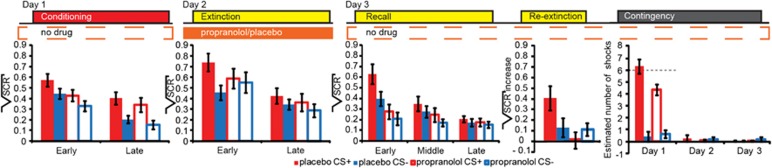
Next, we focused on effects of beta-blockade during extinction learning on sympathetic fear responses as measured using SCR. Both groups acquired differential conditioned fear responses on day 1 (phase (early, late phase of task) × CStype (CS+, CS−) (repeated-measures ANOVA: F1,44=5.503, p=0.024)). There were no significant differences between groups, and, critically, differential responses at the end of conditioning were similar between groups, indicating the acquisition of comparable fear memory in both groups (Figure 1 and Supplementary Table S1 for additional statistics). Note, we only included non-reinforced CS+ trials in our analyses to prevent potential bias by shock delivery. For day 2, we observed an interaction of drug (propranolol, placebo) × phase (early,late) × CStype (CS+, CS−) (F1,44=7.779, p=0.008), but no main effect of drug. Specifically, during the early phase of extinction the placebo group showed retention of learned fear, whereas the propranolol group did not show significant differences in response to CS+ and CS− presentations. In the late phase of extinction, we no longer observed a group effect and neither group showed significant differential conditioned responses, indicating successful extinction training. On day 3, after the drug had washed out, we still observed between-group differences (recall: drug × phase (F1.676,73.739=7.565, p=0.002); re-extinction: drug × CStype (F1,43=5.560, p=0.023)). Although the placebo group showed both spontaneous recovery and reinstatement of fear, the propranolol group showed no spontaneous recovery and no fear reinstatement. Hence, a single dose of propranolol before extinction learning eliminated learned fear responses in a new context, resulted in a subsequent loss of fear, and prevented the return of fear one day later in the absence of drug.
Figure 1.
Results: sympathetic fear responses and explicit memory for fearful events. A single dose of propranolol before extinction learning eliminated learned fear responses, resulted in a subsequent loss of fear, prevented the return of fear, and attenuated explicit memory of the fearful events one day later in the absence of drug. Placebo group (solid bars), propranolol group (open bars), CS+ (red), CS− (blue), error bars reflect SEM. Critical test scores per task are: Conditioning (panel 1): Late phase (trials 7–12) over both groups (paired-samples T-test t(45)=−2.356, p=0.023); Extinction (panel 2): Early phase (trials 1–6) placebo group (paired-samples T-test t(23)=5.127, p<0.001), propranolol group (t(21)=1.054, p=0.308), and Late phase (trials 7–12) placebo group (t(23)=1.147, p=0.263), propranolol group (t(21)=1.341, p=0.194), and no absolute group differences in responses to CS+ or CS− trials in the early nor in the late phase were revealed by independent-samples T-tests; early phase CS+ (t(44)=−1.138, p=0.261), early phase CS− (t(44)=0.493, p=0.624), late phase CS+ (t(44)=−0.813, p=0.420), and late phase CS− (t(44)=−1.065, p=0.293); Recall (panel 3): As the recall paradigm is principally a second extinction session, extinction learning can be expected to occur rapidly. To maximize sensitivity to detect spontaneous recovery effects, we therefore calculated for each CS type (CS+, CS−) the average skin conductance for the early phase (trials 1–4), middle phase (trials 5–8), and late phase (9–12). Early phase placebo group (paired T-test t(23)=5.127, p<0.001), propranolol group (t(21)=1.515, p=0.145)); Re-extinction (fourth panel): a reinstatement score was calculated as the difference between the first re-extinction trial and the last trial of the recall task for the CS+ and CS− for each participant. Note, reinstatement (4 unsignaled shocks) occurred between recall and re-extinction. Placebo group (paired T-test t(23)=2.005, p=0.057), propranolol group (t(20)=−1.371, p=0.186), independent-samples T-tests for CS+ (t(37.021)=2.991, p=0.004) and CS− (t(43)=0.232, p=0.818). Contingency questionnaire (Panel 5): At the end of day 3, participants who had received propranolol on day 2 underestimated the number of shocks they had received following CS+ presentations on day 1 (independent samples T-tests day 1 CS+ t(43)=−2.560, p=0.014, CS−t(43)=0.453, p=0.653). Dotted line represents the actual number of received shocks on day 1. CS, conditioned stimulus.
Beta-Adrenergic Blockade Attenuates Explicit Memory of Fearful Events
Beyond propranolol eliminating sympathetic fear responses, we also found that beta-blockade affected explicit memory. At the end of the experiment on Day 3, participants estimated the number of shocks they had received following the presentation of each type of CS on each day (Figure 1). To avoid explicit recall from influencing SCR (Phelps et al, 2001; Raio et al, 2012), explicit estimation was probed only at the end of day 3. All participants received the same number of shocks, and learning as measured by SCR on day 1 was indistinguishable between groups, suggesting that both groups acquired comparable fear memories. Nevertheless, participants who had received propranolol on day 2 underestimated the number of shocks they had received following CS+ presentations on day 1 (day (days 1, 2, 3) × CStype (CS+, CS−) × drug (propranolol, placebo) (F2,84=3.518, p=0.034)). No significant differences for the CS− were observed. Beta-blockade did not significantly affect subjective fear measures (Supplementary Results). Thus, beta-blockade during extinction eliminated not only learned sympathetic fear responses but also reduced explicit memory of the fearful events.
Beta-Adrenergic Blockade Affects the Fear and Safety Neurocircuitry
In view of the positive effects of propranolol on eliminating sympathetic fear responses and reducing explicit memory, we next examined propranolol effects on neuronal responses in the fear and safety neurocircuitry. To increase specificity, we followed previous region of interest analyses in the field (Kalisch et al, 2006; Milad et al, 2007; Phelps et al, 2004). As such, we restricted all our BOLD-fMRI analyses to our previously defined regions of interest (amygdala, dmPFC, midbrain, vmPFC, hippocampus, and insula) that also showed differential responses [CS+ vs CS−] across both groups. This approach ensured that selection of regions of interest was orthogonal to potential drug interactions.
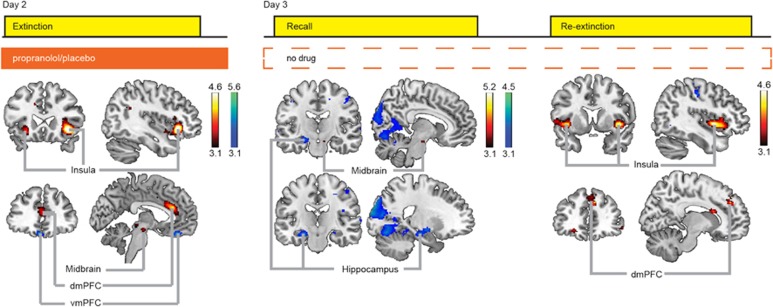
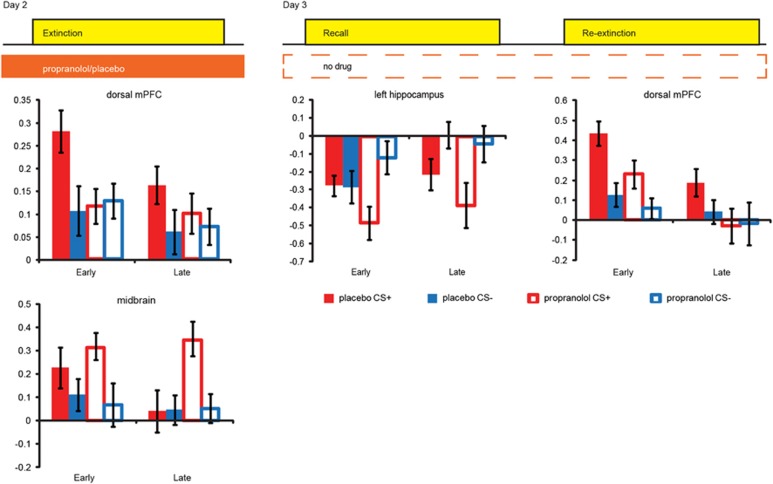
On day 2, propranolol eliminated differential conditioned SCR. BOLD-fMRI data analyses comparing CS+ trials to the CS− trials during extinction revealed differential neural responses in the insula, dmPFC, vmPFC, and midbrain across both groups (Figure 2 and Supplementary Table S2). We observed group differences in dmPFC and midbrain activity (drug × CStype dmPFC: F1,44=4.894, p=0.032; midbrain: F1,44=4.509, p=0.039; Figure 3 and Supplementary Table S3). Specifically, the placebo group, but not the propranolol group, exhibited greater dmPFC responses [CS+ vs CS−]. This differential dmPFC responses in the placebo group disappeared during extinction learning. In contrast, the propranolol group, but not the placebo group, exhibited greater midbrain responses [CS+ vs CS−] throughout the task.
Figure 2.
Blood oxygenation level-dependent functional magnetic resonance imaging (BOLD-fMRI) analyses revealed neural regions responsive during fear extinction, recall and re-extinction. Red: CS+>CS− (‘activation'); blue: CS−>CS+ (‘deactivation'). During extinction activation of the dorsal medial prefrontal cortex (dmPFC), bilateral insula, and midbrain was detected, and deactivation of the ventromedial prefrontal cortex (vmPFC). At recall, activation of the dmPFC and midbrain, and deactivation of the hippocampus and amygdala was evident. During the re-extinction task, we found activation of the bilateral insula and dmPFC. Bars indicate T-values of main effects, activation clusters are displayed overlaid on selective slices of a template brain, and thresholded at p<0.001 uncorrected. CS, conditioned stimulus.
Figure 3.
Beta-blockade effects on blood oxygenation level-dependent functional magnetic resonance imaging (BOLD-fMRI). Propranolol administration affected the neural network of extinction learning. Analyses on the extracted data from regions revealed by the main effects of task showed that during extinction learning propranolol eliminated differential conditioned responses in the dorsal medial prefrontal cortex (dmPFC) while increasing differential responses in the midbrain (paired t-tests CS+ vs CS− within the placebo group for dmPFC: t(23)=2.395, p=0.025, and midbrain: t(23)=1.509, p=0.145; and the propranolol group dmPFC: t(21)=−0.222, p=0.826, and midbrain: t(21)=2.163, p=0.042). During the recall task, the propranolol group showed differential conditioned responses in the hippocampus, an effect not observed in the placebo group (paired T-tests placebo group CS+ vs CS− early phase: t(23)=0.097, p=0.924, and late phase: t(23)=−2.039, p=0.053; propranolol group early phase: t(21)=−3.085, p=0.006, late phase: t(21)=−2.615, p=0.016). During the re-extinction task, the propranolol group showed reduced responses in the dmPFC (independent sample T-test averaging over all conditions: t(43)=2.091, p=0.042). Placebo group (solid bars), propranolol group (open bars), CS+ (red), CS− (blue), early=average over the first half of the trials, late=average over second half of the trials, and error bars reflect SEM. CS, conditioned stimulus.
On day 3, the placebo group showed spontaneous recovery and reinstatement of fear, whereas the propranolol group did not. BOLD-fMRI data analyses for the recall task revealed differential neural responses [CS+ vs CS−] in the midbrain, hippocampus, amygdala, and dmPFC across both groups (Figure 2 and Supplementary Table S3). We observed a group difference in hippocampal responses that just fell short of being significant (Drug × CStype: F1,44=3.820, p=0.053), yet this between-group trend effect was corroborated by significant across-group analyses probing the relationship between neural responses and SCR (see below). Specifically, the propranolol group showed greater differential hippocampal responses during the early phase of the recall task (testing spontaneous recovery), whereas evidence for this differential response only arose in the late phase for the placebo group (Figure 3 and Supplementary Table S3). During the re-extinction task (ie, after fear reinstatement), we observed BOLD-fMRI responses [CS+ vs CS−] in regions including the dmPFC and insula across both groups (Figure 2 and Supplementary Table S3). The dmPFC responses were greater in the placebo group compared with that in the propranolol group (main effect drug: F1,43=4.371, p=0.042; Figure 3 and Supplementary Table S3). Other regions exhibiting differential responses were not significantly affected by beta-blockade (Supplementary Figure S2 and Table S3). Thus, beta-blockade during extinction affected neural processing in the dmPFC and midbrain on day 2, and altered subsequent neural processing during the recall and re-extinction task in the hippocampus and dmPFC on day 3, respectively.
Previous research has indicated a relationship between the expression of differential SCR and dmPFC responses (Klumpers et al, 2015), and between hippocampus responses and an SCR index of subsequent fear recovery (Milad et al, 2007). In addition, the hippocampus has been implicated in explicit knowledge of conditioned contingencies (Bechara, 1995; Knight et al, 2009). We replicated these findings and observed that smaller dmPFC responses were associated with less fear, whereas greater hippocampal responses were associated with less fear and reduced explicit memory of fearful events (see Supplementary Results and Supplementary Figure S3).
Ventral Striatal Prediction Error Responses Persist during Beta-Blockade and Show No Significant Relationship with the Loss of Fear
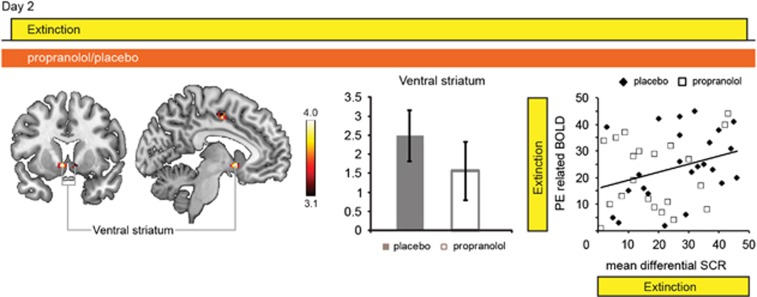
In subsequent analyses, we aimed to test our two hypotheses that beta-blockade might affect a neural signature of prediction errors and that this neural signature might be correlated with absence of the return of fear. We first fitted a Rescorla–Wagner model (Rescorla and Wagner, 1972) to the SCR data and observed that neural responses in the ventral striatum reflected the behavioral estimates of the prediction errors (Figure 4 and Supplementary Table S4), replicating previous findings (O'Doherty et al, 2003; Schiller et al, 2008). Testing our first hypothesis, we found that this well-established ventral striatal signature of prediction error responses remained intact during beta-blockade (Figure 4). Moreover, rank-correlation analyses across participants from both groups revealed that participants with greater mean differential SCR responses, that is, those whose SCR reflected most learning, exhibited greater ventral striatal prediction errors (Figure 4). Testing our second hypothesis, we detected no significant correlation between ventral striatal prediction error-related responses and spontaneous recovery or the reinstatement of fear on day 3 (Figure 4). We also observed prediction error-related responses in the ventral striatum when we fitted the model's free parameters to the SCR data of the extinction task separately for each group. Assessing the optimal solution for each individual subject did not reveal group differences in learning rate or a relationship with the return of fear (see Supplementary Results). Thus, we found evidence for learning-related ventral striatal prediction error responses that persisted during beta-blockade and that were unrelated to the modification of retrieved memories.
Figure 4.
Prediction error analyses results. Left: Analyses of prediction error-related neural signals revealed an area in the ventral striatum (small-volume correction (SVC) nucleus accumbens based on Adcock et al, 2006; Carter et al, 2009). MNI (Montreal Neurological Institute): −6, 8, 6. Z-value=4.93. Cluster size is the number of significant voxels at p<0.001; uncorrected: 58. Middle: Ventral–striatal prediction error-related activity was not modulated by beta-blockade (independent-samples T-test t(43)=1.022, P=0.313; placebo mean: 2.585, SEM, 0.672, propranolol mean: 1.432, SEM, 0.790). Right: Ventral striatal prediction error-related activity was associated with greater differential skin conductance response (SCR) during extinction (rs=0.396, P=0.007), but critically showed no correlation with spontaneous recovery (rs=0.039, P=0.801) or reinstatement of fear (rs=0.136, P=0.379). Bar indicates T-values of main effects. Activation clusters are displayed overlaid on selective slices of a template brain, and thresholded at p<0.001. Display view follows neurological convention, that is, right hemisphere is depicted on the right. Placebo group (solid bars), propranolol group (open bars), and error bars reflect SEM.
Discussion
Here we aimed to investigate the effects of beta-blockade on extinction and the subsequent return of fear in humans. (1) We show that a single dose of the beta-blocker propranolol administered before extinction learning eliminated differential fear responses during extinction, and prevented spontaneous recovery and reinstatement of fear in the absence of drug one day later. (2) Beta-blockade also reduced subsequent explicit memory, but not the subjective feelings, for the fearful events in the absence of drug. (3) We found beta-blockade to affect the fear and safety neurocircuitry. During extinction, we observed a loss of fear-related responses in the dmPFC and increased responses in the midbrain. The loss of fear on day 3 was accompanied by increased differential responses in the hippocampus during recall, whereas the absence of the return of fear was reflected by reduced responses in the dmPFC. (4) We identified that neural responses in beta-blockade-affected regions of the fear and safety neurocircuitry correlated with observed changes in behavior. The dmPFC responses correlated positively with sympathetic fear responses, whereas hippocampal responses correlated negatively with fear responses and explicit memory of the fearful events. In complementary analyses, we found prediction error responses in the ventral striatum that persisted during beta-blockade and bore no relationship with the loss of fear (Figure 4).
Mechanistic Implications of Physiological Results
For day 2, analyses of SCR during extinction indicated that beta-blockade affected extinction learning. For day 3 after the drug had washed out, our SCR results indicate that beta-blockade resulted in a loss of fear and prevented the return of fear. We conceive that our results could be explained by an effect on several mechanisms, namely (i) retrieval, (ii) reconsolidation and/or (iii) new contextual safety learning. First, our results accord with studies reporting a role for noradrenaline in retrieval of aversive and appetitive memory (Kroes et al, 2010; Muravieva and Alberini, 2010; Otis et al, 2013, 2014; Otis and Mueller, 2011; Ouyang and Thomas, 2005). We observed an interaction effect between drug and CS type but not a main effect of drug on day 2, indicating an inability to discriminate between the CS+ and CS− and a generalization of fear to both stimuli, but not a general reduction in fear responses. The detection of this effect was only possible because we used a differential conditioning (CS+ vs CS−) paradigm that is standard in most human conditioning studies, but has not been used in previous animals studies and a previous human study investigating the role of beta-blockade during extinction learning (Bos et al, 2012; Cain et al, 2004; Mueller et al, 2008). Beta-blockade-induced retrieval impairments have been found to persist (Otis et al, 2013, 2014) and may thus also have contributed to the loss of fear and absence of fear recovery we observed on day 3.
Second, our results of day 3 are similar to those of previous studies combining beta-blockade with single brief memory reactivation that have attributed to the loss of fear and absence of the return of fear to disrupted reconsolidation, that is, an attenuation of the original fear memory trace (Debiec and Ledoux, 2004; Kindt et al, 2009; Misanin et al, 1968; Nader et al, 2000; Przybyslawski and Sara, 1997; Schwabe et al, 2012; Sevenster et al, 2013). Reconsolidation and extinction have been suggested to be mutually exclusive processes where interventions only affect the dominant memory process (Eisenberg et al, 2003). The initiation of reconsolidation requires a single brief memory reactivation, whereas we repeatedly reactivated memory likely causing extinction to be the dominant memory process effectively preventing reconsolidation to occur. Furthermore, reconsolidation is a time-dependent process (Nader and Hardt, 2009), yet we already observe an effect of beta-blockade over the multiple extinction trials, which might not be powerful enough to be detected by a single reactivation trial, adding to our consideration that an effect on reconsolidation is unlikely to explain our results.
Third, our results of day 3 contrast with studies that report impaired consolidation of the extinction memory trace due to beta-blockade resulting in increased fear one day later (Bos et al, 2012; Mueller et al, 2008; Ouyang and Thomas, 2005). The role of noradrenaline in strengthening learning and consolidation including that of extinction training is well-established (Cain et al, 2004; Davis et al, 1979; McGaugh, 2004). Further, central neural signals can function as intrinsic reinforcer and drive fear-related neural plasticity (Clugnet and LeDoux, 1990). We therefore conceive the possibility that phasic noradrenaline release associated with fear expression functions as an intrinsic US resulting in the association of conditioned fear with a novel context effectively supporting fear generalization. Thus, eliminating expression of differential conditioned responses during extinction may have prevented formation of an association between the new context and the retrieved fear memory. In support of this idea, an effect of beta-blockade on retrieval may result in a sustained reduction of emotional memory because of new learning at the time of retrieval (Kroes et al, 2010). In contrast, studies that have reported impaired consolidation of extinction due to beta-blockade have tested extinction memory in the same context as in which conditioning occurred (Bos et al, 2012; Mueller et al, 2008; Ouyang and Thomas, 2005), potentially explaining discrepancies.
Linking Beta-Blockade Effects on Brain and Behavior
Our results indicate that beta-blockade before extinction learning affected neural functioning in the dmPFC, midbrain, and hippocampus. First, the dmPFC is considered to be the human homologue of the rodent prelimbic cortex (Heidbreder and Groenewegen, 2003; Ongur et al, 2003), suggested to support retention and retrieval of fear (Burgos-Robles et al, 2009; Gilmartin and McEchron, 2005; Klavir et al, 2012; Schiller and Johansen, 2009), and be affected by beta-blockade resulting in reduced retrieval of fear (Burgos-Robles et al, 2009; Gilmartin and McEchron, 2005; Muravieva and Alberini, 2010; Otis et al, 2013; Rodriguez-Romaguera et al, 2009). Similarly, we found that propranolol reduced, but not permanently eliminated, responses to the CS+ in the dmPFC. Further, neural responses in this propranolol-sensitive dmPFC region were related to the reduction of the simultaneous expression of fear responses, congruent with previous reports (Klavir et al, 2012; Klumpers et al, 2012, 2015). The drop in blood pressure on day 2 induced by propranolol was associated with reduced dmPFC responses, but not SCR, which implies that beta-blockade effects on behavioral indices of fear are likely centrally mediated and not the result of nonspecific peripheral effects (Supplementary Figure S4 and Supplementary Discussion). Considering the occurrence of phasic noradrenaline responses to significant cues (Aston-Jones and Cohen, 2005) and the role of the dmPFC in retrieval of fear memories (Burgos-Robles et al, 2009; Gilmartin and McEchron, 2005; Schiller and Johansen, 2009), we suggest that our findings align with an interpretation where cue-evoked phasic rises in noradrenaline regulate retrieval of fear memories via the dmPFC. Second, we observed greater conditioned responses for the propranolol group in a midbrain region, but as we had no a prior hypothesis for a direct relation between responses in this region and behavioral measures, we will limit discussion of this region to the Supplementary Information. Third, beta-blockade induced greater differential hippocampal responses during the recall task on the next day. Previous studies have suggested that the hippocampus is critical for context conditioning and extinction (Bouton and King, 1983; Kalisch et al, 2006; Milad et al, 2007), thus we propose that reduced noradrenergic signaling may prevent fear generalization and result in the formation of new contextual safety memories. In support of this suggestion, we found the emergence of a differential hippocampal conditioned response in the propranolol group associated with both reduced measures of subsequent sympathetic recovery of fear and subsequent explicit memory of the fearful events. Hence, our findings suggest that the hippocampus provides a contextual safety signal resulting in reduced fear. Fourth, we found that a well-established neural signature of prediction errors in the ventral striatum (O'Doherty et al, 2003; Schiller et al, 2008), which has been found to be affected by dopamine manipulation (Pessiglione et al, 2006), persisted during beta-blockade. We found greater neural prediction error responses to be associated with greater learning as expressed in SCR, but not to be related to the loss of fear or return of fear even though prediction-error-related responses have been implied to initiate reconsolidation (Sevenster et al, 2013). Collectively, this pattern of results is most consistent with a model where beta-blockade can prevent the return of fear by reducing retrieval of fear memory via the dmPFC and by increasing contextual safety learning via the hippocampus.
Noradrenaline and Multiple Memory Systems
Recent human studies report differential effects of beta-blockers on explicit vs implicit measures of fear expression (Kindt et al, 2009; Bos et al, 2012; Sevenster et al, 2013), supporting the idea that memory of multiple systems (Henke, 2010) may be affected differently by beta-blockade (Muravieva and Alberini, 2010). Our results support these ideas, as we found that beta-blockade caused a loss of sympathetic fear responses, merely attenuate explicit memory, but left the subjective experience of fear unaffected. Moreover, while we detected a loss of fear memory representations in the dmPFC, several other brain areas retained these representations (Supplementary Figure S2 and Table S3). A critical question for future research pertains to the contribution of these regions to fear memory, and the possibility for expression of fear to recover (Phelps et al, 2001; Raio et al, 2012), or the persistence of a cognitive representation of fear that may be less sensitive to alteration (Kroes et al, 2014; Kroes and Fernández, 2012; Schiller and Phelps, 2011).
Future Directions and Conclusions
Beta-blockade might prevent output structures from driving fear responses (Cecchi et al, 2002; Schulz et al, 2002), enhance extinction (Eisenberg et al, 2003), or disrupt reconsolidation (Agren et al, 2012; Debiec and Ledoux, 2004; Nader et al, 2000; Schiller et al, 2013), but our results are not consistent with such alternative explanations (see Supplementary Discussion). Yet, a true exclusion of a contribution of these mechanisms to our results requires directed investigations beyond the scope of this study. In addition, if the loss of fear and absence of the return of fear in our study is, in fact, due to new contextual safety learning, then fear should renew in the original conditioning context (Bouton and King, 1983). Further, we found beta-blockade to affect sympathetic fear responses and explicit memory for fearful events, but how beta-blockade during extinction influences interactions between different types of memory remains an open question. Finally, recent computational latent cause models have tried to capture the difference between updating an old memory and the formation of a new memory (Gershman and Niv, 2012). The idea is that if prediction errors change slowly, new learning experiences are assigned as being generated from the same underlying latent cause and effectively update the predictions of an old memory. If prediction errors increase rapidly or if the context changes, new learning experiences are inferred to be generated from a different underlying latent cause and effectively form a new memory. In addition, recent experimental studies imply that high phasic noradrenergic responses increase the likelihood that a memory is updated and low phasic responses that a novel memory is formed (Eldar et al, 2013; Nassar et al, 2012). Based on this, we speculate that beta-blockade before extinction training in a different context could have ensured that participants formed a new latent cause and learned that a situation was different and safe, and when confronted with the same context the next day, they may have retrieved this novel contextual safety memory preventing fear. It would be worthwhile for future research to test the effect of beta-blockade on prediction errors in contextual safety learning and compare computational models that formalize this function (Gershman and Niv, 2012; Redish et al, 2007). Regardless of these limitations and questions for future investigations, the novelty of our findings is twofold. First, we demonstrate a novel method to reduce fear and prevent fear recovery by administering beta-blockers before extinction learning in humans. Second, by investigating neural mechanisms, we reveal a role for noradrenaline in fear retrieval and new contextual safety learning dependent on the dmPFC and hippocampus to be the most parsimonious explanation for our findings. Our results provide support to studies investigating the combined use of beta-blockers and exposure therapy aiming to improve treatment of anxiety disorders.
Funding and Disclosure
The authors declare no conflict of interest.
Acknowledgments
We thank S Kooijman for additional participant recruitment, screening, and clinical coordination, A Takashima and N ter Huurne for medical assistance, N Müller for contributions to reinforcement learning analyses, and SJ Fallon for providing stimulus material in the form of his living room. We acknowledge helpful discussion of this work and comments on previous versions of this manuscript from Christina Alberini, Nathaniel Daw, Joseph Dunsmoor, Erno Hermans, Floris Klumpers, Elizabeth Phelps, and Bryan Strange. Guillén Fernández is supported by European Research Council advanced investigator grant (ERC R0001075), Hanneke den Ouden is supported by a Veni grant of the Netherlands Organisation for Scientific Research (NWO) and a consultant for Eleusis Benefit Corp, but is not an employee. The funding agencies or corporation had no role in the design of the study, collection, and analysis of data and publication decision.
Author contributions
MCWK, GAvW, and GF designed the study. MCWK, KDT, and SV acquired data. MCWK and KDT analyzed data. MCWK and HdO conducted the reinforcement learning analyses. MCWK, HdO, and GF wrote the manuscript. All authors commented and agreed on the final manuscript.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JDE (2006). Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron 50: 507–517. [DOI] [PubMed] [Google Scholar]
- Agren T, Engman J, Frick A, Björkstrand J, Larsson E-M, Furmark T et al (2012). Disruption of reconsolidation erases a fear memory trace in the human amygdala. Science 337: 1550–1552. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD (2005). An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28: 403–450. [DOI] [PubMed] [Google Scholar]
- Bach DR, Weiskopf N, Dolan RJ (2011). A stable sparse fear memory trace in human amygdala. J Neurosci 31: 9383–9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A (1995). Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science 269: 1115. [DOI] [PubMed] [Google Scholar]
- Bos MGN, Beckers T, Kindt M (2012). The effects of noradrenergic blockade on extinction in humans. Biol Psychol 89: 598–605. [DOI] [PubMed] [Google Scholar]
- Bouton ME, King DA (1983). Contextual control of the extinction of conditioned fear: tests for the associative value of the context. J Exp Psychol Anim Behav Process 9: 248–265. [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ (2009). Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci 29: 8474–8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain CK, Blouin AM, Barad M (2004). Adrenergic transmission facilitates extinction of conditional fear in mice. Learn Mem 11: 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RM, MacInnes JJ, Huettel SA, Adcock RA (2009). Activation in the VTA and nucleus accumbens increases in anticipation of both gains and losses. Front Behav Neurosci 3: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Morilak DA (2002). Modulatory effects of norepinephrine, acting on alpha1 receptors in the central nucleus of the amygdala, on behavioral and neuroendocrine responses to acute immobilization stress. Neuropharmacology 43: 1139–1147. [DOI] [PubMed] [Google Scholar]
- Clugnet M, LeDoux J (1990). Synaptic plasticity in fear conditioning circuits: induction of LTP in the lateral nucleus of the amygdala by stimulation of the medial geniculate body. J Neurosci 10: 2818–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Redmond DE, Baraban JM (1979). Noradrenergic agonists and antagonists: effects on conditioned fear as measured by the potentiated startle paradigm. Psychopharmacology (Berl) 65: 111–118. [DOI] [PubMed] [Google Scholar]
- Dayan L, Finberg JPM (2003). L-DOPA increases noradrenaline turnover in central and peripheral nervous systems. Neuropharmacology 45: 524–533. [DOI] [PubMed] [Google Scholar]
- Debiec J, Ledoux JE (2004). Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience 129: 267–272. [DOI] [PubMed] [Google Scholar]
- Eisenberg M, Kobilo T, Berman DE, Dudai Y (2003). Stability of retrieved memory: inverse correlation with trace dominance. Science 301: 1102–1104. [DOI] [PubMed] [Google Scholar]
- Eldar E, Cohen JD, Niv Y (2013). The effects of neural gain on attention and learning. Nat Neurosci 16: 1146–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Yu PH (1995). Effect of haloperidol and its metabolites on dopamine and noradrenaline uptake in rat brain slices. Psychopharmacology (Berl) 121: 379–384. [DOI] [PubMed] [Google Scholar]
- Gershman SJ, Niv Y (2012). Exploring a latent cause theory of classical conditioning. Learn Behav 40: 255–268. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, McEchron MD (2005). Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behav Neurosci 119: 1496–1510. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ (2003). The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev 27: 555–579. [DOI] [PubMed] [Google Scholar]
- Henke K (2010). A model for memory systems based on processing modes rather than consciousness. Nat Rev Neursci 11: 523–532. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, van Marle HJF, Ossewaarde L, Henckens MJAG, Qin S, van Kesteren MTR et al (2011). Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science 334: 1151–1153. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ (2006). Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci 26: 9503–9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt M, Soeter M, Vervliet B (2009). Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci 12: 256–258. [DOI] [PubMed] [Google Scholar]
- Klavir O, Genud-Gabai R, Paz R (2012). Low-frequency stimulation depresses the primate anterior-cingulate-cortex and prevents spontaneous recovery of aversive memories. J Neurosci 32: 8589–8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpers F, Heitland I, Oosting RS, Kenemans JL, Baas JMP (2012). Genetic variation in serotonin transporter function affects human fear expression indexed by fear-potentiated startle. Biol Psychol 89: 277–282. [DOI] [PubMed] [Google Scholar]
- Klumpers F, Kroes MC, Heitland I, Everaerd D, Akkermans SEA, Oosting RS et al (2015). Dorsomedial prefrontal cortex mediates the impact of serotonin transporter linked polymorphic region genotype on anticipatory threat reactions. Biol Psychiatry. 78: 582–589. [DOI] [PubMed] [Google Scholar]
- Knight DC, Waters NS, Bandettini PA (2009). Neural substrates of explicit and implicit fear memory. Neuroimage 45: 208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroes MC, Tendolkar I, van Wingen GA, van Waarde JA, Strange BA, Fernandez G (2014). An electroconvulsive therapy procedure impairs reconsolidation of episodic memories in humans. Nat Neurosci 17: 204–206. [DOI] [PubMed] [Google Scholar]
- Kroes MCW, Fernández G (2012). Dynamic neural systems enable adaptive, flexible memories. Neurosci Biobehav Rev 36: 1646–1666. [DOI] [PubMed] [Google Scholar]
- Kroes MCW, Strange BA, Dolan RJ (2010). β-adrenergic blockade during memory retrieval in humans evokes a sustained reduction of declarative emotional memory enhancement. J Neurosci 30: 3959–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner A, Kalisch R, Vervliet B, Vansteenwegen D, Buchel C (2008). Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. J Neurosci 28: 9030–9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh J (2004). The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci 27: 1–28. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL (2007). Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry 62: 446–454. [DOI] [PubMed] [Google Scholar]
- Misanin JR, Miller RR, Lewis DJ (1968). Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science 160: 554–555. [DOI] [PubMed] [Google Scholar]
- Mueller D, Porter JT, Quirk GJ (2008). Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci 28: 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muravieva EV, Alberini CM (2010). Limited efficacy of propranolol on the reconsolidation of fear memories. Learn Mem 17: 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M (2007). Mechanisms of fear extinction. Mol Psychiatry 12: 120–150. [DOI] [PubMed] [Google Scholar]
- Nader K, Hardt O (2009). A single standard for memory: the case for reconsolidation. Nat Rev Neurosci 10: 224–234. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE (2000). Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406: 722–726. [DOI] [PubMed] [Google Scholar]
- Nassar MR, Rumsey KM, Wilson RC, Parikh K, Heasly B, Gold JI (2012). Rational regulation of learning dynamics by pupil-linked arousal systems. Nat Neurosci 15: 1040–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty JP, Dayan P, Friston KJ, Critchley HD, Dolan RJ (2003). Temporal difference models and reward-related learning in the human brain. Neuron 38: 329–337. [DOI] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL (2003). Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol 460: 425–449. [DOI] [PubMed] [Google Scholar]
- Otis JM, Dashew KB, Mueller D (2013). Neurobiological dissociation of retrieval and reconsolidation of cocaine-associated memory. J Neurosci 33: 1271–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis JM, Fitzgerald MK, Mueller D (2014). Inhibition of hippocampal [beta]-adrenergic receptors impairs retrieval but not reconsolidation of cocaine-associated memory and prevents subsequent reinstatement. Neuropsychopharmacology 39: 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis JM, Mueller D (2011). Inhibition of β-adrenergic receptors induces a persistent deficit in retrieval of a cocaine-associated memory providing protection against reinstatement. Neuropsychopharmacology 36: 1912–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang M, Thomas SA (2005). A requirement for memory retrieval during and after long-term extinction learning. Proc Natl Acad Sci USA 102: 9347–9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD (2006). Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature 442: 1042–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE (2004). Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43: 897–905. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O'Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M (2001). Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci 4: 437–441. [DOI] [PubMed] [Google Scholar]
- Przybyslawski J, Sara SJ (1997). Reconsolidation of memory after its reactivation. Behav Brain Res 84: 241–246. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D (2008). Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33: 56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raio CM, Carmel D, Carrasco M, Phelps EA (2012). Nonconscious fear is quickly acquired but swiftly forgotten. Curr Biol 22: R477–R479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD, Jensen S, Johnson A, Kurth-Nelson Z (2007). Reconciling reinforcement learning models with behavioral extinction and renewal: Implications for addiction, relapse, and problem gambling. Psychol Rev 114: 784–805. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR (1972) A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF (eds). Classical Conditioning II. Appleton-Century-Crofts: New York, NY, pp 64–99. [Google Scholar]
- Rodriguez-Romaguera J, Sotres-Bayon F, Mueller D, Quirk GJ (2009). Systemic propranolol acts centrally to reduce conditioned fear in rats without impairing extinction. Biol Psychiatry 65: 887–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ (2000). Retrieval and reconsolidation: toward a neurobiology of remembering. Learn Mem 7: 73–84. [DOI] [PubMed] [Google Scholar]
- Schiller D, Johansen J (2009). Prelimbic prefrontal neurons drive fear expression: a clue for extinction–reconsolidation interactions. J Neurosci 29: 13432–13434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Kanen JW, LeDoux JE, Monfils M-H, Phelps EA (2013). Extinction during reconsolidation of threat memory diminishes prefrontal cortex involvement. Proc Natl Acad Sci USA 110: 20040–20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA (2008). From fear to safety and back: reversal of fear in the human brain. J Neurosci 28: 11517–11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Phelps EA (2011). Does reconsolidation occur in humans? Front Behav Neurosci 5: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz B, Fendt M, Schnitzler H-U (2002). Clonidine injections into the lateral nucleus of the amygdala block acquisition and expression of fear-potentiated startle. Eur J Neurosci 15: 151–157. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Nader K, Pruessner JC (2013). Beta-adrenergic blockade during reactivation reduces the subjective feeling of remembering associated with emotional episodic memories. Biol Psychol 92: 227–232. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Nader K, Wolf OT, Beaudry T, Pruessner JC (2012). Neural signature of reconsolidation impairments by propranolol in humans. Biol Psychiatry 71: 380–386. [DOI] [PubMed] [Google Scholar]
- Sevenster D, Beckers T, Kindt M (2013). Prediction error governs pharmacologically induced amnesia for learned fear. Science 339: 830–833. [DOI] [PubMed] [Google Scholar]
- Smith CC, Greene RW (2012). CNS dopamine transmission mediated by noradrenergic innervation. J Neurosci 32: 6072–6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervliet B, Craske MG, Hermans D (2013). Fear extinction and relapse: state of the art. Annu Rev Clin Psychol 9: 215–248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.